Abstract
It has been more than 15 years since the identification of individual interleukin-27 (IL-27) and IL-27 receptor components. The last decade has seen the description of the signaling pathways engaged by IL-27 and an appreciation has emerged that this cytokine can modulate the intensity and duration of many classes of T cell responses. The goal of this article is to provide an overview of the immunobiology of IL-27 and to review advances in understanding the functions of individual IL-27 and IL-27 receptor sub-units and the role of IL-27 in dictating the balance between protective and pathological immunity. Additionally, this cytokine has been proposed as a therapy to modify inflammatory conditions or promote anti-tumor responses and situations where experimental and clinical data sets implicate IL-27 in the outcome of disease are highlighted.
Introduction
Interleukin-27 (IL-27) is a heterodimeric cytokine composed of the Epstein-Barr virus-induced gene 3 (EBi3) and IL-27p28, which engages a receptor composed of gp130 and the IL-27Rα that activates Janus kinase (JAK)-signal transducer and activator of transcription (STAT) and mitogen activated protein kinase (MAPK) signaling (see Figure 1) (Kastelein et al., 2007). Although the Ebi3 subunit and IL-27Rα chain were first described in 1996 and 1998 respectively, it was not until 2001 that a combination of in silico and biochemical approaches provided the main framework for understanding how IL-27 functioned, and in 2004, that the full receptor composition was described (Pflanz et al., 2004; Pflanz, 2002). There are a number of structural motifs that characterize the IL-27 receptor and sub-units that highlight its structural relationship with IL-6, IL-12 and IL-23 and which help explain their use of similar signaling pathways and overlapping activities (Kastelein et al., 2007). These latter cytokines have emerged as critical determinants in the development of T helper 1 (Th1) and Th17 cell responses and represent major targets for drug development to manage inflammatory conditions associated with aberrant T cell responses. Because IL-27 is a member of this family and utilizes JAK-STAT signaling associated with T cell activation, when this cytokine was first described there was an expectation that it would be pro-inflammatory. This notion was reinforced by reports on mice that lacked the IL-27Rα and in vitro studies which emphasized the ability of IL-27 to promote NK and T cell proliferation and production of IFN-γ (Chen et al., 2000; Pflanz, 2002; Yoshida et al., 2001). However, when Il27ra−/− mice were challenged with a number of pathogens or utilized in a variety of autoimmune models, the data sets that emerged suggested that one of the main function of IL-27 in these settings was to limit the intensity and duration of T cell responses (Artis et al., 2004a; Artis et al., 2004b; Batten et al., 2006; Hamano et al., 2003; Holscher et al., 2005; Miyazaki et al., 2005; Stumhofer et al., 2006; Villarino et al., 2003). Since then, multiple studies have addressed the basis for the inhibitory effects of IL-27 on Th1, Th2, and Th17-cell responses and highlighted the many mechanisms engaged by this cytokine (see Figure 2). This includes the ability to antagonize T cell production of IL-2, a direct inhibitory effect on Th2 and Th17 activities and that IL-27 is a major stimulus for T cell production of IL-10. There is now an acceptance that IL-27 can limit many facets of T cell-mediated pathology but also a literature that that it can promote Th1 responses (Cao et al., 2008; Mayer et al., 2008). Nevertheless, ongoing studies continue to identify novel suppressive functions of IL-27 and there has been progress in translating the basic findings from murine models into clinical settings. The goal of this article is to highlight recent advances, frame newer questions that have arisen about this cytokine and provide an overview of the current knowledge of the immunobiology of IL-27 that may inform the development of therapies to limit or enhance immune responses.
Figure 1.
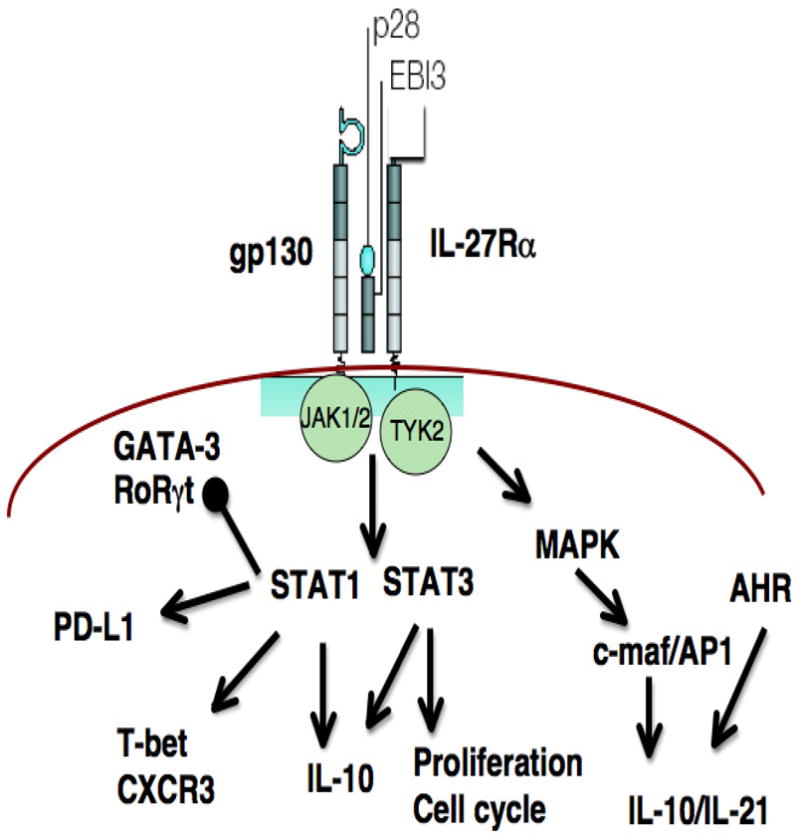
Impact of IL-27 on lymphocyte signaling pathways. Dimerization of gp130 and IL-27Ra engages JAK1, 2 and Tyk2 that engage the MAPK pathway and activation of multiple STATs, most notably STAT1 and STAT3. The activation of STAT1 is linked to inhibition of GATA-3 and RoRγt but upregulation of PD-L1, T-bet and IL-10. The ability to engage STAT3 is linked to increased proliferation as well as IL-10 while the MAPK pathway intersects with AHR to promote IL-10 and IL-21.
Figure 2.
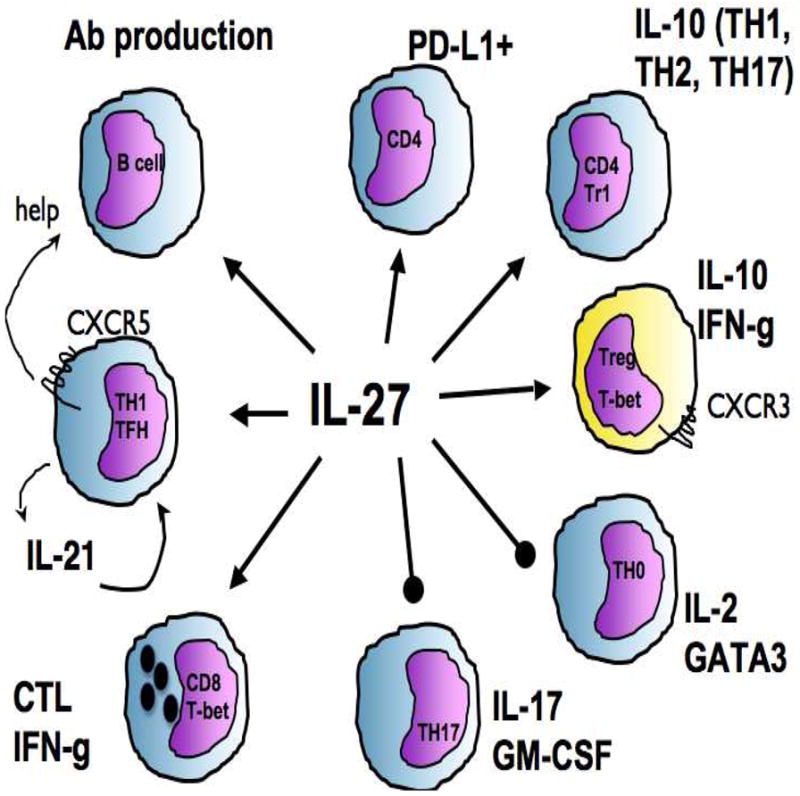
Key regulatory effects of IL-27 on T and B cells. The pro-inflammatory properties attributed to IL-27 include the development of CTL, the promotion of TFH as well as a direct ability to promote B cell production of antibodies. The regulatory activities of IL-27 includes the ability to promote expression of the inhibitory receptor PD-L1 and IL-10 production by multiple helper T cells. The ability to generate a CXCR3+ Treg population is specialized to operate at sites of TH1 inflammation while the ability to control Th2 and Th17 inflammation is due to direct inhibitory effects on GATA3 and RoRgt.
Promiscuity of IL-27 and IL-27 receptor sub-units
Interleukin-12 remains the prototypic hetero-dimeric cytokine and the association of the IL-12p35 and IL-12p40 sub-units is dependent on disulphide interactions (Trinchieri, 2003). In contrast, the nature of the association between IL-27p28 and EBi3 is uncertain and these sub-units can be secreted independently and are differentially expressed in various cell types (Pflanz, 2002). These observations imply that IL-27p28 and EBi3 might associate with other factors to form novel cytokines or have biological functions of their own. Evidence also exists indicating that EBi3 can partner with IL-12p35 to form IL-35, which has been linked to the activities of regulatory T cells (Collison et al., 2007; Devergne et al., 1997) and the biology of this cytokine is reviewed in detail elsewhere (Vignali and Kuchroo, 2012). Similarly, the IL-27p28 subunit can bind to cytokine-like factor 1 (CLF), and this heterodimer promotes T and NK cell production of cytokines (Crabe et al., 2009). This inherent combinatorial biology lends itself to the generation of designer cytokines and although there is currently no evidence that IL-27p28 and IL-12p40 can combine naturally, this recombinant haematopoetin can suppress inflammation in a model of Experimental Autoimmune Uveitis (EAU) associated with the inhibition of Th1 and Th17-cell responses and expansion of Treg cells (Wang et al., 2012). The initial observation that IL-27p28 alone had a modest ability to antagonize Th17 cell responses (Stumhofer et al., 2006) foreshadowed reports that over expression of IL-27p28 can have quite profound biological effects. The generation of a variant of IL-27, in which the IL-27p28 sub-unit could not interact with gp130, revealed that this mutant acted as a receptor antagonist and limited liver damage mediated by Th1 cells (Rousseau et al., 2010). Indeed, IL-27p28 can be secreted independently of EBi3 and, consistent with structural predictions that IL-27p28 would bind to the type I domain of gp130, this sub-unit can block the activity of cytokines (IL-6, IL-11 and IL-27) that utilize this portion of gp130 to signal (Pflanz et al., 2004; Stumhofer et al., 2010). The physiological relevance of these observations was illustrated by the finding that over-expression of IL-27p28 antagonizes gp130-dependent B cell responses (Stumhofer et al., 2010), can block liver damage (Dibra et al., 2012), abrogate anti-tumor responses and suppress graft rejection (Shimozato et al., 2009), and block EAU (Wang et al., 2012). Together, these results have led to the idea that IL-27p28 can act as a naturally occurring low affinity antagonist of signaling through the gp130 receptor that is reminiscent of mechanisms that limit IL-1 activity (Stumhofer et al., 2010). While initial studies that utilized over expression approaches were key to identifying the pairing of IL-27p28 and EBi3, a major knowledge gap remains understanding the factors that govern whether IL-27p28 is secreted alone or as a heterodimer, how these components dimerize and whether there are differences between murine and human cells. Based largely on the over expression data in HEK293T cells, the current paradigm has human EBi3 secreted in excess and human IL-27p28 can only be secreted when co-expressed with EBi3 (Pflanz, 2002). In the mouse the opposite is true, IL-27p28 is secreted in excess whereas EBi3 can only be secreted when co-expressed with p28 (Pflanz, 2002). It is pertinent to acknowledge the current limitations with the commercial reagents to reliably measure the IL-27 heterodimer in human and mouse systems and to distinguish IL-27p28 alone or as part of IL-27. This becomes important if there are efforts to develop IL-27p28 clinically and there needs to be caution in extrapolating the functions of IL-27p28 in mice to the human situation.
Given the close relationship between IL-6, IL-12 and IL-27, the advanced state of knowledge of how IL-6 and IL-12 interact with their receptor components has informed many aspects of IL-27 signaling. Thus, the observation that the gp130 subunit is utilized by a group of related cytokines, including IL-6 and IL-27, while IL-12Rβ1 is employed by IL-12 and IL-23 raises questions about whether the IL-27Rα chain can function as a receptor for other cytokines. Indeed, the IL-27Rα is a component of the receptor (that includes gp130 and IL-6Rα) for the p28-CLF heterodimer (Crabe et al., 2009) and the neuro-protective peptide Humanin also utilizes the IL-27Rα (Hashimoto et al., 2009). There is also evidence that the IL-27Rα can form homo-dimers that activate the JAK-STAT pathway that can promote transformation of haematopetic cells (Pradhan et al., 2007). One additional facet of IL-6 biology that is relevant to IL-27 is the ability of IL-6 to pair with soluble IL-6Rα. This IL-6-IL-6Rα complex is able to bind to gp130 alone and transduce signaling in a process known as trans signaling (Rose-John et al., 2006). This process is thought to underlie the evolution of IL-27 as EBi3, which is analogous to the soluble IL-6Rα receptor, binds to IL-27p28 which is structurally similar to IL-6. At this point, there is no evidence that IL-27 employs trans signaling, but if IL-27 can be engineered to take advantage of this type of biology, it would profoundly alter current perspectives on the cell types that can be targeted by IL-27. Additional structure function studies would be required to inform the development of altered versions of IL-27 that could act as receptor agonists or antagonists. Regardless, the increased understanding of the use and re-use of the IL-27 cytokine and receptor components has already complicated the interpretation of studies with IL-27- and Il27ra−/− animals and the physiological significance of other complexes that contain elements of the IL-27/IL-27R cassette requires further study.
lL-27 regulating regulatory pathways
Although the IL-27Rα chain and IL-27 were initially linked to the development of Th1-cell responses there was a gradual transition to the recognition that this factor acts as a suppressor of many T cell subsets (Kastelein et al., 2007). With that realization, understanding how IL-27 could dampen multiple types of inflammation became a major question (see Figure 2) and the ability of IL-27 to antagonize the production of IL-2 may help to explain some of its broad suppressive effects (Villarino et al., 2006). With the identification of “master regulators” of T helper cell subsets, the observation that IL-27 reduced basal GATA3 expression provided an insight into the mechanism used to limit Th2-cell development (Lucas et al., 2003). Data that IL-27 blocked expression of RoRγt explains the antagonistic effects of IL-27 on the production of IL-17 (Diveu et al., 2009). Given the ability of IL-27 to activate STAT1 and T-bet (Takeda et al., 2003), a transcriptional response associated with Th1 activity, the molecular and cellular basis for the enhanced Th1 cell responses observed in the absence of IL-27 in vivo has been more difficult to comprehend. The section below reviews advances over the last five years that described the ability of IL-27 to promote a series of regulatory pathways that appear secondary to Th1 cell development and which prevent protective responses from becoming pathological.
IL-27 and the production of IL-10
The primary studies that showed that IL-27Rα deficient mice infected with intracellular parasites developed a lethal inflammatory response mediated by CD4+ T cells was reminiscent of work demonstrating that IL-10−/− mice challenged with the same organisms developed similar immune pathology (Gazzinelli et al., 1996; Hamano et al., 2003; Hunter et al., 1997; Villarino et al., 2003). However, in the Il27ra−/− mice, global defects in IL-10 production during the acute phase of these infections were not readily apparent, and these mice do not develop spontaneous colitis or display overt susceptibility to cancer, characteristic of the absence of IL-10 (Berg et al., 1996). These initial reports were interpreted as showing that IL-10 and IL-27 acted in distinct fashions and were consistent with a paradigm that IL-10 mediates its suppressive activities largely through its effects on accessory cell function while IL-27 could directly inhibit T cells. However, in 2007, a series of studies highlighted that Th1, Th2, Th17 and Tr1 cell subsets could be activated by IL-27 to promote the production of IL-10 in the context of infectious and autoimmune conditions and provided an unprecedented insight into the heterogeneity that can be apparent even within T cell responses that are defined by current T helper subset nomenclature (Awasthi et al., 2007; Fitzgerald et al., 2007b; Stumhofer et al., 2007). This phenomenon has been confirmed in a variety of other experimental models and expanded to humans (Anderson et al., 2009; Ansari et al., 2011; Batten et al., 2008; Freitas do Rosario et al., 2012; Murugaiyan et al., 2009; Sun et al., 2011; Wang et al., 2011). Wheras initial reports defining the molecular basis for these events identified the involvement of STAT1 and STAT3 signaling (Stumhofer et al., 2007) and of the inducible costimulator (ICOS) (Pot et al., 2009), the complex molecular events that underpin IL-10 production in T cells are still emerging (See Figure 1). For example, the ability of IL-27 to activate MAPK signaling and to induce expression of the AP-1 transcription factor promotes production of IL-21 that sustains IL-10 expression (Pot et al., 2009; Xu et al., 2009). Furthermore, the aryl hydrocarbon receptor and its ability to partner with c-Maf to optimize interactions with the Il10 and Il21 promoters has been implicated in these events (Apetoh et al., 2010). Much of this more recent work has been framed in the context of Tr1 cells, but since IL-10 can be produced by all T cell subsets it seems likely that these principles for the control of IL-10 will be broadly relevant.
IL-27 and PD-L1
The section above focused on the role of IL-27 in promoting IL-10 as one mechanism to limit inflammatory responses but there are regulatory networks mediated by other cytokines and receptor-ligand interactions that limit different facets of an immune response. The PD1-PD-L1 interaction has emerged as a major mediator of exhaustion in T cells, most prominently in the setting of chronic viral infections and cancer (Barber et al., 2006; Topalian et al., 2012), and so studies in which micro-array analysis of CD4+ T cells identified PD-L1 as a target of IL-27 provide an important link between two apparently distinct regulatory mechanisms. There are reports in which IL-27 was shown to inhibit the development of Th17 cells, but had less of an effect on established responses (El-behi et al., 2009) but the ability of IL-27 to promote PD-L1 by CD4+ T cells allows these cells to act in trans to limit Th17-cell responses and ameliorate the development of EAE, thus providing another strategy for IL-27 to indirectly limit inflammation mediated by Th17 cells (Hirahara et al., 2012). Given the prominent role of PD-1-PD-L1 in viral infections and T cell exhaustion (Wherry, 2011), these studies also highlight the gap in our knowledge of the role of IL-27 in the regulation of anti-viral immunity and provide the impetus to determine whether this cytokine is involved in co-ordinating the expression of other inhibitory pathways associated with exhaustion.
IL-27 and Treg cells
A major paradox for the last five years has been the disparate findings that IL-27 can limit inflammation, but there are reports that it antagonizes Treg-cell development or conversion, a major arm of the immune system devoted to operational tolerance. Several groups have observed that IL-27 antagonizes the ability of TGF-β and IL-2 to generate inducible Tregs (Huber et al., 2008; Neufert et al., 2007; Stumhofer et al., 2007) and in a transfer model of colitis, the absence of the IL-27Rα led to increased conversion of Treg cells and this population ameliorated disease more efficiently than wild-type cells (Cox et al., 2010). Moreover, mice transgenically overexpressing IL-27 lack Treg cell populations and develop an autoimmune disease analogous to the scurfy disease present in mice that lack Foxp3 (Wojno et al., 2011). Taken together, these findings would be interpreted as showing that IL-27 negatively impacts on Treg cell homeostasis. However, in the transgenic model, the loss of Treg cells appears to be secondary to the ability of IL-27 to limit the production of IL-2, which is required to maintain Treg populations. Moreover, Il27ra−/− mice have normal Treg cell frequencies and IL-27 does not down-regulate Foxp3 expression nor does it antagonize the ability of Treg cells to function in suppression assays (Cox et al., 2010; Villarino et al., 2005) and more recent studies have found that IL-27 promotes Treg cell growth and survival (Hall et al., 2012).
The studies described above highlight the contradictory literature on the effects of IL-27 on Tregs and stress the heightened production of IL-2 observed in the absence of IL-27 signaling can complicate the interpretation of these studies. An alternative view on the interaction of IL-27 and Treg cells began to emerge with reports in 2009 that during Th1-cell responses to the intracellular pathogens Mycobacteria or Toxoplasma gondii, a population of T-bet+ Treg cells emerged that could also produce IFN-γ (Koch et al., 2009; Oldenhove et al., 2009). During mycobacterial challenge, the development of this population was dependent on Treg expression of STAT1 that induced Tbet mediated expression of CXCR3 (Koch et al., 2009). The model that emerged from these latter studies was that this Th1-like subset of Treg cells developed in response to IFN-γ signals and was specialized to control Th1 responses. A role for IL-27 in these events was established when it was shown that IL-27 can engage this same STAT1-T-bet transcriptional pathway in Tregs and that following challenge with T. gondii, L. major or Salmonella, IL-27 is required for the emergence of a T-bet+, CXCR3+ Treg populations at local sites of inflammation, which produce IL-10 and suppress parasite-specific effector response (Hall et al., 2012) This led to a modified model in which the production of IFN-γ and IL-27 in distinct anatomical sites can drive sub-populations of Treg cells to express CXCR3, which allows these populations to operate at distinct sites of Th1 inflammation. These findings raise new questions about the precise impact of CXCR3 on Treg cell function and, when combined with reports that IL-27 upregulates cellular expression of LFA-1, ICAM-1 and sphingosine 1 phosphate (Liao et al., 2007; Owaki et al., 2006), suggests the need for additional studies to understand the impact of IL-27 on trafficking and behavior of different lymphocytes.
Translating models to clinical disease
With data sets emerging that IL-27 suppresses human Th17 cell responses and promotes the production of IL-10 (Amadi-Obi et al., 2007; Apetoh et al., 2010; Murugaiyan et al., 2009), several groups have already proposed that these properties of IL-27 may be useful therapeutically (Batten and Ghilardi, 2007; Pan et al., 2010) and there are multiple proof of principle studies in murine models that support this idea. The opportunity to apply this information to human disease is invariably complicated by data sets in which the pro- and anti-inflammatory properties of IL-27 are debated and by questions about how to interpret aberrant IL-27 levels in different disease states. In this section, situations where experimental and clinical data sets implicate IL-27 in the outcome of disease are highlighted.
IL-27 and infectious disease
Parasitic organisms have provided some of the strongest phenotypes for IL-27 in murine systems; there are now reports in which IL-27 is linked to the outcome of these infections in humans. For example, Il27ra−/− mice challenged with visceral leishmaniasis develop enhanced immune pathology (Rosas et al., 2006) and in patients with visceral disease, serum titers of IL-27 are elevated and have been linked to the production of IL-10 that may provide a feedback loop that limits inflammation but allows parasite persistence (Ansari et al., 2011). In different murine models of malaria, endogenous IL-27 promotes IL-10, limits T cell responses and prevents immune pathology (Findlay et al., 2011; Freitas do Rosario et al., 2012). In the human setting, those patients with the most severe clinical malaria have reduced amounts of IL-27, consistent with the elevated inflammatory responses (Ayimba et al., 2011). In contrast, very little is known about the impact of IL-27 on models of fungal infection. However, in elegant bedside to bench studies, Casanova and colleagues characterized a group of patients with gain of function mutations in STAT1 that were susceptible to mucocutaneous candidiasis (Liu et al., 2011). Because IL-17F and IL-17RA are required for resistance to candida, one explanation for this increased susceptibility of these patients is provided by the enhanced ability of IL-27 and type I IFNs to activate STAT1, and to suppress Th17-cell responses in these patients (Liu et al., 2011). These results suggest that reduced production of IL-27 may improve control of fungal organisms, wheras excessive IL-27 activity would promote susceptibility, an idea that has yet to be tested in murine systems.
IL-27 and cancer
Although much of this article focuses on the suppressive effects of IL-27 in infectious and autoimmune settings, IL-27 can also act as a potent stimulus for lymphocyte expansion and survival and there are reports that address the impact of IL-27 on haematopoesis (Seita et al., 2008) and proliferation (Charlot-Rabiega et al., 2011). As for many growth factors, IL-27 has also been linked to tumor progression and in human patients with acute myeloid leukemia (AML), the ability of the IL-27Rα to dimerize has been linked to transformation (Pradhan et al., 2007). However, IL-27 can have a direct inhibitory effect on tumor cells, even in the context of AML (Ho et al., 2009; Zorzoli et al., 2012). Thus, the role of IL-27Rα in cancer biology is complex, with a well developed literature that implicates IL-27 in the regulation of anti-tumor immunity mediated by CD8+ T cells (Chiyo et al., 2005; Hisada et al., 2004; Salcedo et al., 2009; Salcedo et al., 2004). Consistent with the ability of IL-27 to activate a transcriptional response reminiscent of Th1 cells, the ectopic expression of IL-27 promoted anti-tumor cytotoxic T lymphocyte (CTL) responses in mice associated with increased proliferation, expression of T-bet and IL-12Rβ2 and the production of IFN-γ (Salcedo et al., 2009). In that context, there is an increasing appreciation of the role of the PD-1-PD-L1 interaction in cancer and the observation that IL-27 induces PD-L1 (Hirahara et al., 2012) implies that IL-27 may be an important molecule in controlling immune checkpoint mechanisms that operate in cancer. The IL-27Rα chain is also expressed by epithelial tumor lines (Dibra et al., 2009) where IL-27 has been linked to promoting expression of MHC class I-related chain A (MICA), a ligand for NKG2D, which is an activating receptor expressed on NK and some CD8+ T cells that promotes cytotoxicity (Dibra et al., 2009). Taken together, these findings make it difficult to interpret how a polymorphism in IL-27p28 that is associated with colorectal cancer (Huang et al., 2012) can impact on tumor formation or surveillance. Given the contributory role of inflammation to the development of cancer, and in particular the links to the IL-23-Th17 axis (Langowski et al., 2006), it seems likely that IL-27 would have some impact on the immunological processes that contribute to these events, but there is a paucity of studies that address the impact of endogenous IL-27 on tumorogenesis.
IL-27 at barrier surfaces – colitis, asthma and psoriasis
Maladaptive Th2 and Th17 cell inflammatory responses are characteristic of several diseases that affect barrier sites that include asthma, colitis and psoriasis (Kay, 2006; McKinley et al., 2008; Pene et al., 2008). These are spectral conditions and grouping them together represents a gross simplification that ignores their complex relationship to conditions like lupus, ankylosing spondylitis and arthritis. In the setting of human psoriasis, IL-27 has been associated with promoting disease (Kanda and Watanabe, 2008; Shibata et al., 2012; Shibata et al., 2010) although studies that link IL-27 to keratinocyte and intestinal epithelial cell biology may alter how we view IL-27 acting at this local barrier site (Diegelmann et al., 2011; Kanda and Watanabe, 2008). Nevertheless, IL-27 represents an attractive candidate for a therapeutic approach to manage some of these diseases. In murine models of asthma, the absence of the IL-27Rα results in exacerbated lung pathology, characterized by goblet cell hyperplasia, infiltration of eosinophils, elevated serum IgE titers and airway hyper-responsiveness (Miyazaki et al., 2005; Yoshimoto et al., 2007). Similarly IL-27Rα deficient mice treated with a high dose of DSS develop more severe colitis associated with elevated Th17 cell activity (Troy et al., 2009) and in a TNBS model of acute colitis treatment with IL-27 can ameliorate disease (Sasaoka et al., 2011). However, there is a paradox that when low doses of DSS have been used to induce colitis IL-27 is thought to contribute to the development of disease (Honda et al., 2005). An alternative way to consider some of these findings is that the production of IL-17 is not solely pathogenic and has a role in promoting barrier function and limiting tissue damage (Esplugues et al., 2012; Kinugasa et al., 2000; O’Connor et al., 2009; Ogawa et al., 2004). Indeed, at low doses of DSS, IL-17 plays a role in protecting from disease (Ogawa et al., 2004) and in this type of situation the well characterized ability of IL-27 to antagonize IL-17 production could explain its pro-inflammatory activities.
Recognizing that the interpretation of the biological properties of IL-27 will be context dependent, a series of studies have emerged in the last 5 years that have highlighted polymorphisms in IL-27p28 that are connected with these conditions in humans. One of the first SNPs identified in IL-27p28 that was associated with disease was linked with susceptibility to asthma and increased IgE and eosinophilia (Chae et al., 2007) and similar linkages are reported for polymorphisms in IL-27p28 in chronic obstructive pulmonary disease (COPD) and IBD (Huang et al., 2008; Li et al., 2009). Perhaps the most comprehensive study surveyed a pediatric cohort, utilizing genome-wide association studies and high-density SNP analysis, identified IL-27p28 as a candidate gene for Crohn’s disease susceptibility (Imielinski et al., 2009). Data were presented that suggested that this was a consequence of reduced IL-27 production and was consistent with the idea that IL-27 (or IL-27p28) may play a role in preventing disease. These are all intriguing studies and suggest that IL-27p28 may be useful as a biomarker or in a select group of patients may be useful as a therapy, analogous to the use of IL-1Ra.
IL-27 and multiple sclerosis
In murine models of MS, treatment with IL-27 could delay the onset of Experimental Allergic Encephalmyelitis (EAE) and ameliorate established CNS disease and in certain models of EAE, the absence of the IL-27Rα results in more severe disease (Batten et al., 2006; Fitzgerald et al., 2007a; Fitzgerald et al., 2007b). Since then, a series of studies have provided a link between type I IFNs and IL-27 that may be clinically relevant. The first suggestion that these two cytokines were linked in MS was the realization that type I IFNs, which are used to treat MS, were potent inducers of IL-27 (Molle et al., 2007; Pirhonen et al., 2007b). Studies from different groups then linked the ability of type interferons to block disease in EAE to their ability to promote IL-27 (Guo et al., 2008; Shinohara et al., 2008). A clinical correlate of this finding was provided by a report that in patients with MS, the ability to produce IL-27 in response to type I interferons predicts the efficacy of interferon therapy (Sweeney et al., 2011b). Together, these findings suggest that the clinical efficacy of IFN-β in patients with MS may be attributed to its ability to induce IL-27 (or the IL-27p28 monomer) and it has been proposed that IL-27 may represent an alternative strategy to manage this condition.
IL-27 and the regulation of humoral responses in arthritis and lupus
Systemic Lupus Erythematosus (SLE) and arthritis are complex spectral diseases associated with the development of auto-antibodies that include pathognomonic levels of anti-dsDNA and rheumatoid factor respectively. In the clinical setting, increased levels of IL-27 have been observed in the synovial fluid of rheumatoid arthritis patients, which correlates with reduced local levels of IL-17 and IL-6, consistent with the inhibitory properties of IL-27 (Niedbala et al., 2008; Tanida et al., 2011). There are a plethora of models that reflect different facets of these clinical entities and some of the earliest studies that showed that treatment with IL-27 can attenuate disease was performed in collagen induced arthritis (Niedbala et al., 2008; Pickens et al., 2011). In contrast, during proteogylcan-induced arthritis endogenous IL-27 appears to promote disease (Cao et al., 2008).
In MRL/lpr mice, which exhibit a spontaneous disease similar to SLE, over-expression of the IL-27Rα results in decreased titers of self-reactive antibody and reduced skin disease (Kido et al., 2011; Sugiyama et al., 2008), likely a consequence of increased IL-27 signals. Further support for the idea that IL-27 affects auto-antibody responses is found in studies in which deletion of EBi3 in MRL/lpr mice resulted in increased titers of auto-antibodies, but surprisingly improved disease scores (Igawa et al., 2009). The report that there is a strong inverse correlation between serum amounts of IL-27 and active disease in SLE patients (Li et al., 2010b) has led to speculation that reduced IL-27 in some lupus patients can allow for the emergence of pathological T and B cell responses. One paradox with these findings is that SLE is associated with high amounts of type I interferons (Banchereau and Pascual, 2006), which are known to promote IL-27 (Pirhonen et al., 2007a; Remoli et al., 2007; Sweeney et al., 2011a). Thus, it is unclear why lupus patients exhibit lower amounts of IL-27 (Li et al., 2010a). Of course, the inherent heterogeneity that is apparent in this disease setting may mask direct associations between relative levels of IL-27 and the type I IFNs.
Given the critical role of antibodies in these disease states, there has been a focus on understanding the contribution of Tfh cells to the development of auto-antibody responses and IL-17 has been linked to these events (Doreau et al., 2009). The effects of IL-27 in this area have been understudied, but IL-27 can activate c-Maf signaling and upregulate IL-21 and these events are considered critical for Tfh cell responses(Bauquet et al., 2009; Nurieva et al., 2008; Pot et al., 2009). Indeed, culturing CD4+ T cells with IL-27 led to production IL-21 and vaccination of IL-27Rα−/− mice revealed that they had impaired IL-21 levels, decreased numbers of Tfh, and reduced production of high affinity class-switched antibody(Batten et al., 2010). It should be noted that IL-27 signaling is not required for the generation of antibody responses in models of infection, allergy and autoimmunity (Artis et al., 2004a; Miyazaki et al., 2005; Shimizu et al., 2005; Yoshida et al., 2001) and that IL-27 also has direct stimulatory effects on human B cells (Boumendjel et al., 2006; Larousserie et al., 2006; Yoshimoto et al., 2004) and can directly inhibit the growth of leukemic B cells (Canale et al., 2011). Understanding the context of when IL-27 promotes or limits humoral responses remains an important area of investigation.
Conclusions and Future Directions
One of the challenges that the field now faces is to determine whether manipulation of IL-27 could be used therapeutically to modulate inflammation that occurs during various human disease states. The difficulty in this area is making decisions about which diseases to target and the type of intervention that would be most appropriate. This could be in a direct fashion using agonists of the IL-27R while indirect strategies are suggested by studies showing that the ability of pro-biotic bacteria to induce IL-10, which can ameliorate experimental colitis, is dependent on IL-27 (Jeon et al., 2012). There may also be situations where the blockade of IL-27 would prove beneficial and appreciating how the role of IL-27 is likely to vary depending on the underlying disease cause will be important. Many of the human studies described above are informative in thinking about how the immunobiology of IL-27 may be translated, but questions remain about how individual polymorphisms might impact on the expression patterns of IL-27. Intriguingly, all of the polymorphisms reported to date have been associated with IL-27p28 and it is unclear whether this represents effects on the cytokine IL-27 and/or the ability of the IL-27p28 sub-unit to act as a receptor antagonist. Regardless, clinical studies have emerged, such as those with patients that express gain of function mutations of STAT1, that highlight the ability of IL-27 to suppress Th17 cells in humans(Liu et al., 2011). Another area in which further insights into the significance of IL-27 in humans may be gained are the clinical trials that target the Janus Kinases (JAK), which mediate the effects of IL-6, IL-12 and IL-23. Currently, JAK inhibitors that have been designed to target inflammatory pathways mediated by these latter cytokines are in clinical trials for arthritis and lupus (Fleischmann et al., 2012; Kremer et al., 2012). These inhibitors should also affect the JAKS utilized by IL-27 (as well as IL-10, and type I IFNS) and it is possible that treatment with these compounds may lead to dysregulation of the natural regulatory networks that limit inflammation. A lack of adverse inflammatory events would suggest that the endogenous regulatory pathways provided by IL-27 represent secondary responses to ongoing inflammation, rather than mechanisms that continuously enforce operational tolerance.
The last ten years have seen an evolution of our understanding of IL-27: from acting as a driver of Th1 cell responses, to the broad inhibitory effects of IL-27, to a realization that this is a factor that engages multiple lymphocyte populations to participate in a program of diverse regulatory mechanisms that are responsible for returning the immune response to homeostasis. From a signaling perspective, the growing appreciation of the distinct biological properties of IL-6, IL-12, IL-23 and IL-27 raises basic questions about how similar downstream signals are integrated to provide functionally disparate transcriptional programs. This is a question relevant to many biological systems and the fact that IL-6 and IL-27 both prominently activate STAT1 and STAT3 but have profoundly different effects on Th17 and Treg cells provides a system to dissect how apparently similar signals provide distinct functions. The more recent studies that have linked IL-27 to the production of IL-10, the activation of Treg cells and expression of PD-L1 highlight our lack of knowledge about how the suppressive activities of IL-27 are coordinated. These pathways may be redundant, act independently or may represent complementary cassettes that are coordinated in a parallel or linear fashion. Understanding the underlying biology of IL-27 can provide a template to understand how the immune system approaches the problem of tempering appropriate and aberrant responses.
Acknowledgments
Past and present members of the Hunter and Kastelein laboratory, the NIH, the American Asthma Foundation and the Commonwealth of Pennsylvania. Apologies for omissions necessitated by the brief nature of the article
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. T(H)17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183:4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, Sacks D. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol. 2011;186:3977–3985. doi: 10.4049/jimmunol.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D, Johnson LM, Joyce K, Saris C, Villarino A, Hunter CA, Scott P. Cutting Edge: Early IL-4 production governs the requirement for IL-27-WSX-1 signaling in the development of protective Th1 cytokine responses following Leishmania major infection. J Immunol. 2004a;172:4672–4675. doi: 10.4049/jimmunol.172.8.4672. [DOI] [PubMed] [Google Scholar]
- Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004b;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Ayimba E, Hegewald J, Segbena AY, Gantin RG, Lechner CJ, Agosssou A, Banla M, Soboslay PT. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol. 2011;166:218–226. doi: 10.1111/j.1365-2249.2011.04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007 doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting Edge: IL-27 Is a Potent Inducer of IL-10 but Not FoxP3 in Murine T Cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. Journal Clinical Investigation. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumendjel A, Tawk L, de Malefijt RW, Boulay V, Yssel H, Pene J. IL-27 induces the production of IgG1 by human B cells. Eur Cytokine Netw. 2006;17:281–289. [PubMed] [Google Scholar]
- Canale S, Cocco C, Frasson C, Seganfreddo E, Di Carlo E, Ognio E, Sorrentino C, Ribatti D, Zorzoli A, Basso G, et al. Interleukin-27 inhibits pediatric B-acute lymphoblastic leukemia cell spreading in a preclinical model. Leukemia. 2011;25:1815–1824. doi: 10.1038/leu.2011.158. [DOI] [PubMed] [Google Scholar]
- Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 Induces a Th1 Immune Response and Susceptibility to Experimental Arthritis. J Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, Yang YS, Chung HT. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- Charlot-Rabiega P, Bardel E, Dietrich C, Kastelein R, Devergne O. Signaling events involved in interleukin 27 (IL-27)-induced proliferation of human naive CD4+ T cells and B cells. J Biol Chem. 2011;286:27350–27362. doi: 10.1074/jbc.M111.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005 doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2010 doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabe S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, Mavoungou-Bigouagou U, Lefouili F, Cognet I, Ferlin W, et al. The IL-27 p28 Subunit Binds Cytokine-Like Factor 1 to Form a Cytokine Regulating NK and T Cell Activities Requiring IL-6R for Signaling. J Immunol. 2009 doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proceedings National Academy Science USA. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. Interleukin-30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology. 2012;55:1204–1214. doi: 10.1002/hep.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibra D, Cutrera JJ, Xia X, Birkenbach MP, Li S. Expression of WSX1 in tumors sensitizes IL-27 signaling-independent natural killer cell surveillance. Cancer Res. 2009;69:5505–5513. doi: 10.1158/0008-5472.CAN-08-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann J, Olszak T, Goke B, Blumberg RS, Brand S. A novel role for IL-27 as mediator of intestinal epithelial barrier protection mediated via differential STAT signaling and induction of antibacterial and anti-inflammatory proteins. J Biol Chem. 2011 doi: 10.1074/jbc.M111.294355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W, Jr, Rongvaux A, Van Rooijen N, Haberman AM, et al. Control of TH17 cells occurs in the small intestine. Nature. 2012;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay EG, Greig R, Stumhofer JS, Hafalla JC, de Souza JB, Saris CJ, Hunter CA, Riley EM, Couper KN. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. J Immunol. 2011;185:2482–2492. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007a;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007b;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, Gruben D, Krishnaswami S, Wallenstein G, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis and rheumatism. 2012;64:617–629. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- Freitas do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O’Garra A, Langhorne J. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188:1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10 mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. Journal Immunology. 1996;157:798–805. [PubMed] [Google Scholar]
- Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, et al. The Cytokines Interleukin 27 and Interferon-gamma Promote Distinct Treg Cell Populations Required to Limit Infection-Induced Pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, Sciume G, Hall AO, Dupont CD, Francisco LM, et al. Interleukin-27 Priming of T Cells Controls IL-17 Production In trans via Induction of the Ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Research. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J Immunol. 2009;183:6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 Receptor Chain WSX-1 Differentially Regulates Antibacterial Immunity and Survival during Experimental Tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- Honda K, Nakamura K, Matsui N, Takahashi M, Kitamura Y, Mizutani T, Harada N, Nawata H, Hamano S, Yoshida H. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm Bowel Dis. 2005;11:1044–1052. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- Huang N, Liu L, Wang XZ, Liu D, Yin SY, Yang XD. Association of interleukin (IL)-12 and IL-27 gene polymorphisms with chronic obstructive pulmonary disease in a Chinese population. DNA Cell Biol. 2008;27:527–531. doi: 10.1089/dna.2007.0715. [DOI] [PubMed] [Google Scholar]
- Huang ZQ, Wang JL, Pan GG, Wei YS. Association of single nucleotide polymorphisms in IL-12 and IL-27 genes with colorectal cancer risk. Clin Biochem. 2012;45:54–59. doi: 10.1016/j.clinbiochem.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- Hunter CA, Ellis L, Slifer T, Kanaly S, Grunig G, Rennick D, Araujo F. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. Journal Immunology. 1997;158:3311–3316. [PubMed] [Google Scholar]
- Igawa T, Nakashima H, Sadanaga A, Masutani K, Miyake K, Shimizu S, Takeda A, Hamano S, Yoshida H. Deficiency in EBV-induced gene 3 (EBI3) in MRL/lpr mice results in pathological alteration of autoimmune glomerulonephritis and sialadenitis. Modern rheumatology / the Japan Rheumatism Association. 2009;19:33–74. doi: 10.1007/s10165-008-0117-1. [DOI] [PubMed] [Google Scholar]
- Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, Kugathasan S, Bradfield JP, Walters TD, Sleiman P, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8:e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. IL-12, IL-23, and IL-27 enhance human beta-defensin-2 production in human keratinocytes. Eur J Immunol. 2008;38:1287–1296. doi: 10.1002/eji.200738051. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Kay AB. The role of T lymphocytes in asthma. Chem Immunol Allergy. 2006;91:59–75. doi: 10.1159/000090230. [DOI] [PubMed] [Google Scholar]
- Kido M, Takeuchi S, Sugiyama N, Esaki H, Nakashima H, Yoshida H, Furue M. T cell-specific overexpression of interleukin-27 receptor α subunit (WSX-1) prevents spontaneous skin inflammation in MRL/lpr mice. The British journal of dermatology. 2011;164:1214–1234. doi: 10.1111/j.1365-2133.2011.10244.x. [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis and rheumatism. 2012;64:970–981. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- Li CS, Zhang Q, Lee KJ, Cho SW, Lee KM, Hahm KB, Choi SC, Yun KJ, Chung HT, Chae SC. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol. 2009;24:1692–1696. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- Li TT, Zhang T, Chen GM, Zhu QQ, Tao JH, Pan HF, Ye DQ. Low level of serum interleukin 27 in patients with systemic lupus erythematosus. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2010a;58:737–746. doi: 10.231/JIM.0b013e3181d88f7b. [DOI] [PubMed] [Google Scholar]
- Li TT, Zhang T, Chen GM, Zhu QQ, Tao JH, Pan HF, Ye DQ. Low level of serum interleukin 27 in patients with systemic lupus erythematosus. J Investig Med. 2010b;58:737–739. doi: 10.231/JIM.0b013e3181d88f7b. [DOI] [PubMed] [Google Scholar]
- Liao JJ, Huang MC, Goetzl EJ. Cutting edge: Alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–5428. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KD, Mohrs K, Reiley W, Wittmer S, Kohlmeier JE, Pearl JE, Cooper AM, Johnson LL, Woodland DL, Mohrs M. Cutting Edge: T-bet and IL-27R Are Critical for In Vivo IFN-{gamma} Production by CD8 T Cells during Infection. J Immunol. 2008;180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, Hamano S, Yoshimura A, Yoshida H. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- Molle C, Nguyen M, Flamand V, Renneson J, Trottein F, De Wit D, Willems F, Goldman M, Goriely S. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178:7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, Liew FY. Interleukin 27 attenuates collagen-induced arthritis. Annals of the rheumatic diseases. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 Induces Th1 Differentiation via p38 MAPK/T-bet- and Intercellular Adhesion Molecule-1/LFA-1/ERK1/2-Dependent Pathways. J Immunol. 2006;177:7579–7587. doi: 10.4049/jimmunol.177.11.7579. [DOI] [PubMed] [Google Scholar]
- Pan HF, Tao JH, Ye DQ. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert Opin Ther Targets. 2010;14:479–484. doi: 10.1517/14728221003769911. [DOI] [PubMed] [Google Scholar]
- Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S, Moles JP, Danger Y, Ravon E, Lesaux S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis and rheumatism. 2011;63:2289–2298. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. Journal of leukocyte biology. 2007a;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007b;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A, Lambert QT, Reuther GW. Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor. Proc Natl Acad Sci U S A. 2007;104:18502–18507. doi: 10.1073/pnas.0702388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. European journal of immunology. 2007;37:3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-Cell Cytokine Receptor) gene-deficient mice display enhanced resistance to Leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Basset L, Froger J, Dinguirard N, Chevalier S, Gascan H. IL-27 structural analysis demonstrates similarities with ciliary neurotrophic factor (CNTF) and leads to the identification of antagonistic variants. Proc Natl Acad Sci U S A. 2010;107:19420–19425. doi: 10.1073/pnas.1005793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Hixon JA, Stauffer JK, Jalah R, Brooks AD, Khan T, Dai RM, Scheetz L, Lincoln E, Back TC, et al. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol. 2009;182:4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- Sasaoka T, Ito M, Yamashita J, Nakajima K, Tanaka I, Narita M, Hara Y, Hada K, Takahashi M, Ohno Y, et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G568–576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- Seita J, Asakawa M, Ooehara J, Takayanagi S, Morita Y, Watanabe N, Fujita K, Kudo M, Mizuguchi J, Ema H, et al. Interleukin-27 directly induces differentiation in hematopoietic stem cells. Blood. 2008;111:1903–1912. doi: 10.1182/blood-2007-06-093328. [DOI] [PubMed] [Google Scholar]
- Shibata S, Tada Y, Asano Y, Yanaba K, Sugaya M, Kadono T, Kanda N, Watanabe S, Sato S. IL-27 Activates Th1-Mediated Responses in Imiquimod-Induced Psoriasis-Like Skin Lesions. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.313. [DOI] [PubMed] [Google Scholar]
- Shibata S, Tada Y, Kanda N, Nashiro K, Kamata M, Karakawa M, Miyagaki T, Kai H, Saeki H, Shirakata Y, et al. Possible roles of IL-27 in the pathogenesis of psoriasis. J Invest Dermatol. 2010;130:1034–1039. doi: 10.1038/jid.2009.349. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, et al. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- Shimozato O, Sato A, Kawamura K, Chiyo M, Ma G, Li Q, Tagawa M. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology. 2009;128:e816–825. doi: 10.1111/j.1365-2567.2009.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, Fielding CA, O’Hara AC, Chen Y, Jones ML, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Nakashima H, Yoshimura T, Sadanaga A, Shimizu S, Masutani K, Igawa T, Akahoshi M, Miyake K, Takeda A, et al. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of interleukin 27 receptor alpha (WSX-1) Annals of the rheumatic diseases. 2008;67:1461–1468. doi: 10.1136/ard.2007.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4(+) T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011 doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Lonergan R, Basdeo S, Kinsella K, Dungan L, Higgins S, Kelly P, Costelloe L, Tubridy N, Mills K, Fletcher J. IL-27 mediates the response to IFN-β therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain, behavior, and immunity. 2011a;25:1170–1251. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011b;25:1170–1181. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. Journal Immunology. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- Tanida S, Yoshitomi H, Ishikawa M, Kasahara T, Murata K, Shibuya H, Ito H, Nakamura T. IL-27-producing CD14(+) cells infiltrate inflamed joints of rheumatoid arthritis and regulate inflammation and chemotactic migration. Cytokine. 2011;55:237–244. doi: 10.1016/j.cyto.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Troy AE, Zaph C, Du Y, Taylor BC, Guild KJ, Hunter CA, Saris CJ, Artis D. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 Limits IL-2 Production during Th1 Differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- Wang H, Meng R, Li Z, Yang B, Liu Y, Huang F, Zhang J, Chen H, Wu C. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol Lett. 2011;136:21–28. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis (EAU) by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem. 2012 doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wojno ED, Hosken N, Stumhofer JS, O’Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- Zorzoli A, Di Carlo E, Cocco C, Ognio E, Ribatti D, Ferretti E, Dufour C, Locatelli F, Montagna D, Airoldi I. Interleukin-27 inhibits the growth of pediatric acute myeloid leukemia in NOD/SCID/Il2rg-/- mice. Clin Cancer Res. 2012;18:1630–1640. doi: 10.1158/1078-0432.CCR-11-2432. [DOI] [PubMed] [Google Scholar]


