Summary
Voltage-gated ion channels are diverse and fundamental determinants of neuronal intrinsic excitability. Voltage-gated K+ (Kv) and Na+ (Nav) channels play complex yet fundamentally important roles in determining intrinsic excitability. The Kv and Nav channels located at the axon initial segment (AIS) play a unique and especially important role in generating neuronal output in the form of anterograde axonal and backpropagating action potentials, Aberrant intrinsic excitability in individual neurons within networks contributes to synchronous neuronal activity leading to seizures. Mutations in ion channel genes gives rise to a variety of seizure-related “Channelopathies”, and many of the ion channel subunits associated with epilepsy mutations are localized at the AIS, making this a hotspot for epileptogenesis. Here we review the cellular mechanisms that underlie the trafficking of Kv and Nav channels found at the AIS, and how Kv and Nav channel mutations associated with epilepsy can alter these processes.
Keywords: Potassium, channel-Sodium, channel- Neuron-Subcellular, localization-Seizures
Introduction
Voltage-gated ion channels are fundamental determinants of intrinsic excitability in neurons (Hille, 2001). Changes in expression, localization and function of ion channels underlie changes in neuronal excitability (Nusser, 2011). Epilepsy is characterized by the occurrence of spontaneous seizures, which consist of large populations of brain neurons exhibiting bursts of synchronous firing. Aberrant intrinsic excitability in individual neurons within networks contributes to synchronous neuronal activity leading to seizures. A variety of mechanisms working to alter expression, localization and function of voltage-gated ion channels lead to aberrant intrinsic excitability. Among the intrinsic ionic conductances that govern neuronal excitability, voltage-gated K+ (Kv) and voltage-gated Na+ (Nav) currents play complex and fundamentally important roles in fine-tuning cellular and network activity. While Kv and Nav channels are found throughout neurons, those located at the axon initial segment (AIS) play a unique and especially important role in generating neuronal output in the form of axonal action potentials (Bender & Trussell, 2012), and backpropagating action potentials that invade the soma and dendritic tree to influence computational and integrative events (Hu et al., 2009). Moreover, many of the ion channel subunits associated with epilepsy mutations are localized at the AIS, making this a hotspot for epileptogenesis (Wimmer et al., 2010b). We note that recent reviews have focused on both general aspects of ion channel trafficking (Jensen et al., 2011, Leterrier et al., 2011, Vacher & Trimmer, 2011) and on the generation and maintenance of the AIS (Grubb & Burrone, 2010b, Rasband, 2010, 2011). Here we review the cellular mechanisms that underlie the trafficking of Kv and Nav channels found at the AIS, and how Kv and Nav channel mutations associated with epilepsy can alter these processes. Mutations involved in numerous human diseases can cause protein trafficking defects via diverse mechanisms, in some cases due to mutations in bona fide trafficking motifs, and in others due to a more general misfolding of the mutant protein that cannot pass the rough endoplasmic reticulum (ER) quality control systems (Braakman & Bulleid, 2011) leading to degradation (Cobbold et al., 2003, Sitia & Braakman, 2003). There is increased interest in identifying molecules can rescue trafficking defective mutants and restore expression and function (Bernier et al., 2004). Note that a series of recent papers have highlighted cell-specific differences in the composition of the AIS [e.g., (Duflocq et al., 2008, Lorincz & Nusser, 2008), etc.], here we focus primarily on the AIS in glutamatergic neurons.
I- Voltage-gated potassium (Kv) channels
I.1- Structure and expression of principal and auxiliary subunits of native Kv channels in mammalian brain
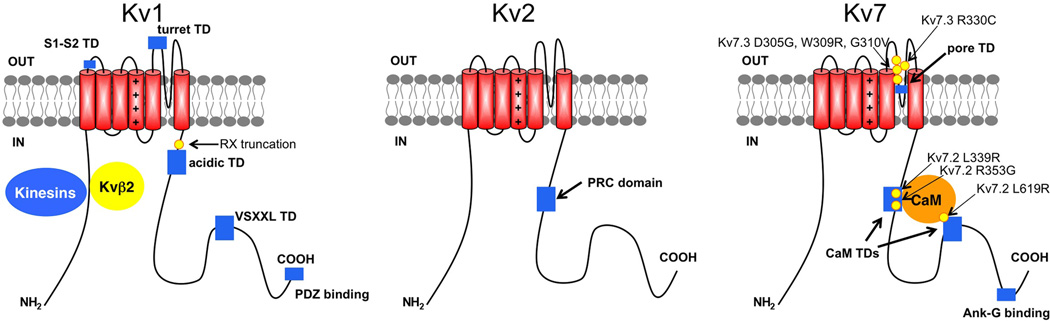
Kv channels are the most diverse subfamily of voltage-gated ion channel α subunits, with over 40 human genes in 12 subfamilies, termed Kv1-Kv12 (Yu & Catterall, 2004). Kv channel α subunits have six transmembrane segments, the first four (S1-S4) form the voltage-sensing module and the region between transmembrane segments S5-S6 the pore module (Figure 1). Four independent α subunits from the same subfamily assemble to form functional homo- or hetero-meric Kv channels, and coassembly with auxiliary subunits further enhances Kv channel diversity. Subunit composition determines the trafficking, localization, biophysical, pharmacological and modulation characteristics of a given Kv channel.
Figure 1.
Cartoon showing transmembrane topology of a prototypical Kv1 (left) and Kv7 (right) channel α subunit, with the voltage-sensing module comprising transmembrane segments S1-S4 (positive charges shown in S4) to the left, and the pore module comprising S5-S6 to the right. Colored rectangles show approximate locations of identified trafficking determinants or TDs. Colored ovals show approximate binding sites of interacting proteins implicated in trafficking to and/or scaffolding at the AIS. Yellow circles denote specific sites of epilepsy-associated mutations known to impact trafficking.
In general, Kv channels exhibit subfamily-specific patterns of subcellular localization (Vacher et al., 2008), with Kv1, Kv2 and Kv7 family members unique in that they are found at the AIS (Figure 2) (Vacher et al., 2008, Clark et al., 2009). Kv1 channels at the AIS play crucial roles in determining action potential initiation and propagation (Kole & Stuart, 2012). These channels generally contain Kv1.1 and Kv1.2 α subunits and auxiliary Kvβ subunits (Van Wart et al., 2007, Lorincz & Nusser, 2008, Ogawa et al., 2010, Duflocq et al., 2011, Vacher et al., 2011), although Kv1.4 is found at the AIS in younger animals (Ogawa et al., 2008). Delayed rectifier-type Kv2.1 and Kv2.2 channels (Lim et al., 2000, Johnston et al., 2008, Sarmiere et al., 2008) and M-type Kv7.2 and Kv7.3 channels (Cooper et al., 2001, Cooper, 2011, Klinger et al., 2011) are present at high density at the AIS. Other Kv channel subunits have not been described at the AIS (Vacher et al., 2008).
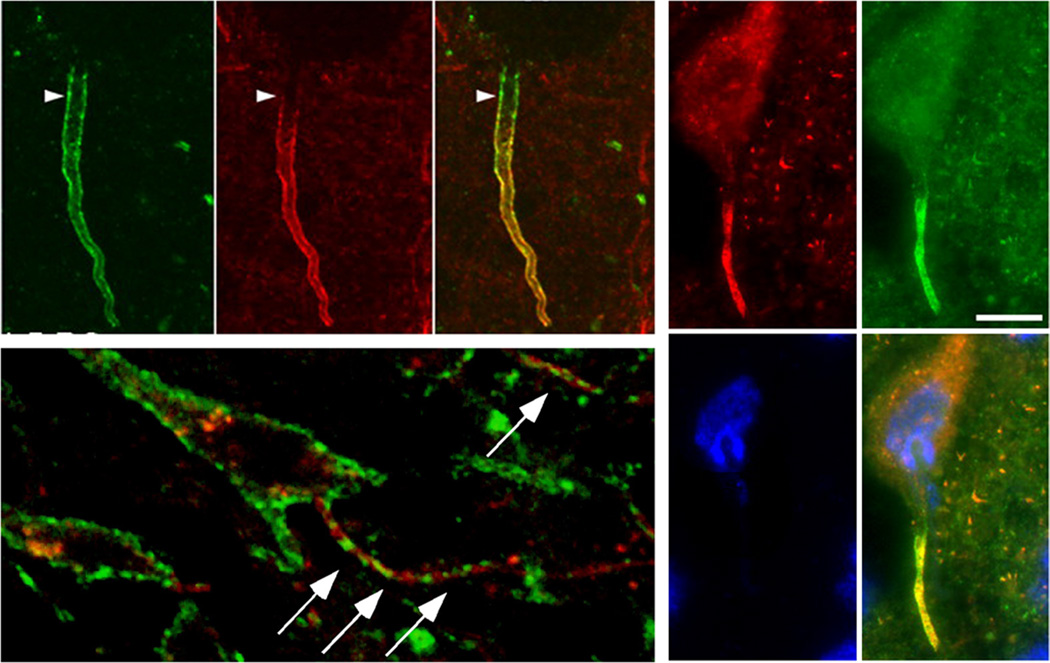
Figure 2.
Localization of Kv1 α subunits at the AIS in the mammalian nervous system. Top left panel: Nav1.6 (green) and Kv1.2 (red) α subunits in a layer 5 pyramidal neuron in rat neocortex (Lorincz & Nusser, 2008). Bottom left panel: Kv2.1 (green) α subunits and ANK-G (red) in neuron within the CA1 region of rat hippocampus (Sarmiere et al., 2008). Right panel. KCNQ2 (red) and KCNQ3 (green) α subunits in the axon initial segment of a mouse spinal cord ventral horn motoneuron; blue is the nucleic acid stain DAPI (Pan et al., 2006).
I.2- Kv channel trafficking and clustering determinants and mechanisms
Specific amino acid sequence motifs acting as trafficking determinants (TDs) have been identified within Kv1, Kv2 and Kv7 α and Kvβ auxiliary subunits, and direct the establishment and maintenance of expression and localization of these channels at the AIS. Many of these TDs are located within the intrinsically disordered C-terminal domains, which are thought to act as “intermolecular fishing rods” for interacting proteins important in Kv channel trafficking (Magidovich et al., 2006). These C-terminal domains are highly conserved in mammalian channel orthologs (e.g., human and rat Kv1.2 are 100% identical in the cytoplasmic C-terminus and 99% identical overall), yet are the most divergent regions within the α subunits in mammalian paralogs (e.g., human Kv2.1 and Kv2.2 are only 39% identical in the C-terminus, yet are otherwise 92% identical, and 62% identical overall).
A number of TDs regulate biogenic trafficking of Kv channels through the endomembrane system, from their sites of translation in the ER, to their sites of action in the plasma membrane. These include an anterograde TD sequence VXXSL present within the Kv1.4 cytoplasmic C-terminus, but not in other Kv1 family members (Li et al., 2000), and a potent ER retention TD in the turret domain external to the Kv1.1 channel pore that is dominant to the VXXSL TD (Manganas et al., 2001b), and which acts dictate the subunit composition-dependent trafficking of heteromeric Kv1 channels (Manganas & Trimmer, 2000). These TDs can act interdependently to regulate trafficking and plasma membrane expression of Kv1 channels (Zhu et al., 2003).
The same amino acids that form the potent turret TD confer sensitivity to dendrotoxin or DTX, which suggests that a DTX-like molecule within the ER could mediate ER retention by binding to this TD (Manganas et al., 2001b). Expressing soluble DTX within the ER lumen enhances cell surface expression of homomeric and heteromeric channels containing wild-type DTX-sensitive Kv1.1, but not those containing Kv1.1 mutants with reduced DTX binding (Vacher et al., 2007). The matrix metalloprotease MMP23 has a domain similar to a toxin with a Kv1 channel binding selectivity distinct from DTX. Overexpression of MMP23 leads to intracellular retention of Kv1 channels in order of the strength of their MMP23-binding (Rangaraju et al., 2010), suggesting that other MMP23-like mammalian proteins that contain a DTX-like domain could mediate intracellular trafficking by binding to the turret TD. Other Kv1 channel TDs include an acidic motif in a membrane-proximal region of the cytoplasmic C-terminus of Kv1 α subunits (Manganas et al., 2001a), and a specific motif within the extracellular loop between transmembrane segments S1 and S2 (McKeown et al., 2008), although the impact of altering these TDs is likely due to folding defects and ER quality control (Braakman & Bulleid, 2011).
The Kv1.2 α subunit contains weak versions of these TDs, and as such has trafficking characteristics sensitive to modulation by coassembly with Kv1 α subunits containing stronger TDs (Manganas & Trimmer, 2000) and with auxiliary Kvβ subunits (Shi et al., 1996, Campomanes et al., 2002, Gu et al., 2003). Phosphorylation of Kv1.2 also regulates its trafficking, for example upon acute suppression of Kv1.2 current upon muscarinic stimulation (Huang et al., 1993), mediated by endocytosis triggered by phosphorylation of specific C-terminal tyrosine residues (Nesti et al., 2004)., and leading to disruption of Kv1.2 interaction with the cytoskeletal protein cortactin (Williams et al., 2007). Note that phosphorylation at a different C-terminal tyrosine residue regulates Kv1.2 clustering (Gu & Gu, 2011, Smith et al., 2012). C-terminal serine phosphorylation sites regulate biogenic trafficking of Kv1.2-containing channels (Yang et al., 2007), and PKA-mediated enhancement of Kv1.2 currents (Johnson et al., 2009).
Polarized trafficking of Kv1 channels to axons is dependent upon the T1 domain within the cytoplasmic N-terminus (Rivera et al., 2005) that serves as a binding site for Kif5b subunits of the microtubule motor kinesin 1 (Rivera et al., 2007). Another kinesin subunit, KIF3A, a component of kinesin 2, is involved in polarized trafficking of Kv1.2 channels to axons (Gu et al., 2006, Gu & Gu, 2010). Kinesin-based trafficking of Kv1 channels is strongly influenced by cytoplasmic Kvβ auxiliary subunits, which also bind to the T1 domain. All three Kvβ subunit isoforms (Kvβ1-3) can promote cell surface expression of associated Kv1 channel complexes via effects on biogenic ER export (Shi et al., 1996, Campomanes et al., 2002), while co-expression of Kvβ2 with intact Kv1.2 (Campomanes et al., 2002) or reporter constructs containing the Kv1.2 T1 domain (Gu et al., 2003) increases their polarized expression in axons versus dendrites. Kvβ2 directly interacts with the microtubule binding protein EB1 (Gu et al., 2006, Vacher et al., 2011), and knockdown of EB1 suppresses Kv1.2 trafficking to axons (Gu & Gu, 2010). Phosphorylation of Kvβ2 regulates its interaction with EB1, and alters trafficking of Kv1 channels to axons and specifically to the AIS (Vacher et al., 2011), suggesting a mechanism for the dynamic regulation of ion channel localization at the AIS (Kuba et al., 2006, Grubb & Burrone, 2010a, Kuba et al., 2010, Grubb et al., 2011). Kv1 α subunits contain a PDZ-binding motif (Figure 1) on their C-termini (Kim et al., 1995), and specific PDZ domain-containing proteins of the MAGUK family, specifically PSD-93, are found associated and colocalized with Kv1 channels at the AIS (Ogawa et al., 2008). The precise targeting mechanisms that generate the observed colocalization of these proteins to the AIS are not known.
Unlike Kv1 channels, biogenic intracellular trafficking of Kv2.1 channels to the plasma membrane is not strongly influenced by TDs (Shi et al., 1994, Lim et al., 2000). However, increased insertion of Kv2.1 into the plasma membrane occurs acutely in response to stimuli that induce neuronal apoptosis (Yu et al., 1999, Pal et al., 2003, Pal et al., 2006, Redman et al., 2007) via increased Kv2.1 phosphorylation at specific C-terminal sites (Redman et al., 2007), and in response to monocular deprivation, leading to changes in neuronal excitability and intrinsic plasticity in visual cortical neurons (Nataraj et al., 2010). Changes in Kv2.1 phosphorylation at other C-terminal sites regulate whether Kv2.1 is clustered or is dispersed across the somatodendritic membrane (Misonou et al., 2004, Misonou et al., 2005, Misonou et al., 2006), although Kv2.1 channels at the AIS of cultured neurons are somewhat refractory to dephosphorylation-dependent dispersal (Misonou et al., 2004). The clustering of Kv2.1 in neurons is mediated by a specific C-terminal domain (the PRC domain, Figure 1) that also restricts the localization of clustered Kv2.1 to proximal dendrites, and to the AIS (Lim et al., 2000). Phosphorylation of Kv2.1 by CDK5 is required for maintenance of AIS clustering (Cerda & Trimmer, 2011). Recent studies suggest that Kv2.2 exists in somatodendritic clusters in certain brain neurons (Hermanstyne et al., 2010), in some cases associated with Kv2.1 (Kihira et al., 2010). Kv2.2 has been proposed to act at the AIS of auditory neurons (Johnston et al., 2008). The precise mechanism of Kv2 channel clustering at the AIS has not been determined, although compelling data have been provided supporting a role for a perimeter fence-based mechanism underlying the somatodendritic clustering of Kv2.1 (O'Connell et al., 2006, Tamkun et al., 2007).
Neuronal M-type Kv7 channels at the AIS exist as heteromers of Kv7.2 and Kv7.3 α subunits (Figure 2) (Cooper, 2011). Coassembly of Kv7.2 and Kv7.3 is crucial to their efficient trafficking, as the respective homomeric channels exhibit poor cell surface expression due to ER retention (Schwake et al., 2003, Rasmussen et al., 2007). Heteromeric Kv7.2/Kv7.3 assembly is also required for efficient localization at the AIS (Rasmussen et al., 2007). Trafficking of homomeric Kv7.3 is especially deficient, a characteristic that has been related to a Kv7.3-specific Ala at position 315 (as opposed to a Thr at this position in all other Kv channels) that acts as a potent pore TD, such that its mutation to Thr allows for expression of homomeric Kv7.3 channels (Gomez-Posada et al., 2010)(Figure 1). The location of this residue deep within the channel pore makes it unlikely that retention is mediated by interaction with a luminal ER protein, and more likely due to misfolding of homomeric Kv7.3 channels and retention via ER quality control (Braakman & Bulleid, 2011).
Numerous protein-protein interactions are mediated by the C-terminus of Kv7 α subunits (Haitin & Attali, 2008) (Figure 1). This includes interaction of Kv7.2 and Kv7.3 with ankyrin-G (Ank-G), via a C-terminal binding motif, which localizes Kv7 channels to the AIS (Cooper, 2011), and of Kv7.2 with calmodulin (CaM), which plays a particularly critical role in trafficking (Etxeberria et al., 2008, Alaimo et al., 2009). The C-terminus of Kv7 α subunits is also extensively modified by phosphorylation, with proteomic analyses identifying at least fourteen in vivo sites in Kv7.2, and four sites in Kv7.3 (Cerda et al., 2011), providing a potential mechanism for dynamic regulation of Kv7 channel trafficking. Trafficking of Kv7 channels is dynamically regulated in epithelial cells, with intracellular retention in unpolarized cells, and plasma membrane expression upon establishment of epithelial polarity (Andersen et al., 2011).
1I.3- Defects in Kv trafficking in epilepsy
Among the Kv1 and Kv2 α and Kvβ subunits known to be associated with the AIS, only the KCNA1 gene (http://omim.org/entry/176260) encoding the Kv1.1 α subunit has mutant alleles associated with neurological disorders, specifically episodic ataxia type 1 or EA-1, often associated with seizures, which arises due to loss-of-function of Kv1.1-containing channels. Numerous missense mutations have been identified that reduce or eliminate trafficking of Kv1.1-containing channels, as well as those that affect channel gating (Kullmann, 2010). A truncated mutant generated from a specific EA-1 nonsense mutation “RX” (Rea et al., 2002) lacks the membrane proximal acidic TD in the Kv1.1 C-terminus (Figure 1), causing misfolding and intracellular retention of the mutated Kv1.1 α subunits (Manganas et al., 2001a). Importantly, co-assembly of these mutated RX subunits with otherwise normal Kv1 α subunits acts as a dominant negative for their folding and trafficking (Manganas et al., 2001a, Rea et al., 2002), and expression of RX in neurons leads to reduced Kv current levels, and increased glutamate release, consistent with loss of expression of RX-containing channels, including those at the AIS (Heeroma et al., 2009), and providing a possible basis for the severe EA-1 symptoms associated with this mutation (Eunson et al., 2000).
The KCNA2 gene encoding Kv1.2 is not known to harbor mutations resulting in neurological or other human diseases (http://omim.org/entry/176262), however, Kv1.2 knockout (KO) mice have enhanced susceptibility to flurothyl-induced seizures at postnatal day 14 (P14), at P15 begin to have spontaneous running-bouncing seizures with tonic extension, and by P19 are dead from seizures (Brew et al., 2007). A mouse ENU-induced mutant that exhibits chronic motor uncoordination, named Pingu, carries a KCNA2 missense mutation yielding an I402T mutation in the S6 transmembrane segment, resulting in decreased Kv1.1 and Kv1.2 expression in cerebellum, and heterologous cells expressing this mutant have decreased expression, suggesting a trafficking defect (Xie et al., 2010). The KCNAB2 gene encoding Kvβ2 does not harbor disease-causing mutations (http://omim.org/entry/601142), but certain patients with 1p36 deletion syndrome lack the chromosomal region containing the Kvβ2 gene (Shapira et al., 1997), and there exists a strong association between seizure severity and deletion of KCNAB2 (Heilstedt et al., 2001, Kurosawa et al., 2005). Disease-associated mutations in Kv2.1 and Kv2.2 (http://omim.org/entry 600397 and 607738, respectively) have not been described.
Eleven distinct allelic variants of Kv7.2 are associated with Benign Familial Neonatal Epilepsy (BFNE) (http://omim.org/entry/602235), as are mutations in Kv7.3 (http://omim.org/entry/602232). Kv7 channels are also important as targets for retigabine, a novel anti-epileptic drug that acts as a positive allosteric modulator in promoting the enhanced opening of neuronal Kv7 channels (Gunthorpe et al., 2012). One set of BFNE-associated mutations cluster in the Kv7.3 primary sequence just up- (D305G, W309R, G310V) and down- (R330C) stream of the critical pore Ala-315 TD (Figure 1). It is not known whether these mutants exhibit reduced current due to reduced single channel conductance or reduced expression (Maljevic et al., 2010), although the G310V mutant has reduced levels of axonal surface expression in cultured neurons (Chung et al., 2006). A mutation in the pore region of Kv7.2 results in misfolding and reduced trafficking, however the channels that appear at the cell surface have normal single channel conductance (Maljevic et al., 2011). It is intriguing that a large number of BFNE mutations cluster within the Kv7.2 C-terminus, including missense, nonsense and frame shift mutations. Not surprisingly, many of these yield reduced expression via effects on trafficking of homomeric and heteromeric Kv7.2-containing channels (Maljevic et al., 2010). Some of these (Figure 1) appear to act through decreasing binding of CaM (Richards et al., 2004) such as Kv7.2 mutations R353G (Etxeberria et al., 2008) and L339R (Alaimo et al., 2009), which impact the exit of Kv7.2-containing channels from the ER, as well as L619R (Richards et al., 2004), whose trafficking properties have not been determined. While the role of CaM in Kv7 channel function is complex (Haitin & Attali, 2008), and includes effects on gating, and binding to other interacting proteins such as AKAPs (Bal et al., 2010), CaM binding, and its disruption by mutations found in epilepsy, impact trafficking. BFNE mutations have also been found to alter the polarized localization and plasma membrane expression of Kv7.2/Kv7.3 channels in axons (Chung et al., 2006).
II- Voltage-gated sodium channels
II.1- Structure and expression of principal and auxiliary subunits of native Nav channels in mammalian brain
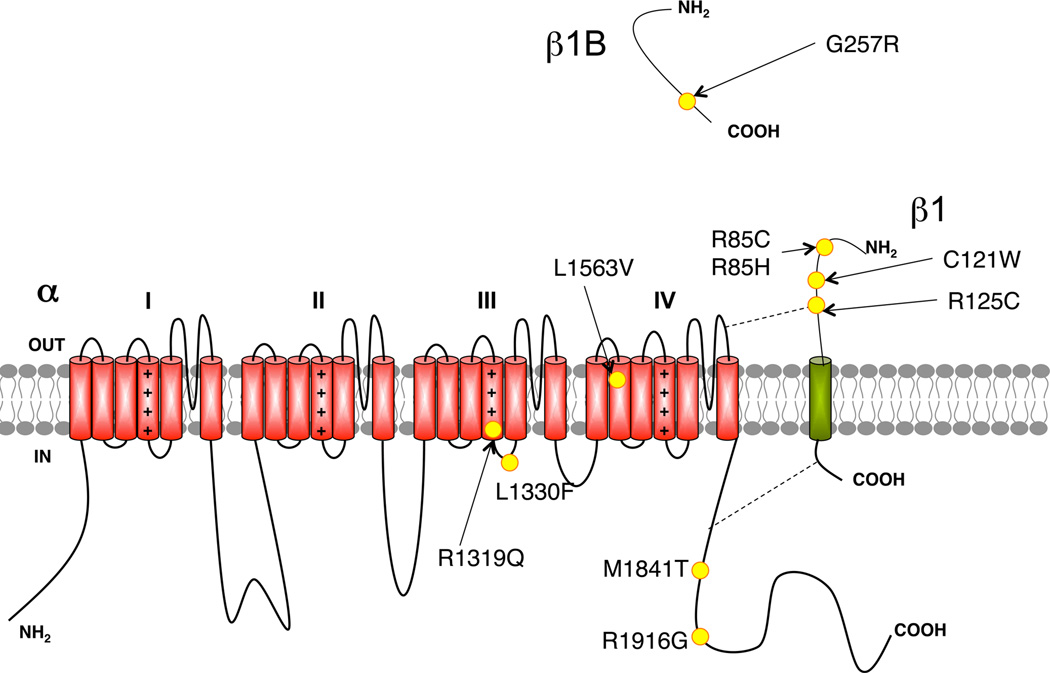
Nav channels consist of a highly posttranslationally modified α subunit, approximately 260 kDa in size, associated with auxiliary β subunits (~30 KDa) through either covalent (β2, β4) or non-covalent (β1, β3) linkages (Catterall, 2000). Nav α subunits have four internally repeated homologous domains (I-IV), each resembling a Kv channel α subunit (Figure 3). The voltage-sensing and pore-forming α subunit is sufficient for functional expression, but the kinetics and voltage-dependence of channel gating are impacted by β subunits. Auxiliary subunits are also involved in channel trafficking, localization, and interaction of Nav channels with cell adhesion molecules, extracellular matrix, and intracellular cytoskeleton (Qu et al., 2001, McEwen et al., 2004). Nav1.1 (Duflocq et al., 2008, Lorincz & Nusser, 2008), Nav1.2 (Boiko et al., 2003, Lorincz & Nusser, 2008) and Nav1.6 (Caldwell et al., 2000, Krzemien et al., 2000, Boiko et al., 2003) are present at the AIS (Figure 4). Specific functions have been attributed to the different AIS Nav channels (Van Wart & Matthews, 2006, Hu et al., 2009). Nearly 700 mutations of Nav1.1 (http://omim.org/entry/182389) have been identified in patients with inherited and sporadic epilepsy, making this the most commonly mutated gene in human epilepsy (Catterall et al., 2010, Meisler et al., 2010). A small number of mutations have been found in Nav1.2 (http://omim.org/entry/182390) and Nav1.3 (http://omim.org/entry/182391) (Meisler et al., 2010), and studies in mice suggest that Nav1.6 (http://omim.org/entry/600702) may contribute to seizure disorders (Meisler et al., 2010). Of the four β subunit genes (SCN1B-4B), to date only mutations in SCN1B (http://omim.org/entry/600235) are associated with epilepsy (Patino & Isom, 2010).
Figure 3.
Cartoon showing transmembrane topology of an Nav channel, with the domains 1–4 arranged from left to right. The colored oval shows approximate binding sites of ANK-G that mediates localization at the AIS. Yellow circles denote specific sites of epilepsy-associated mutations known to impact trafficking.
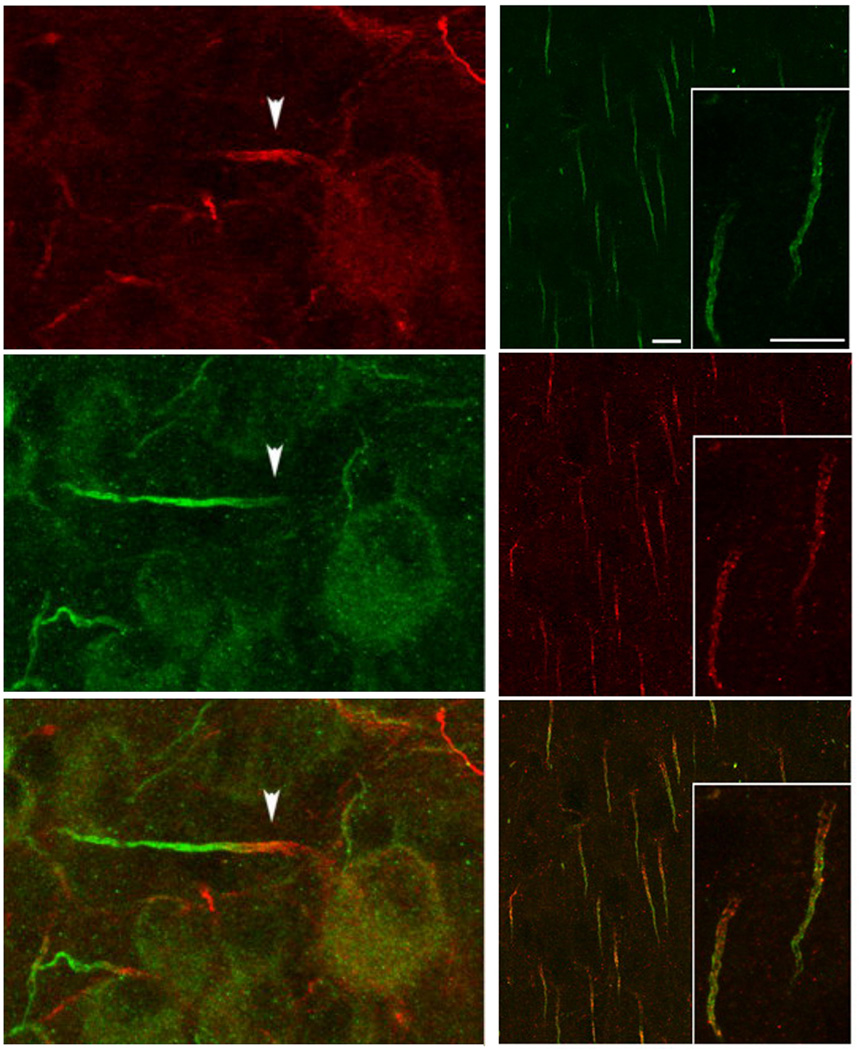
Figure 4.
Localization of Nav α subunits at the AIS in the mammalian nervous system. Left. Nav1.1 (red) and Nav1.6 (green) in the AIS of rat retinal ganglion cells. (Van Wart et al., 2007). Right. Nav1.6 (green) and ANK-G (red) in layer 2/3 pyramidal neurons in rat neocortex. (Lorincz & Nusser, 2008).
II.2- Nav channel trafficking determinants and mechanisms
In mammalian neurons, dense clusters of Nav channels at the AIS underlie action potential generation (Clark et al., 2009, Kress & Mennerick, 2009). Similar to Kv7 channels, Nav clustering at the AIS occurs via binding to AnkG (Leterrier et al., 2011), mediated by phosphorylation of the ankyrin-binding motif of Nav by the protein kinase CK2 (Garrido et al., 2003, Brechet et al., 2008), suggesting a mechanism for dynamic changes in AIS localization (Grubb & Burrone, 2010a, Grubb et al., 2011). β subunits, especially β2, increase sodium current density in some heterologous systems by enhancing α subunit cell surface expression (Isom et al., 1995). Compared to wild type, Scn2b null hippocampal cultures have an 50% reduction in cell surface 3H-saxitoxin binding (Chen et al., 2002), and the CA3 region of Scn1b null hippocampus expresses decreased levels of Nav1.1 and increased levels of Nav1.3 compared to wild type (Chen et al., 2004). However, the ability of β1 and β2 to increase Nav current density appears to be cell-type specific (Patino & Isom, 2010).
II.3- Defects in Nav trafficking and epilepsy
Improper localization or expression of Nav channels has been implicated in a number of pathological conditions linked to altered neuronal action potential generation and conduction (Mantegazza et al., 2010). Strikingly, defects in Nav channel function can lead to either neurological hyperexcitability (Meisler et al., 2010) or conduction failure/axonal degeneration (Smith, 2007) depending on the pathological context. Analyses of Nav mutations performed in non-neuronal cells or in mouse genetic models reveal a mixture of loss-of-function and gain-of-function effects due to altered channel gating (Catterall et al., 2010). Other mutations lead to loss-of-function resulting from folding and/or trafficking defects that reduce channel expression, which is exacerbated in the absence of auxiliary β subunits (Rusconi et al., 2007, Misra et al., 2008, Rusconi et al., 2009). However in contrast to Cav channels (Pietrobon, 2010), mutated Nav1 channels do not seem to impair expression or function of wild-type Nav1 channels. A recent study showed that coexpression of two Dravet syndrome Nav1.1 truncation mutants (R222* and R1234*) did not impact expression of wild-type Nav1.1, in either heterologous cells or neurons (Bechi et al., 2012). Moreover, mutations in β1 subunits also impair cell surface expression of Nav channels (Patino & Isom, 2010).
In the case of generalized epilepsy with febrile seizures (GEFS+), two mutations in the Nav1.1 C-terminal cytoplasmic domain cause improper folding/trafficking. The M1841T mutant (Rusconi et al., 2007) exhibits impaired trafficking, which can be partially rescued by coexpression of β1 subunits, yielding channels with biophysical properties identical to those of wild-type Nav1.1. Incubation of cells at permissive temperatures (temperatures <30°C) and interactions with CaM, G-protein βγ subunits and pore blocker drugs (phenytoin) all partially rescue M1841T cell surface expression (Rusconi et al., 2007). The R1916G mutation (Rusconi et al., 2009) occurs within the IQ motif critical to CaM binding (Bahler & Rhoads, 2002) and yields loss of function due to folding and trafficking defects (Bernier et al., 2004). Cell surface trafficking of R1916G can be rescued by β1 subunit coexpression, revealing channels with altered gating properties, but not by other β subunits, CaM or Gβγ (Rusconi et al., 2009). Although numerous mutations of Nav1.2 channels (M252V, V261M, A263V, R233Q, R1319Q, L1330F, and L1563V) associated with benign familial neonatal epilepsy (BFNIS) affect Nav1.2 gating, leading to a gain-of-function and consequently neuronal hyperexcitability, only a few of these mutations are also associated with changes in trafficking (Scalmani et al., 2006, Xu et al., 2007b, Liao et al., 2010a, Liao et al., 2010b). To date, only one study shows that R1319Q, L1330F, and L1563V mutations yield reduced plasma membrane trafficking in addition to loss- (R1319Q, L1330F) or gain- (L1563V) of-function gating phenotypes (Misra et al., 2008). These findings have led to several hypotheses regarding how the developmental expression of Nav1.2 impacts the age dependence of BFNIS.
All epileptic syndromes associated with β1 subunit mutations are included in GEFS+ as well as in Dravet Syndrome. The majority of SCN1B epilepsy mutations are loss-of-function, with many yielding reduced Nav channel plasma membrane trafficking. Interestingly, the majority of reported disease-causing mutations in β1 (C121W, R85C, R85H, R125C) occur within the extracellular immunoglobulin-like domain (Meadows et al., 2002, Xu et al., 2007a, Patino et al., 2009). This domain is important in mediating interaction of β1 with cell adhesion molecules and extracellular matrix. β1(C121W) knock-in mice exhibit a complete loss of β1 targeting to the AIS, although the subcellular localization of Nav channel α subunits appears surprisingly unaffected (Wimmer et al., 2010a), consistent with previous studies showing that AnkG-binding motifs in Nav α subunits are both necessary and sufficient for their AIS targeting. This suggests that the increase in excitability in β1(C121W) neurons is mediated by a “gain-of-function” in channel gating, due to loss of β1 subunits in the AIS channels. It is intriguing that these results from the β1(C121W) knock-in mice (Wimmer et al., 2010a) conflict with those from heterologous cells, where β1-C121W mutants exhibit normal cell surface trafficking (Meadows et al., 2002). The R125C mutation results in β1 subunits that are synthesized normally but not trafficked to the cell surface, although this can be rescued by lower temperatures, or by expression in Xenopus oocytes maintained at 18°C (Patino et al., 2009). Other GEFS+ β1 mutations (R85C and R85H) yield reduced plasma membrane expression, and coexpressed Nav channels exhibit altered gating relative to wild-type β1 (Xu et al., 2007a). A recently identified GEFS+ mutation G257R occurs in a soluble secreted splice variant of β1B that promotes neurite outgrowth in vitro, and based on its prominent expression during embryonic development, has been suggested to play a role in axonal pathfinding, although subtle effects on Nav1.3 gating are observed (Brackenbury & Isom, 2011). The G257R mutant has defective trafficking resulting in its intracellular retention, generating a functional null cellular phenotype that may impact neuronal function via effects on Nav channel gating, or through altered neuronal pathfinding (Patino et al., 2011).
Increases in Nav α subunit expression and channel activity have been observed after status epilepticus in the rat model of temporal lobe epilepsy (TLE), due to increased expression of specific Nav mRNAs (Aronica et al., 2001, Ketelaars et al., 2001). For example, entorhinal cortex layer II neurons have increased Nav channel expression and activity (Hargus et al., 2011), leading to their hyperexcitability, and a resultant increase in excitatory input into hippocampus via the perforant path (Kumar & Buckmaster, 2006). Interestingly, these neurons exhibit increased staining of Nav1.6 at the AIS and Nav1.2 (Hargus et al., 2011). Expression of Nav1.6 and Ank-G, but not Nav1.1, is upregulated in the CA1 region of the hippocampus following status epilepticus, while Nav1.1 expression remains similar to the age-matched controls, however, the staining presented was not at normal sites of expression of Nav1.6 and Ank-G at the AIS, but was intracellular (Chen et al., 2009), such that the relationship of the increased expression to neuronal excitability remains unclear. Note that Nav a subunits are extensively phosphorylated (Berendt et al., 2010), providing a potential mechanism for dynamic changes in expression, localization, and function.
Conclusions
Trafficking of Kv and Nav channels to the AIS is a crucial determinant of neuronal excitability. Many of the Kv and Nav channels associated with epilepsy mutations are localized at the AIS, making this a hotspot for epileptogenesis (Wimmer et al., 2010b), and many of these mutations yield defects in trafficking. Moreover, dynamic regulation of the expression and localization of Kv and Nav channels at the AIS results in altered excitability (Kuba et al., 2006, Grubb & Burrone, 2010a, Kuba et al., 2010), suggesting that activity-dependent modulation of trafficking to these sites could impact neuronal and network function.
Acknowledgments
The authors would like to thank the NIH (grants NS34383 and NS42225 to J.S. Trimmer) and the Institut National de la Santé et de la Recherche Médicale and Marie Curie 7th framework program (grant IRG-2008-239499 to H. Vacher) for generous support.
Footnotes
Disclosures
The authors have no conflicts to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this review is consistent with those guidelines.
References
- Alaimo A, Gomez-Posada JC, Aivar P, Etxeberria A, Rodriguez-Alfaro JA, Areso P, Villarroel A. Calmodulin activation limits the rate of KCNQ2 K+ channel exit from the endoplasmic reticulum. J Biol Chem. 2009;284:20668–20675. doi: 10.1074/jbc.M109.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MN, Olesen SP, Rasmussen HB. Kv7.1 surface expression is regulated by epithelial cell polarization. Am J Physiol Cell Physiol. 2011;300:C814–C824. doi: 10.1152/ajpcell.00390.2010. [DOI] [PubMed] [Google Scholar]
- Aronica E, Yankaya B, Troost D, van Vliet EA, Lopes da Silva FH, Gorter JA. Induction of neonatal sodium channel II and III alpha-isoform mRNAs in neurons and microglia after status epilepticus in the rat hippocampus. Eur J Neurosci. 2001;13:1261–1266. doi: 10.1046/j.0953-816x.2001.01502.x. [DOI] [PubMed] [Google Scholar]
- Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- Bal M, Zhang J, Hernandez CC, Zaika O, Shapiro MS. Ca2+/calmodulin disrupts AKAP79/150 interactions with KCNQ (M-Type) K+ channels. J Neurosci. 2010;30:2311–2323. doi: 10.1523/JNEUROSCI.5175-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechi G, Scalmani P, Schiavon E, Rusconi R, Franceschetti S, Mantegazza M. Pure haploinsufficiency for Dravet syndrome Na(V)1.1 (SCN1A) sodium channel truncating mutations. Epilepsia. 2012;53:87–100. doi: 10.1111/j.1528-1167.2011.03346.x. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. The physiology of the axon initial segment. Annu Rev Neurosci. 2012;35:249–265. doi: 10.1146/annurev-neuro-062111-150339. [DOI] [PubMed] [Google Scholar]
- Berendt FJ, Park KS, Trimmer JS. Multisite phosphorylation of voltage-gated sodium channel alpha subunits from rat brain. J Proteome Res. 2010;9:1976–1984. doi: 10.1021/pr901171q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–228. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. Na channel beta subunits: overachievers of the ion channel family. Front Pharmacol. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, Robbins CA, McKee-Johnson J, Chiu SY, Messing A, Tempel BL. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol. 2007;98:1501–1525. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Na(v)1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campomanes CR, Carroll KI, Manganas LN, Hershberger ME, Gong B, Antonucci DE, Rhodes KJ, Trimmer JS. Kv beta subunit oxidoreductase activity and Kv1 potassium channel trafficking. J Biol Chem. 2002;277:8298–8305. doi: 10.1074/jbc.M110276200. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. J Physiol. 2010;588:1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda O, Baek JH, Trimmer JS. Mining recent brain proteomic databases for ion channel phosphosite nuggets. J Gen Physiol. 2011;137:3–16. doi: 10.1085/jgp.201010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda O, Trimmer JS. Activity-dependent phosphorylation of neuronal Kv2.1 potassium channels by CDK5. J Biol Chem. 2011;286:28738–28748. doi: 10.1074/jbc.M111.251942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, 3rd, Kazen-Gillespie KA, Kazarinova-Noyes K, Shrager P, Saunders TL, Macdonald RL, Ransom BR, Scheuer T, Catterall WA, Isom LL. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O'Malley HA, Bharucha V, Meadows LS, Knudsen GA, Vilaythong A, Noebels JL, Saunders TL, Scheuer T, Shrager P, Catterall WA, Isom LL. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Chen S, Chen L, Zhou J, Dai Q, Yang L, Li X, Zhou L. Long-term increasing co-localization of SCN8A and ankyrin-G in rat hippocampal cornu ammonis 1 after pilocarpine induced status epilepticus. Brain Res. 2009;1270:112–120. doi: 10.1016/j.brainres.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci U S A. 2006;103:8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Goldberg EM, Rudy B. Electrogenic tuning of the axon initial segment. Neuroscientist. 2009;15:651–668. doi: 10.1177/1073858409341973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold C, Monaco AP, Sivaprasadarao A, Ponnambalam S. Aberrant trafficking of transmembrane proteins in human disease. Trends Cell Biol. 2003;13:639–647. doi: 10.1016/j.tcb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Cooper EC. Made for "anchorin": Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons. Seminars in cell & developmental biology. 2011;22:185–192. doi: 10.1016/j.semcdb.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflocq A, Chareyre F, Giovannini M, Couraud F, Davenne M. Characterization of the axon initial segment (AIS) of motor neurons and identification of a para-AIS and a juxtapara-AIS, organized by protein 4.1B. BMC Biol. 2011;9:66. doi: 10.1186/1741-7007-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflocq A, Le Bras B, Bullier E, Couraud F, Davenne M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol Cell Neurosci. 2008;39:180–192. doi: 10.1016/j.mcn.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Aivar P, Rodriguez-Alfaro JA, Alaimo A, Villace P, Gomez-Posada JC, Areso P, Villarroel A. Calmodulin regulates the trafficking of KCNQ2 potassium channels. FASEB J. 2008;22:1135–1143. doi: 10.1096/fj.07-9712com. [DOI] [PubMed] [Google Scholar]
- Eunson LH, Rea R, Zuberi SM, Youroukos S, Panayiotopoulos CP, Liguori R, Avoni P, McWilliam RC, Stephenson JB, Hanna MG, Kullmann DM, Spauschus A. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48:647–656. [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- Gomez-Posada JC, Etxeberria A, Roura-Ferrer M, Areso P, Masin M, Murrell-Lagnado RD, Villarroel A. A pore residue of the KCNQ3 potassium M-channel subunit controls surface expression. J Neurosci. 2010;30:9316–9323. doi: 10.1523/JNEUROSCI.0851-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010a;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Building and maintaining the axon initial segment. Curr Opin Neurobiol. 2010b;20:481–488. doi: 10.1016/j.conb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Shu Y, Kuba H, Rasband MN, Wimmer VC, Bender KJ. Short- and long-term plasticity at the axon initial segment. J Neurosci. 2011;31:16049–16055. doi: 10.1523/JNEUROSCI.4064-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J Biol Chem. 2011;286:25835–25847. doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301:646–649. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52:803–816. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Gu Y, Gu C. Dynamics of Kv1 channel transport in axons. PLoS One. 2010;5:e11931. doi: 10.1371/journal.pone.0011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- Haitin Y, Attali B. The C-terminus of Kv7 channels: a multifunctional module. J Physiol. 2008;586:1803–1810. doi: 10.1113/jphysiol.2007.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargus NJ, Merrick EC, Nigam A, Kalmar CL, Baheti AR, Bertram EH, 3rd, Patel MK. Temporal lobe epilepsy induces intrinsic alterations in Na channel gating in layer II medial entorhinal cortex neurons. Neurobiol Dis. 2011;41:361–376. doi: 10.1016/j.nbd.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeroma JH, Henneberger C, Rajakulendran S, Hanna MG, Schorge S, Kullmann DM. Episodic ataxia type 1 mutations differentially affect neuronal excitability and transmitter release. Dis Model Mech. 2009;2:612–619. doi: 10.1242/dmm.003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilstedt HA, Burgess DL, Anderson AE, Chedrawi A, Tharp B, Lee O, Kashork CD, Starkey DE, Wu YQ, Noebels JL, Shaffer LG, Shapira SK. Loss of the potassium channel beta-subunit gene, KCNAB2, is associated with epilepsy in patients with 1p36 deletion syndrome. Epilepsia. 2001;42:1103–1111. doi: 10.1046/j.1528-1157.2001.08801.x. [DOI] [PubMed] [Google Scholar]
- Hermanstyne TO, Kihira Y, Misono K, Deitchler A, Yanagawa Y, Misonou H. Immunolocalization of the voltage-gated potassium channel Kv2.2 in GABAergic neurons in the basal forebrain of rats and mice. J Comp Neurol. 2010;518:4298–4310. doi: 10.1002/cne.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic channels of excitable membranes. Sinauer, Sunderland, MA: 2001. [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Huang XY, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Isom LL, Scheuer T, Brownstein AB, Ragsdale DS, Murphy BJ, Catterall WA. Functional co-expression of the beta 1 and type IIA alpha subunits of sodium channels in a mammalian cell line. J Biol Chem. 1995;270:3306–3312. doi: 10.1074/jbc.270.7.3306. [DOI] [PubMed] [Google Scholar]
- Jensen CS, Rasmussen HB, Misonou H. Neuronal trafficking of voltage-gated potassium channels. Mol Cell Neurosci. 2011;48:288–297. doi: 10.1016/j.mcn.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP, Cole WC. Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449. J Biol Chem. 2009;284:16562–16574. doi: 10.1074/jbc.M109.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J Physiol. 2008;586:3493–3509. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelaars SO, Gorter JA, van Vliet EA, Lopes da Silva FH, Wadman WJ. Sodium currents in isolated rat CA1 pyramidal and dentate granule neurones in the post-status epilepticus model of epilepsy. Neuroscience. 2001;105:109–120. doi: 10.1016/s0306-4522(01)00176-2. [DOI] [PubMed] [Google Scholar]
- Kihira Y, Hermanstyne TO, Misonou H. Formation of heteromeric Kv2 channels in mammalian brain neurons. J Biol Chem. 2010;285:15048–15055. doi: 10.1074/jbc.M109.074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Klinger F, Gould G, Boehm S, Shapiro MS. Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat hippocampus. Neuroimage. 2011;58:761–769. doi: 10.1016/j.neuroimage.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Mennerick S. Action potential initiation and propagation: upstream influences on neurotransmission. Neuroscience. 2009;158:211–222. doi: 10.1016/j.neuroscience.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien DM, Schaller KL, Levinson SR, Caldwell JH. Immunolocalization of sodium channel isoform NaCh6 in the nervous system. J Comp Neurol. 2000;420:70–83. [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Neurological channelopathies. Annu Rev Neurosci. 2010;33:151–172. doi: 10.1146/annurev-neuro-060909-153122. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa K, Kawame H, Okamoto N, Ochiai Y, Akatsuka A, Kobayashi M, Shimohira M, Mizuno S, Wada K, Fukushima Y, Kawawaki H, Yamamoto T, Masuno M, Imaizumi K, Kuroki Y. Epilepsy and neurological findings in 11 individuals with 1p36 deletion syndrome. Brain Dev. 2005;27:378–382. doi: 10.1016/j.braindev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Leterrier C, Brachet A, Dargent B, Vacher H. Determinants of voltage-gated sodium channel clustering in neurons. Seminars in cell & developmental biology. 2011;22:171–177. doi: 10.1016/j.semcdb.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Li D, Takimoto K, Levitan ES. Surface expression of Kv1 channels is governed by a C-terminal motif. J Biol Chem. 2000;275:11597–11602. doi: 10.1074/jbc.275.16.11597. [DOI] [PubMed] [Google Scholar]
- Liao Y, Anttonen AK, Liukkonen E, Gaily E, Maljevic S, Schubert S, Bellan-Koch A, Petrou S, Ahonen VE, Lerche H, Lehesjoki AE. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010a;75:1454–1458. doi: 10.1212/WNL.0b013e3181f8812e. [DOI] [PubMed] [Google Scholar]
- Liao Y, Deprez L, Maljevic S, Pitsch J, Claes L, Hristova D, Jordanova A, Ala-Mello S, Bellan-Koch A, Blazevic D, Schubert S, Thomas EA, Petrou S, Becker AJ, De Jonghe P, Lerche H. Molecular correlates of age-dependent seizures in an inherited neonatal-infantile epilepsy. Brain. 2010b;133:1403–1414. doi: 10.1093/brain/awq057. [DOI] [PubMed] [Google Scholar]
- Lim ST, Antonucci DE, Scannevin RH, Trimmer JS. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron. 2000;25:385–397. doi: 10.1016/s0896-6273(00)80902-2. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidovich E, Fleishman SJ, Yifrach O. Intrinsically disordered C-terminal segments of voltage-activated potassium channels: a possible fishing rod-like mechanism for channel binding to scaffold proteins. Bioinformatics. 2006;22:1546–1550. doi: 10.1093/bioinformatics/btl137. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Naros G, Yalcin O, Blazevic D, Loeffler H, Caglayan H, Steinlein OK, Lerche H. Temperature and pharmacological rescue of a folding-defective, dominant-negative KV 7.2 mutation associated with neonatal seizures. Hum Mutat. 2011;32:E2283–E2293. doi: 10.1002/humu.21554. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Wuttke TV, Seebohm G, Lerche H. KV7 channelopathies. Pflugers Arch. 2010;460:277–288. doi: 10.1007/s00424-010-0831-3. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Akhtar S, Antonucci DE, Campomanes CR, Dolly JO, Trimmer JS. Episodic ataxia type-1 mutations in the Kv1.1 potassium channel display distinct folding and intracellular trafficking properties. J Biol Chem. 2001a;276:49427–49434. doi: 10.1074/jbc.M109325200. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem. 2000;275:29685–29693. doi: 10.1074/jbc.M005010200. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Wang Q, Scannevin RH, Antonucci DE, Rhodes KJ, Trimmer JS. Identification of a trafficking determinant localized to the Kv1 potassium channel pore. Proc Natl Acad Sci U S A. 2001b;98:14055–14059. doi: 10.1073/pnas.241403898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–424. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Meadows LS, Chen C, Thyagarajan V, Isom LL. Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J Biol Chem. 2004;279:16044–16049. doi: 10.1074/jbc.M400856200. [DOI] [PubMed] [Google Scholar]
- McKeown L, Burnham MP, Hodson C, Jones OT. Identification of an evolutionarily conserved extracellular threonine residue critical for surface expression and its potential coupling of adjacent voltage-sensing and gating domains in voltage-gated potassium channels. J Biol Chem. 2008;283:30421–30432. doi: 10.1074/jbc.M708921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, Koopman MC, Kriegler S, Isom LL, Ragsdale DS. Functional and biochemical analysis of a sodium channel beta1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J Neurosci. 2002;22:10699–10709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, O'Brien JE, Sharkey LM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. J Physiol. 2010;588:1841–1848. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci. 2006;26:13505–13514. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005;25:11184–11193. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Misra SN, Kahlig KM, George AL., Jr Impaired NaV1.2 function and reduced cell surface expression in benign familial neonatal-infantile seizures. Epilepsia. 2008;49:1535–1545. doi: 10.1111/j.1528-1167.2008.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj K, Le Roux N, Nahmani M, Lefort S, Turrigiano G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron. 2010;68:750–762. doi: 10.1016/j.neuron.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesti E, Everill B, Morielli AD. Endocytosis as a mechanism for tyrosine kinase-dependent suppression of a voltage-gated potassium channel. Mol Biol Cell. 2004;15:4073–4088. doi: 10.1091/mbc.E03-11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z. Differential subcellular distribution of ion channels and the diversity of neuronal function. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.10.006. [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J Neurosci. 2006;26:9609–9618. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal SK, Takimoto K, Aizenman E, Levitan ES. Apoptotic surface delivery of K+ channels. Cell Death Differ. 2006;13:661–667. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O'Malley HA, Chen C, Calhoun JD, Lafreniere RG, Cossette P, Rouleau GA, Isom LL. Voltage-gated Na+ channel beta1B: a secreted cell adhesion molecule involved in human epilepsy. J Neurosci. 2011;31:14577–14591. doi: 10.1523/JNEUROSCI.0361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, Chen C, O'Malley HA, Gray CB, Miyazaki H, Nukina N, Oyama F, De Jonghe P, Isom LL. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–10778. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino GA, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel beta subunits in development and disease. Neurosci Lett. 2010;486:53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D. CaV2.1 channelopathies. Pflugers Arch. 2010;460:375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- Qu Y, Curtis R, Lawson D, Gilbride K, Ge P, DiStefano PS, Silos-Santiago I, Catterall WA, Scheuer T. Differential modulation of sodium channel gating and persistent sodium currents by the beta1, beta2, and beta3 subunits. Mol Cell Neurosci. 2001;18:570–580. doi: 10.1006/mcne.2001.1039. [DOI] [PubMed] [Google Scholar]
- Rangaraju S, Khoo KK, Feng ZP, Crossley G, Nugent D, Khaytin I, Chi V, Pham C, Calabresi P, Pennington MW, Norton RS, Chandy KG. Potassium channel modulation by a toxin domain in matrix metalloprotease 23. J Biol Chem. 2010;285:9124–9136. doi: 10.1074/jbc.M109.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Seminars in cell & developmental biology. 2011;22:178–184. doi: 10.1016/j.semcdb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- Rea R, Spauschus A, Eunson LH, Hanna MG, Kullmann DM. Variable K(+) channel subunit dysfunction in inherited mutations of KCNA1. J Physiol. 2002;538:5–23. doi: 10.1113/jphysiol.2001.013242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MC, Heron SE, Spendlove HE, Scheffer IE, Grinton B, Berkovic SF, Mulley JC, Davy A. Novel mutations in the KCNQ2 gene link epilepsy to a dysfunction of the KCNQ2-calmodulin interaction. J Med Genet. 2004;41:e35. doi: 10.1136/jmg.2003.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Chu PJ, Lewis TL, Jr, Arnold DB. The role of Kif5B in axonal localization of Kv1 K(+) channels. Eur J Neurosci. 2007;25:136–146. doi: 10.1111/j.1460-9568.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- Rivera JF, Chu PJ, Arnold DB. The T1 domain of Kv1.3 mediates intracellular targeting to axons. Eur J Neurosci. 2005;22:1853–1862. doi: 10.1111/j.1460-9568.2005.04384.x. [DOI] [PubMed] [Google Scholar]
- Rusconi R, Combi R, Cestele S, Grioni D, Franceschetti S, Dalpra L, Mantegazza M. A rescuable folding defective Nav1.1 (SCN1A) sodium channel mutant causes GEFS+: common mechanism in Nav1.1 related epilepsies? Hum Mutat. 2009;30:E747–E760. doi: 10.1002/humu.21041. [DOI] [PubMed] [Google Scholar]
- Rusconi R, Scalmani P, Cassulini RR, Giunti G, Gambardella A, Franceschetti S, Annesi G, Wanke E, Mantegazza M. Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J Neurosci. 2007;27:11037–11046. doi: 10.1523/JNEUROSCI.3515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalmani P, Rusconi R, Armatura E, Zara F, Avanzini G, Franceschetti S, Mantegazza M. Effects in neocortical neurons of mutations of the Na(v)1.2 Na+ channel causing benign familial neonatal-infantile seizures. J Neurosci. 2006;26:10100–10109. doi: 10.1523/JNEUROSCI.2476-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Jentsch TJ, Friedrich T. A carboxy-terminal domain determines the subunit specificity of KCNQ K(+) channel assembly. EMBO Rep. 2003;4:76–81. doi: 10.1038/sj.embor.embor715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira SK, McCaskill C, Northrup H, Spikes AS, Elder FF, Sutton VR, Korenberg JR, Greenberg F, Shaffer LG. Chromosome 1p36 deletions: the clinical phenotype and molecular characterization of a common newly delineated syndrome. American journal of human genetics. 1997;61:642–650. doi: 10.1086/515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem. 1994;269:23204–23211. [PubMed] [Google Scholar]
- Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- Smith KJ. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathol. 2007;17:230–242. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Xu L, Kasten MR, Anderson MP. Mutant LGI1 inhibits seizure-induced trafficking of Kv4.2 potassium channels. J Neurochem. 2012;120:611–621. doi: 10.1111/j.1471-4159.2011.07605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun MM, O'Connell KM, Rolig AS. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J Cell Sci. 2007;120:2413–2423. doi: 10.1242/jcs.007351. [DOI] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Misonou H, Trimmer JS. Regulation of Kv1 channel trafficking by the mamba snake neurotoxin dendrotoxin K. FASEB J. 2007;21:906–914. doi: 10.1096/fj.06-7229com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Trimmer JS. Diverse roles for auxiliary subunits in phosphorylation-dependent regulation of mammalian brain voltage-gated potassium channels. Pflugers Arch. 2011;462:631–643. doi: 10.1007/s00424-011-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kvβ2 auxiliary subunit regulates Kv1 channel axonal targeting. J Cell Biol. 2011;192:813–824. doi: 10.1083/jcb.201007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking Nav1.6 sodium channels. J Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Williams MR, Markey JC, Doczi MA, Morielli AD. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc Natl Acad Sci U S A. 2007;104:17412–17417. doi: 10.1073/pnas.0703865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, Mitchell S, Richards KL, Scaf BB, Leaw BT, Hill EL, Royeck M, Horstmann MT, Cromer BA, Davies PJ, Xu R, Lerche H, Berkovic SF, Beck H, Petrou S. Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. J Clin Invest. 2010a;120:2661–2671. doi: 10.1172/JCI42219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, So EY, Berkovic SF, Petrou S. Axon initial segment dysfunction in epilepsy. J Physiol. 2010b;588:1829–1840. doi: 10.1113/jphysiol.2010.188417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Harrison J, Clapcote SJ, Huang Y, Zhang JY, Wang LY, Roder JC. A new Kv1.2 channelopathy underlying cerebellar ataxia. J Biol Chem. 2010;285:32160–32173. doi: 10.1074/jbc.M110.153676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Thomas EA, Gazina EV, Richards KL, Quick M, Wallace RH, Harkin LA, Heron SE, Berkovic SF, Scheffer IE, Mulley JC, Petrou S. Generalized epilepsy with febrile seizures plus-associated sodium channel beta1 subunit mutations severely reduce beta subunit-mediated modulation of sodium channel function. Neuroscience. 2007a;148:164–174. doi: 10.1016/j.neuroscience.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Xu R, Thomas EA, Jenkins M, Gazina EV, Chiu C, Heron SE, Mulley JC, Scheffer IE, Berkovic SF, Petrou S. A childhood epilepsy mutation reveals a role for developmentally regulated splicing of a sodium channel. Mol Cell Neurosci. 2007b;35:292–301. doi: 10.1016/j.mcn.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A. 2007;104:20055–20060. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh C, Strasser U, Tian M, Choi DW. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science. 1999;284:336–339. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- Zhu J, Watanabe I, Gomez B, Thornhill WB. Trafficking of Kv1.4 potassium channels: interdependence of a pore region determinant and a cytoplasmic C-terminal VXXSL determinant in regulating cell-surface trafficking. Biochem J. 2003;375:761–768. doi: 10.1042/BJ20030885. [DOI] [PMC free article] [PubMed] [Google Scholar]