Abstract
Genetic variation can have important consequences for populations: high population genetic diversity is typically associated with ecological success. Some mechanisms that account for these benefits assume that local social groups with high genetic diversity are more successful than low-diversity groups. At the same time, active decision-making by individuals can influence group genetic diversity. Here, we examine how maternal decisions that determine group genetic diversity influence the viability of Drosophila melanogaster larvae. Our groups contained wild-type larvae, whose genetic diversity we manipulated, and genetically marked ‘tester’ larvae, whose genotype and frequency were identical in all trials. We measured wild-type and tester viability for each group. Surprisingly, the viability of wild-type larvae was neither augmented nor reduced when group genetic diversity was altered. However, the viability of the tester genotype was substantially depressed in large, high-diversity groups. Further, not all high-diversity groups produced this effect: certain combinations of wild-type genotypes were deleterious to tester viability, while other groups of the same diversity—but containing different wild-type genotypes—were not deleterious. These deleterious combinations of wild-type genotypes could not be predicted by observing the performance of the same tester and wild-type genotypes in low-diversity groups. Taken together, these results suggest that nonadditive interactions among genotypes, rather than genetic diversity per se, account for between-group differences in viability in D. melanogaster and that predicting the consequences of genetic diversity at the population level may not be straightforward.
Keywords: D. melanogaster, diversity, group size, nonadditive, social interactions, viability
Introduction
Intrapopulation genetic variation is fundamental to bio-diversity. Recently, studies have uncovered pervasive effects of genetic diversity on key ecological processes. Populations with high genetic diversity may enjoy greater productivity and abundance (reviewed in Hughes et al. 2008; Bolnick et al. 2011); improved colonization success (Crawford & Whitney 2010; but see Tsutsui et al. 2000); greater likelihood of adaptive evolution in response to new ecological challenges (Lesica & Allendorf 1995; Fisher 2000; Agashe 2009; Agashe & Bolnick 2010; Agashe et al. 2011; Strasburg et al. 2011); and improved resistance to disturbance (Hughes & Stachowicz 2004). Understanding the mechanisms of these diversity benefits is critical to making evolutionary predictions and to conservation efforts targeted at increasing diversity.
Ecological benefits of diversity are evident at the population level, yet many proposed mechanisms of such benefits rely on local interactions. For example, benefits of niche partitioning occur only when organisms are interacting enough to potentially influence each other’s resource availability. Similarly, low diversity may cause deleterious effects by forcing individuals to inbreed; these effects only apply to organisms close enough to potentially mate. Thus, many benefits of genetic diversity at the population level may be a direct outcome of diversity within groups, that is, among individuals who interact most frequently. For organisms with limited opportunities to move among groups, including many juvenile animals, conspecifics in close spatial proximity are most likely to engage in competition, facilitation and other processes influencing performance.
Given the potential importance of group genetic diversity, it is critical to understand the processes that determine how genotypes are distributed among groups within populations. Organisms play an active role in determining the identities of their associates, and different active group formation processes have dramatically different impacts on group genetic diversity (Storz 1999). For example, some patterns of group formation cause kin to interact more frequently with each other than with other members of the population. These processes include limited dispersal (Wilson 1975; Archie et al. 2008) and kin preference (Grosberg & Quinn 1986; Waldman 1988). Because relatives are genetically similar, groups in which individuals are closely related have low genetic diversity, even when population-level diversity is relatively high. By contrast, some processes—including long-distance dispersal and inbreeding avoidance—cause kin to interact infrequently (reviewed in Pusey & Wolf 1996; Johnson & Woollacott 2010). When these processes operate, groups should be more genetically diverse than in the former case, even at the same population level of genetic diversity.
If group genetic diversity plays an important causal role in the observed beneficial outcomes of population genetic diversity, then group formation processes that promote high group genetic diversity should be associated with more productive groups. By contrast, if group genetic diversity is not related to beneficial population outcomes, then group-level genetic diversity should be unrelated to group productivity—or even negatively related, if kin groups enjoy higher viability than groups of unrelated individuals (Smith 1964; Wilson 1975). Testing this hypothesis in nature is often infeasible, because the various effects of group formation processes, population-level genetic diversity, selection, chance and abiotic factors on group genetic diversity and success are difficult to disentangle.
To circumvent these problems, we studied groups of Drosophila melanogaster larvae whose genotypic composition we controlled. D. melanogaster is an ideal system to test the effects of group genetic diversity. Flies live socially in nature and have many opportunities to make social decisions. In the wild, females can mate multiply and unrelated females may lay eggs on the same substrate (Atkinson 1979; Hoffmann & Nielsen 1985; Harshman & Clark 1998). In the laboratory, natural, heterozygous genotypes may be replicated, enabling precise control over group genetic diversity.
The frequency of movement among fruits by larval D. melanogaster has not been directly measured, but a number of factors strongly suggest that larval dispersal is rare. Sokolowski (1985) showed under semi-natural conditions that pupal survival is substantially lower off-food than on-food, except under very wet soil conditions (soil moisture >50%; Sokolowski 1985). First-instar larvae are much smaller than pupae and desiccate rapidly (J.B.S., personal observation), suggesting that larvae who leave the food substrate may suffer high rates of mortality. Most importantly, direct measurement of the relatedness of flies eclosing from single fruits collected in nature have revealed that individuals from the same fruit are generally related; on average, newly eclosed adults represent the progeny of 2–3 females (Hoffmann & Nielsen 1985).
Taken together, the available data indicate that larval groups are defined primarily by spatial proximity and that female egg-laying decisions are the primary determinant of group genetic diversity. As such, Drosophila populations can be considered as a set of relatively isolated groups of larvae connected by highly mobile adults who can freely interbreed.
To assess the effects of group genetic diversity on larval viability, we formed groups designed to mimic the genetic effects of three different group formation processes (Table 1) and measured the number of individuals who survived to adulthood. The group formation processes we experimentally simulated were based on two simple, common maternal decisions: the decision to re-mate, and the decision to share rearing sites with other females. These maternal decisions have important consequences for the genetic diversity of their off-spring’s group. When a female re-mates during a single breeding season and produces multiple offspring, her progeny develop with both full- and half-siblings. Thus, the offspring of females who re-mate are likely to develop in an environment with high genetic diversity, relative to the offspring of females who do not re-mate (Jennions & Petrie 2000; Mattila & Seeley 2007; Caesar et al. 2010; Aguirre & Marshall 2011). Similarly, females of some species may increase the genetic diversity of their progeny’s environment by choosing to share or parasitize on breeding or rearing sites with other females (Hoffmann & Nielsen 1985; Krakauer & Kimball 2009). When females share breeding sites, their offspring develop with both siblings and nonsiblings; when females do not share breeding sites, their offspring develop only with siblings.
Table 1.
Description of experiments
| Small groups contained 72 larvae, large groups contained 144 larvae | |||||
|---|---|---|---|---|---|
| Experiment | Number of genotypes per group |
Patterns of relatedness among wild-type genotypes |
Representative natural situation |
Number of genotype combinations |
Total number of groups analysed |
| Low diversity | 1 wild type + tester | N/A | Singly-mated females | 20 | 348 (178 small groups, 170 large groups) |
| Medium diversity | 3 wild type + tester | Maternal half-sibs | Multiply-mated female | 20 | 354 (181 small groups, 173 large groups) |
| High diversity | 9 wild type + tester | Unrelated, maternal half-sibs and paternal half-sibs. | Several unrelated females | 20 | 353 (177 small groups, 176 large groups) |
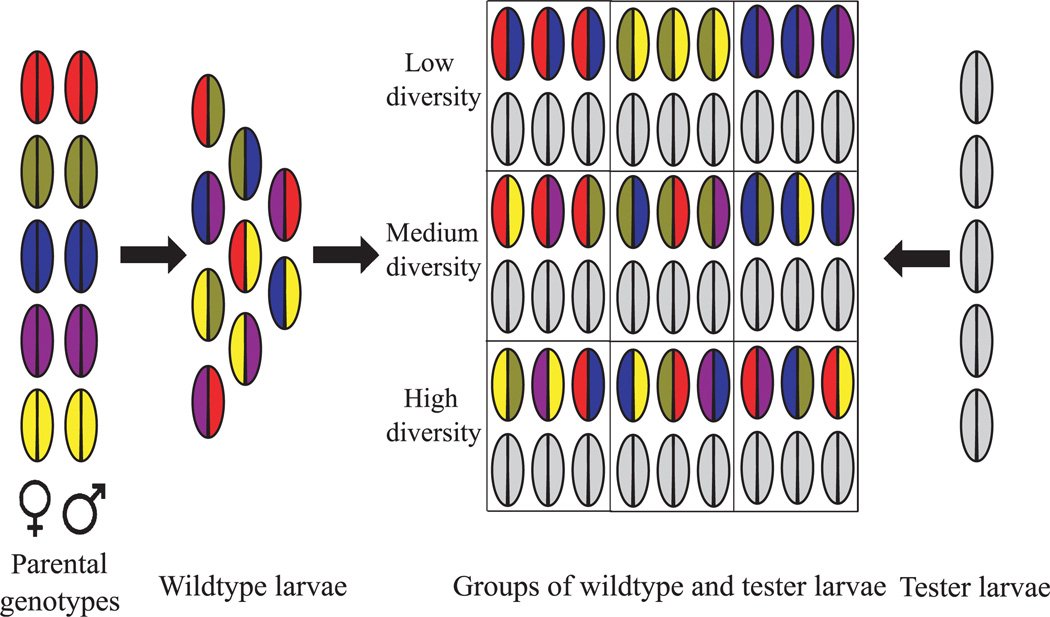
Our three levels of group genetic diversity—low, medium and high—reproduced the potential genetic outcomes of these maternal decisions (Table 1, Fig. 1). In all groups, half of the larvae present were from a standard ‘tester’ genotype (described in more detail below); we directly manipulated the genetic diversity of the remaining, wild-type larvae. In the low-diversity experiment, one heterozygous wild-type genotype interacted with the tester genotype. This mimicked the situation in which two singly-mated females (one wild type, one tester) laid eggs on a food patch. The medium-diversity experiment included three wild-type genotypes, all maternal half-sibs, plus the tester genotype; this mimicked the situation in which one multiply-mated wild-type female laid eggs on a food patch with just one other female (the tester genotype). In the high-diversity experiment, the tester genotype interacted with nine wild-type genotypes; some were half-sibs while others were unrelated. The high-diversity experiment thus mimicked the situation in which several unrelated, multiply-mated females laid eggs on the same food patch. Our treatments thus simulated scenarios that are relevant to the natural history of fly larvae (Table 1, Fig. 1). To measure the outcome of group genetic diversity, we recorded the number of wild-type and tester adults that eclosed from each group.
Fig. 1.
Schematic representation of experimental design. Wild-type inbred lines are crossed to produce heterozygous wild-type progeny (left), which are then combined into groups of different diversities (centre). All groups also contained 50% tester larvae (right) which were marked with a visible mutation. We generated 20 genotype combinations at each level of diversity (here only three examples are depicted) and high-diversity groups contained nine wild-type genotypes (here only three example genotypes are depicted). For more details about the experimental design, see the text, Table 1 and Appendix S1, Supporting Information.
We used the same 20 heterozygous genotypes in all experiments, and these genotypes were represented at the same population-level frequencies (i.e. in low-, medium- and high-diversity experiments, each genotype made up 5% of the total population). Thus, our manipulations of group-level diversity represent groups formed by different maternal decisions, at the same level of population genetic diversity. If group-level genetic diversity influences population-level outcomes, as described above, then we expect differences in average viability among our three levels of diversity.
Each level of diversity included 20 different combinations of genotypes. By comparing genotype combinations, we could determine whether viability was influenced by diversity per se or by specific genotypes or genotype combinations. By comparing viability effects associated with each genotype at different diversities, we could further evaluate whether effects of group diversity were because of additive or nonadditive effects. If wild-type genotypes affect viability additively, then the viability of wild-type larvae in high-diversity groups should be predictable, based on the performance of each genotype in the low-diversity experiment (Hughes et al. 2008).
As described above, half of the larvae in every group were from the tester genotype, which is marked with a visible eye mutation (Table 1, Fig. 1). The marker identified tester individuals who survived to adulthood, which allowed us to separately measure tester viability and wild-type viability in each group. As tester larvae were present at the same frequency (i.e. 50%) in all groups, tester viability offers a sensitive measure of how interactions among wild-type genotypes influenced the environment experienced by the group. Specifically, if variation in diversity affects only the wild-type genotypes whose frequencies we manipulated, tester viability should be identical in all experiments. By contrast, if the diversity or genotypic combination of wild-type larvae affects the environment experienced by all larvae, then tester viability may vary across groups.
We repeated all experiments at two group sizes (72 larvae and 144 larvae), and larvae interacted in a food-limited environment, which maximizes the potential to detect competitive or facilitative effects of diversity (Pérez-Tomé & Toro 1982; Fowler & Partridge 1986; Martin et al. 1988; Santos et al. 1992; Lopez-Suarez et al. 1993; Fitzpatrick et al. 2007).
Materials and methods
Experimental methods
Genotypes
Parental genotypes were from a natural population in Raleigh, NC. Genotypes were randomly chosen from the collection of lines that were recently resequenced as part of the Drosophila Population Genomics Project (www.dpgp.org). The genotypes were 208, 313, 335, 360 and 765. All wild-type flies used in the experiment were F1s generated by crossing these parental lines. Thus, flies were heterozygous in all treatments, ensuring that inbreeding effects are not confounded with effects of group diversity.
The tester genotype was the Oregon-R line, which contains the visible eye mutation sparkling-poliert (Nuzhdin et al. 1998; similar to Santos et al. 1992). This allowed us to distinguish wild-type and tester adults.
Fly rearing
Wild-type and tester larvae were generated by adding 75 virgin females of a single genotype, and 50 males from a single, different genotype to a bottle. For example, to generate larvae of the genotype 208/313 (where genotypes are written as maternal/paternal), 75 virgin females of genotype 208 were added to a bottle with 50, 313-genotype males. After 1 day, females were allowed to lay eggs on grape medium (consisting of 30 g agar, 700 mL deionized water, 0.5 g tegocept, 10.0 mL ethanol, 300 mL grape juice; to each plate we also added a small smear of live yeast) for a 2-hour period in the morning. Eggs laid during the 2-hour period were allowed to develop for 27 h; and the resulting first-instar larvae were used in the experiment. This design ensures that genetic variability in hatch rate does not confound estimates of larval viability (Fowler & Partridge 1986).
Group formation
Using a paintbrush, first-instar larvae were added to low-food vials. The vials were 28 mm in diameter and contained 2 mL of agar with 1 mL of low-nutrient food on top. The food was made from 0.3 g agar, 4.3 g malt sugar, 4.7 g corn flour, 0.9 g dead yeast and 0.15 g tegocept. This represents a severe reduction in food availability, compared to standard Drosophila medium (similar to Santos et al. 1992). Because the vials were small and the 1 mL of food was confined to the region atop the agar, it is likely larvae had the opportunity to interact with most individuals in their group (at least indirectly) during development.
Eclosing adults were removed daily to prevent newly eclosed adults from laying additional eggs in the food. All experiments were run concurrently over a 9-month period, in a room maintained at 72°F on a 12:12 L:D cycle.
Treatments
All vials contained 50% tester larvae and 50% wild-type larvae. We varied the total number of flies, and the genotypes of the wild-type flies, as follows.
Group size
Small groups contained 72 larvae (36 wild type, 36 tester) per vial. Large groups contained 144 larvae (72 wild type, 72 tester) per vial. This manipulation influences the number and the density of larvae in the vials, and the amount of food per larva.
Diversities
In the low-diversity experiment, groups contained one wild-type genotype and the tester genotype. The medium-diversity experiment included the tester and three wild-type genotypes, all maternal half-sibs; this mimicked the situation in which the wild-type female mated multiply. The high-diversity experiment included tester larvae and nine wild-type genotypes; some were half-sibs while others were unrelated. The high-diversity experiment thus mimics the situation in which several unrelated, multiply-mated females lay eggs on the same food patch. All 20 heterozygous genotypes were present in all three experiments, in equal proportions. Thus, any differences between diversity experiments are because of variation in group-level genetic diversity.
Genotype combinations
The five parental genotypes permit production of 20 heterozygous progeny genotypes (i.e. if all parental lines are crossed to all other parental lines, excluding self-crosses). Thus, there were 20 genotype combinations—one for each heterozygous genotype—in the low-diversity experiment.
In the medium-diversity experiment, the three wild-type genotypes represented were maternal half-sibs. Each wild-type maternal genotype was represented in four genotype combinations, representing matings with all possible sets of three male genotypes. All wild-type genotypes were represented at equal frequency. For example, a large-group, medium-diversity vial might contain 24 208/313 larvae, 24 208/335 larvae, 24 208/360 larvae and 72 tester larvae. A different treatment included 24 208/313 larvae, 24 208/335 larvae, 24 208/765 larvae and 72 tester larvae. Thus, there were 20 genotype combinations (five maternal genotypes times four sets of paternal genotypes) in the medium-diversity experiment.
In the high-diversity experiment, there were nine wild-type genotypes in each genotype combination. To determine the genotype combinations, we divided the 20 genotypes into blocks of 10 genotypes; each incomplete diallel was a block. Each genotype combination contained nine of the 10 genotypes from one block. Thus, every genotype was ‘left out’ once, generating 10 combinations per block for a total of 20 treatments. Wild-type genotypes were represented at equal frequency. Note that this design is not a full factorial, because genotypes in separate blocks did not co-occur in any genotype combinations (see Appendix S1, Supporting Information).
Because the design was balanced, wild-type genotypes were represented at equal frequencies in the low-, medium- and high-diversity experiments. All genotype combination × diversity × group size combinations were replicated 7–10 times (mean = 8.67 times), for a total of 1055 vials (Table 1).
Analysis
Estimating viability
For each vial, we calculated two measures of viability.
Absolute viability
Wild type absolute viability was calculated as the number of wild-type flies that eclosed divided by the number of wild-type larvae that we originally added to the vial (i.e. 36 for small groups, 72 for large groups). Tester absolute viability was calculated equivalently. These two metrics describe the proportion of larvae of each type that survived to eclosion.
Composite index of relative viability
To describe the viability of the wild-type larvae relative to the tester larvae in each vial, we constructed a composite index:
Number of wild-type flies that eclosed/total number of flies that eclosed
This metric thus describes, for each group, the proportion of survivors that were wild type. A composite index of 0.5 indicates that equal number of tester and wild-type larvae survived to eclosion; a composite index of 1 indicates that only wild-type individuals survived.
Statistical analysis
Analyses were conducted using sas version 9.2 (SAS Institute, Cary, NC). Generalized linear mixed models were performed using Proc Glimmix. Where applicable, group size, diversity, and the date the vial was created were modelled as fixed factors. As the data were proportions, we used a binomial distribution function and logit link function. All fixed-effect models included an overdispersion parameter to avoid inflation of F statistics (Schabenberger 2007).
To evaluate differences between genotype combinations, we used likelihood ratio tests (LRTs). For each level of group size × diversity, we fit a model with date as a fixed effect and genotype combination as a random effect. Models with and without the random effect were compared to calculate likelihood ratios (LRs).
Results
Flies in larger groups suffered significantly reduced absolute viability
Group size had profound effects on both tester (F1,919 = 348.35, P < 0.0001) and wild-type (F1,888 = 59.48, P < 0.0001) absolute viability. In both cases, absolute viability was substantially reduced in the large group size treatment (Fig. 3). Specifically, for tester larvae, the odds of surviving were 3.06 times greater in small groups, compared to large groups. For wild-type larvae, the odds of surviving were 1.58 times greater in small groups, relative to large groups. Given that wild-type and tester larvae developed in the same environment, these figures demonstrate that the effects of group size were genotype-dependent.
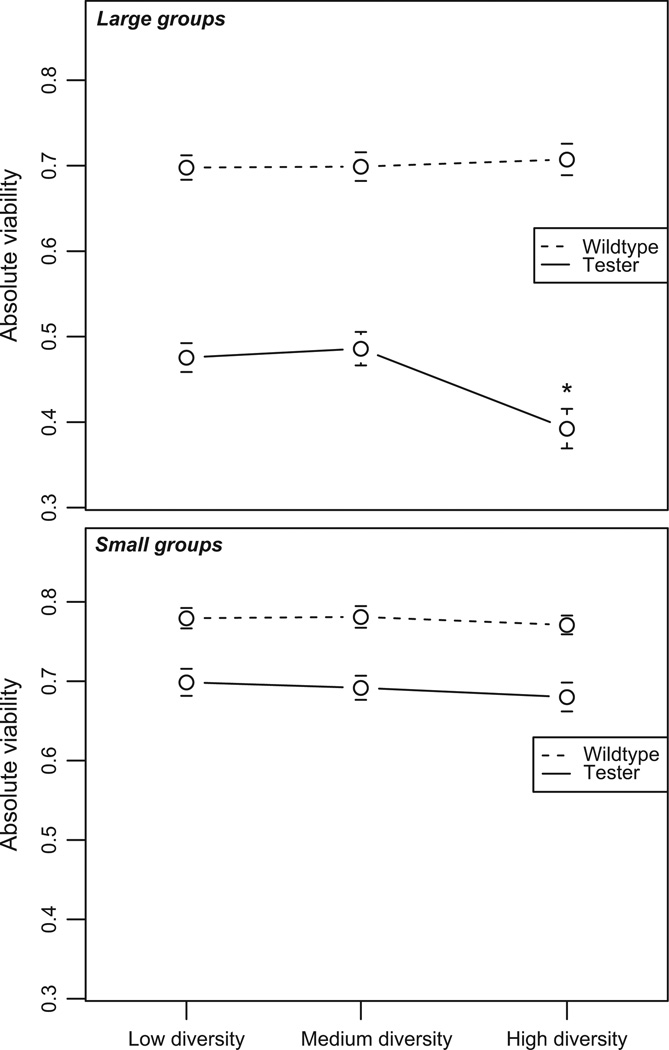
Fig. 3.
Absolute viability for wild-type and tester individuals at each level of diversity. The x-axis describes group diversity; the y-axis represents the absolute proportion of larvae of each type that survived to eclosion. Dots represent viability means ± SEM. In small groups (lower graph), diversity has no effect on wild-type or tester absolute viability. In large groups (upper graph), tester viability is significantly depressed (indicated by *) at high diversity.
Group-level diversity alters relative viability by disproportionately impacting tester larvae
Relative viability is highest in large, high-diversity groups
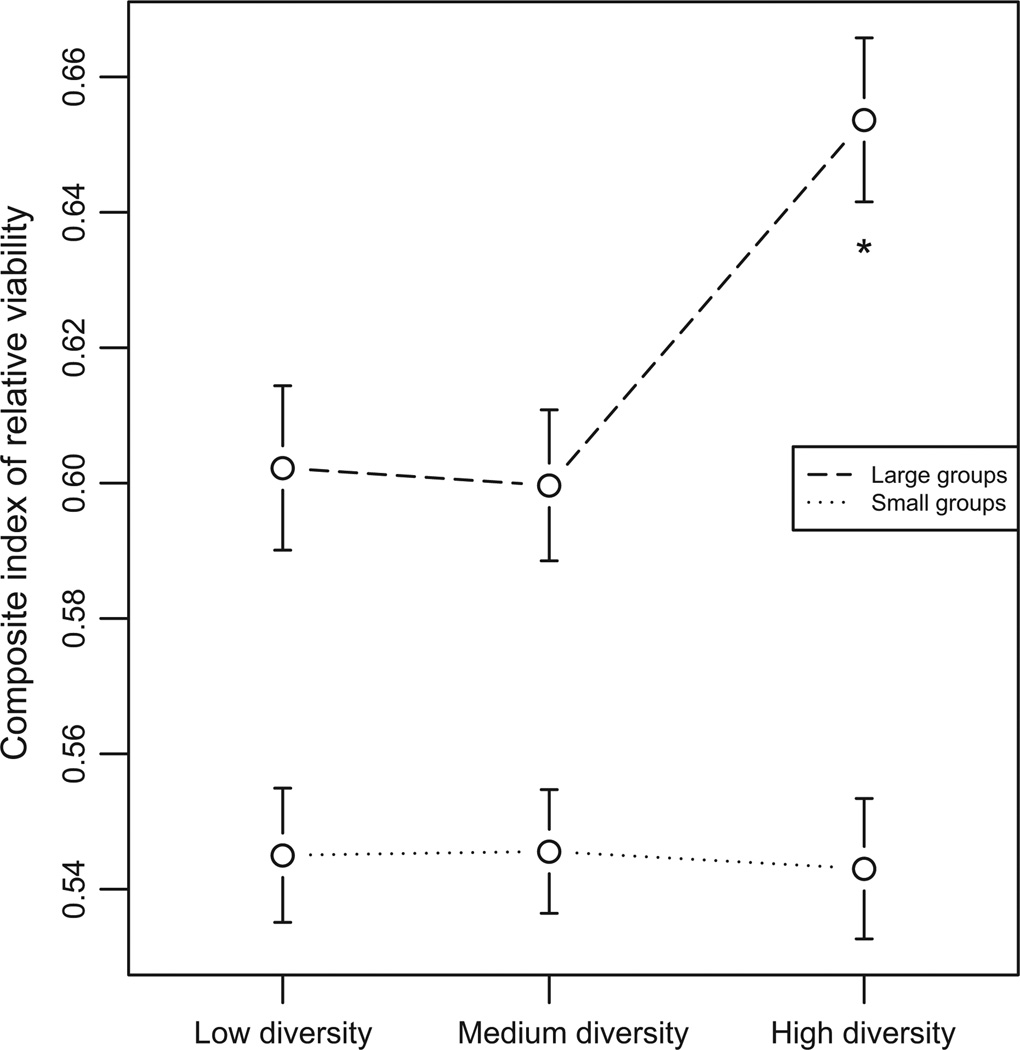
We modelled the effects of diversity on viability using a generalized linear mixed model as described above. We found no evidence that diversity affected the composite index of relative viability in small groups (F2,425 = 0.03, P = 0.973). By contrast, diversity significantly predicted relative viability in large groups (F2,415 = 5.71, P = 0.0036) (Fig. 2). Examination of the mean values of relative viability at each level of diversity indicated that relative viability was similar at low and medium diversities (low vs. medium: t415 = 0.28, P = 1 after Bonferroni correction for three tests); but relative viability at high diversity significantly exceeded relative viability at the other two levels of diversity (high vs. low: t415 = 2.91, corrected P = 0.0114; high vs. medium: t415 = 3.26, corrected P = 0.0036). These results indicate that, in large groups, wild-type larvae represented a larger proportion of surviving flies at high diversity, compared to the other two diversities.
Fig. 2.
Composite index of relative viability at each level of diversity. The x-axis describes group diversity. The y-axis describes the number of surviving wild-type flies from each group, divided by the total number of survivors. Dots represent viability means ± SEM. Dotted line represents small groups; dashed line represents large groups. In small groups, relative viability is not affected by genetic diversity. In large groups, relative viability is significantly higher (indicated by *) at high diversity, compared to other diversities.
Group-level diversity affects tester, but not wild type, absolute viability
Again using a generalized linear mixed model, we found no evidence of a diversity effect on wild-type absolute viability at either group size (large: F2,409 = 0.06, P = 0.9381; small: F2,387 = 0.19, P = 0.827) (Fig. 3).
For tester absolute viability, we found an effect of diversity only in large groups [large: F2,415 = 5.03, P = 0.007 (Fig. 3, upper); small: F2,411 = 0.26, P = 0.768 (Fig. 3, lower)]. Comparing the mean absolute viability at each level of diversity revealed that tester viability in the low-diversity and medium-diversity experiments was indistinguishable (low vs. medium: t415 = −0.43, P = 1 after Bonferroni correction for three tests). By contrast, tester larvae experienced significantly greater mortality at high diversity, compared to the other two diversities (high vs. low: t415 = −2.65, corrected P = 0.0252; high vs. medium: t415 = −3.09, corrected P = 0.0063). In fact, the odds of tester larvae surviving were 1.4 times greater in the low-diversity experiment than in the high-diversity experiment and 1.5 times greater in the medium-diversity experiment than in the high-diversity experiment. Thus, tester larvae were strongly affected by the wild-type genotypes present in their environment.
Taken together, these results demonstrate that the difference in relative viability in large, high-diversity groups was caused by comparatively greater tester mortality in those groups.
Genotype combinations influence absolute viability
The finding that wild-type and tester larvae were affected differently by diversity indicates that the effects of the group genetic diversity depend on larval genotype. To assess whether this was also true within levels of diversity, we used LRTs as described above. These tests evaluate whether including ‘genotype combination’ as a random effect significantly improves the fit of the model. The LRTs were significant for both wild-type and tester absolute viability, at all levels of group size and diversity (Table 2).
Table 2.
Results of likelihood ratio (LR) tests establishing that the combination of wild-type genotypes present in a group influence wild-type and tester absolute viability
| Wild-type absolute viability |
Tester absolute viability |
||||
|---|---|---|---|---|---|
| Diversity | Group size | LR | P | LR | P |
| Low | Small | 137.96 | <0.0001 | 77.63 | <0.0001 |
| Low | Large | 167.48 | <0.0001 | 195.51 | <0.0001 |
| Medium | Small | 58.72 | <0.0001 | 66.59 | <0.0001 |
| Medium | Large | 111.92 | <0.0001 | 201.23 | <0.0001 |
| High | Small | 4.68 | 0.0305 | 29.77 | <0.0001 |
| High | Large | 93.48 | <0.0001 | 47.59 | <0.0001 |
This result indicates that the combination of wild-type genotypes present in a group predict wild-type larval survival. As seen for the diversity manipulations, the combination of wild-type genotypes also exerted strong effects on tester viability, even though tester genotype and frequency was identical in all trials.
Which genotype combinations contribute to the deleterious effects on tester viability at high diversity?
Given that different genotype combinations resulted in different tester absolute viabilities, we wished to identify specific combinations contributing to the decline in tester fitness in the large-group, high-diversity treatment. To do so, we modelled tester absolute viability with genotype combination and date as fixed effects, and a random residual to account for overdispersion. We extracted least-squared means and confidence intervals for each genotype combination in the large-group, high-diversity experiment. We compared these to mean tester viability in the overall experiment at low diversity, reasoning that high-diversity genotype combinations with low tester viability (relative to the population mean in the low-diversity experiment) would be most likely to account for the high-diversity effects on tester viability.
We found eight high-diversity genotype combinations (three from block 1, five from block 2) matching this description, with confidence intervals for tester absolute viability that did not overlap with the confidence interval for low-diversity tester viability. The combinations were as follows: A, B, K, L, M, N, Q and T (see Appendix S1, Supporting Information).
We found no high-diversity genotype combinations with confidence intervals for tester viability that exceeded tester viability at low diversity. This is not surprising given the overall population-level results (Fig. 3).
Deleterious effects on tester viability are because of nonadditive effects of wild-type genotypes
Why did the eight high-diversity genotype combinations identified above have especially deleterious effects on tester absolute viability? Perhaps these combinations simply include many of the most harmful (to the tester) wild-type genotypes; if so, the especially deleterious effects are simply an additive outcome that can be predicted from each genotype’s effect on tester viability at low diversity. By contrast, the especially deleterious effects could arise from nonadditive interactions among wild-type genotypes.
We used contrasts to discriminate among these hypotheses. These contrasts compare the absolute viability of the tester larvae when interacting with a combination of wild-type genotypes at high diversity, to the absolute viability of tester larvae when interacting with the same wild-type genotypes at low diversity. The contrasts test the null hypothesis that the viability effects on the tester of a given genotype combination can be predicted using the average of each genotype’s individual effect on the tester, as measured in the low-diversity experiment. Significant parameter estimates for contrasts would indicate that some genotype combinations exerted nonadditive effects on tester viability, that is, effects that differed from the prediction based on the low-diversity experiment.
We performed two contrasts of this type: (A) using the 12 genotype combinations that were not associated with especially low tester viability and (B) using the eight genotype combinations that were associated with especially low tester viability (as identified above).
We elected to perform contrasts using these two sets of genotype combinations (A and B), rather than performing individual contrasts for all 20 genotype combinations, for two reasons. First, we had different hypotheses for the two contrasts: we predicted that contrast B would show evidence for nonadditivity, but contrast A would not. Second, because data from the low-diversity experiment are used to form the null hypothesis for all contrasts, testing 20 genotype combinations could severely inflate our experiment-wise error rate. Our method thus maximizes our power to test the most biologically relevant hypotheses. Because low-diversity viability data were used in both contrasts, all P-values were Bonferonni corrected for two tests. We repeated this analysis separately for each block.
The analysis supported our predictions. In both blocks, contrast A was not significant (block 1: estimate = −8.8448, t = −0.86, corrected P = 0.7852; block 2: estimate = −5.1250, t = −0.62, corrected P = 1.0676), but contrast B was significant after Bonferroni correction for two tests (block 1: estimate = −15.7678, t = −2.65, corrected P = 0.017, block 2: estimate = −21.0046, t = −2.51, corrected P = 0.0246). Overall, these results indicate that the genotype combinations contributing to especially low tester viability at high diversity did so because of nonadditive interactions among wild-type genotypes.
Effects of genotype combinations on tester viability do not generalize to wild-type viability
If wild-type larvae are interacting nonadditively to produce especially low tester viability, we might expect that wild-type absolute viability in these groups would be unusual as well. If these genotype combinations depress tester viability by toxic or harmful means, all larvae (both tester and wild type) may show reduced viability. By contrast, tester fitness may be reduced if the wild-type larvae in the genotype combinations out-compete the tester larvae; in this case, the wild-type larvae should show unusually high absolute viability.
To test these hypothesis, we performed the same contrasts as above, but using wild-type absolute viability as the response variable. We found no support for either hypothesis: no contrasts were significant in any block (block 1: contrast A: estimate = 0.2160, t = −0.02, corrected P = 1, contrast B: estimate = −10.5913, t = −1.96, corrected P = 0.1008; block 2: contrast A: estimate = 15.1849, t = 1.93, corrected P = 0.109, contrast B: estimate = −1.9464, t = −0.26, corrected P = 1). This finding suggests that wild-type viability is additive across diversities, even among genotype combinations with nonadditive effects on tester viability.
Effects of genotype combinations on tester viability do not generalize to smaller groups
Given the above result, we predicted that genotype combinations with especially deleterious effects on tester viability in large groups would have qualitatively the same type of effect in small groups. To test this prediction, we performed contrasts exactly as described above, using the same groups of genotype combinations in the A and B contrasts, and tester viability as the response variable. In this case, however, we used data from small groups.
Surprisingly, the results of these contrasts were inconsistent with the results from the large groups. In block 1, neither contrast was significant (A: estimate = 11.1286, t = 1.00, corrected P = 0.635, B: estimate = −4.0802, t = −0.72, corrected P = 0.9488). However, the parameter estimates indicated that the effects were in the same direction as large groups—that is, positive for contrast A, negative for contrast B. In block 2, contrast A was significant (estimate = −17.9751, t = −2.38, corrected P = 0.036). The direction of the parameter estimate indicates that these genotype combinations were more harmful to tester viability than expected under additivity. Contrast B for this block was not significant (estimate = −12.8785, t = −1.69, corrected P = 0.1832). Thus, the effects of genotype combinations identified as especially harmful to tester larvae in large groups were different—even reversed—in small groups.
Discussion
Genetic diversity is a critical property of all populations and, increasingly, the focus of ecological, evolutionary and conservation research. However, individual population members experience only the diversity of their local group. How do these local interactions influence patterns at the population level?
To examine this question, we formed groups of Drosophila melanogaster larvae with different combinations of wild-type genotypes. Our results demonstrate that group-level diversity influences viability patterns: when groups were large, the population containing high-diversity groups had lower average viability for tester individuals, relative to the low- and medium-diversity treatments (Figs 2 and 3). This result suggests that maternal decisions that define larval group genetic diversity may have important ecological and evolutionary implications in D. melanogaster. One way that genetic diversity may cause ecological impacts is by enabling populations to respond rapidly to selection (Fisher 2000; Hughes et al. 2008). In our experiments, large high-diversity groups exerted stronger selection against the lower-quality tester genotype (Fig. 2). This finding is consistent with the idea that group-level genetic diversity can enhance a population’s evolutionary potential, although not in the manner usually described.
Our results are partially consistent with social evolution theory, which predicts that groups with high relatedness should have highest average fitness (Smith 1964; Wilson 1975; Jasieński et al. 1988). However, in our experiments, the viability benefits of highly related groups (i.e., low- and medium-diversity experiments) were confined to the tester larvae; we found no effect of group diversity on the viability of wild-type individuals. As the tester larvae were derived from a different population than the wild-type larvae, the relatedness between tester larvae and wild-type larvae was independent of group genetic diversity. Thus, low relatedness cannot directly explain the reduced viability suffered by tester flies at high diversity.
Why were so many of our results specific to the tester genotype? One answer is that the tester larvae were present in every trial, and because tester larvae who survived to adulthood had an identifiable eye mutation, we had precise measurements of tester viability. By contrast, we did not identify individual wild-type genotypes, so our viability estimates for wild-type genotypes were at the group level. In this sense, the tester larvae served the function we intended, providing an indication that manipulating the composition of wild-type genotypes can influence the social or abiotic environment experienced by the entire group. However, one concern with using laboratory-adapted, mutated genotypes is that these individuals may be generally of low quality and fail to reflect interactions that are likely to occur in nature (Brakefield 2003). Our tester larvae did indeed have lower absolute viability than wild-type larvae in all experiments (Fig. 3). Still, intrinsic differences in tester viability do not fully explain why differences in tester and wild-type viability were profoundly exacerbated in the large, high-diversity groups. As inbred individuals, tester larvae may have been especially sensitive to the increased competition, toxicity and/or stress exerted by high-diversity groups of wild-type larvae; this hypothesis requires further testing.
Further, not all high-diversity groups were deleterious to tester larval viability—only eight of the 20 high-diversity genotype combinations had strongly deleterious effects on tester larvae. The effects of especially deleterious genotype combinations were not additive, that is, we could not predict which high-diversity genotype combinations would be deleterious, based on each genotype’s effect on tester viability at low diversity. Further, the deleterious effects of these eight genotype combinations was highly situation specific: the tester larvae suffered depressed viability, while the average viability of wild-type larvae in the same groups was unaffected, and the effects were completely different—in some cases, even reversed—between large and small groups. Such pervasive nonadditivity indicates that analyses of one group size or genotype combination cannot be extrapolated to other groups (Lewontin 1955; Becerra et al. 1999; Jousset et al. 2011). This extreme specificity may explain why previous studies of larval group diversity produced a wide array of results (Pérez-Tomé & Toro 1982; Fowler & Partridge 1986; Martin et al. 1988; Santos et al. 1992; Lopez-Suarez et al. 1993). If different experiments (or different replicates of the same experiment) included different genotypes by chance, our results suggest that this variation in genotypic composition can produce systematic differences in viability among groups. Ubiquitous nonadditive effects may further explain why we did not detect an effect of genetic diversity on wild-type viability: strong genotype-by-genotype interaction effects on wild-type viability would ‘cancel out’ at the population level.
Overall, these results suggest that complex interactions among wild-type genotypes, rather than high diversity per se, accounted for depressed tester viability in groups with high genetic diversity. A wide variety of behavioural and physiological mechanisms may underlie these nonadditive effects. For example, the well-known ‘rover/sitter’ polymorphism, which describes the effect of foraging alleles on the distance larvae move while feeding (Sokolowski 1980), has been shown to cause frequency-dependent selection when larvae interact in food-limited conditions (Fitzpatrick et al. 2007). In addition, the ‘biotic residues’ (presumably, metabolic waste products) of a given genotype may influence the viability of other genotypes, even when the individual larvae do not interact directly (Weisbrot 1966; Dawood & Strickberger 1969). To our knowledge, such mechanistic analysis of larval interactions has not yet been extended to describe interactions among multiple (more than 2) genotypes; this additional complexity may be necessary to explain the surprisingly varied viability effects of our different high-diversity genotype combinations.
Among wild-type genotypes, we found no evidence that female ‘re-mating’ had any influence on viability. Critically, females in our experiment did not actually re-mate. Rather, we simulated the genetic effects of re-mating by comparing groups containing full siblings only (low diversity) to those containing both full- and half-sibs (medium diversity). This design allowed us to examine the potential benefits of genetic diversity on offspring viability without the confounding influence of sperm competition or female fertilization bias (Simmons 2005; Aguirre & Marshall 2011). In some insects, progeny of females who re-mate enjoy fitness benefits, because more diverse groups are more successful (Mattila & Seeley 2007; Caesar et al. 2010). We found no evidence that high group genetic diversity is beneficial to wild-type larvae, which may explain why we did not uncover a similar benefit to re-mating in this species. Of course, our results may be different under alternate conditions, or if we examined a fitness component other than viability (Promislow et al. 1998; Sørensen & Loeschcke 2001; Fellous & Lazzaro 2010). Drosophila melanogaster females are able to choose mates that maximize their progeny’s competitive ability as larvae (Partridge 1980); and re-mating reduces female lifespan and lifetime fecundity (Fowler & Partridge 1989). In light of this existing literature, our results are consistent with the idea that females re-mate to try to secure a high-quality mate, not because re-mating is advantageous per se (Jennions & Petrie 2000; Foerster et al. 2003; Priest et al. 2008).
Similarly, a key feature of our study was that no individuals were able to choose their associates. In mimicking the effects of maternal decision-making on the genotypic composition of groups, we used the best available evidence and focused on maternal decisions with relatively straightforward consequences. However, it is likely that females in nature make much more complex decisions than those studied here. For example, our experiment revealed that large, dense groups are associated with lower viability (Fig. 3), but such groups are common in nature (Wertheim et al. 2002b). In fact, female flies seek out (Wertheim et al. 2002a) and prefer (Sarin & Dukas 2009) egg-laying substrates that have already been used by other females, suggesting that benefits of social information may partially or fully compensate the costs of laying eggs in high-density sites. Oviposition substrate preference is heritable in D. melanogaster (Takamura & Fuyama 1980; Ruiz-Dubreuil et al. 1994), including in the Raleigh population (Miller et al. 2011); oviposition behaviour is also influenced by environmental (Sheeba et al. 1998; Joseph et al. 2009; Miller et al. 2011) and social (del Solar & Palomino 1966; Wertheim et al. 2002a; Sarin & Dukas 2009) factors. The evidence we present in the current study suggests that investigating these more complex decisions may be ecologically and evolutionarily relevant.
In general, our results suggest that the organization of genetic diversity within populations may be as important as differences in genetic diversity among populations. Group formation processes such as the maternal decisions we examined are expected to occur in many species; specifically, whenever juveniles have limited dispersal potential, maternal decisions are the most important influence on the genetic composition of the juvenile social environment. In mice, for example, the genotypic composition of littermates can affect an individual’s later behaviour (Crews 2008). Thus, maternal mating decisions can determine both the paternity and the developmental environment of their progeny (Wolf et al. 1999; Miller & Moore 2007). Further, nonrandom association among genotypes may occur even among unrelated individuals (e.g. in humans: Fowler et al. 2011). Finally, increasing evidence suggests that behaviours directly related to group formation have a genetic basis (Brown & Brown 2000; Serrano & Tella 2007; Fowler et al. 2011; Saltz 2011; Saltz & Foley 2011). The expected prevalence of nonrandom association among genotypes suggests that the ecological effects of group genotype composition may warrant investigation in a wide range of species.
Supplementary Material
Acknowledgements
We would like to thank Andy Sih, Judy Stamps, and Gail Patricelli for useful comments on the manuscript and Courtney Fjeldsted for logistical assistance. J.B.S. was supported by the UC Davis Center for Population Biology and by the USC Center for Excellence in Genome Science. E.T.A., J.G., M.H., and N.M. were supported by the USC Undergraduate Research Associates Program.
Footnotes
J.B.S. and S.V.N. are interested in how genetic variation in social behaviors influences ecology and the maintenance of genetic diversity. M.K.H. is interested in Biology and Public Policy. E.T.A., J.G., and N.M. are pursuing careers in medicine.
Data accessibility
Data from all experiments have been deposited in Dryad (doi: 10.5061/dryad.7384037h).
Supporting information
Additional supporting information may be found in the online version of this article.
Appendix S1 Genotype combinations.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agashe D. The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. The American Naturalist. 2009;174:255–267. doi: 10.1086/600085. [DOI] [PubMed] [Google Scholar]
- Agashe D, Bolnick DI. Intraspecific genetic variation and competition interact to influence niche expansion. Proceedings of the Royal Society B. 2010;277:2915–2924. doi: 10.1098/rspb.2010.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agashe D, Falk JJ, Bolnick DI. Effects of founding genetic variation on adaptation to a novel resource. Evolution. 2011;65:2481–2491. doi: 10.1111/j.1558-5646.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- Aguirre JD, Marshall JD. Does genetic diversity reduce sibling competition? Evolution. 2011;66:94–102. doi: 10.1111/j.1558-5646.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- Archie EA, Maldonado JE, Hollister-Smith JA, et al. Fine-scale population genetic structure in a fission-fusion society. Molecular Ecology. 2008;17:2666–2679. doi: 10.1111/j.1365-294X.2008.03797.x. [DOI] [PubMed] [Google Scholar]
- Atkinson WD. A field investigation of larval competition in domestic Drosophila. Journal of Animal Ecology. 1979;48:91–102. [Google Scholar]
- Becerra M, Brichette I, Garcia C. Short-term evolution of competition between genetically homogeneous and heterogeneous populations of Drosophila melanogaster. Evolutionary Ecology Research. 1999;1:567–579. [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, et al. Why intraspecific trait variation matters in community ecology. Trends in Evolution & Ecology. 2011;26:183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakefield PM. Artificial selection and the development of ecologically relevant phenotypes. Ecology. 2003;84:1661–1671. [Google Scholar]
- Brown CR, Brown MB. Heritable basis for choice of group size in a colonial bird. Proceedings of the National Academy of Sciences, USA. 2000;97:14825–14830. doi: 10.1073/pnas.97.26.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar S, Karlsson M, Forsman A. Diversity and relatedness enhance survival in colour polymorphic grasshoppers. PLoS ONE. 2010;5:e10880. doi: 10.1371/journal.pone.0010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KM, Whitney KD. Population genetic diversity influences colonization success. Molecular Ecology. 2010;19:1253–1263. doi: 10.1111/j.1365-294X.2010.04550.x. [DOI] [PubMed] [Google Scholar]
- Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Frontiers in Neuroendocrinology. 2008;29:344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood MM, Strickberger MW. The effect of larval interaction on viability in Drosophila melanogaster. III. Effects of biotic residues. Genetics. 1969;63:213–220. doi: 10.1093/genetics/63.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous S, Lazzaro BP. Larval food quality affects adult (but not larval) immune gene expression independent of effects on general condition. Molecular ecology. 2010;19:1462–1468. doi: 10.1111/j.1365-294X.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- Fisher RA. In: The Genetical Theory of Natural Selection: A Complete Variorum Edition. Bennett JH, editor. Oxford: Oxford University Press; 2000. pp. 22–47. [Google Scholar]
- Fitzpatrick MJ, Feder E, Rowe L, Sokolowski MB. Maintaining a behavior polymorphism by frequency-dependent selection on a single gene. Nature. 2007;447:210–213. doi: 10.1038/nature05764. [DOI] [PubMed] [Google Scholar]
- Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. [DOI] [PubMed] [Google Scholar]
- Fowler K, Partridge L. Variation in male fertility explains an apparent effect of genotypic diversity on success in larval competition in Drosophila melanogaster. Heredity. 1986;57:31–36. [Google Scholar]
- Fowler K, Partridge L. A cost of mating in fruitflies. Nature. 1989;338:760–761. [Google Scholar]
- Fowler JH, Settle JE, Christakis NA. Correlated genotypes in friendship networks. Proceedings of the National Academy of Sciences, USA. 2011;108:1993–1997. doi: 10.1073/pnas.1011687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosberg RK, Quinn JF. The genetic control and consequences of kin recognition by the larvae of a colonial marine invertebrate. Nature. 1986;322:456–459. [Google Scholar]
- Harshman LG, Clark AG. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998;52:1334–1341. doi: 10.1111/j.1558-5646.1998.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Nielsen KM. The effect of resource subdivision on genetic variation in Drosophila. The American Naturalist. 1985;125:421–430. [Google Scholar]
- Hughes AR, Stachowicz JJ. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proceedings of the National Academy of Sciences, USA. 2004;101:8998–9002. doi: 10.1073/pnas.0402642101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M. Ecological consequences of genetic diversity. Ecology Letters. 2008;11:609–623. doi: 10.1111/j.1461-0248.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- Jasieński M, Korzeniak U, Lomnicki A. Ecology of kin and nonkin larval interactions in Tribolium beetles. Behavioral Ecology and Sociobiology. 1988;22:277–284. [Google Scholar]
- Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biological Reviews. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Woollacott RM. Larval settlement preference maximizes genetic mixing in an inbreeding population of a simultaneous hermaphrodite (Bugula stolonifera, Bryozoa) Molecular Ecology. 2010;19:5511–5520. doi: 10.1111/j.1365-294X.2010.04887.x. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Devineni AV, King IFG, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proceedings of the National Academy of Sciences, USA. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousset A, Schmid B, Scheu S, Eisenhauer N. Genotypic richness and dissimilarity opposingly affect ecosystem functioning. Ecology Letters. 2011;14:537–545. doi: 10.1111/j.1461-0248.2011.01613.x. [DOI] [PubMed] [Google Scholar]
- Krakauer AH, Kimball RT. Interspecific brood parasitism in galliform birds. The Ibis. 2009;151:373–381. [Google Scholar]
- Lesica P, Allendorf FW. When are peripheral populations valuable for conservation? Conservation Biology. 1995;9:753–760. [Google Scholar]
- Lewontin R. The effects of population density and composition on viability in Drosophila melanogaster. Evolution. 1955;9:27–41. [Google Scholar]
- Lopez-Suarez C, Toro MA, Garcia C. Genetic heterogeneity increases viability in competing groups of Drosophila hydei. Evolution. 1993;47:977–981. doi: 10.1111/j.1558-5646.1993.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Pérez-Tomé JM, Toro MA. Competition and genotypic variability in Drosophila melanogaster. Heredity. 1988;60:119–123. doi: 10.1038/hdy.1988.17. [DOI] [PubMed] [Google Scholar]
- Mattila HR, Seeley TD. Genetic diversity in honey bee colonies enhances productivity and fitness. Science. 2007;317:362–364. doi: 10.1126/science.1143046. [DOI] [PubMed] [Google Scholar]
- Miller CW, Moore AJ. A potential resolution of the lek paradox through indirect genetic effects. Proceedings of the Royal Society B. 2007;274:1279–1286. doi: 10.1098/rspb.2006.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PM, Saltz JB, Cochrane VA, Marcinkowski CM, Mobin R, Turner TL. Natural variation in decision-making behavior in Drosophila melanogaster. PLoS ONE. 2011;6:e16436. doi: 10.1371/journal.pone.0016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzhdin SV, Keightley PD, Pasyukova EG, Morozova EA. Mapping quantitative trait loci affecting sternopleural bristle number in Drosophila melanogaster using changes of marker allele frequencies in divergently selected lines. Genetical Research. 1998;72:79–91. doi: 10.1017/s001667239800336x. [DOI] [PubMed] [Google Scholar]
- Partridge L. Mate choice increases a component of offspring fitness in fruit flies. Nature. 1980;283:290–291. [Google Scholar]
- Pérez-Tomé JM, Toro MA. Competition of similar and non-similar genotypes. Nature. 1982;299:153–154. [Google Scholar]
- Priest NK, Galloway LF, Roach DA. Mating frequency and inclusive fitness in Drosophila melanogaster. The American Naturalist. 2008;171:10–21. doi: 10.1086/523944. [DOI] [PubMed] [Google Scholar]
- Promislow DE, Smith EA, Pearse L. Adult fitness consequences of sexual selection in Drosophila melanogaster. Proceedings of the National Academy of Sciences, USA. 1998;95:10687–10692. doi: 10.1073/pnas.95.18.10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey A, Wolf M. Inbreeding avoidance in animals. Trends in Ecology and Evolution. 1996;11:201–206. doi: 10.1016/0169-5347(96)10028-8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Dubreuil G, Burnet B, Connolly K. Behavioural correlates of selection for oviposition by Drosophila melanogaster females in a patchy environment. Heredity. 1994;73:103–110. doi: 10.1038/hdy.1994.105. [DOI] [PubMed] [Google Scholar]
- Saltz JB. Natural genetic variation in social environment choice: context-dependent gene-environment correlation in Drosophila melanogaster. Evolution. 2011;65:2325–2334. doi: 10.1111/j.1558-5646.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- Saltz JB, Foley BR. Natural genetic variation in social niche construction: social effects of aggression drive disruptive selection in Drosophila melanogaster. The American Naturalist. 2011;177:645–654. doi: 10.1086/659631. [DOI] [PubMed] [Google Scholar]
- Santos M, Fowler K, Partridge L. On the use of tester stocks to predict the competitive ability of genotypes. Heredity. 1992;69:489–495. doi: 10.1038/hdy.1992.163. [DOI] [PubMed] [Google Scholar]
- Sarin S, Dukas R. Social learning about egg-laying substrates in fruitflies. Proceedings of the Royal Society B. 2009;276:4323–4328. doi: 10.1098/rspb.2009.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabenberger O. Introducing the GLIMMIX Procedure for Generalized Linear Models. SUGI (SAS User’s Group International) North Carolina: SAS Institute, Cary; 2007. pp. 196–130. [Google Scholar]
- Serrano D, Tella J. The role of despotism and heritability in determining settlement patterns in the colonial lesser kestrel. The American Naturalist. 2007;169:E53–E67. doi: 10.1086/510598. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Nadhyastha NAA, Joshi A. Oviposition preference for novel versus normal food resources in laboratory populations of Drosophila melanogaster. Journal of Biosciences. 1998;23:93–100. [Google Scholar]
- Simmons LW. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annual Review of Ecology, Evolution, and Systematics. 2005;36:125–146. [Google Scholar]
- Smith JM. Group selection and kin selection. Nature. 1964;201:1145–1147. [Google Scholar]
- Sokolowski MB. Foraging strategy of Drosophila melanogaster: a chromosomal analysis. Behavior Genetics. 1980;10:291–302. doi: 10.1007/BF01067774. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB. Genetics and ecology of Drosophila melanogaster larval foraging and pupation behavior. Journal of Insect Physiology. 1985;31:857–864. [Google Scholar]
- del Solar E, Palomino H. Choice of oviposition in Drosophila melanogaster. The American Naturalist. 1966;100:127–133. [Google Scholar]
- Sørensen JG, Loeschcke V. Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. Journal of Insect Physiology. 2001;47:1301–1307. doi: 10.1016/s0022-1910(01)00119-6. [DOI] [PubMed] [Google Scholar]
- Storz JF. Genetic consequences of mammalian social structure. Journal of Mammalogy. 1999;80:553–569. [Google Scholar]
- Strasburg JL, Kane NC, Raduski AR, Bonin A, Michelmore R, Rieseberg LH. Effective population size is positively correlated with levels of adaptive divergence among annual sunflowers. Molecular Biology and Evolution. 2011;28:1569–1580. doi: 10.1093/molbev/msq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura T, Fuyama Y. Behavior genetics of choice of oviposition sites in Drosophila melanogaster. I. Genetic variability and analysis of behavior. Behavior Genetics. 1980;10:105–120. doi: 10.1007/BF01067322. [DOI] [PubMed] [Google Scholar]
- Tsutsui ND, Suarez AV, Holway DA, Case TJ. Reduced genetic variation and the success of an invasive species. Proceedings of the National Academy of Sciences, USA. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman B. The ecology of kin recognition. Annual Review of Ecology and Systematics. 1988;19:543–571. [Google Scholar]
- Weisbrot DR. Genotypic interactions among competing strains of and species of Drosophila. Genetics. 1966;53:427–435. doi: 10.1093/genetics/53.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim B, Dicke M, Vet LEM. Behavioural plasticity in support of a benefit for aggregation pheromone use in Drosophila melanogaster. Entomologia Experimentalis et Applicata. 2002a;103(1):61–71. [Google Scholar]
- Wertheim B, Marchais J, Vet LEM, Dicke M. Allee effect in larval resource exploitation in Drosophila: an interaction among density of adults, larvae, and micro-organisms. Ecological Entomology. 2002b;27:608–617. [Google Scholar]
- Wilson DS. A theory of group selection. Proceedings of the National Academy of Sciences, USA. 1975;72:143–146. doi: 10.1073/pnas.72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JB, Brodie ED, Moore AJ. Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. The American Naturalist. 1999;153:254–266. doi: 10.1086/303168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.