Background: The FoxO1 transcription factor, which controls the hepatic metabolism, is regulated through Akt-mediated phosphorylation.
Results: We show that stimulation of hepatocytes with the pro-inflammatory cytokine IL-1β leads to increased FoxO1 nuclear content through an Akt-independent mechanism involving neutral sphingomyelinase-2 and ceramide.
Conclusion: IL-1β is a regulator of FoxO1.
Significance: IL-1β may adversely affect hepatic metabolism by modulating FoxO1 regulation.
Keywords: Ceramide, FoxO, Insulin, Insulin Resistance, Interleukin, Jun N-terminal Kinase (JNK), Liver, Sphingomyelinase, Akt
Abstract
FoxO1 transcription factor controls the glucose and lipid metabolism, as well as cell proliferation and stress response. Akt, activated by insulin and other growth factors, phosphorylates FoxO1 causing its nuclear export and activity suppression. In this manuscript, we show that IL-1β, a pro-inflammatory cytokine, has the opposite effects on FoxO1. IL-1β stimulation of primary rat hepatocytes and HEK293 cells overexpressing the IL-1β receptor (293-IL-1RI) results in increased nuclear and cytosolic FoxO1 protein but not mRNA levels. IL-1β stimulation also elevates the levels of a mutant FoxO1 that is resistant to Akt phosphorylation. This suggests that an Akt-independent mechanism is involved. Co-stimulation with insulin does not affect the IL-1β induction of FoxO1. The IL-1β effects on FoxO1 are counteracted, however, by the silencing or inhibition of neutral sphingomyelinase 2 (nSMase-2) using shRNAi, scyphostatin, or GW4869, as well as by the pharmacological inhibition of JNK and ERK. Reversely, the overexpression of nSMase-2 through adenovirus-mediated gene transfer potentiates, in a JNK- and ERK-dependent manner, the IL-1β effects. We also show that transcription of insulin-like growth factor-binding protein-1 mRNA, which requires active FoxO1, is stimulated by IL-1β and is suppressed by the inhibition of nSMase-2 and JNK. In conclusion, we propose that IL-1β regulates FoxO1 activity through a novel nSMase-2-dependent pathway.
Introduction
IL-1β coordinates the systemic and local responses of the body to infection, injury, or antigen challenge. The liver is one of the main organs affected by these factors. The “acute phase response” is the most prominent hepatic reaction to IL-1β stimulation. However, this cytokine also influences numerous hepatic housekeeping functions, including the control of glucose, lipid, and protein metabolism. IL-1β has consequently been implicated in the deregulation of glucose metabolism in type II diabetes (1, 2) and in the development of hypercholesterolemia in atherosclerosis (3).
IL-1β regulation of hepatic function is achieved through the activation of the Toll-like/IL-β receptor signaling pathway. The binding of IL-1β to the IL-1β receptor forms an active receptor complex involving the IL-1 receptor accessory protein and several adaptor proteins (4, 5). Furthermore, a signaling complex consisting of IL-1R-associated kinase-1 (IRAK-1),3 IRAK-4, and TNF-associated factor-6 is formed, ultimately leading to activation of IκB kinase and the MAP kinase pathway (6). The mechanism of MAP kinase activation by IL-1β also involves activation of neutral sphingomyelinase 2 (nSMase-2) and the generation of ceramide at the plasma membrane (7, 8). The latter is required for the proper activation of the JNK arm of the IL-1β signaling pathway because it regulates the phosphorylation and ubiquitination of IRAK-1 (9). The IL-1β signaling cascade ends with the stimulation of NF-κB and activator protein-1 transcription factors that launch the hepatic acute phase response.
On the other hand, the FoxO1 transcription factor regulates the transcription of genes involved in various hepatic housekeeping functions such as gluconeogenesis and protein and lipid synthesis (10–12). The members of the FoxO family have been the subject of extensive studies because of their key roles in the stress response, type II diabetes, and cancer (Refs. 13 and 14 and the references therein). FoxO1 activity is regulated through reversible changes in subcellular localizations. In its active state, FoxO1 is localized in the nucleus, and it regulates gene transcription through its interactions with co-stimulators or with co-repressors. Newly synthesized FoxO1 is targeted directly to the nucleus because of a nuclear localization signal. Activation of Akt by insulin or by other growth factors leads to the phosphorylation of FoxO1 at the Thr24, Ser256, and Ser319 sites and leads to FoxO1 nuclear export. The related serum- and glucocorticoid-inducible kinase can phosphorylate FoxO1 at the same sites with similar results. The phosphorylated FoxO1 is exported to the cytosol by interacting with adapter 14-3-3 proteins, and it can undergo ubiquitination and proteosomal degradation, depending on the phosphorylation status at the FoxO1 Ser256 site and its binding to the E3 ubiquitin ligase complex. Phosphatases such as PP2A can act on phosphorylated FoxO1 and/or its binding partners and add more complexity to the mechanism behind its regulation.
Akt-dependent phosphorylation is a major factor that regulates FoxO1 nuclear export; however, antagonistic pathways that promote FoxO1 nuclear retention also exist. One study reports that oxidative stress is associated with increased nuclear retention of FoxO1, which requires active JNK (15). In that particular study, the effects of JNK were considered indirect and were attributed to the down-regulation of Akt (15). Other studies, however, seem to suggest that JNK may directly regulate FoxO1 activity. FoxO1 phosphorylation and nuclear retention is caused by a variety of stress stimuli that activate JNK and include oxidative stress, heat shock, and UV radiation (14). Recombinant JNK can phosphorylate FoxO1 in vitro. However, a JNK phosphorylation site has not been identified in the FoxO1 molecule. JNK-mediated signaling has nevertheless emerged as an alternative pathway for FoxO1 regulation and apparently counteracts insulin- and growth factor-mediated pathways. It is unknown whether physiological inducers of JNK such as pro-inflammatory cytokines can similarly regulate FoxO1 nuclear export and/or retention.
The experiments we performed show that IL-1β elevates FoxO1 protein content and induces the nuclear retention of FoxO1 in hepatocytes. We also found that the underlying mechanism is Akt-independent and involves JNK, nSMase-2, and ceramide. Our study suggests that IL-1β effectively interferes with the normal regulation of FoxO1 in healthy, insulin-responsive cells. Considering the role of FoxO1 in regulating hepatic lipid protein and glucose metabolism, our study indicates the existence of novel pathways by which IL-1β (and inflammation in general) can influence the basic metabolic functions of an organism.
EXPERIMENTAL PROCEDURES
Materials
Male Fisher 344 rats (150–200 g) were purchased from Harlan, Inc. (Indianapolis, IN). The animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. HEK293 cells overexpressing the IL-1β receptor type I (293-IL-1RI) were a gift from Dr. X. Li (Cleveland Clinic, Cleveland, OH). Rat and human recombinant IL-1β were from Invitrogen. Tet system-approved FBS was from Clontech, and growth factor-reduced Matrigel® was from BD Bioscience Discovery Labware (Bedford, MA). Scyphostatin was kindly provided by Sankyo (Tokyo, Japan). SP600125 and PD 980059 were from Sigma. GW4869 was from EMD Biosciences (Billerica, MA). Anti-FoxO1 antibodies were from Cell Signaling (Danvers, MA), anti-GFP antibodies were from Invitrogen, whereas anti-β-actin, and all secondary antibodies were from Sigma. IGFBP-1 antibody was from Upstate (Lake Placid, NY). N-Acetyl sphingosine was from Avanti Polar Lipids (Alabaster, AL).
Adenoviral and Plasmid Constructs
GFP-tagged human FoxO1 (FoxO1 WT), GFP-tagged mutant FoxO1 caring three mutations of Thr24, Ser256, and Ser319 to Ala (FoxO1 (AAA)) (plasmid 9023) (17), and the corresponding empty vector expressing only GFP were purchased from AddGene (Cambridge, MA). Adenovirus expressing FLAG-tagged mouse nSMase-2 (Ad-nSMase-2) was purified as previously described (8). DNA oligonucleotides encoding a sense loop antisense sequence (GCCCTCATCTTCCCATGTTACTTCAAGAGAGTACATGGGAAGATGAGGGC) against the rat nSMase-2 and a scrambled sense loop antisense RNA sequence (scr) were cloned into pENTR/U6 entry vector (Invitrogen) creating an RNAi cassette that was subcloned into pAdTrack vector that also encodes GFP (gift from Dr. George Smith, University of Kentucky). Homologous recombination between the pAdTrack and the adenoviral backbone plasmid (pAdEasy-1) was done in Escherichia coli strain BJ5183 to produce adenovirus expressing the shRNA against nSMase-2 (Ad-sh-nSMase-2) or the corresponding scrambled sequence (Ad-scr). Plasmid 12146 (IGFBP1 promoter/pGL3) containing the canonical insulin response sequence and driving a luciferase reporter was obtained from Addgene (Cambridge, MA), whereas pRL-TK plasmid expressing the Renilla reporter was gift from Dr. Karyn Esser (University of Kentucky).
Cell Cultures and Treatments
Hepatocytes were isolated from ether-anesthetized male Fisher 344 rats and cultured in Matrigel-coated dishes as described previously (7, 19). 293-IL-1RI cells were maintained in DMEM supplemented with 10% FBS (standard conditions). In some instances indicated in the text, the cells were kept in DMEM without serum for 2 h before treatments, as well as during treatments. Infections with Ad-nSMase-2, Ad-sh-nSMase-2, or Ad-scr were performed 48 h after hepatocyte isolation, at a multiplicity of infection between 2 and 5. When necessary, the expression of the transgene was induced by the addition of doxycycline at the day of infection and again 48 h later (for additional details see Refs. 7 and 20). Transfections of 293-IL-1RI cells with FoxO1 plasmids were done using Lipofectamine Plus reagent when cells reached ∼75–90% confluency. Hepatocytes were treated with IL-1β 72 h after infection, whereas 293-IL-1RI at 24 h post-transfection. Inhibitors (or appropriate vehicles) were added 30 min before the treatment with IL-1β at the indicated concentrations.
Luciferase Assay
HepG2 cells were transfected with IGFBP1 promoter/pGL3 containing the canonical insulin response sequence and driving a firefly luciferase reporter, as well as with pRL-TK Renilla luciferase reporter vector (Promega) as an internal control. After 48–72 h, the cells were harvested, and firefly and Renilla luciferase activities were measured using the dual luciferase reporter assay system (Promega). The results were presented as ratios of firefly and Renilla luciferase activity.
Preparation of Cell Extracts
To harvest cultured primary hepatocytes, the medium was first aspirated, and the Matrigel was reliquidified by incubating with PBS containing 5 mm EDTA for 30 min at 4 °C. 293-IL-1RI cells were harvested in cold PBS using cell scrapers. The collected cells were pelleted by centrifugation at 500 × g for 4 min, rinsed, and incubated with 50–200 μl of lysis buffer (1 mm EDTA, 0.5% Triton X-100, 1 mm Na2VO4, 1 mm NaF, 1:200 (v/v) protease inhibitor mixture, 10 mm Tris-HCl, pH 7.4) on ice for 30 min. Cell lysates were centrifuged at 16,000 × g for 10 min at 4 °C. The clear supernatant was used for SDS-PAGE and Western blot analyses. IGFBP-1 protein levels were assessed in the cell medium. Equal volumes of medium (1 ml) were aspired from the culture dishes containing equal cell numbers, and 20 μl/line were subsequently used for Western blotting.
SDS-PAGE and Western Blotting
The proteins were resolved by 10% SDS-PAGE and transferred to Immobilon-P polyvinylidene fluoride membrane by semidry blotting. Specified proteins were detected using the antibodies described under “Materials.” Protein-antibody interactions were visualized using the ECF kit (Amersham Biosciences) and Storm 860 PhosphorImager (Molecular Dynamics) and analyzed using ImageQuant5.0 software (Molecular Dynamics).
Fluorescent Imaging
293-IL-1RI cells were cultured on coverslips to 90% confluency and treated as indicated. The cells were mounted on slides in glycerol buffer containing Hoechst 33342 or DAPI for nuclear staining. GFP fluorescence was examined on a Leica LCS confocal laser scanning microscope.
nSMase Activity Assay
Cells from each dish were harvested, resuspended in lysis buffer without Triton X-100, lysed with three consecutive freeze-thaw cycles, and homogenized by sonication for 5 min. nSMase activity was determined as described previously using 6-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino-sphingomyelin as a substrate (19, 21).
Statistical Analysis
After proving the assumption of equal variance across groups, statistical significance was determined by Student's t test. Each experiment was reproduced at least three times. The data are reported as means ± S.D. A p value of less then 0.05 was considered significant.
RESULTS
IL-1β Stimulation Increases the Nuclear and Total Cellular Content of FoxO1
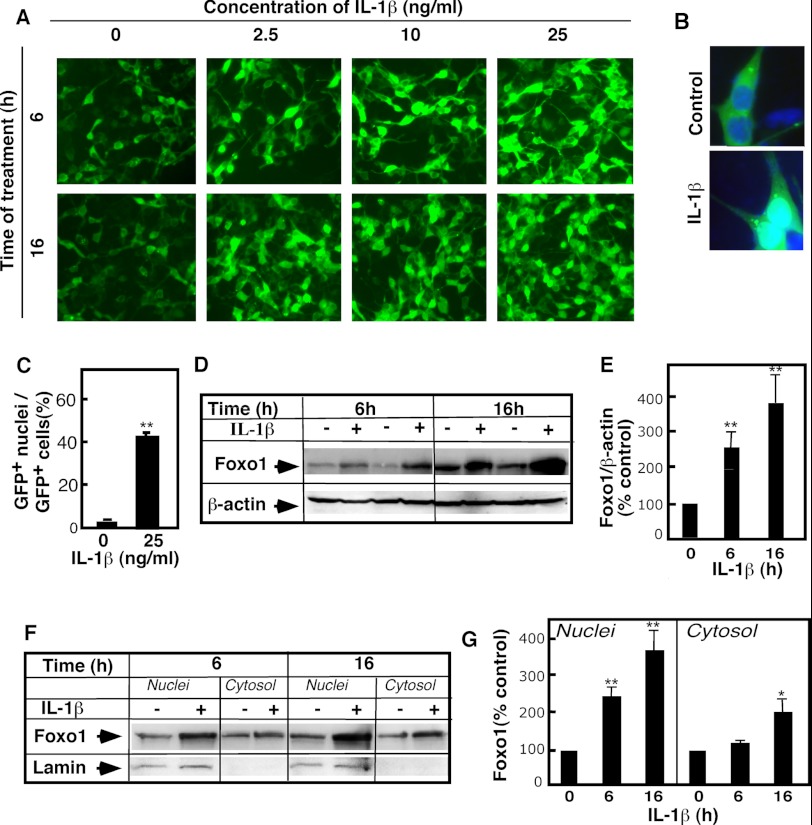
The effects of IL-1β on FoxO1 were first studied in 293-IL-1RI cells that transiently overexpressed GFP-tagged human FoxO1. IL-1β-stimulated cells exhibited significantly higher levels of GFP fluorescence, compared with control cells. The increased fluorescence was seemingly correlated with IL-1β stimulation in a time- and dose-dependent manner (Fig. 1A). In the control cells, co-staining with DAPI showed that most of the GFP fluorescence was cytoplasmic. This was anticipated because the cells were grown in the presence of serum. In IL-1β-treated cells, however, GFP fluorescence was ubiquitously localized throughout the cells with enhanced fluorescence in the nuclei (Fig. 1, B and C). This was paralleled by an overall increase in the cellular levels of FoxO1, based on Western blot analyses (Fig. 1, D and E). These effects were present as early as 2 h (data not shown) and 6 h after IL-1β stimulation and were even greater after 16 h. To test directly whether the increases in FoxO1 levels were the results of increased nuclear FoxO1 content, we performed subcellular fractionation (Fig. 1F). Western blot analysis of the nuclear and cytosolic fractions indeed revealed that the levels of FoxO1 in the nuclei increased 3–4-fold (Fig. 1G). Some elevation in cytosolic FoxO1 was also noticeable, but only after 16 h of IL-1β stimulation.
FIGURE 1.
IL-1β treatment leads to increased FoxO1 levels. GFP-FoxO1-transfected 293-IL-1RI cells were cultured for 24 h under standard conditions. The cells were then treated with IL-1β at the indicated doses and times. A, GFP-FoxO1 was monitored by direct live fluorescent microscopy. B, nuclear localization of GFP-FoxO1 was assessed using DAPI nuclear staining of fixed cells. The green (GFP) and blue (DAPI) fluorescence was monitored by confocal microscopy. C, quantification of the changes shown in B. The data were calculated by dividing the number of cells with GFP-positive nuclei to the number of GFP-positive cells. The results are the averages ± S.D. (n = 3) and are representative of multiple independent experiments. D, GFP-FoxO1 level was determined in whole cell lysates by Western blotting using anti-GFP antibody. The abundance of β-actin was used to control for equal loading. E, quantification of the changes shown in D. The results are expressed as percentages of the FoxO1 levels (normalized for β-actin content) in untreated cells. The data are representative of at least five independent experiments. F, levels of GFP-FoxO1 were monitored in purified nuclear and cytosolic fractions of 293-IL-1RI cells by Western blotting using anti-GFP antibody. 20 μg of each subcellular fraction were loaded per line. Lamin A/C served as control for the purity of nuclear fraction. G, quantification of the changes shown in F. The results are expressed as percentages of the FoxO1 levels in untreated cells. The values are the means ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
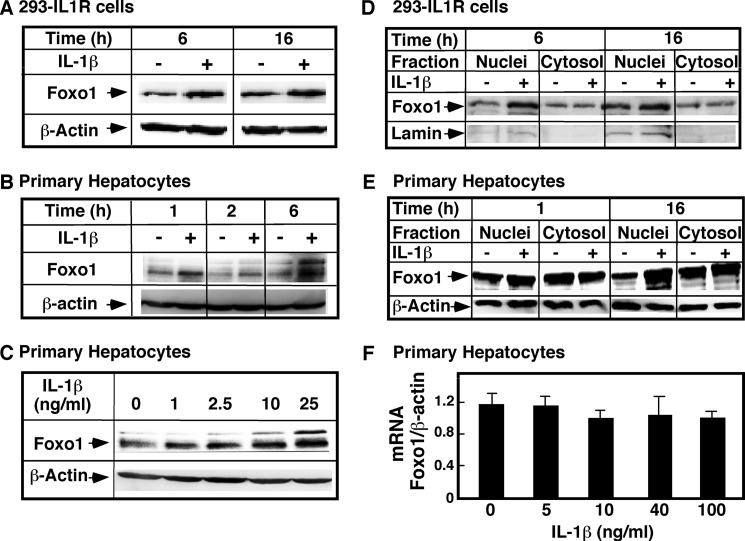
To ascertain that these effects were not limited to the heterologous expression system and that they may be physiologically relevant, the levels of endogenous FoxO1 were measured in 293-IL-1RI cells (Fig. 2, A and D) and in primary rat hepatocytes (Fig. 2, B and C). IL-1β stimulation of 293-IL-1RI cells led to a significant increase in endogenous FoxO1, which was similar to the increase occurring with the overexpressed protein. In hepatic cells, the endogenous FoxO1 appeared as a doublet on the Western blot test, and the IL-1β stimulation increased the intensity of both bands. Separation of cellular homogenates into nuclear and cytosolic fractions confirmed that the nuclear FoxO1 was significantly elevated in response to IL-1β stimulation (Fig. 2, D and E). The levels of endogenous FoxO1 mRNA in the control cells and in the IL-1β-treated cells were similar, based on real time PCR assay (Fig. 2F). This precluded the possibility that the higher FoxO1 levels resulted from increases in the transcription and/or stabilization of mRNA. These results altogether indicate that IL-1β stimulation in various cell types increases the nuclear and total cellular FoxO1 content, which is likely the result of changes in the post-transcriptional processing of the protein.
FIGURE 2.
Effects of IL-1β on FoxO1 mRNA and proteins levels in 293-IL-1RI cells and primary hepatocytes. The levels of endogenous FoxO1 were assessed by Western blotting using an antibody specific for FoxO1 or by RT-PCR. A, 293-IL-1RI cells were treated with human IL-1β (25 ng/ml) in DMEM without serum for the indicated times. B, primary rat hepatocytes were cultured for 5 days on Matrigel® and treated with rat IL-1β (25 ng/ml) for the indicated times. C, primary rat hepatocytes were cultured for 5 days and treated with rat IL-1β at the indicated concentrations for 6 h. D, 293-IL-1RI cells were treated with human IL-1β (25 ng/ml) in DMEM without serum for the indicated times. Nuclear and cytosolic subfractions were purified from total cell homogenates. E, primary rat hepatocytes were cultured for 5 days on Matrigel® and treated with rat IL-1β (25 ng/ml) for the indicated times. The cells were harvested, and nucleus and cytosolic fractions were prepared. F, primary hepatocytes were treated with IL-1β at the indicated doses for 6 h. The cells were harvested, and mRNA was isolated. The abundance of FoxO1 mRNA was measured by real time PCR and normalized for the levels of β-actin. The data are the averages ± S.D. (n = 3). The data are representatives from at least three independent experiments.
IL-1β Effects on FoxO1 Are Independent of Akt-1
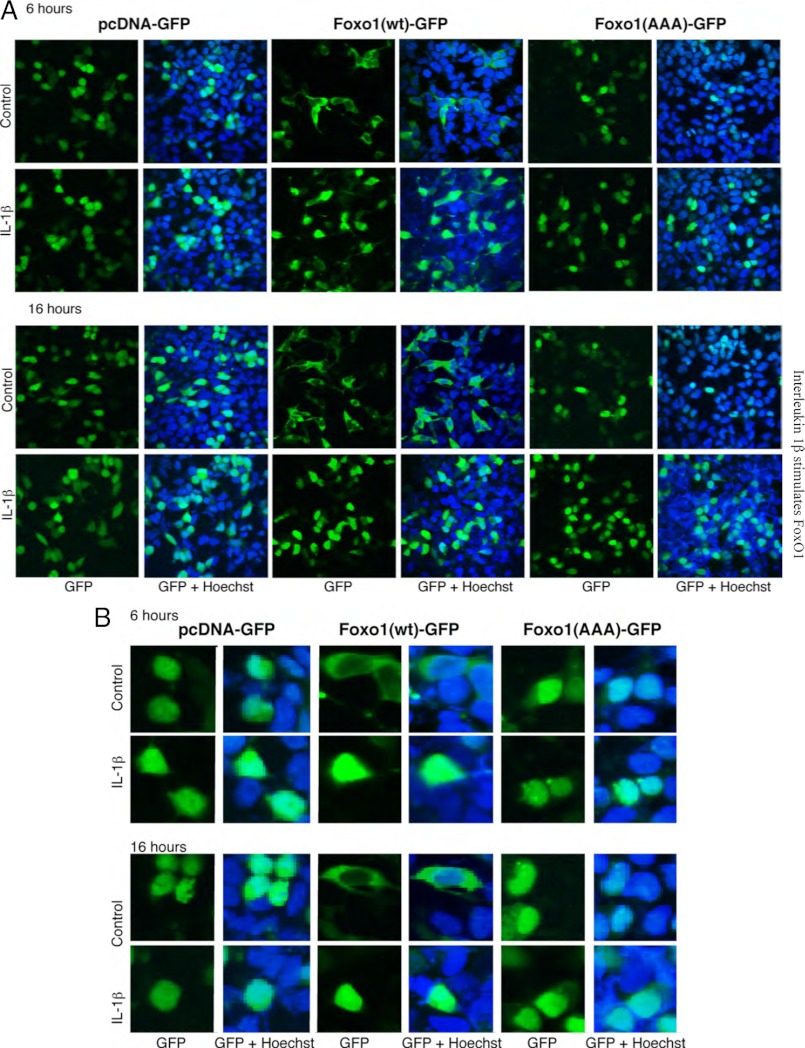
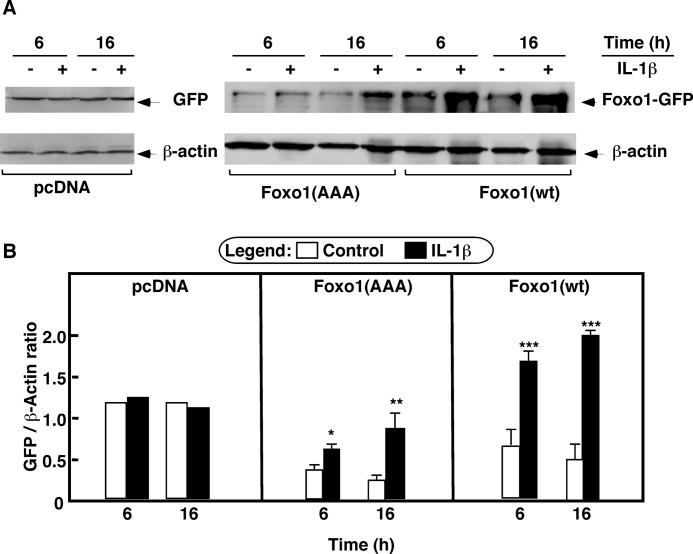
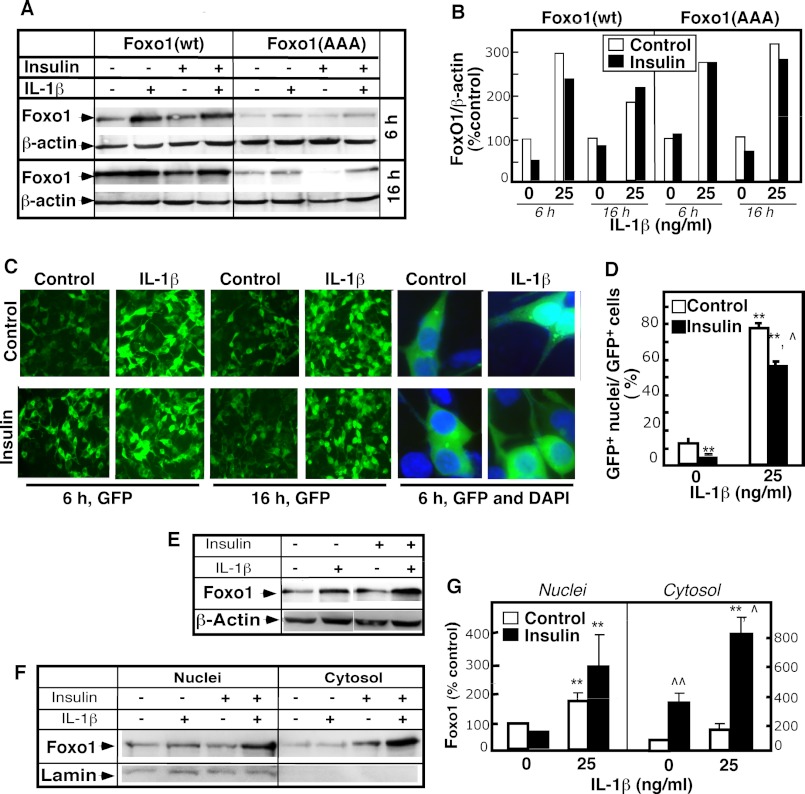
Newly synthesized FoxO1 protein is targeted to the nucleus. However, in the presence of serum and/or growth factors, FoxO1 is localized in the cytosol because Akt-mediated phosphorylation at the Thr24, Ser256, and Ser319 sites causes nuclear export. Depending on other protein interactions in the cytosol, the phosphorylated FoxO1 is ubiquitinated and degraded. Some inflammatory agents are shown to inhibit Akt phosphorylation. The suppression of Akt-dependent FoxO1 nuclear export and degradation may therefore explain the increased FoxO1 content in IL-1β-treated cells. To test this hypothesis, a GFP-tagged mutant construct, FoxO1 (AAA), was used. In this construct all three Akt-1 phosphorylation sites are replaced with Ala. This construct is resistant to Akt-dependent regulation (17). The 293-IL1-RI cells were transiently transfected with FoxO1 (AAA), with FoxO1 WT, or with a control pcDNA3 vector that expresses only GFP. Confocal microscopy (Fig. 3) and Western blotting (Fig. 4) were used to monitor GFP fluorescence and the levels of overexpressed proteins. As we anticipated, in the presence of serum, FoxO1 WT was localized in the cytosol, whereas FoxO1 (AAA) was retained in the nucleus. Stimulation with IL-1β seemed to intensify fluorescence in the nuclei in the FoxO1 WT-transfected cells and in the FoxO1 (AAA)-transfected cells (Fig. 3, A and B). Western blot analyses confirmed that the increased nuclear fluorescence resulted from elevated levels of total FoxO1 wild-type and mutant proteins (Fig. 4A). Statistically significant increases were present after 6 h of treatment and were even more pronounced after 16 h of treatment (Fig. 4B). By contrast, there were no differences in the protein levels in the GFP-only control cells (Fig. 4B). This ruled out the possibility that IL-1β affected transfection efficiency and/or transgene expression. IL-1β apparently regulates FoxO1 levels through Akt-independent pathways.
FIGURE 3.
IL-1β effects on FoxO1 are Akt-independent: cell localization assays. 293-IL-1RI cells were transfected with plasmids expressing either GFP alone (pcDNA), human GFP-tagged FoxO1 (Foxo1(wt)), or mutant GFP-tagged FoxO1 in which the three Akt phosphorylation sites (Thr24, Ser256, and Ser319) of FoxO1 were replaced with alanines (Foxo1(AAA)). The cells were cultured for 24 h under standard conditions and then treated with IL-1β (25 ng/ml) for 6 or 16 h. Fluorescence from the overexpressed GFP-tagged proteins (green) and from the Hoechst nuclear stain (blue) was monitored by confocal microscopy. The results are representative of at least four independent experiments. A, pictures captured on a Leica LCS confocal laser-scanning microscope. B, 5-fold magnified excerpts from the pictures shown in A.
FIGURE 4.
IL-1β effects on FoxO1 are Akt-independent: quantitative assays. 293-IL-1RI cells were transfected with plasmids expressing either GFP alone (pcDNA), human GFP-tagged FoxO1 (Foxo1(wt)), or mutant GFP-tagged FoxO1 in which the three Akt phosphorylation sites (Thr24, Ser256, and Ser319) of FoxO1 were replaced with alanines (Foxo1(AAA)). The cells were cultured for 24 h under standard conditions and then treated with IL-1β (25 ng/ml) for 6 or 16 h. A, the levels of the overexpressed GFP, FoxO1 (WT), and FoxO1 (AAA) were determined by Western blotting using anti-GFP antibody. The abundance of β-actin was used to control for equal loading and for normalization. B, quantification of the results from A. The data are the averages ± S.D. (n = 3). The results are representative of at least three independent experiments.
Cross-talk between Insulin and IL-1β in FoxO1 Regulation
The influence of insulin and/or serum on the effects of IL-1β on FoxO1 was tested to elucidate the physiological role of IL-1β in FoxO1 regulation. Experiments were first performed in the presence of serum (Fig. 5, A–D). IL-1β stimulation elevated the total FoxO1 level. The magnitude of the increase was not affected by adding insulin (Fig. 5, A and B). Insulin co-stimulation, however, seems to have a slight and statistically significant effect on the subcellular distribution of FoxO1 (Fig. 5, C and D). Direct immunofluorescence observation of the control cells showed that FoxO1 was localized mostly in the cytosol and that ∼10% of the cells exhibited nuclear FoxO1. Less than 1% of the insulin-treated cells exhibited nuclear FoxO1, confirming that insulin stimulation induced the nuclear export of Fox O1. By contrast, 80% of the IL-1β-stimulated cells had GFP-positive nuclei. A small but statistically significant decline in this number occurred with insulin. This suggests that the effects of IL-1β and insulin on FoxO1 are additive, albeit independent, with respect to the distribution of FoxO1 between the nucleus and cytosol.
FIGURE 5.
Effect of insulin on IL-1β regulation of FoxO1. 293-IL-1RI cells were transfected with plasmids expressing either the human GFP-tagged FoxO1 (Foxo1(wt)) or mutant GFP-tagged FoxO1 in which the three Akt phosphorylation sites (Thr24, Ser256, and Ser319) of FoxO1 were replaced with alanines (Foxo1(AAA)). The cells were cultured for 24 h in DMEM containing 10% serum. Then the medium was exchanged to DMEM containing 10% serum (A–D) or to DMEM containing no serum (E–G). The cells were cultured for an additional 1 h and then treated with IL-1β (25 ng/ml) and/or insulin (100 ng/ml) either for the indicated times (A–D) or for 6 h (E–G). A, the levels of the overexpressed FoxO1 (WT) and FoxO1 (AAA) were assessed by Western blotting using anti-GFP antibody. The abundance of β-actin was used to control for equal loading and for normalization. B, quantification of the results from A. The intensity of FoxO1 was normalized for the amount of β-actin. The results are presented as percentages of the normalized FoxO1 level in the untreated cells. C, fluorescence from the overexpressed GFP-tagged proteins (green) and from the DAPI nuclear stain (blue) was monitored by confocal microscopy. Pictures were captured on a Leica LCS confocal laser-scanning microscope. The last four panels on the right represent 10-fold magnified excerpts of DAPI/GFP overlay. D, quantification of the changes shown in C. The data were calculated by dividing the number of cells with GFP-positive nuclei to the number of GFP-positive cells. The results are the averages ± S.D. (n = 3) and are representative of multiple independent experiments. E, the levels of the overexpressed FoxO1 (WT) were assessed in whole cell lysates by Western blotting using anti-GFP antibody. F, the levels of GFP-FoxO1 (WT) were monitored in purified nuclear and cytosolic fractions of 293-IL-1RI cells by Western blotting using anti-GFP antibody. 20 μg of each subcellular fraction were loaded per line. Lamin A/C served as control for the purity of nuclear fraction. G, quantification of the changes shown in F. The results are expressed as percentages of the FoxO1 levels in untreated cells. The values are the means ± S.D. (n = 3). * or ^, p < 0.05; ** or ^^, p < 0.01. Asterisks represent the significance of IL-1β effects; carets represent the significance of insulin effect.
In a second cohort of experiments, the cells were serum-starved before stimulation with IL-1β or insulin (Fig. 5, E–G). Similar results were observed with these cells. IL-1β induced a potent increase in the total FoxO1 protein content. However, the magnitude of the induction surprisingly was somewhat larger in the presence of insulin (Fig. 5E). To address this further, FoxO1 content was measured in purified nuclear and cytosolic cell fractions. In the absence of serum, FoxO1 was detected mostly in the nuclear fraction, as anticipated (Fig. 5, F and G). IL-1β treatment increased the nuclear FoxO1 content and had no effect on FoxO1 cytosolic levels. Adding insulin alone elevated the cytosolic FoxO1 content, which is consistent with the expected export of FoxO1 to the cytosol. The FoxO1 cytosolic levels interestingly increased even further in cells stimulated with the combination of insulin and IL-1β (Fig. 5, F and G). Insulin seems to potentiate IL-1β-induced increases in FoxO1, but only in the absence of serum. A trivial explanation for the serum-dependent effect of insulin is that insulin signaling in the presence of serum is weak because Akt is already active. An alternate explanation is that growth factors present in the serum may induce the ubiquitination and degradation of cytosolic FoxO1, thus lessening insulin-dependent increases. These results altogether show that 1) the stimulation of FoxO1 in response to IL-1β is not affected by the presence of insulin or serum and 2) insulin may affect, to some extent, the distribution of FoxO1 in IL-1β-stimulated cells.
MAP Kinase Pathway Mediates the Effects of IL-1β on FoxO1
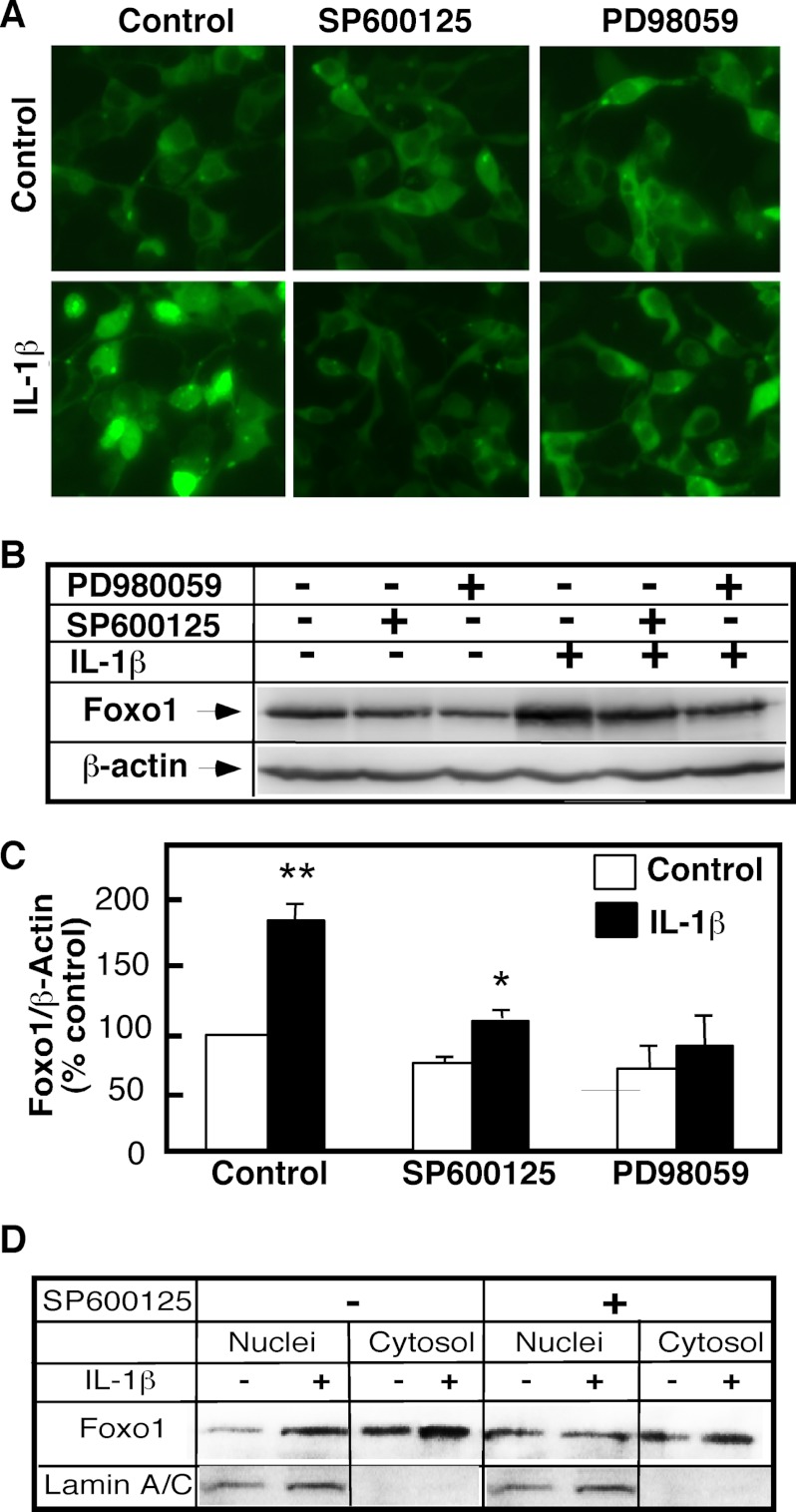
Activation of the MAP kinases—specifically ERK1/2 and JNK—mediates many cellular effects of IL-1β. The JNK1/2 inhibitor SP600125 and the ERK1/2 inhibitor PD98069 were used to ascertain whether the increases in the nuclear FoxO1 levels occurring after IL-1β stimulation are MAP kinase-dependent. The FoxO1 status in FoxO1-overexpressing 293-IL1-RI cells was examined by direct fluorescence observation (Fig. 6A), and the FoxO1 status of total cell homogenates (Fig. 6, B and C) and purified nuclear and cytosolic fractions (Fig. 6D) was examined by Western blot analysis. In comparison with cells treated with IL-1β alone, the 293-IL1-RI cells treated with the inhibitors exhibited a lower FoxO1 nuclear level (Fig. 6, A and D) and lower total FoxO1 level (Fig. 6, B and C), whereas the cytosolic FoxO1 levels were not affected (Fig. 6D). These results confirmed that the mechanism for the elevation of nuclear FoxO1 content in response to IL-1β involved the MAP kinase pathway.
FIGURE 6.
JNK and ERK mediate IL-1β effects on FoxO1. GFP-FoxO1-transfected 293-IL-1RI cells were cultured for 24 h under standard conditions. The cells were then pretreated for 30 min with SP600125 (20 μm), an inhibitor of JNK, PD98059 (50 μm), an inhibitor of ERK, or Me2SO. IL-1β (25 ng/ml) treatments were done in the presence of the respective inhibitors/vehicle for 6 h. A, the presence of GFP-FoxO1 was monitored by direct fluorescent microscopy of live cells. B, levels of FoxO1 were determined in whole cell lysates by Western blotting using anti-GFP antibody. The abundance of β-actin was used to control for equal loading and to normalize the data. The data are representative of at least three independent experiments. C, quantification of the changes shown in B. The results are expressed as percentages of the FoxO1 levels in untreated cells. The values are the means ± S.D. (n = 3). *, p < 0.05; **, p < 0.01. D, the levels of GFP-FoxO1 (WT) in purified nuclear and cytosolic fractions of 293-IL-1RI cells were analyzed by Western blotting using anti-GFP antibody. 20 μg of each subcellular fraction were loaded per line. Lamin A/C served as control for the purity of nuclear fraction.
The Role of nSMase-2 in FoxO1 Regulation
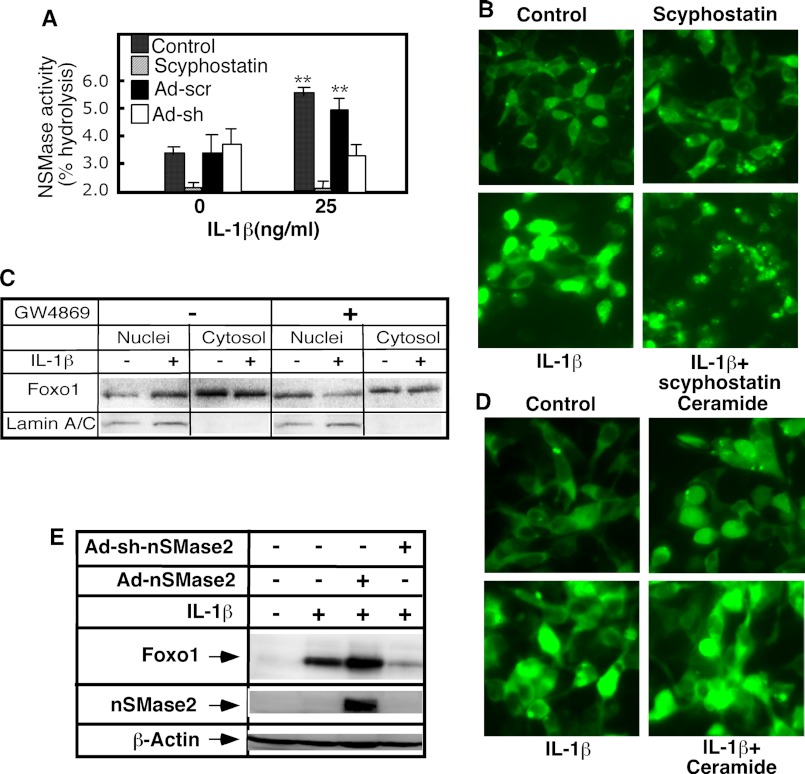
Our earlier studies have identified nSMase-2 and ceramide as mediators of IL-1β responses that act upstream of the ERK/JNK arm of the signaling pathway (8). Because ceramide has been shown to induce apoptosis and cell growth arrest (22)—two responses that are typically associated with increased FoxO1 activity—we decided to test whether nSMase-2 and ceramide play a role in FoxO1 regulation. IL-1β-induced activation of nSMase was first characterized in primary hepatocytes. As reported previously, the total cellular nSMase activity increased within 30 min of IL-1β stimulation. The short hairpin RNA interference prevented an increase in the nSMase activity, confirming that IL-1β activated nSMase-2 (Fig. 6A). The pharmacological nSMase inhibitors scyphostatin (Fig. 6A) and GW4869 (data not shown; see Ref. 20) had similar effects.
A pharmacological and genetic approach was then used to test how changes in nSMase-2 activity affect FoxO1 regulation. The 293-IL-1R cells were first treated with scyphostatin and GW4869. In the presence of scyphostatin, IL-1β failed to induce an increase in nuclear FoxO1 fluorescence, indicating that nSMase activity and ceramide generation are required for this effect (Fig. 7B). A similar inhibition was observed in the presence of GW4868. Subcellular fractionation studies furthermore confirmed that GW4868 prevented increases in nuclear FoxO1 but has no effect on cytosolic levels (Fig. 7C). By contrast, stimulation of cells with C2-ceramide, a short chain membrane-permeable analog of ceramide, potentiated the magnitude of the IL-1β effect (Fig. 7D).
FIGURE 7.
nSMase-2 and ceramide mediate the effects of IL-1β on FoxO1. A, primary rat hepatocytes were infected with adenovirus expressing shRNA against nMSase2 or scrambled control for 48 h or with scyphostatin for 30 min. The cells were treated with IL-1β (25 ng/ml) for 30 min, and the activity of nMSase was measured using NBD-SM as a substrate. B, GFP-FoxO1-transfected 293-IL-1RI cells were cultured for 24 h under standard conditions. The cells were then treated with IL-1β (25 ng/ml) and/or an inhibitor of sphingomyelinase, scyphostatin. The presence of GFP-FoxO1 was monitored by direct fluorescent microscopy of live cells. C, GFP-FoxO1-transfected 293-IL-1RI cells were cultured for 24 h under standard conditions. The cells were then treated with IL-1β (25 ng/ml) and/or an inhibitor of sphingomyelinase, GW4869 (10 μm). The levels of GFP-FoxO1 (WT) in purified nuclear and cytosolic fractions of 293-IL-1RI cells were analyzed by Western blotting using anti-GFP antibody. 20 μg of each subcellular fraction were loaded per line. Lamin A/C served as control for the purity of nuclear fraction. D, GFP-FoxO1-transfected 293-IL-1RI cells were cultured for 24 h under standard conditions. The cells were then treated with IL-1β (25 ng/ml) and/or C2-ceramide (30 μm). The presence of GFP-FoxO1 was monitored by direct fluorescent microscopy of live cells. E, primary rat hepatocytes were cultured for 3 days, and one group was infected with adenovirus overexpressing the rat nSMase-2 (Ad-nSMase2), and expression of nSMase-2 was induced with doxycycline. A second group of infected, noninduced hepatocytes serves as control. A third group was infected with adenovirus expressing shRNA against nSMase-2 (Ad-sh-nSMase2). The levels of FoxO1 were determined in whole cell lysates by Western blotting using anti-FoxO1 antibody. The abundance of β-actin was used to control for equal loading. The efficiency of nSMase-2 overexpression was tested using anti-FLAG antibody. The data are representative of two independent experiments.
Similar effects were observed when testing the role of nSMase-2 in regulating endogenous FoxO1 levels in primary rat hepatocytes (Fig. 7E). In this cell culture system, a genetic approach modulated nSMase-2 activity through the adenovirus-mediated transfer of shRNAi or cDNA for the rat nSMase-2 (8, 9). Suppressing nSMase-2 activity—which prevented the IL-1β-induced stimulation of sphingomyelin turnover, ceramide generation, and JNK activation (Fig. 6A and Ref. 9)—also blocked increases in the FoxO1 level in IL-1β-stimulated cells (Fig. 7E). We found that the overexpression of nSMase-2, which elevates the hepatocyte ceramide content by 30% and augments JNK activation (20), conversely increased the magnitude of IL-1β-induced stimulation of FoxO1 (Fig. 7E). This outcome is in agreement with the role nSMase-2 has in the IL-1β signaling cascade, which was described in detail in our earlier studies (8, 9, 20).
MAP Kinase Pathway Is Downstream of nSMase-2 in the Pathway Leading to FoxO1 Stimulation
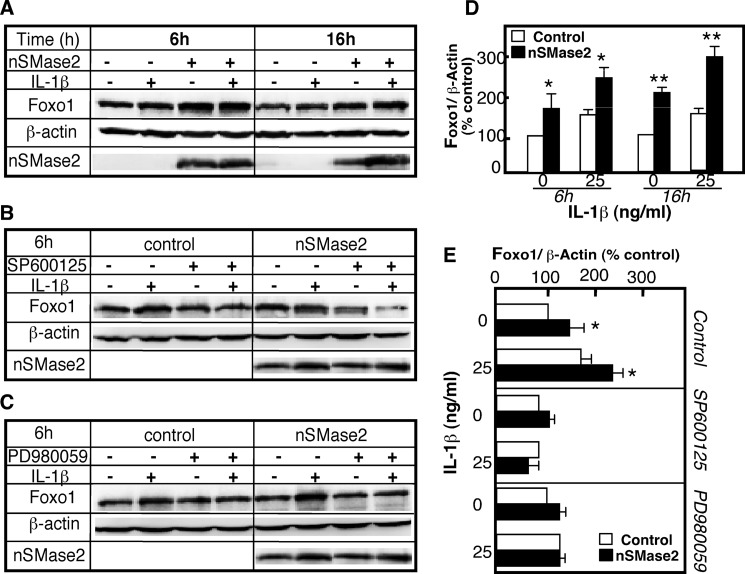
The overexpression of nSMase-2 in 293-IL-1R cells caused a 2-fold increase in FoxO1 levels in nonstimulated cells and in IL-1β-stimulated cells after 6 and 16 h (Fig. 8, A and D). Furthermore, JNK and ERK inhibitors, which reversed IL-1β-induced FoxO1 increases (compare Fig. 6), also blocked the effects occurring after nSMase-2 overexpression (Figs. 8B and 5, C and E). These results confirmed that, with respect to FoxO1 stimulation, nSMase-2 activation is upstream of the JNK/ERK pathways, and it is part of the mechanism by which IL-1β elevates FoxO1 content and induces its nuclear retention.
FIGURE 8.
JNK and ERK mediate the effects of nSMase-2 on FoxO1. 293-IL-1RI cells were infected with adenovirus expressing nSMase-2 for 48 h and then treated with IL-1β (25 ng/ml) in the presence of absence of SP600125 (20 μm) or PD980059 (20 μm) for the indicated times (A and D) or for 6 h (B, C, and E). A–C, the levels of the endogenous FoxO1 were assayed by Western blotting using antibody against FoxO1. The expression of nMSase2 was monitored using Anti-nSMase-2 antibody. The abundance of β-actin was used to control for equal loading and to normalize the results. D and E, quantification of the changes shown in A–C. The results are expressed as percentages of the FoxO1 levels in untreated cells. The values are the means ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
IL-1β, nSMase-2, and JNK Regulate the Expression of IGFBP1, a FoxO1-responsive Target
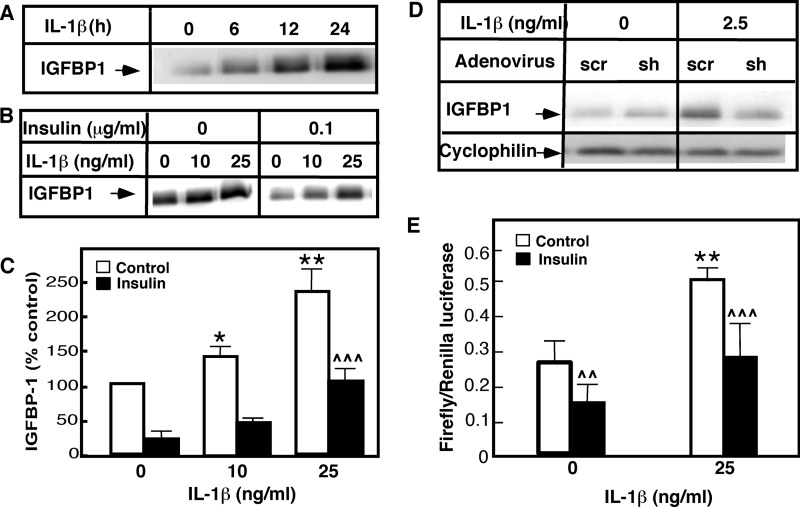
Hepatocytes secrete insulin-like growth factor-binding protein 1 (IGFBP1), which binds to and neutralizes IGF in the plasma. In the absence of insulin, high IGFBP1 mRNA expression is maintained because FoxO1 transcription factor is bound to the classical insulin response element in the 1-kb IGFBP1 promoter. Insulin-initiated FoxO1 nuclear export, however, stops the transcription of IGFBP1 mRNA, causing a rapid drop in IGFBP1 mRNA and IGFBP1 protein levels. To understand better the physiological significance of IL-1β-induced increases in FoxO1, we used primary rat hepatocytes to test the effects of IL-1β, nSMase-2, and JNK on IGFBP1 production (Fig. 9, A–D). The cells were initially treated with IL-1β alone or with IL-1β in combination with insulin. The IGFBP1 level in the medium was monitored by Western blotting. As recently reported, IL-1β stimulated IGFBP1 production in a time- (Fig. 9A) and dose-dependent manner (Fig. 1, B and C). The level of IGFBP1 in the medium began to increase after 6 h of IL-1β treatment and continued to rise after 12 and 24 h. This IL-1β-related effect occurred in the absence of insulin or other growth factors. Insulin alone significantly suppressed the basal expression of IGFBP1 (Fig. 9, B and C). Nevertheless, IL-1β stimulation effectively increased IGFBP1 secretion 5-fold, even in the presence of insulin. Therefore, IL-1β seemed to efficiently counteract the insulin-induced suppression of IGFBP1 production.
FIGURE 9.
Coordinated regulation of IGFBP1 expression and secretion by IL-1β and insulin. A–C, primary rat hepatocytes were cultured on Matrigel for 5 days and treated with IL-1β (25 ng/ml) at the indicated times (A) or with the indicated doses in the presence or absence of insulin for 6 h (B). The levels of IGFBP1 were determined in the medium by Western blotting using antibody against the rat IGFBP1 (A and B). The results were quantified and expressed as percentages of the IGFBP1 levels secreted by untreated cells (C). D, primary rat hepatocytes were infected with adenovirus expressing shRNA against nSMase-2 or scrambled control for 48 h and then treated with IL-1β for 6 h. The levels of IGFBP1 were determined in the medium by Western blotting using antibody against the rat IGFBP1. E, HepG2 cells were transfected with plasmids expressing the firefly and the Renilla luciferase and treated with IL-1β and/or insulin (100 ng/ml) for 6 h. The results were presented as ratios of firefly and Renilla luciferase activity.
The dual luciferase system was employed to elucidate whether IL-1β had a direct effect on the activity of the IGFBP1 promoter. In these experiments, HepG2 cells were transfected with a plasmid encoding firefly luciferase under the 1-kb IGFBP1 promoter or transfected with a control plasmid expressing Renilla luciferase. IL-1β treatment stimulated the firefly luciferase activity 2–3-fold, whereas insulin had the opposite effect (Fig. 9D). The combined stimulation of IL-1β and insulin had a significantly smaller effect, compared with the effect of IL-1β alone. This indicates that IL-1β and insulin likely acted on the same response element in the IGFBP1 promoter.
The suppression of nSMase-2 expression in primary rat hepatocytes (Fig. 9D) or the pharmacological inhibition of JNK activity (data not shown; see Ref. 23) completely blocked IL-1β-induced IGFBP1 secretion. This confirmed that the same mechanism or mechanisms likely mediate IL-1β regulation of FoxO1 and IGFBP1.
DISCUSSION
This study makes the novel observation that stimulation of hepatocytes with IL-1β leads to increases in nuclear FoxO1 through a mechanism that is Akt-independent and involves nSMase-2, ceramide, and the MAP kinase pathway. Data are also presented implicating IL-1β in the negative regulation of cellular insulin responses in respect to FoxO1 activity and FoxO1-mediated processes.
The pro-inflammatory cytokines and inflammation in general are well known factors in the onset of insulin resistance and hyperglycemia. IL-1β in particular, plays a key role, as underscored by studies showing significant improvement of hyperglycemia in mice and humans following the administration of IL-1β receptor antagonist, IL-1RA (1, 2, 24–27). The IL-1β action has been associated with down-regulation of the insulin signaling pathway at the insulin receptor level (28). Indeed, several key mediators of IL-1β signaling pathway, namely ceramide (29–34), IRAK-1 (35), and JNK (36, 37) have been shown to inhibit IRS-1 and/or Akt-1 activation. These studies indicate that IL-1β may suppress the cellular insulin response. Our experiments however, show that IL-1β also has a direct, Akt-independent, effect on FoxO1 as evidenced by 1) FoxO1 mutant lacking all three Akt phosphorylation sites being regulated similarly to the wild type FoxO1 and 2) the effects of IL-1β that were clearly observed even when Akt was not active, i.e., in the absence of insulin and other growth factors.
Akt-1 is known to be the main factor regulating FoxO1 nuclear localization and activity. The current paradigm states that insulin activates Akt, which then is translocated to the nucleus and phosphorylates FoxO1 at Thr24, Ser256, and Ser319 (13, 14). The phosphorylated FoxO1 is exported to the cytosol, terminating its transcriptional activity. This same mechanism accounts for the insulin-induced down-regulation of gluconeogenesis in the liver, which is central for the systemic regulation of glucose homeostasis. The identification of FoxO1 as a direct target of IL-1β in the presented data implies that the inflammatory and metabolic signaling pathways in the liver converge on FoxO1 regulating its transcriptional activity.
The mechanism by which IL-1β modulates FoxO1 levels is not entirely clear. We show that the increases in nuclear FoxO1 are not a consequence of higher transcription levels. TNFα, by contrast, has been shown to induce the expression of FoxO1 in asymptomatic plaques smooth muscle cells by affecting the FoxO1 mRNA levels (38). The data presented here also clearly show that IL-1β does not act to redistribute the existing FoxO1 between cytosol and nucleus, because the levels of cytosolic FoxO1 also actually rose. We therefore conclude that the observed IL-1β-induced increases in FoxO1 nuclear levels are probably due to the retention of FoxO1 in the nucleus and, consequently, its delayed cytosolic degradation.
We further show that nSMase-2 activation and the consequent increases in ceramide levels are required steps in the pathway leading to FoxO1 accumulation in IL-1β-stimulated cells. This leads us to hypothesize that ceramide-activated phosphates (PP2A or PP1) are probably involved. PP2A has been shown to have a central role in the regulation of the phosphorylation status of FoxO1 (39) and the interactions with its chaperon binding proteins, the 14-3-3 proteins. Furthermore, our previous studies have shown that IL-1β activates PP2A in nSMase-2-dependent manner (9). It is thus possible that activation of PP2A and subsequent dephosphorylation of 14-3-3 proteins or FoxO1 (at an Akt-independent site) may be involved in the IL-1β response. Currently we are conducting studies aimed at assessment of the changes in FoxO1 and 14-3-3 proteins phosphorylation.
The direct effect of IL-1β on FoxO1 also seems to involve the activation of JNK/ERK in an nSMase-2/ceramide-dependent manner. In previous studies we identified nSMase-2 as being IL-1β-inducible signaling sphingomyelinase, responsible for the transient accumulation of ceramide and the activation of JNK activation following IL-1β stimulation (9, 19, 20). The role of nSMase-2 and ceramide in JNK activation was shown to be intricate. nSMase-2 activity was required, but not sufficient, for the phosphorylation and activation of JNK in response to IL-1β. Also, in the absence of IL-1β, neither increased nSMase-2 activity nor ceramide supplementation alone induced JNK phosphorylation. nSMase-2, however, potently increased the magnitude of JNK phosphorylation following IL-1β treatment, suggesting that nSMase-2 may be part of a positive feed forward mechanism for control of JNK activation. The effects of nSMase-2 on FoxO1 seen in this study closely recall those nSMase-2 has on JNK. This further underscores the important connection between nSMase-2, JNK, and FoxO1 in the IL-1β signaling pathway.
The mechanism of action of JNK on FoxO1 is not clear. Another member of the FoxO family, FoxO4, is phosphorylated by JNK at Thr447 and Thr451 (40–42), which supersedes the Akt-1-dependent regulation and induces nuclear retention. These phosphorylation sites, however, are not conserved in FoxO1, FoxO3, and DAF-16. Nevertheless, direct JNK phosphorylation of FoxO1 purified protein by JNK has been shown in vitro, but the target phosphorylation sites have not been identified. It is also plausible that JNK prevents FoxO1 nuclear export by disrupting its association with the 14-3-3 proteins. JNK phosphorylation of the 14-3-3 proteins has been shown to impede their interaction with other downstream target proteins, including Bad, FoxO3A, BAX, and c-Abl (43–45).
We show that ERK1/2 inhibition also blocks the IL-1β regulation of FoxO1. In contrast to JNK, the ERK pathway has not been associated with the regulation of FoxO1 phosphorylation and/or its localization. Recent studies have, however, shown that ERK activation results in direct phosphorylation of the p300 co-activator ( at Ser2279, Ser2315, and Ser2366), resulting in stimulation of its intrinsic acethylase activity (16). This is relevant because FoxO1 is a p300 substrate but also more importantly because the p300/CBP-dependent FoxO1 acethylation is known to play a role in regulating FoxO1 signaling by promoting the nuclear localization of FoxO1 (18).
The data presented clearly show that IL-1β counteracts the Akt-mediated insulin signals by increasing the overall cellular FoxO1 content and augmenting its nuclear localization. Stimulation of hepatocytes with IL-1β apparently mimics the consequences of insulin resistance (at least in respect to FoxO1) but without the associated pathologies such as hepatic steatosis. These direct effects of IL-1β on FoxO1, together with the previously reported IL-1β down-regulation of IRS-1 and Akt, may constitute the key elements of a very complex, intricate mechanism for the deregulation of hepatic metabolic functions during inflammation.
This work was supported, in whole or in part, by National Institutes of Health Grant AG019223 (to M. N. K.).
- IRAK-1
- interleukin-1 receptor-associated kinase 1
- IGFBP1
- insulin-like growth factor-binding protein-1
- nSMase-2
- neutral sphingomyelinase-2
- PP2A
- protein phosphatase 2A
- E3
- ubiquitin-protein isopeptide ligase.
REFERENCES
- 1. Larsen C. M., Faulenbach M., Vaag A., Vølund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., Donath M. Y. (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 [DOI] [PubMed] [Google Scholar]
- 2. Sauter N. S., Schulthess F. T., Galasso R., Castellani L. W., Maedler K. (2008) The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 149, 2208–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartolomé N., Arteta B., Martínez M. J., Chico Y., Ochoa B. (2008) Kupffer cell products and interleukin 1β directly promote VLDL secretion and apoB mRNA up-regulation in rodent hepatocytes. Innate Immun. 14, 255–266 [DOI] [PubMed] [Google Scholar]
- 4. Muzio M., Ni J., Feng P., Dixit V. M. (1997) IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278, 1612–1615 [DOI] [PubMed] [Google Scholar]
- 5. Wesche H., Henzel W. J., Shillinglaw W., Li S., Cao Z. (1997) MyD88. An adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7, 837–847 [DOI] [PubMed] [Google Scholar]
- 6. Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J., Cao Z., Matsumoto K. (1999) The kinase TAK1 can activate the NIK-I κB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 [DOI] [PubMed] [Google Scholar]
- 7. Karakashian A. A., Giltiay N. V., Smith G. M., Nikolova-Karakashian M. N. (2004) Expression of neutral sphingomyelinase-2 (nSMase-2) in primary rat hepatocytes modulates IL-β-induced JNK activation. FASEB J. 18, 968–970 [DOI] [PubMed] [Google Scholar]
- 8. Giltiay N. V., Karakashian A. A., Alimov A. P., Ligthle S., Nikolova-Karakashian M. N. (2005) Ceramide- and ERK-dependent pathway for the activation of CCAAT/enhancer binding protein by interleukin-1β in hepatocytes. J. Lipid Res. 46, 2497–2505 [DOI] [PubMed] [Google Scholar]
- 9. Dobierzewska A., Giltiay N. V., Sabapathi S., Karakashian A. A., Nikolova-Karakashian M. N. (2011) Protein phosphatase 2A and neutral sphingomyelinase 2 regulate IRAK-1 protein ubiquitination and degradation in response to interleukin-1β. J. Biol. Chem. 286, 32064–32073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamagate A., Qu S., Perdomo G., Su D., Kim D. H., Slusher S., Meseck M., Dong H. H. (2008) FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118, 2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsumoto M., Pocai A., Rossetti L., Depinho R. A., Accili D. (2007) Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor FoxO1 in liver. Cell Metab 6, 208–216 [DOI] [PubMed] [Google Scholar]
- 12. Cheng Z., White M. F. (2011) Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid. Redox Signal. 14, 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang H., Tindall D. J. (2007) Dynamic FoxO transcription factors. J. Cell Sci. 120, 2479–2487 [DOI] [PubMed] [Google Scholar]
- 14. Greer E. L., Brunet A. (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24, 7410–7425 [DOI] [PubMed] [Google Scholar]
- 15. Kawamori D., Kaneto H., Nakatani Y., Matsuoka T. A., Matsuhisa M., Hori M., Yamasaki Y. (2006) The forkhead transcription factor FoxO1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 281, 1091–1098 [DOI] [PubMed] [Google Scholar]
- 16. Chen Y.-J., Wang Y.-N., Chang W.-C. (2007) ERK2-mediated C-terminal serine phosphorylation of p300 is vital to the regulation of epidermal growth factor-induced keratin 16 gene expression. J. Biol. Chem. 282, 27215–27228 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura N., Ramaswamy S., Vazquez F., Signoretti S., Loda M., Sellers W. R. (2000) Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 20, 8969–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senf S. M., Sandesara P. B., Reed S. A., Judge A. R. (2011) p300 acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. Am. J. Physiol. Cell Physiol. 300, C1490–C1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nikolova-Karakashian M., Morgan E. T., Alexander C., Liotta D. C., Merrill A. H., Jr. (1997) Bimodal regulation of ceramidase by interleukin-1β. Implications for the regulation of cytochrome p450 2C11. J. Biol. Chem. 272, 18718–18724 [DOI] [PubMed] [Google Scholar]
- 20. Rutkute K., Karakashian A. A., Giltiay N. V., Dobierzewska A., Nikolova-Karakashian M. N. (2007) Aging in rat causes hepatic hyperresposiveness to interleukin-1β which is mediated by neutral sphingomyelinase-2. Hepatology 46, 1166–1176 [DOI] [PubMed] [Google Scholar]
- 21. Rutkute K., Asmis R. H., Nikolova-Karakashian M. N. (2007) Regulation of neutral sphingomyelinase-2 by GSH. A new insight to the role of oxidative stress in aging-associated inflammation. J. Lipid Res. 48, 2443–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hannun Y. A., Obeid L. M. (2002) The ceramide-centric universe of lipid-mediated cell regulation. Stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 [DOI] [PubMed] [Google Scholar]
- 23. Rutkute K., Nikolova-Karakashian M. N. (2007) Regulation of insulin-like growth factor binding protein-1 expression during aging. Biochem. Biophys. Res. Commun. 361, 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandberg J. O., Andersson A., Eizirik D. L., Sandler S. (1994) Interleukin-1 receptor antagonist prevents low dose streptozotocin induced diabetes in mice. Biochem. Biophys. Res. Commun. 202, 543–548 [DOI] [PubMed] [Google Scholar]
- 25. Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z.-W., Karin M., Shoelson S. E. (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science 293, 1673–1677 [DOI] [PubMed] [Google Scholar]
- 26. Kanety H., Feinstein R., Papa M. Z., Hemi R., Karasik A. (1995) Tumor necrosis factor α-induced phosphorylation of insulin receptor substrate-1 (IRS-1). J. Biol. Chem. 270, 23780–23784 [DOI] [PubMed] [Google Scholar]
- 27. Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 28. Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J. F. (2007) Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148, 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arboleda G., Huang T. J., Waters C., Verkhratsky A., Fernyhough P., Gibson R. M. (2007) Insulin-like growth factor-1-dependent maintenance of neuronal metabolism through the phosphatidylinositol 3-kinase-Akt pathway is inhibited by C2-ceramide in CAD cells. Eur. J. Neurosci. 25, 3030–3038 [DOI] [PubMed] [Google Scholar]
- 30. Hajduch E., Turban S., Le Liepvre X., Le Lay S., Lipina C., Dimopoulos N., Dugail I., Hundal H. S. (2008) Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J. 410, 369–379 [DOI] [PubMed] [Google Scholar]
- 31. Fox T. E., Houck K. L., O'Neill S. M., Nagarajan M., Stover T. C., Pomianowski P. T., Unal O., Yun J. K., Naides S. J., Kester M. (2007) Ceramide recruits and activates protein kinase Cζ (PKCζ) within structured membrane microdomains. J. Biol. Chem. 282, 12450–12457 [DOI] [PubMed] [Google Scholar]
- 32. Stratford S., Hoehn K. L., Liu F., Summers S. A. (2004) Regulation of insulin action by ceramide. Dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279, 36608–36615 [DOI] [PubMed] [Google Scholar]
- 33. Schubert K. M., Scheid M. P., Duronio V. (2000) Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 275, 13330–13335 [DOI] [PubMed] [Google Scholar]
- 34. Stratford S., DeWald D. B., Summers S. A. (2001) Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem. J. 354, 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim J.-A., Yeh D. C., Ver M., Li Y., Carranza A., Conrads T. P., Veenstra T. D., Harrington M. A., Quon M. J. (2005) Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by mouse Pelle-like kinase/interleukin-1 receptor-associated kinase. J. Biol. Chem. 280, 23173–23183 [DOI] [PubMed] [Google Scholar]
- 36. Copps K. D., White M. F. (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Werner E. D., Lee J., Hansen L., Yuan M., Shoelson S. E. (2004) Insulin resistance Due to phosphorylation of insulin receptor substrate-1 at serine 302. J. Biol. Chem. 279, 35298–35305 [DOI] [PubMed] [Google Scholar]
- 38. Jia G., Aggarwal A., Tyndall S. H., Agrawal D. K. (2011) Tumor necrosis factor-α regulates p27 kip expression and apoptosis in smooth muscle cells of human carotid plaques via forkhead transcription factor O1. Exp. Mol. Pathol. 90, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yan L., Lavin V. A., Moser L. R., Cui Q., Kanies C., Yang E. (2008) PP2A regulates the pro-apoptotic activity of FOXO1. J. Biol. Chem. 283, 7411–7420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oh S. W., Mukhopadhyay A., Svrzikapa N., Jiang F., Davis R. J., Tissenbaum H. A. (2005) JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. U.S.A. 102, 4494–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang M. C., Bohmann D., Jasper H. (2005) JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125 [DOI] [PubMed] [Google Scholar]
- 42. Essers M. A., Weijzen S., de Vries-Smits A. M., Saarloos I., de Ruiter N. D., Bos J. L., Burgering B. M. (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sunayama J., Tsuruta F., Masuyama N., Gotoh Y. (2005) JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J. Cell Biol. 170, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsuruta F., Sunayama J., Mori Y., Hattori S., Shimizu S., Tsujimoto Y., Yoshioka K., Masuyama N., Gotoh Y. (2004) JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 23, 1889–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshida K., Yamaguchi T., Natsume T., Kufe D., Miki Y. (2005) JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat. Cell Biol. 7, 278–285 [DOI] [PubMed] [Google Scholar]