Abstract
Activation of the Nlrp3 inflammasome has been proposed to require two signals based on studies in mouse macrophages. The first signal is provided by Toll-like receptor stimulation and triggers the synthesis of the IL-1β precursor and Nlrp3. The second signal can be mediated by stimulation of the purinergic P2X7 receptor by millimolar concentrations of ATP. However, these high concentrations of ATP are not found normally in the in vivo extracellular milieu, raising concern about the physiological relevance of the ATP-P2X7 pathway of inflammasome activation. Here, we show that unlike macrophages, murine bone marrow derived and splenic DCs can secrete substantial amounts of mature IL-1β upon stimulation with TLR ligands in the absence of ATP stimulation. The differential ability of DCs to release IL-1β and activate caspase-1 was associated with increased expression of Nlrp3 under steady state conditions and of pro-IL-1β and Nlrp3 after stimulation with TLR agonists. IL-1β secretion from stimulated DCs was largely dependent on the Nlrp3 inflammasome, but independent of P2X7 and unaffected by incubation with apyrase. More importantly, intraperitoneal administration of LPS induced IL-1β production in serum which was abrogated in Nlrp3-null mice but was unaffected in P2X7-deficient mice. These results demonstrate differential regulation of the Nlrp3 inflammasome in macrophages and dendritic cells. Furthermore, they challenge the notion that the ATP-P2X7 axis is critical for TLR-induced IL-1β production via the Nlrp3 inflammasome in vivo.
Keywords: Dendritic cells, IL-1β, Inflammasome, TLRs, P2X7
Introduction
IL-1β is a critical mediator in the induction of immune responses and the development of inflammatory diseases (1). Blood monocytes, tissue macrophages and dendritic cells are the primary sources of IL-1β. As a potent proinflammatory factor, the production of IL-1β is tightly regulated at both transcriptional and translational levels. Under normal conditions, IL-1β is not constitutively expressed, but its expression is induced in response to stimulation with microbial products including Toll-like receptor (TLR) ligands or certain endogenous stimuli such as TNF-β or IL-1β itself (2, 3). Unlike its family member IL-1α, the IL-1β precursor is synthesized as an inactive precursor (pro-IL-1β) that is cleaved into its biologically active product by activated caspase-1, also known as IL-1β-converting enzyme (4). Caspase-1 activation occurs through autoproteolytic cleavage of pro-caspase-1, which can be initiated by inflammasomes, multi-protein complexes that include a member of the NOD (nucleotide-binding oligomerization domain)-like receptor (NLR) family as a sensor and the adaptor protein apoptosis-associated speck-like protein (Asc) (5).
It has been proposed that activation of the NLR pyrin domain-containing 3 (Nlrp3) inflammasome requires two signals based on studies in mouse macrophages. The first signal, referred to as priming, is the NF-κB-dependent production of pro-IL-1β and Nlrp3, through the stimulation with microbial products or certain cytokines (6, 7). The second signal activates Nlrp3 and is induced by ATP, certain bacterial toxins, or particulate matter (8). ATP induces Nlrp3 activation through stimulation of the purinergic receptor P2X ligand-gated ion channel 7 (P2X7) that induces K+ efflux (9). In contrast, human monocytes secrete active IL-1β in response to TLR ligands alone, which has been suggested to be dependent on autocrine stimulation by extracellular ATP and the Nlrp3 inflammasome (10, 11). The concentration of ATP required for the P2X7-mediated caspase-1 activation is in the millimolar range (12). These high concentrations of ATP are not found normally in the in vivo extracellular milieu, although they could perhaps be reached under certain situations in the context of cell lysis or injury.
Murine bone marrow derived DCs have been used for studying of inflammasome activation (13, 14), although whether DCs behave like macrophages or monocytes in terms of IL-1β secretion upon stimulation with TLR ligands is largely unknown. In this study, we used murine bone marrow derived DCs and splenic DCs to investigate the IL-1β secretion from DCs in response to TLR ligands. We found DCs stimulated by TLR ligands can secrete substantial amount of mature IL-1β (p17), which was dependent on the Nlrp3 inflammasome, but independent of the purinergic P2X7 receptor. Importantly, we demonstrated that P2X7 is not required for IL-1β production in response to LPS administration in vivo.
Materials and Methods
Mice
Mice deficient in P2X7, Nlrp3, Nlrc4, caspase-1 or Asc on the C57BL6 background have been previously described (15); wild-type (WT) C57BL6 mice were originally purchased from The Jackson Laboratory and bred in our animal facility. All mice were maintained in a specific pathogen-free facility. All protocols of animal studies were approved by the University of Michigan Committee on Use and Care of Animals.
Reagents
Ultrapure LPS from E. coli 0111:B4, synthetic monophosphoryl lipid A (Lipid A), Pam2CSK4, Pam3CSK4, poly (I:C) LMW, R848 and CpG (ODN 1826) were purchased from Invivogen. ATP was from Sigma-Aldrich. Murine IL-1β antibody (AF-401-NA) was purchased from R&D Systems. Gapdh antibody was purchased from GenScript. Caspase-1 antibody (sc-514) was purchased from Santa Cruz. IL-18 antibody (5180R-100) was purchased from BioVision. Antibody for mouse Asc has been described (16). Rabbit anti mouse-Nlrp3 antibody was generated by immunizing rabbits with mouse Nlrp3 protein (amino acids 1–194) expressed in E. coli and purified by affinity chromatography using a nickel column.
Cell culture and splenic DC isolation
Mouse bone-marrow-derived dendritic cells (BMDCs) and macrophages (BMDMs) were prepared as previously described (6). DCs were used for experiments after 7–8 days of culture when CD11c expression, analyzed by flow cytometry, was higher than 90%. Cells were seeded at 4 × 105 cells per well in 48-well plates or 2 × 106 per well in 12-well plates the day before the experiment. Splenic DCs (CD11c+CD11b−) and macrophages (CD11c−CD11b+) were freshly isolated from mouse spleens by positive and negative selection using MACS beads (Miltenyi Biotec). Cells were suspended in RPMI 1640 medium containing glutamine, sodium pyruvate, 10% heat-inactivated fetal bovine serum (GIBCO-BRL) and seeded at 2 × 105 cells per well in 96-well plates.
Cytotoxicity Assay
The percentage of cell death was determined using the LDH release assay (Promega). The absorbance at 490 nm was measured, and the percentage of cytotoxicity was calculated relative to the 100% release value obtained by lysis of cells with a solution of 0.1% triton-X-100.
Measurements of cytokines
Mouse IL-1β and TNF-α in culture supernatants or serum were measured by ELISA kits (R&D Systems). Assays were performed in triplicate for each independent experiment.
Immunoblotting
Cells were lysed in ice-cold PBS buffer containing 1% NP-40 supplemented with complete protease inhibitor cocktail (Roche, Mannheim, Germany). Mature IL-1β in the culture supernatant was precipitated by 7.7% trichloroacetic acid. Protein samples were separated by SDS-PAGE and transferred to PVDF membranes by electroblotting (Bio-Rad) and membranes were immunoblotted with respective antibodies.
cDNA Synthesis and Real-Time RT-PCR
BMDCs and BMDMs were stimulated with LPS (100 ngml−1) for the indicated periods or left unstimulated. Total RNA extraction, cDNA synthesis and Real-time PCR were carried out as previously described (17). The primers sequence was: IL-1β forward, TGTAATGAAAGACGGCACACC; IL-1β reverse, TCTTCTTTGGGTATTGCTTGG; Nlrp3 forward,CCCTTGGAGACACAGGACTC; Nlrp3 reverse, GAGGCTGCAGTTGTCTAATTCC; TNF-α forward, TCTTCTCATTCCTGCTTGTGG; and TNF-α reverse, GGTCTGGGCCATAGAACTGA; GAPDH forward, AGCTTGTCATCAACGGGAAG; GAPDH reverse, TTTGATGTTAGTGGGGTCTCG; IL-1β, NLRP3 or TNF-α to GAPDH relative expression was calculated using the 2(−ct) method and normalized to the level of unstimulated BMDCs.
Endotoxemia
For the measurement of cytokines in serum, mice were injected i.p. with 25 mgkg−1 of LPS (E.coli 0111:B4, Sigma-Aldrich) and mouse serum were collected 1 and 3 hours after injection.
Statistical analysis
Student's t test was used to determine statistically significant difference between two groups. One way ANOVA was used to analyze differences among multiple groups. A p value of <0.05 was considered significant.
Results
Activation of TLRs is sufficient for IL-1β secretion in murine DCs
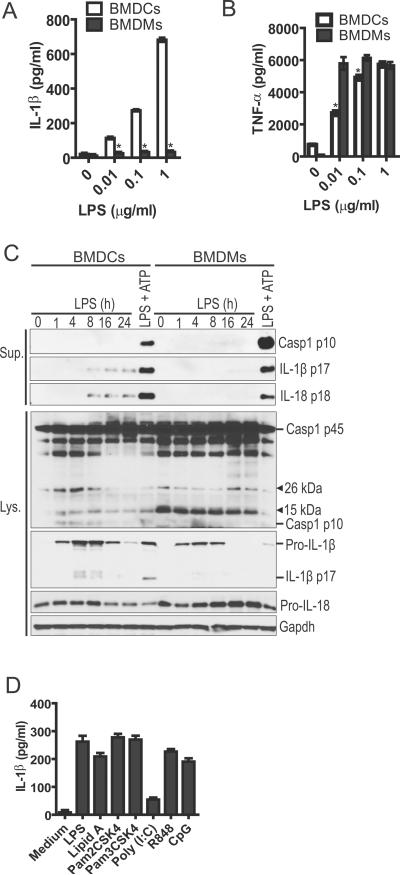
We initially compared the ability of mouse BMDMs and BMDCs to release IL-1β in response to different concentrations of LPS in the absence of exogenous ATP. Consistent with previous results, stimulation with LPS alone did not induce the release of IL-1β in BMDMs, but it triggered secretion of TNF-β (Fig. 1A,B). In contrast, BMDCs produced robust amounts of both IL-1β and TNF-β in response to LPS alone (Fig. 1A,B). To examine the kinetics of IL-1β release in DCs after LPS exposure, culture supernatants and cell extracts were collected at different time points and immunoblotted for IL-1β (Fig. 1C). After LPS stimulation, production of pro-IL-1β in cell extracts were first detected at 1 hr, peaked by 4–8 hrs and decreased at later time points (Fig. 1C). In contrast, the mature form of IL-1β (p17) was detected 4 hrs after LPS stimulation in cell extracts and accumulated over time in culture supernatants (Fig. 1C). Consistently, caspase-1 activation was detected in the cell extract by 1 hr after LPS stimulation, as assessed by the presence of the p10 subunit of active caspase-1 (Fig. 1C). Likewise, processing of pro-IL-18 into the mature form of IL-18 (p18) was detected in the cell supernatant at 8 hrs post LPS stimulation (Fig. 1C). In contrast, neither caspase-1 cleavage, nor IL-1β or IL-18 maturation was detected in BMDMs stimulated with LPS alone (Fig. 1C). As expected, stimulation of BMDCs and BMDMs with LPS followed by ATP was associated with toxicity (supplemental Fig. 1A). However, the levels of LDH release induced by LPS alone were comparable to those observed in cells cultured in medium alone (supplemental Fig. 1A). We also examined the secretion of IL-1β in DCs stimulated with other TLR ligands. All tested ligands including Pam2- and Pam3-CSK4 (TLR2 ligand), poly (I:C) (TLR3 ligand), R848 (TLR7 ligand) and CpG (TLR9 ligand) triggered significant release of IL-1β in BMDCs in the absence of exogenous ATP (Fig. 1D). Collectively, these results indicate that unlike in BMDMs, TLR stimulation can trigger caspase-1 activation and IL-1β/IL-18 processing and release in murine BMDCs in the absence of exogenous ATP.
Figure 1.
Activation of TLRs triggers robust IL-1β secretion in murine DCs in the absence of exogenous ATP. (A) IL-1β or TNF-α (B) secretion was analyzed in BMDCs and BMDMs in response to different doses of LPS. Culture supernatant was collected 24 hours after LPS stimulation and the amounts of IL-1β or TNF-α were determined by ELISA. (C) The kinetics of caspase-1 activation, IL-1β and IL-18 processing in LPS-stimulated DCs and macrophages. Cell-free culture supernatants (Sup.) and cell lysates (Lys.) were collected at the indicated time points after LPS (1 μg/ml) or LPS plus 5 mM ATP stimulation, and analyzed for caspase-1 activation, IL-1β and IL-18 processing as described in Material and Methods. Gapdh was a loading control. (D) DCs were treated with different TLR ligands (LPS, 100 ng/ml; Lipid A, 100 ng/ml; Pam2CSK4, 100 ng/ml; Pam3CSK4, 100 ng/ml; Poly (I:C), 50 μg/ml; R848, 10 μg/ml; CpG, 10 μg/ml) for 24 hours and the amounts of IL-1β present in culture supernatant were determined by ELISA. *, p<0.05.
LPS induces IL-1β secretion from murine splenic DCs
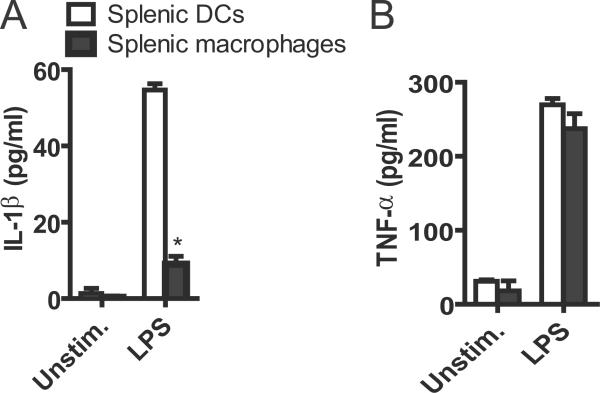
Next we examined whether primary DCs could also secrete IL-1β in response to TLR ligands, we isolated splenic mouse DCs and macrophages and challenged the cells with LPS. Although splenic DCs and macrophages secreted comparable amounts of TNF-α in response to LPS, splenic DCs released about five times more IL-1β (~60 pg/ml) than splenic macrophages (~10 pg/ml) after LPS stimulation (Fig. 2A,B). These results indicate that primary DCs produce more IL-1β than macrophages after LPS challenge.
Figure 2.
LPS induces IL-1β secretion from murine splenic DCs. Isolated murine splenic DCs and macrophages were stimulated with 1 μg/ml LPS for 24 hours or left unstimulated. The culture supernatants were analyzed for IL-1β (A) or TNF-α (B) by ELISA. Data are representative of three independent experiments. Bar graphs shown were the mean ± s.d. of triplicate wells. *, p<0.05.
DCs express more pro-IL-1β and Nlrp3 proteins than macrophages in response to LPS
We sought to compare the protein levels of pro-IL-1β and Nlrp3 in DCs and macrophages. In accord with the increased production of IL-1β in the culture supernatant, BMDCs produced higher amounts of both pro-IL-1β and Nlrp3 proteins in response to LPS than BMDMs (Fig. 3A).
Figure 3.
DCs express more pro-IL-1β and NLRP3 proteins than macrophages in response to LPS. (A) Cell extracts from murine DCs or macrophages stimulated with 100 ng/ml LPS for indicated time were immunoblotted for pro-IL-1β and Nlrp3. Gapdh was a loading control. (B) Murine DCs and macrophages were stimulated with 100 ng/ml LPS for indicated time. Total RNAs from each condition were extracted, reversely transcripted and quantified by qPCR. (C) Cell extracts from murine DCs or macrophages stimulated with 100 ng/ml LPS for indicated time were immunoblotted for phosphorylation of IκB, JNK, ERK or p38. The immunoblots were stripped and re-probed for proteins IκB, JNK, ERK or p38. Data shown are representative of two or three independent experiments. *, p<0.05.
Furthermore, Nlrp3 was detected in unstimulated BMDCs but not BMDMs (Fig. 3A). Analysis of mRNA expression by quantitative PCR showed that the induction of pro-IL-1β by LPS was comparable in BMDCs and BMDMs at 1 hr, but was significantly higher in BMDCs than in BMDMs at 4 and 8 hrs post LPS stimulation (Fig. 3B). In contrast, the induction of Nlrp3 mRNA by LPS was similar in BMDCs and BMDMs (Fig. 3B). While the expression of Nlrp3 mRNA was low in unstimulated BMDCs and BMDMs, it was higher in BMDCs than in BMDMs (Fig. 3B). To determine whether LPS induces differential signaling in BMDCs and BMDMs, we stimulated both cell populations with LPS and assessed NF-κB and MAPK signaling events at different times. Notably, phosphorylation of IκB-α and p38 in response to LPS occurred earlier and was slightly increased in BMDCs when compared to BMDMs (Fig. 3C). Thus, the differential ability of DCs to activate caspase-1 and secrete IL-1β in response to LPS is associated with increased expression of the proteins Nlrp3 and pro-IL-1β as well as enhanced LPS-induced IκB-α and p38 phosphorylation. However, the increased expression of Nlrp3 protein in DCs cannot be explained by differential induction of Nlrp3 mRNA, suggesting that post-transcriptional or other mechanisms can regulate the expression of Nlrp3.
IL-1β secretion by LPS-stimulated DCs requires caspase-1 and Nlrp3, but is insensitive to extracellular K+
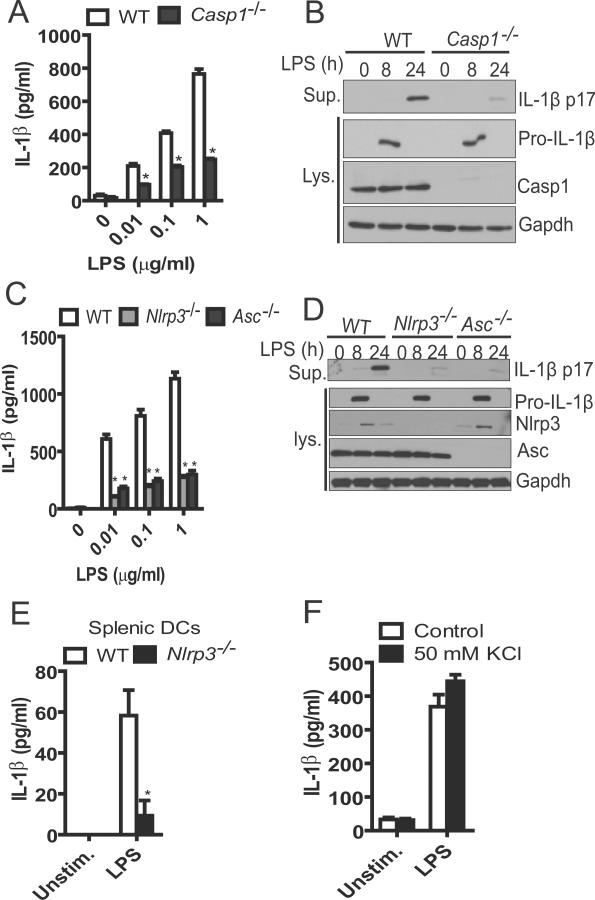
We sought to investigate the role of caspase-1 in IL-1β secretion by murine DCs in response to LPS. The release of IL-1β induced by LPS stimulation was impaired in mouse DCs deficient in caspase-1 when compared to WT DCs (Fig. 4A). In contrast, the production of TNF-α was comparable in WT and caspase-1−/− DCs (supplemental Fig 1B). Wild-type and caspase-1−/− DCs produced similar amounts of cytosolic pro-IL-1β, while the mature IL-1β (p17) in the culture supernatant of caspase-1−/− DCs was barely detectable after 24 hrs of LPS stimulation (Fig. 4B). When murine DCs were co-treated with YVAD-cmk, a caspase-1 inhibitory peptide, a similar reduction of IL-1β was observed (data not shown). Next, we assessed the role of the different inflammasomes in LPS-induced production of IL-1β in murine DCs. To this end, we treated murine DCs deficient in Nlrc4, Nlrp3 and Asc with LPS for 24 hours and analyzed culture supernatants for IL-1β secretion. WT and Nlrc4−/− DCs secreted comparable amounts of IL-1β (supplemental Fig. 1C). In contrast, the release of IL-1β induced by LPS was impaired in DCs deficient in Nlrp3 or Asc (Fig. 4C). The reduction of IL-1β secretion in Nlrp3 or Asc deficient DCs was further confirmed by immublotting which revealed that Nlrp3 or Asc deficiency did not affect the levels of cytosolic pro-IL-1β (Fig. 4D), but impaired LPS-induced production of mature IL-1β (p17) in the cell supernatant (Fig. 4D). Furthermore, primary splenic DCs with Nlrp3 deficiency produced similar level of TNF-α but much less IL-1β in response to LPS (Fig. 4E and supplemental Fig. 1D). High concentrations of extracellular K+ inhibit the activation of the Nlrp3 inflammasome induced by ATP, bacterial toxins and particulate matter (6). Notably, addition of KCl (50 mM) to the medium did not affect IL-1β secretion induced by LPS in DCs (Fig. 4F). In control experiments performed in parallel, ATP robustly enhanced the release of IL-1β in LPS-primed DCs and the enhancement was almost abrogated by 50 mM extracellular KCl (Supplemental Fig. 1E). These results indicate that stimulation with LPS alone induces IL-1β secretion largely via the Nlrp3 inflammasome in DCs, but this pathway is insensitive to high concentrations of extracellular K+.
Figure 4.
IL-1β secretion by LPS-stimulated murine DCs requires caspase-1 and the Nlrp3 inflammasome. (A) IL-1β secretion by WT or caspase-1−/− murine DCs in response to different doses of LPS. Culture supernatants were collected 24 hours after the treatment of LPS and analyzed for IL-1β by ELISA. (B) WT or caspase-1−/− DCs were stimulated by 1 μg/ml LPS for the indicated time or left unstimulated. Culture supernatants and cell extracts were immunoblotted for IL-1β. Gapdh was used as a loading control. (C) IL-1β secretion by WT, Nlrp3−/− or Asc−/− DCs in response to different doses of LPS. Culture supernatants were collected 24 hours after the treatment of LPS and analyzed for IL-1β by ELISA. (D) WT, Nlrp3−/− or Asc−/−DCs were stimulated by 1 μg/ml LPS for the indicated time or left unstimulated. Culture supernatants and cell extracts were immunoblotted for IL-1β, Nlrp3 or Asc. Gapdh was used as a loading control. (E) Isolated murine splenic DCs from WT or Nlrp3−/− were stimulated with 1 μg/ml LPS for 24 hours or left unstimulated. The culture supernatants were analyzed for IL-1β by ELISA. (F) BMDCs were stimulated with LPS as indicated in panel A in the presence or absence of 50 mM KCl and IL-1β in culture supernatants was analyzed by ELISA. Data are representative of three independent experiments. *, p<0.05.
P2×7 signaling is not required for LPS-induced IL-1β production in vitro and in vivo
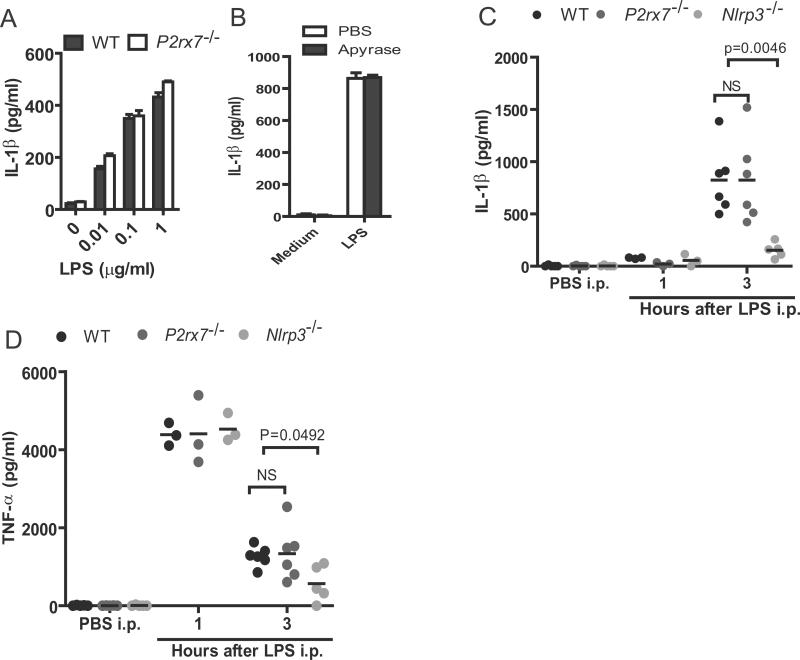
Previous studies have shown that human monocytes can release mature IL-1β with LPS stimulation alone, which is dependent on autocrine stimulation by ATP (10). To test whether IL-1β secretion by LPS-stimulated BMDCs is also dependent on signaling via P2×7, we used DCs from P2×7−/− mice and determined the effects of P2×7 deficiency on IL-1β release. In response to different doses of LPS, DCs from WT and P2×7−/− mice released comparable amounts of IL-1β and TNF-α (Fig. 5A and supplemental Fig. 1F). Addition of apyrase, an enzyme that hydrolyzes extracellular ATP, did not affect IL-1β secretion in LPS-stimulated DCs (Fig. 5B), which is consistent with the lack of requirement for P2×7. Furthermore, intraperitoneal administration of LPS induced comparable amounts of IL-1β in the serum of wild-type and P2×7−/− mice (Fig. 5C). In agreement with previous findings (18, 19), production of IL-1β after intraperitoneal LPS challenge was reduced in Nlrp3−/− mice when compared to WT mice (Fig. 5C). In contrast, the production of TNF-α induced by LPS administration was comparable in WT and P2×7−/− mice (Fig. 5D). Nlrp3 deficiency did not alter the peak induction of TNF-α 1 hr after LPS administration, although the amounts of TNF-α in serum were lower at 3 hrs in Nlrp3−/− mice (Fig. 5D). Taken together, these results indicate that stimulation via an ATP-P2×7 axis is not required for LPS-induced Nlrp3-dependent IL-1β production in vivo.
Figure 5.
P2X7 signaling is dispensable for Nlrp3-dependent IL-1β secretion induced by LPS in in vivo. (A) IL-1β secretion in WT and P2X7−/− DCs stimulated by indicated doses of LPS. Cell culture supernatants were collected 24 hours after LPS stimulation. Data are Data are the mean ± s.d. of triplicate wells and representative of three independent experiments. (B) The IL-1β secretion from LPS-stimulated DCs in the presence of apyrase. PBS or apyrase (10 u/ml) was added to the culture medium of DCs with or without LPS. The amounts of IL-1β in culture supernatant were analyzed 24 hours after treatment. Data are the mean ± s.d. of triplicate wells and representative of three independent experiments. (C–D) Serum from WT, P2X7−/− or Nlrp3−/−mice (n=3–6 per genotype) were collected at 1 and 3 hrs after intraperitoneal injection of 25 mgkg−1 LPS and the amounts of IL-1β and TNF-α were determined by ELISA. Data are representative of two independent experiments.
Discussion
IL-1β is a critical inflammatory mediator of host immune responses. The precursor pro-IL-1β is inactive and requires processing by caspase-1 or serine proteases for maturation and induction of biological activities. The activation of caspase-1 is mediated by inflammasomes of which Nlrp3 has received significant attention due to its role in both physiological and pathological conditions. Most studies of the Nlrp3 inflammasome have been performed in macrophages which showed that stimulation with TLR ligands alone is not sufficient to trigger Nlpr3 inflammasome activation. In contrast, stimulation with TLR ligands can activate Nlrp3 in human monocytes which may require autocrine P2×7 stimulation by ATP (11). Our results indicate that unlike mouse BMDMs, BMDCs and splenic DCs can produce substantial amounts of IL-1β in the culture supernatant in response to TLR ligands, which depended on the Nlrp3 inflammasome. In contrast to human monocytes, apyrase treatment and P2×7 ablation did not affect IL-1β secretion induced by LPS stimulated in murine DCs. More importantly, Nlrp3, but not P2×7, was required for LPS-induced IL-1β production in vivo.
How might DCs and macrophages differ in the regulation of Nlrp3 inflammasome activation and IL-1β maturation in response to TLR ligands? Under steady state conditions and after LPS stimulation, we found that DCs express more Nlrp3 protein than macrophages. Furthermore, the induction of pro-IL-1β by LPS was more marked in DCs than in macrophages. Our findings appear to be relevant in vivo because studies with reporter eGFP-Nlrp3 knockin mice revealed that splenic DCs have higher Nlrp3 promoter activity than splenic macrophages (20). Although downstream signaling events upon TLR4 ligation were more rapid and robust in DCs than that in macrophages, the induction of TNF-α and Nlrp3 mRNA was comparable in both cell types. Likewise, the production of pro-IL-β in response to LPS was comparable at 1 hr, but more pro-IL-1β mRNA was accumulated at later points in DCs than in macrophages. Because the latter correlated with the release of IL-1β in DCs, it is possible that the increase in IL-1β mRNA in DCs at later time points is caused by self-induction of pro-IL-1β by mature IL-1β. Although the increased production of pro-IL-1β in DCs contributes to increase production of mature IL-1β, increase release of mature IL-1β is also caused by the induction of caspase-1 activation by LPS in DCs. The latter correlated with processing and released of pro-IL-1β and IL-18 in DCs indicating that stimulation with LPS is sufficient to trigger caspase-1 activation in DCs but not macrophages. The increased expression of Nlrp3 protein may explain, at least in part, the differential ability of DCs to activate the Nlrp3 inflammasome in response to TLR agonists. Notably, the induction of Nlrp3 mRNA levels by LPS was comparable in DCs and macrophages. The findings suggest that post-transcriptional mechanisms are important in the regulation of Nlrp3 protein expression. Consistently, recent studies showed that microRNAs and in particular miR-223 control Nlrp3 expression and Nlrp3 inflamamsome activation in human monocytes and mouse macrophages and DCs (21, 22). Consistent with our observations, Bauernfeind et al report that the Nlrp3 protein is expressed at higher levels in DCs than in macrophages which correlated with higher expression of miR-233 in macrophages (22). The elevated amounts of Nlrp3 protein in DCs after TLR stimulation might be sufficient to achieve an activation threshold for the Nlrp3 inflammasome. This mechanism of LPS-induced Nlrp3 activation in DCs could be similar to conditions in non-immune HEK293 cells in which overexpression of inflammasome components can trigger caspase-1 activation (23). Alternatively, the TLR signaling pathway may be physically linked to Nlrp3 activation in DCs. Regardless of the mechanism, increased Nlrp3 activation in DCs may contribute to their ability to activate certain immune responses against microbial stimuli that involve IL-1β and IL-18.
The amounts of ATP necessary for Nlrp3-mediated caspase-1 activation to be detected in macrophages in vitro are much greater than physiological concentrations (24, 25) and, therefore, the relevance of the ATP-P2X7 pathway in vivo has remained uncertain. Our results indicate that the accepted two-signal model necessary for activation of the Nlrp3 inflammasome in response to TLR ligands in mouse macrophages (8) does not apply to DCs. More importantly, P2X7 signaling was not required for IL-1β production in response to LPS administration in vivo. Thus, DCs and/or other cells can produce mature IL-1β via the Nlrp3 inflamasome in vivo in the absence of P2X7 stimulation. Although our studies do not rule out the possibility of a very minor contribution of P2X7 signaling, they challenge the physiological relevance of ATP-P2X7 axis for the induction of IL-1β production in response to TLR agonists such as LPS.
Supplementary Material
Acknowledgments
We would like to thank Millennium Pharmaceuticals for providing mutant mice. We would also like to thank Peter Kuffa for help in generating anti-Nlrp3 antibody, Jessica Werner for reviewing the manuscript and Sharon Koonse for animal husbandry.
∥This work was supported by grants R01AI063331 and R01DK091191 from the National Institutes of Health. L. F. was supported by a Research Career Development Award from the Crohn's and Colitis Foundation of America.
Abbreviations used in this paper
- BMDCs
bone marrow derived dendritic cells
- BMDMs
bone marrow derived macrophages
- DCs
dendritic cells
- NLRs
nucleotide-binding oligomerization domain-like receptors
- Nlrp3
NLR pyrin domain-containing 3
- P2X7
purinergic receptor P2X ligand-gated ion channel 7
- TLRs
Toll-like receptors
- WT
wild-type
References
- 1.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Schindler R, Clark BD, Dinarello CA. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J Biol Chem. 1990;265:10232–10237. [PubMed] [Google Scholar]
- 3.Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 4.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–615. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 10.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 13.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 14.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome Activators Induce Interleukin-1alpha Secretion via Distinct Pathways with Differential Requirement for the Protease Function of Caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Harder J, Franchi L, Munoz-Planillo R, Park JH, Reimer T, Nunez G. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol. 2009;183:5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozoren N, Masumoto J, Franchi L, Kanneganti TD, Body-Malapel M, Erturk I, Jagirdar R, Zhu L, Inohara N, Bertin J, Coyle A, Grant EP, Nunez G. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J Immunol. 2006;176:4337–4342. doi: 10.4049/jimmunol.176.7.4337. [DOI] [PubMed] [Google Scholar]
- 17.Kim YG, Park JH, Reimer T, Baker DP, Kawai T, Kumar H, Akira S, Wobus C, Nunez G. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 19.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, Tardivel A, Mattmann C, Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 21.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA, Masters SL. Cutting Edge: miR-223 and EBV miR-BART15 Regulate the NLRP3 Inflammasome and IL-1beta Production. J Immunol. 2012 doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 22.Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 Inflammasome Activity Is Negatively Controlled by miR-223. J Immunol. 2012 doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 23.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 24.Beigi RD, Dubyak GR. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J Immunol. 2000;165:7189–7198. doi: 10.4049/jimmunol.165.12.7189. [DOI] [PubMed] [Google Scholar]
- 25.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.