Abstract
Forkhead box P3 (FOXP3)+ is a transcription factor necessary for the function of regulatory T cells (Treg cells). Treg cells maintain immune homeostasis and self-tolerance, and play an important role in the prevention of autoimmune disease. Here, we discuss the role of Treg cells in the pathogenesis of myasthenia gravis (MG) and review evidence indicating that a significant defect in Treg cell in vitro suppressive function exists in MG patients, without an alteration in circulating frequency. This functional defect is associated with a reduced expression of key functional molecules such as FOXP3 on isolated Treg cells and appears to be more pronounced in immunosuppression-naive MG patients. In vitro administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) enhanced the suppressive function of Treg cells and up-regulated FOXP3 expression. These findings indicate a clinically relevant Treg cell–intrinsic defect in immune regulation in MG that may reveal a novel therapeutic target.
Keywords: myasthenia gravis, regulatory T cells, FOXP3, GM-CSF
Introduction
Acquired myasthenia gravis (MG) is an autoimmune disorder of the neuromuscular junction in which patients experience fluctuating skeletal muscle weakness that often affects selected muscle groups preferentially. The target of the autoimmune attack in most cases is the skeletal muscle acetylcholine receptor (AChR), but in others, non-AChR components of the neuromuscular junction, such as the muscle-specific receptor tyrosine kinase, are targeted (reviewed in Ref. 1). The precise origin of the autoimmune response in MG is unknown, but abnormalities of the thymus gland (hyperplasia and neoplasia) almost certainly play a role in patients with anti-AChR antibodies(2,3) and genetic predisposition is also likely to influence which patients develop the disorder.(4–6) High-affinity, anti-AChR antibodies bind to the neuromuscular junction, activate complement and accelerate AChR destruction, while causing failure of neuromuscular transmission and the resulting myasthenic symptoms (reviewed in Ref. 1). However, auto reactive AChR-specific CD4+ T cells, which can be detected in most MG patients,(7–10) likely play an important role in MG, through cognate interactions with B cells leading to and the synthesis of anti-AChR antibodies.(11)
Regulatory T cells and immune tolerance
Immune tolerance to self antigens is initially achieved during thymic development by the clonal deletion of potentially autoreactive T cells. However, some of these pathogenic cells, including some with reactivity to skeletal muscle AChR, survive clonal deletion in normal individuals, and are kept in check by peripheral tolerance mechanisms, most notably by a specialized subset of CD4+ T cells called regulatory T cells (Treg cells).(12) Treg cells are a subpopulation of T cells that act to suppress activation of other immune cells and thereby maintain immune system homeostasis and self-tolerance.(13) Current theories regarding the pathogenesis of autoimmune disease hypothesize that a functional deficiency in Treg cells could result in a failure to suppress autoreactive T cell responses.(14,15)
The thymus gland is the primary source of Treg cells which constitute approximately 5–10% of the peripheral CD4+ T cell population, and play a crucial role in the maintenance of immune homeostasis.(13) Treg cells actively mediate self-tolerance and thus control autoimmunity by suppressing the activation, proliferation and cytokine production of effector autoreactive T cells that arise de novo or escape thymic deletion.(13,16) The transcription factor forkhead box protein P3 (FOXP3) is expressed in CD4+ CD25+ Treg cells and is the master regulator for the development and function of these cells. (17,18) FOXP3 gene transfer has been shown to convert naive CD4+ CD25− T cells into a functional regulatory lymphocyte population, demonstrating the pivotal role of FOXP3 in Treg cell biology.(18)
A deficiency or dysfunction of Treg cells has been shown to contribute to the pathogenesis and development of many autoimmune diseases.(19–22) Functional defects have been demonstrated in Treg cells isolated from patients with diverse autoimmune diseases, suggesting that in autoimmunity, Treg cell suppressive capacity may be diminished. It has also recently been reported that the source of the apparent Treg cell dysfunction may not necessarily be primarily intrinsic to Treg cells, and that effector T cells may acquire resistance to Treg cell suppression in certain autoimmune diseases. (19, 23–25)
Regulatory T cells and immune regulation in myasthenia gravis
While it is likely that Treg cells exert their effects on T helper cells, thereby regulating B cell function and antibody secretion, there is also evidence that Treg cells may have a direct effect on B cells.(26) Specifically, in MG, it may be hypothesized that dysfunctional Treg cells permit the production of anti-AChR autoantibodies, a hypothesis that remains to be specifically tested. Investigators examining the relative frequencies and function of Treg cells in the peripheral circulation and thymi of MG patients have reported conflicting results including reductions in Treg cell numbers,(27–29) impaired regulatory function,(30) or no defect.(31,32) Aside from measuring Treg cell numbers, and ability to suppress non-specific T cell proliferation, more detailed analyses of Treg cell– intrinsic (Treg phenotype, cytokine production, and suppressive function) versus extrinsic factors (T helper/effector cells, dendritic cells), and ability to suppress specific AChR-induced T proliferation have not been performed. The reported demonstration of a functional impairment of thymic Treg cells in MG patients is an interesting finding and suggests an origin for Treg cell dysfunction in the thymus, possibly owing to thymic pathology (hyperplasia or neoplasia). (33)
Importantly, in all of the studies referenced above, investigators utilized a single-step enrichment protocol in which Treg cells were identified and isolated by high surface expression of CD25. Unfortunately, the level of expression of CD25 that defines Treg cells has been inconsistently reported in the literature, and CD25 is also expressed by recently activated T cells, resulting in the possible inclusion of effector T cells in isolated Treg cell populations.(34) More recent studies have shown that Treg cells express low or absent levels of the IL-7 receptor α-chain (CD127) and that this expression is inversely correlated with FOXP3 expression and with suppressive function,(35,36) and that it may provide a more precise method for the isolation of a pure Treg cell population.
Impaired suppressive function of isolated MG Treg cells
We have investigated the existence of a functional defect in CD4+ CD25high CD127low/− Treg cells in MG patients by isolating this population of cells using flow-based sorting and co-culturing them with autologous CD4+ CD25− T responder cells (Tresp) at different Treg:Tresp ratios, in the presence of irradiated autologous antigen presenting cells (APCs). We performed T cell proliferation studies (CFSE dilution) stimulating the cell cultures with anti-CD3 Abs and analyzed cytokine profiles from cell culture supernatants. We found that the suppressive ability of isolated Treg cells from MG patients (MG Treg cells) was consistently reduced compared to age-matched controls analyzed concurrently.(37) As expected, Tresp cells from MG patients produced higher levels of pro-inflammatory cytokines (IL-6 and IFN-γ) and lower levels of IL-10, compared with controls. Furthermore, when isolated CD4+ CD25high CD127low/− cells (Treg cells) were added to the cultures, IL-6 and IL-17 levels actually increased, without altering IL-10 levels in MG patients.(37) This result suggests not only a functional inability of MG Treg cells to suppress the production of pro-inflammatory cytokines, but also the possible production of IL-6 and IL-17 by CD4+ CD25high CD127low/− cells in MG. Along these lines, it has been recently reported that peripheral Treg cells may differentiate into IL-17–producing cells upon T cell receptor stimulation in the presence of IL-6.(38) Thus, enhanced Treg cell plasticity in MG may underlie the observed disturbance in Treg cell suppressive function in MG, through functional conversion of cells with a Treg cell phenotype into pathogenic Th17 cells. This phenomenon may be associated with the level of FOXP3 expression within a T cell (see below).
Dysfunctional MG Treg cells express attenuated FOXP3 levels
The level of expression of FOXP3 plays a critical role in the development and function of Treg cells. A number of factors, some positive, some negative, interact to collectively drive FOXP3 gene expression and then maintain its expression in Treg cells. Reduced FOXP3 expression in circulating CD4+ CD25+ T cells(33,39) and CD4+ CD25+ thymocytes(33) has been observed in MG patients. We isolated CD4+ CD25high CD127low/− Treg cells from peripheral blood mononuclear cells (PBMCs) and found no alteration in the reservoir of Treg cells in the peripheral blood of MG patients compared to controls. We confirmed that these cells were largely (>90%) FOXP3-expressing cells, by flow cytometry–based phenotypic analysis, and were highly suppressive in functional suppressor assays.(37) However, we observed a significant down-regulation of the relative expression of FOXP3 at the protein and mRNA levels in isolated CD4+ CD25high CD127low/− cells in MG patients, compared with healthy controls. (37)
We went on to evaluate the expression of FOXP3-associated proteins including those responsible for key features of Treg function such as cytotoxic T lymphocyte antigen-4 (CTLA-4). We have shown that a significantly lower percentage of isolated Treg cells from MG patients express CTLA-4,(37) consistent with the impairment in suppressive function when these cells are used in suppressive assays. MHC II expression on human Treg cells has been reported to identify a distinct population of Treg cells with high FOXP3 expression and with the capability to mediate strong contact-dependent suppression.(40) HLA-DR expressing Treg cells have also been hypothesized to be involved in homeostatic maintenance of Treg cells in vivo via presentation of self- antigens,(40) so that a deficiency in this subset of Treg cells may impact on tolerance to self-antigens like the AChR. Accordingly, we have found that a reduced number of CD4+ CD25high CD127low/− cells express HLA-DR in MG patients.(37)
It should be pointed out that the demonstrated functional defect in Treg cells that has been reported in MG to date appears to be a global one (non-specific) since there is a failure to normally suppress T cell proliferation in response to non-specific T cell receptor engagement (anti-CD3 antibodies). One may hypothesize that Treg cell suppression of AChR-specific T cells is perturbed in MG, and that restoration of function in this population of Treg cells may be a focused strategy for treatment. The first step to address this possibility would be to investigate the question of whether Treg cells from MG patients have a reduced ability to suppress AChR-reactive T cells. While the number of circulating AChR-specific T cells is obviously low, previous investigators have identified in many patients with MG myasthenogenic AChR peptides that induce T cell proliferation.(41) Based on this work, we synthesized two peptides representing sequences of the human AChR-α subunit (p195–212 and p257–259), in which at least one of the two peptides have been determined to induce proliferation of peripheral blood lymphocytes in approximately 70% of patients with MG.(41) These peptides were used in suppression assay performed as described above, using AChR peptide rather than anti-CD3 stimulation. To assess Treg cell suppression of AChR-stimulated T cell proliferation, previously activated CD4+ CD25high CD127low/− cells were added to the culture. T cell proliferation of 4.2–15% in response to one of the two AChR peptides was observed, none of which were significantly suppressed by the addition of Treg cells.(37) Little or no proliferation in response to the AChR-α peptide was seen in the age-matched healthy control population. These studies indicate that the observed defect in suppressive function of Treg cells in MG extends to AChR-specific T cell responses.
Defects in in vitro Treg cell suppression in autoimmune disease may potentially be explained by Treg cell–intrinsic defects, resistance of Tresp cells, or the pro-inflammatory properties of antigen presenting cells (APCs).(23–25) Our data showing reduced cellular expression of FOXP3 in MG Treg cells argues for a Treg cell–intrinsic defect. To further test this, we have co-cultured MG Treg cells from individuals with Tresp from healthy controls, and have also performed mirror image experiments, determining the degree of in vitro suppression of both of polyclonal and AChR-stimulated T cell proliferation. Both polyclonal and AChR-activated Tresp cells from MG patients could be effectively suppressed using Treg cells isolated from healthy controls, while polyclonal-activated Tresp cells from controls were not effectively suppressed using Treg cells isolated from MG patients, which strongly suggests (37) a primary intrinsic defect in isolated Treg cells in MG.(37)
Imbalanced homeostatic composition of circulating Treg cells in MG
While the existing data suggests that the relative numbers of circulating Treg cells is unchanged in MG patients, the observed Treg cell dysfunction may be linked to an imbalanced homeostatic composition of circulating Treg cell subsets. There are two major types of CD4+ FOXP3+ Treg cells.(42) Natural Treg cells (nTreg cells) constitute a stable subset derived from the thymus and are thought to control reactivity to self-antigens. Induced or adaptive Treg cells (iTreg cells) are less stable, being derived from CD4+ CD25− T cells in the periphery upon antigen exposure. There is currently no good method for isolating viable nTreg cells for functional studies, since the most reliable method relies on the presence of an epigenetic signature. However, cells recently exiting the thymus, including nTreg cells, may be identified by expression of CD45RA and CD31 (recent thymic emigrants). In addition, important biological differences between naive (CD45RA+) and memory (CD45RO+) CD4+ CD25high Treg cells have been described.(43,44) Differences in Treg cell subsets in patients with autoimmune disease (SLE, T1D) compared to controls have also been reported when CD45RA is used to differentiate Treg cell subsets,(45) suggesting that alterations in the relative composition of Treg cell subsets may underlie autoimmunity. Therefore, we have analyzed the frequency of circulating recent thymic emigrant (CD31+) and naive (CD45RA+) CD4+ CD25high CD127low/− Treg cells in MG patients. We have found that the prevalence of recent thymic emigrant and naive Treg cells is significantly decreased in MG patients compared to healthy controls. (37)
This finding raises the question of whether Treg cells may exhibit multiple differences from healthy controls that are likely to play roles in the complex pathogenesis of MG, including reduced thymic output of naive Treg cells, impaired naive Treg cell suppression, and distinct impairments in specific subsets of memory Treg cells. The reduced percentage of circulating naive and RTE Treg cells is particularly intriguing given the high incidence of thymic pathology (hyperplasia and neoplasia) in MG. Further studies correlating Treg cell subset phenotype and function with thymic pathology will help to better characterize the relationship of circulating Treg cell alterations and thymic Treg cell development.
Are CD4+ CD25high CD127low/− Treg cells affected by immunotherapy?
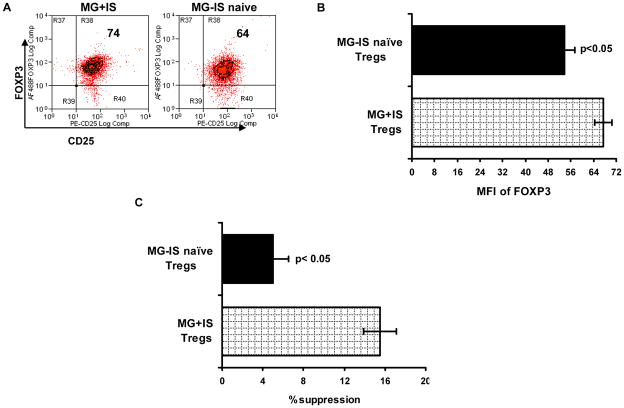
Most MG patients in our study were treated with prednisone or other immunosuppressive drugs, and it is conceivable that these drugs may have an effect on Treg cell function. Subset analyses of Treg cell suppressive function and FOXP3 expression in MG patients treated with immunosuppressants (IS) compared to those that were immunsuppression-naive at the time of blood collection, showed that Treg cells from MG patients treated with IS more effectively suppressed T cell proliferation and expressed higher levels of FOXP3 (Fig. 1). In addition, a correlation was also found between manual muscle testing (MMT) scores and Treg cell suppressive function.(37) These findings agree with those of other investigators,(46,47) and suggest that the observed Treg cell defects are clinically relevant and that the beneficial effects of IS therapy are at least partly mediated through enhancement of Treg cell function.
Figure 1.
FOXP3 expression and suppressive function of Treg cells in treated versus untreated MG patients. Intensity of FOXP3 expression (MFI = mean fluorescent intensity) in CD4+ CD25high CD127low/− Treg cells. (A) Representative data for immunosuppressive naive (MG - IS naive) and immunosuppressed (MG + IS) subjects. (B) MFI of FOXP3 expression in isolated CD4+ CD25high CD127low/− cells. (C) Percent suppression of Tresp cell proliferation by Treg cells. Data in (B) and (C) represent n = 5 for MG-IS naive subjects and n = 12 for MG-IS subjects. Results are expressed as mean ± SEM.
GM-CSF enhances Treg cell function in MG
The expansion of Treg cells for cellular therapy is a common experimental strategy applied to a number of autoimmune conditions.(48,49) Unfortunately, expansion of dysfunctional MG Treg cells is unlikely to provide a therapeutic benefit, and the identification of agents that enhance the function of Treg cells would be a more appropriate strategy. GM-CSF is an important hematopoietic growth factor and immune modulator that has a profound effect on various circulating immune cells.(50) In vivo administration of GM-CSF has been shown to prevent or attenuate autoimmunity in a number of mouse models of autoimmune disease by expanding dendritic cells and inducing an expansion of Treg cells.(51–55) Furthermore, we have used the murine experimental model of MG to demonstrate that the therapeutic administration of GM-CSF produces clinical improvement, an expansion of the numbers of splenic FOXP3+ cells, a suppression of AChR-induced T cell proliferation, diminished production of anti-AChR antibodies, and a reduction of the deposition of IgG and complement at the muscle endplate, resulting in preservation of the normal postsynaptic endplate morphology and functional AChRs.(53,54) More importantly, adoptively transferred GM-CSF-induced Tregs potently suppressed ongoing MG in recipient mice, confirming their critical role in GM-CSF’s effects.(55)
Based on these preclinical studies, we have investigated the effects of GM-CSF treatment in a case of refractory myasthenic crisis.(56) Clinical improvement in this case was associated with expansion in the circulating numbers of FOXP3+ cells, increase in FOXP3 expression levels in CD4+ CD25high CD127low/− Treg cells, early improvement in Treg cell suppressive capacity for AChR-α–induced T cell proliferation, and subsequent enhancement in Treg cell suppression of polyclonal T cell proliferation.(56) Although the use of multiple immunosuppressive drugs may have contributed to these findings, the observed effects on Treg cells are consistent with preclinical studies.
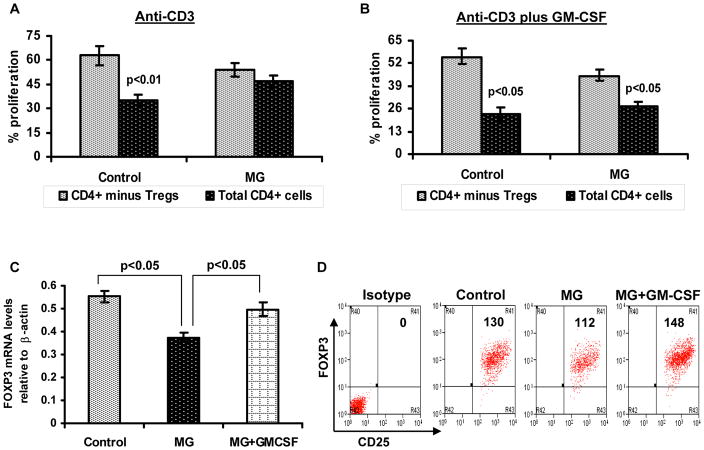
We have also performed studies examining the in vitro effects of GM-CSF in T cell proliferation/suppression assays from MG patients and healthy controls. Total CD4+ and/or CD4+ CD25high CD127low/− Treg cells depleted CD4+ T cells were cultured with autologus APCs in the presence of anti-CD3 and or anti-CD3 plus GM-CSF. We have found that the addition of GM-CSF to lymphocyte cultures ameliorates the suppressive defect in MG Treg cells (Fig. 2A and 2B), and markedly enhances expression of FOXP3 in MG Treg cells at the mRNA and protein level (Fig. 2C and 2D). This suggests that GM-CSF may be effectively used as an enhancer of Treg cell suppressive function, as has been reported for a number of other agents (i.e., rapamycin, vitamin D analogues).(57,58) Additional studies are needed to confirm these findings, define the requirements for GM- CSF’s in vitro effects, and also determine its actions on non-Treg cells.
Figure 2.
In vitro GM-CSF treatment to lymphocytes enhances FOXP3 expression and suppressive function of Treg cells in MG. CFSE-labeled total CD4+ T cells and Treg cell–depleted CD4+ T cells were stimulated with human anti-CD3 alone or anti-CD3 plus GM-CSF in the presence of autologus irradiated APCs. Cultures consisting of total CD4+ cells (black bars) and CD4+ cells after removal of CD4+ CD25high CD127low/− cells (grey bars), were compared to evaluate suppressive function of Treg cells. After 5 days of culture, percentage of T cell proliferation was analyzed based on CFSE dilution. The bar diagram represents percentage Tresp cell proliferation in response to anti-CD3 alone (A) and anit-CD3 plus GM-CSF (B) for four MG patients and four healthy controls.(C) FOXP3 mRNA expression was determined by multiplex PCR using isolated CD4+ CD25high CD127low/−Treg cells obtained from a control subject, MG patient and an MG patient’s PBMCs treated with GM-CSF. Results are expressed as relative FOXP3 mRNA expression. Results are expressed as mean ± SEM. (D) Representative flow cytometry plots illustrate mean florescent intensity of FOXP3 expression within isolated CD4+ CD25high CD127low/− Treg cells.
Conclusions
Defective immune regulation is present in patients with MG, characterized by defects in suppressive function and expression of FOXP3 in CD4+ CD25high CD127low/− cells. Treg cell–mediated suppression of both polyclonal and autoantigen (AChR)-specific T cell responses is impaired and may correlate with disease severity, being notably less severe in the subgroup of patients treated with immunosuppressive drugs. This defect primarily resides within the isolated population of CD4+ CD25high CD127low/− cells which display reduced or unstable FOXP3 expression. While it has been generally thought that FOXP3 expression serves as an on-off switch endowing T lymphocytes with suppressive ability, emerging evidence, including our own work showing an association between attenuated FOXP3 expression in Treg cells and human MG, suggests a paradigm in which alterations in FOXP3 expression in lymphocytes may lead to loss of immune tolerance in a dose-dependent manner (Fig. 3). Furthermore, GM-CSF has a profound in vitro beneficial effect on Treg cell function as well as FOXP3 expression, and further investigations of the mechanisms responsible for these effects are warranted.
Figure 3.
Hypothetical model linking Treg cell suppressive function to FOXP3 expression level. In this model, high expression of FOXP3 in T cells endows them with enhanced suppressive capacity and a characteristic phenotype. Absence of FOXP3 expression is associated with no suppressive capacity, but reduced FOXP3 expression may result in an intermediate phenotype without suppressive function, and possibly a tendency for enhanced functional plasticity.
In sum, investigation of the nature and subset localization of the Treg cell defect in MG may reveal critical factors that regulate immune responses in MG and other autoimmune diseases, leading to the identification of novel therapeutic targets.
Acknowledgments
This work was funded by the NIH (National Institute of Neurologic Disorders and Stroke, K08NS058800, MNM; and National Institute of Allergy and Infectious Diseases, RO1 AI 058190, BSP); and the Muscular Dystrophy Association (MDA 185924, MNM and MDA 157286, JRS); and by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrih S, Morel E, Gaud C, et al. Anti-AChR antibodies, thymic histology, and T cell subsets in myasthenia gravis. Neurology. 1984;34:66–71. doi: 10.1212/wnl.34.1.66. [DOI] [PubMed] [Google Scholar]
- 3.Roxanis I, Micklem K, Willcox N. True epithelial hyperplasia in the thymus of early-onset myasthenia gravis: implications for immunopathogenesis. J Neuroimmunol. 2001;112:163–173. doi: 10.1016/s0165-5728(00)00415-x. [DOI] [PubMed] [Google Scholar]
- 4.Giraud M, Vandiedonck C, Garchon HJ. Genetic factors in autoimmune myasthenia gravis. Ann NY Acad Sci. 2008;1132:180–192. doi: 10.1196/annals.1405.027. [DOI] [PubMed] [Google Scholar]
- 5.Landoure G, Knight MA, Stanescu H, et al. A candidate gene for autoimmune myasthenia gravis. Neurology. 2012;79:342–347. doi: 10.1212/WNL.0b013e318260cbd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniaol AH, Elsais A, Lorentzen AR, et al. Late onset myasthenia gravis is associated with HLA DRB1*15:01 in the Norwegian population. PLoS One. 2012;7(5):e36603. doi: 10.1371/journal.pone.0036603. Epub 2012 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protti MP, Manfredi AA, Straub C, et al. Immunodominant regions for T helper-cell sensitization on the human nicotinic receptor alpha subunit in myasthenia gravis. Proc Natl Acad Sci USA. 1990;87:7792–7796. doi: 10.1073/pnas.87.19.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ZY, Okita DK, Howard J, Jr, et al. T-cell recognition of muscleacetylcholine receptor subunits in generalized and ocular myasthenia gravis. Neurology. 1998;50:1045–1054. doi: 10.1212/wnl.50.4.1045. [DOI] [PubMed] [Google Scholar]
- 9.Abramsky O, Aharonov A, Webb C, Fuchs S. Cellular immune response to acetylcholine receptor rich-fraction, in patients with myasthenia gravis. Clin Exp Immunol. 1975;19:11–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Richman DP, Antel JP, Patrick JW, Arnason BGW. Cellular immunity to acetylcholine receptor in myasthenia gravis: relationship to histocompatibility type and antigenic site. Neurology. 1979;29:291–296. doi: 10.1212/wnl.29.3.291. [DOI] [PubMed] [Google Scholar]
- 11.Hohlfeld R, Kalies I, Kohleisen B, et al. Myasthenia gravis: stimulation of anti-receptor autoantibodies by autoreactive T cell lines. Neurology. 1986;36:618–621. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- 12.Bouneaud C, Kourilsky P, Buosso P. Impact of negative selection on the T cell repertoire reactive to a self peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 13.Workman CJ, Szymczak-Workman AL, Collison LW, et al. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 15.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Gavin MA, Rudensky AY. FOXP3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FOXP3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 19.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moes N, Rieux-Laucat F, Begue B, et al. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology. 2010;139:770–778. doi: 10.1053/j.gastro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Valencia XC, Yarboro G, Illei P, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 22.Venken K, Hellings N, Thewissen M, et al. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reducedFOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venigalla RK, Tretter T, Krienke S, et al. Reduced CD4+, CD25− T cell sensitivity to the suppressive function of CD4+, CD25high, CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 24.Vargas-Rojas MI, Crispín JC, Richaud-Patin Y, et al. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17:289–294. doi: 10.1177/0961203307088307. [DOI] [PubMed] [Google Scholar]
- 25.Walker LS. Regulatory T cells overturned: the effectors fight back. Immunology. 2009;126:466–474. doi: 10.1111/j.1365-2567.2009.03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iikuni N, Lourenco EV, Hahn BH, et al. Regulatory T cells directly suppress B cells in systemic lupus erythematosis. J Immunol. 2009;183:1518–1522. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fattorossi A, Battaglia A, Buzzonetti A, et al. Circulating and thymic CD4+CD25+ T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunol. 2005;116:134–141. doi: 10.1111/j.1365-2567.2005.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Xiao BG, Xi JY, et al. Decrease of Foxp3+ regulatory T cells and elevation of CD19+BAFF-R+ B cells and soluble ICAM-1 in myasthenia gravis. Clin Immunol. 2008;126:180–188. doi: 10.1016/j.clim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Masuda M, Matsumoto M, Tanaka S, et al. Clinical implication of peripheral CD4+ CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimmunol. 2010;225:123–131. doi: 10.1016/j.jneuroim.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang H, Chi L, et al. The role of FoxP3+CD4+CD25hi Tregs in the pathogenesis of myasthenia gravis. Immunol Lett. 2009;122:52–57. doi: 10.1016/j.imlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Huang YM, Pirskanen R, Giscombe R, et al. Circulating CD4+CD25+ and CD4+CD25+T cells in myasthenia gravis and in relation to thymectomy. Scand J Immunol. 2004;59:408–414. doi: 10.1111/j.0300-9475.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsui N, Nakane S, Saito F, et al. Undiminished regulatory T cells in the thymus of patients with myasthenia gravis. Neurology. 2010;74:816–20. doi: 10.1212/WNL.0b013e3181d31e47. [DOI] [PubMed] [Google Scholar]
- 33.Balandina A, Lécart S, Dartevelle P, et al. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim AY, Price P, Beilharz MW, et al. Cell surface markers of regulatory T cells are not associated with increased forkheadbox p3 expression in blood CD4+ T cells from HIV-infected patients responding to antiretroviral therapy. Immunol Cell Biol. 2006;84:530–536. doi: 10.1111/j.1440-1711.2006.01467.x. [DOI] [PubMed] [Google Scholar]
- 35.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Putnam AL, Xu-Yu Z, Szot GL, et al. CD127 expression inversely correlates with FOXP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiruppathi M, Rowin J, Ganesh B, et al. Impaired Regulatory Function in Circulating CD4+CD25highCD127low/− T cells in Patients with Myasthenia Gravis. Clin Immunol. 2012 doi: 10.1016/j.clim2012.09.012. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu WH, Zhang AM, Ren MS, et al. Changes of Treg-associated molecules on CD4(+)CD25(+) Treg cells in myasthenia gravis and effects of immunosuppressants. J Clin Immunol. 2012;32:975–983. doi: 10.1007/s10875-012-9685-0. [DOI] [PubMed] [Google Scholar]
- 40.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 41.Brocke S, Brautbar C, Steinman L, et al. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 45.d’Hennezel E, Yurchenko E, Sgouroudis E, et al. Single-cell analysis of the human T regulatory population uncovers functional heterogeneity and instability within FOXP3+ cells. J Immunol. 2011;186:6788–6797. doi: 10.4049/jimmunol.1100269. [DOI] [PubMed] [Google Scholar]
- 46.Luther C, Adamopoulou E, Stoeckle C, et al. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J Immunol. 2009;183:841–848. doi: 10.4049/jimmunol.0802046. [DOI] [PubMed] [Google Scholar]
- 47.Kessel A, Ammuri H, Peri R, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5575. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman P, Eder R, Kunz-Schughart LA, et al. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 49.Earle KE, Tang Q, Zhou X, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 51.Ganesh BB, Cheatem DM, Sheng JR, et al. GMCSF-induced CD11c+CD8a- -dendritic cells facilitate FOXP3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaudreau S, Guindi C, Menard M, et al. Granulocyte-macrophage colony stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 53.Sheng JR, Li LC, Ganesh BB, et al. Suppression of experimental autoimmune myasthenia gravis (EAMG) by granulocyte-macrophage colony-stimulating factor (GMCSF) is associated with an expansion of FOXP3+ regulatory T cells. J Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 54.Sheng LC, JR, Li BB, Ganesh, et al. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheng JR, Muthusamy T, Prabhakar BS, et al. GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol. 2011;240–241:65–73. doi: 10.1016/j.jneuroim.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowin J, Thiruppathi M, Arhebamen E, et al. Granulocyte macrophage colony-stimulating factor treatment of a patient in myasthenic crisis: Effects on regulatory T cells. Muscle Nerve. 2012;46:449–453. doi: 10.1002/mus.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–352. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 58.Battaglia M, Stabilini A, Migliavacca B, et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]