Abstract
The development of invasive aspergillosis is a feared complication for immunocompromised patients. Despite the use of antifungal agents with excellent bioactivity, the morbidity and mortality rate for invasive aspergillosis remains unacceptably high. Defects within the innate immune response portend the highest risk for patients, but detailed knowledge of molecular pathways in neutrophils and macrophages in response to this fungal pathogen is lacking. Phagocytosis of fungal spores is a key step that places the pathogen into a phagosome, a membrane-delimited compartment that undergoes maturation and ultimately delivers antigenic material to the class II MHC pathway. We review the role of Toll-like receptor 9 (TLR9) in phagosome maturation of Aspergillus fumigates–containing phagosomes. Advanced imaging modalities and the development of fungal like particles are promising tools that will aid in the dissection of the molecular mechanism to fungal immunity.
Keywords: innate immunity, Dectin-1, Toll-like receptor, phagosome, macrophage, TLR9
Introduction
“For breath is life, and if you breathe well you will live long on earth.”
~Sanskrit Proverb
Exposure to Aspergillus fumigatus is a universal phenomenon. In immunocompetent individuals, inhalation of these fungal spores is taken up by neutrophils and macrophages that quickly neutralize any potential invasion or infection.1 However, through the advances of medical care, a growing number of patients have acquired immunological defects either by cellular deficiencies/dysfunction or administration of therapies that modulates the immune response.2 These “immunologically fragile” patients possess a higher risk for development of clinically significant invasive fungal infections including aspergillosis. Indeed, over 10% of patients who receive allogeneic bone marrow transplantation develop invasive aspergillosis during their clinical course.3 Antimicrobial therapies that have excellent in vitro activity against Aspergillus species have been developed and are routinely used for both prophylaxis and treatment in these patients.4 Despite their widespread use and increased awareness of this infectious complication by clinicians, the morbidity and mortality rate for immunocompromised patients with invasive aspergillosis remains unacceptably high. These clinical observations support the following statements:
Persons with normal immune systems successfully keep A. fumigatus from developing invasive infections.
Innate immunity is critical as neutropenic patients have the highest risk of invasive aspergillosis.
Successful recovery from invasive aspergillosis requires antimicrobial therapy coupled with some contribution from the host immune system.
These clinical observations lead to the fundamental question of what components of the immune response are critical to neutralize A. fumigatus quickly and efficiently. Knowledge of the basic pathways of antifungal defense in both normal and immunocompromised animal models will likely elucidate the fundamental pathways necessary to develop novel therapeutics against this invasive fungal pathogen.
Innate Immunity to Aspergillus
To provide a conceptual framework, the immune system can be divided into two principal branches: innate and adaptive immunity.5 The innate immune response includes neutrophils, monocytes/macrophages, and dendritic cells (DCs) whose primary role is to engulf microorganisms before they cause harm.6 To detect their presence, these phagocytic cells express both surface-disposed and endosomal Toll-like receptors (TLRs) that activate the cell in the presence of infectious agents. Instead of relying on highly specific signatures from pathogens, TLRs sense the presence of common building blocks of microorganisms as triggers.7 The outcome of TLR signaling is determined, in part, by cell lineage and the selective use of signaling adaptors.8 Both ligand recognition by TLRs and the functional outcome of binding are governed in part by the subcellular location of the TLRs.9
In addition to TLRs, C-type lectin receptors (CLRs) compose a large family of receptors that bind to carbohydrates.10 The lectin binding activity of these receptors is mediated by conserved carbohydrate-recognition domains, and members of this family of proteins participate directly in the host defense against fungal infections.11 Dectin-1, a surface type II membrane protein and CLR, is highly expressed on phagocytes.12 Dectin-1 recognizes the carbohydrate epitope β-1,3-glucan, which constitutes the major cell wall component of multiple pathogenic fungi, including Candida albicans and A. fumigatus.13 Dectin-1 is required for proper modulation of immune responses against fungal pathogens, and patients with mutations in Dectin-1 are at higher risk for invasive fungal infections.14,15 The cytoplasmic tail of Dectin-1 contains an immunoreceptor tyrosine-based activation (ITAM)-like motif.16 Upon ligation of the extracellular domain of Dectin-1, the tyrosine residue within the cytoplasmic ITAM motif is phosphorylated. This phosphorylation event results in recruitment of spleen tyrosine kinase (Syk). Ultimately, this pathway leads to the activation of NF-κB, production of reactive oxygen species (ROS), and elaboration of pro-inflammatory cytokines, including TNF-α and IL-12, leading to recruitment of additional immune cells to the site of infection.17 While the kinetics of this response is rapid (minutes to hours), the innate immune system does not provide memory to a given infection. In contrast, adaptive immunity consists of T and B lymphocytes that become activated after initiation of innate immunity and focus on specific peptides derived from pathogen-encoded proteins.18 In order to extract pathogen-specific information, newly-enveloped microorganisms are shuttled into membrane-delimited compartments termed phagosomes, delivered through the endocytic pathway by modification of the membrane proteins and intra-phagosomal environment (phagosome maturation), and targeted to lysosomes for degradation.19 Class II major histocompatibility complex (MHC) molecules intersect with these pathogen-containing compartments and relevant peptides derived from the pathogen are loaded into the MHC antigen binding clefts for delivery to the cell surface.5, 20 Much of our understanding of phagosomal biology has come from live cell imaging of professional antigen presenting cells (APCs).21
Novel imaging tools to visualize host-pathogen interactions
Application of live-cell imaging tools to the interface of immunology and infectious disease is in its infancy though preliminary observations challenge our view of long-held beliefs about immune responses to microorganisms. As an example, to control Mycobacterium tuberculosis, the host forms granulomas, histologically-distinct structures regularly seen in clinical tissue of infected persons. Conventional wisdom suggested that macrophages, T cells, and occasionally DCs form a static barrier that envelops organisms to control their spread. The use of intravital two-photon microscopy in the liver of mice infected with M. bovis demonstrated that immune cells showed remarkable dynamic movements to initiate and maintain these granulomas.22 TNF-α–derived signals were required to recruit uninfected macrophages to these structures and preserve the involvement of T cells.
A long-term goal of our work is to understand how initial interactions between pathogens and phagocytic cells influence the degree of success of the host response. In order to understand this first step, it is important to visualize subcellular interactions of host innate immune cells with pathogenic organisms in order to observe phagocytosis. Successful phagocytosis not only neutralizes the threat from the pathogen, but it also generates antigenic material crucial for the ensuing adaptive immune response. The regulation of phagocytosis and phagosome formation/maturation has significant implications in specific immune responses to pathogens.23 To carry out these experiments, we have assembled both spinning-disk confocal microscopy as well as optical trapping on the same platform. The power of spinning disk confocal microscopy lies in ability of the pinhole to exclude out-of-focal-plane fluorescence emission from biological specimens, allowing the high-contrast imaging of an optically sectioned slice. The scanning of confocal excitation light is achieved through two spinning disks in which one contains thousands of pinholes and the other contains an equal number of microlenses to focus the laser beam into the pinholes. This enables fast, multicolor, three-dimensional live cell imaging. In combination with sub-micron Z-plane scanning of the sample by use of a precise motorized stage, this system permits complete sectioning of the specimen to create a three-dimensional image.20, 24–27
While this system generates illustrative images of APCs interacting with pathogens, spinning-disk confocal microscopes lack the capacity to exert control on the precise timing of these interactions. Commonly, investigators rely on serendipitous mixing of immune cells with pathogens to initiate phagocytosis. To overcome these obstacles, we adopted optical trapping to position actively live fungal pathogens at any arbitrary time and at any arbitrary location relative to immune cells, which allows real-time observation of the entire process using spinning disk confocal microscopy housed in an environment to mimic in vivo conditions.28, 29
TLR9 is recruited to phagosomes containing A. fumigatus
The generation of fusion proteins of TLR/CLR appended to fluorescent proteins (including GFP and mCherry) coupled with live cell imaging has provided valuable insight into the dynamic nature of protein redistribution in phagosome formation/maturation.30, 31 While the majority of the data regarding TLR specificity and function has focused on bacterial and viral-derived ligands, our understanding of fungal interactions with TLRs is accumulating.1 Much of our understanding of TLR function is derived from infection experiments in mice lacking specific TLRs.32 TLR2- and TLR4-defective mice show a decreased recruitment of neutrophils and a reduced cytokine response to A. fumigatus conidia and hyphae.33 The complex fungal cell wall component zymosan triggers expression of proinflammatory cytokines via Dectin-1 and crosstalk with TLR2 and TLR4.34 During swelling and germination of A. fumigatus conidia, β-1,3 glucans become exposed to the surface and can be targeted by cells of innate immune system expressing TLR2, TLR6, and Dectin-1, including macrophages and DCs.35, 36
Comprehensive reviews of TLRs and the role of TLRs in fungal infections have been recently published.1, 6, 8, 18, 37 We will focus on the role of TLR9 in the host defense of A. fumigatus. The subcellular localization of TLR9, which engages and signals to unmethylated CpG DNA, is tightly regulated and receptor activation is a multistep process.38 TLR9 is translated into the endoplasmic reticulum in its mature, full-length form and then passes through the Golgi to the endolysosomal compartment where its ectodomain is proteolytically cleaved to generate a functional receptor.39, 40 In the endolysosomal compartment, ligand binding to preassembled TLR9 dimers induces a conformational change that allosterically initiates signal transduction.41 While the truncated form of TLR9 can be found in the endolysosomal compartment of unstimulated cells, TLR9 trafficking has been shown to be highly regulated, dynamic process.31 The extent to which there is dynamic movement of TLR9 between subcompartments and the underlying processes regulating TLR9 trafficking remain poorly understood.
Although the best known ligand for TLR9 is unmethylated bacterial and viral CpG-rich DNA, TLR9 has also been implicated in antifungal defense.42, 43 A role for TLR9 in host defense against A. fumigatus has been suggested by experiments in murine models of invasive pulmonary aspergillosis and allergic bronchopulmonary aspergillosis, where TLR9 modulates the innate immune response in the lung.44 Although most studies have focused on the importance of TLR2 and TLR4 in defense against A. fumigatus, a polymorphism study associated increased susceptibility to ABPA with a polymorphism in the TLR9 gene.45 Intranasal CpG, a known TLR9 ligand, had a therapeutic effect during established murine fungal asthma, implicating a requisite role for TLR9 in this disease process.46 However, the cell biological processes underlying TLR9-mediated A. fumigatus immune responses are still largely unresolved. TLR9-mediated recognition of A. fumigatus DNA by human and murine cells induced proinflammatory cytokines,47 but the intracellular processes that enable A. fumigatus antigen recognition by TLR9 in professional APCs remain uninvestigated.
To gain insight into the intracellular fate of TLR9 when immune cells are exposed to A. fumigatus conidia, we investigated the spatiotemporal regulation of TLR9 compartmentalization after phagocytosis of A. fumigatus conidia by murine macrophages. We found that the presence of A. fumigates phagosomes resulted in a dramatic change of the subcellular distribution of TLR9 to a bright, ring-shaped compartment around the A. fumigates conidia (Fig. 1).25 TLR9 recruitment was specifically induced by A. fumigates but not by bead-containing phagosomes, indicating that the composition of the phagocytosed content dictates recruitment of TLR9 to the phagosomal membrane. We demonstrated that A. fumigatus-induced TLR9 recruitment was independent of A. fumigates spore germination stage. Expression of TLR2, TLR4, and the TLR signaling adaptors MyD88 and TRIF were not required for successful A. fumigatus phagosomal TLR9 recruitment. Further investigation of the requirements for proper intracellular trafficking of TLR9 revealed that the TLR9 N-terminal proteolytic cleavage domain was critical for accumulation of TLR9 in CpG-containing compartments and A. fumigates phagosomal membranes.
Figure 1.
TLR9 is specifically recruited to phagosomes containing Aspergillus fumigatus. Mouse macrophages expressing TLR9-GFP (green) and CD82-mRFP1 (red) were exposed to both live A. fumigatus (Af293) and fluorescent polystyrene beads (blue-5 μm). Twenty minutes after phagocytosis, macrophages had taken up both particles, but only phagosomes containing A. fumigatus recruited both CD82-mRFP1 and TLR9-GFP. Scale bar equals 5μm and arrow denotes internalized polystyrene bead.
In addition to A. fumigatus, we have demonstrated that specific triggering of TLR9 recruitment to the macrophage phagosomal membrane is a conserved feature of fungi of distinct phylogenetic origins, including Candida albicans, Saccharomyces cerevisiae, Malassezia furfur, and Cryptococcus neoformans.27 The capacity to trigger phagosomal TLR9 recruitment was not affected by a loss of fungal viability or cell wall integrity. TLR9 deficiency has been linked to increased resistance to murine candidiasis and to restriction of fungal growth in vivo. Macrophages lacking TLR9 demonstrate a comparable capacity for phagocytosis and normal phagosomal maturation compared to wild-type macrophages. We have shown that TLR9 deficiency increases macrophage TNF-α production in response to C. albicans and S. cerevisiae, independent of yeast viability.27 The increase in TNF-α production was reversible by functional complementation of the TLR9 gene, confirming that TLR9 was responsible for negative modulation of the cytokine response. Consistently, TLR9 deficiency enhanced the macrophage effector response by increasing macrophage nitric oxide production. Moreover, microbicidal activity against C. albicans and S. cerevisiae was more efficient in TLR9 knockout macrophages than in wild-type macrophages.
In conclusion, our data have demonstrated that TLR9 is compartmentalized selectively to fungal phagosomes and negatively modulates macrophage antifungal effector functions. Our data support a model in which orchestration of antifungal innate immunity involves a complex interplay of fungal ligand combinations, host cell machinery rearrangements, and TLR cooperation and antagonism.
Reducing the complexity: development and use of fungal-like particles
The mammalian immune response to invasive fungal pathogens, including A. fumigates, is a complex and dynamic process that serves to protect the host from invasive infection. Indeed, the generation of phagosomes and maturation of these organelles in part dictates the ensuing immune response to the pathogen. Our ability to understand the rules that govern this response is limited by two principal factors. First, the cell wall of A. fumigatus is a dynamic structure that undergoes both constitutional and morphologic changes, triggering additional responses from the APC. A. fumigatus can swell inside the phagosome and reveal new antigenic determinants that are effectively shielded during the engulfment phase. Second, the resulting immune response from a complex particle such as A. fumigatus is difficult to dissect and to attribute specific responses to discrete cell wall components.
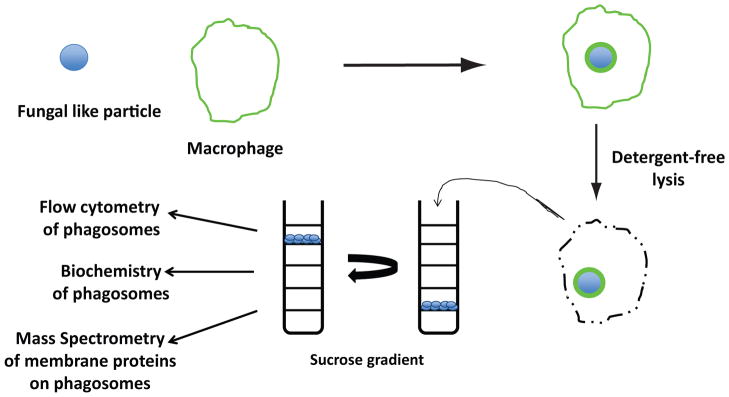
To overcome these obstacles, we set out to develop defined synthetic particles that displayed a single or fixed combination of fungal cell wall constituents on a size-matched polystyrene sphere.48 In this way, we preserve the geometry of the native pathogen, an important feature to phagocytosis.49 Synthetic fungal-like particles have multiple advantages as a substrate to probe the immune system. First, these particles do not vary during phagosomal maturation process. Second, the inflammatory response triggered by these particles can now be attributed to specific fungal ligands. Third, intrinsic properties of polystyrene beads permit the rapid recovery of these particles from APCs permitting direct interrogation of these phagosomes to probe the mammalian contribution of membrane proteins.50 Polystyrene beads have a low buoyant density such that they float in concentrated sucrose solutions, allowing for the efficient separation of phagosomes from other cellular structures after cell lysis. Analysis of these phagosomes can be done by the use of flow cytometry (PhagoFACS),51 Western blot analysis to determine the presence and relative quantity of a specific protein on the phagosome, and proteomic-based analysis to determine the composition of the mammalian phagosome generated by a synthetic ligand (Fig. 2).
Figure 2.
Cartoon depicting method to isolate and analyze phagosomes containing fungal-like particles. Polystyrene beads with fungal-derived carbohydrates covalently attached to the surface are incubated with macrophages. After a defined period, detergent-free lysis releases cellular content including intact phagosomes. Lysates are added to discontinuous sucrose gradients and then are subjected to ultracentrifugation. Phagosomes are easily isolated as a result of the buoyant properties of polystyrene beads and then analyzed by flow cytometry, Western blot, or mass spectrometry of membrane proteins.
As proof of principle, we have generated fungal like particles that display β-1,3 glucan on the cell surface.48 These fungal-like particles were characterized using differential interference contrast microscopy, immunofluorescence, and transmission-electron microscopy. The covalent interaction of the β-1,3-glucan layer to the surface of the bead was confirmed by a series of increasingly stringent detergent treatments. Purity of the β-1,3-glucan layer was also determined by incubating the beads with laminarinase, a specific β-1,3-gluconase. By stimulating bone marrow derived macrophages with conjugated β-1,3-glucan beads, we observed a dose dependent response of TNF-α produced when compared to soluble β-1,3-glucan, uncoated beads, and soluble β-1,3-glucan mixed with uncoated beads. Finally, β-glucan coated fungal-like particles triggered dynamic and specific recruitment of GFP-Dectin-1 to nascent phagosomes in living mouse macrophages, demonstrating that the beads retained the desired biological properties.48
By providing a platform to probe directly immune responses to β-1,3-glucan, we can dissect the critical steps in the early recognition of pathogen-derived fungal carbohydrate antigens in innate immunity. Additionally, this system permits the addition of other relevant pathogen-derived carbohydrates to polymeric beads to analyze the specific contribution of these ligands in the immune response.
Concluding remarks
Three clinical outcomes from inhalation of A. fumigates exist: rapid removal of conidia without overt clinical symptoms (neutralization), exuberant Th2-biased responses characterized as allergic responses (allergic), or local tissue destruction that can lead to systemic infection (invasion). The outcome of this host-pathogen interaction appears to be dictated largely by host factors. Our understanding of the rules that govern the innate immune response to A. fumigatus conidia is relatively simple at this time, but this area is critical to the elucidation of the critical immune pathways that require modulation in order to add to our armamentarium against this clinically relevant pathogen. Novel tools utilizing subcellular imaging and synthetic fungal-like particles are critical developments to elucidate the molecular basis of A. fumigatus-host cell interactions.
Acknowledgments
JMV and JMT are supported by NIH grant R01 AI092084. MKM is supported by NIH grant T32AI007061. The authors thank all of the current and former members of the laboratory.
References
- 1.Brown GD, Netea MG. Exciting developments in the immunology of fungal infections. Cell Host Microbe. 2012;11:422–424. doi: 10.1016/j.chom.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Perfect JR. The impact of the host on fungal infections. Am J Med. 2012;125:S39–51. doi: 10.1016/j.amjmed.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2012;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 4.Herbrecht R, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 5.Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy RM, Klein BS. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe. 2012;11:436–446. doi: 10.1016/j.chom.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- 8.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Berg LM, Gringhuis SI, Geijtenbeek TB. An evolutionary perspective on C-type lectins in infection and immunity. Ann N Y Acad Sci. 2012;1253:149–158. doi: 10.1111/j.1749-6632.2011.06392.x. [DOI] [PubMed] [Google Scholar]
- 12.Kerrigan AM, Brown GD. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011;32:151–156. doi: 10.1016/j.it.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31:346–353. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Cunha C, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 15.Ferwerda B, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariizumi K, et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 17.Drummond RA, et al. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol. 2011;41:276–281. doi: 10.1002/eji.201041252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadevall A, Pirofski LA. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe. 2012;11:447–456. doi: 10.1016/j.chom.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan JC, Iwasaki A. The phagosome as the organelle linking innate and adaptive immunity. Traffic. 2012 doi: 10.1111/j.1600-0854.2012.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyas JM, et al. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–7210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajenoff M, Germain RN. Seeing is believing: a focus on the contribution of microscopic imaging to our understanding of immune system function. Eur J Immunol. 2007;37(Suppl 1):S18–33. doi: 10.1002/eji.200737663. [DOI] [PubMed] [Google Scholar]
- 22.Egen JG, et al. Macrophage and T Cell Dynamics during the Development and Disintegration of Mycobacterial Granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusse O. Biochemistry of the phagosome: the challenge to study a transient organelle. Scientific World Journal. 2011;11:2364–2381. doi: 10.1100/2011/741046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artavanis-Tsakonas K, et al. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc Natl Acad Sci U S A. 2006;103:15945–15950. doi: 10.1073/pnas.0607528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasperkovitz PV, Cardenas ML, Vyas JM. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol. 2010;185:7614–7622. doi: 10.4049/jimmunol.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Artavanis-Tsakonas K, et al. The tetraspanin CD82 is specifically recruited to fungal and bacterial phagosomes prior to acidification. Infect Immun. 2011;79:1098–1106. doi: 10.1128/IAI.01135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasperkovitz PV, et al. Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect Immun. 2011;79:4858–4867. doi: 10.1128/IAI.05626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam JM, et al. Control and manipulation of pathogens with an optical trap for live cell imaging of intercellular interactions. PLoS One. 2010;5:e15215. doi: 10.1371/journal.pone.0015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam JM, et al. Use of an optical trap for study of host-pathogen interactions for dynamic live cell imaging. J Vis Exp. 2011 doi: 10.3791/3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underhill DM, et al. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YM, et al. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter S, O’Neill LA. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 33.Meier A, et al. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5:561–570. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferwerda G, et al. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 35.Faro-Trindade I, et al. Characterisation of innate fungal recognition in the lung. PLoS One. 2012;7:e35675. doi: 10.1371/journal.pone.0035675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hohl TM, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010;49:1–9. doi: 10.1016/j.cyto.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park B, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latz E, et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- 42.Bellocchio S, et al. TLRs govern neutrophil activity in aspergillosis. J Immunol. 2004;173:7406–7415. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- 43.Miyazato A, et al. Toll-like receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect Immun. 2009;77:3056–3064. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramaprakash H, et al. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun. 2009;77:108–119. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho A, et al. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J Infect Dis. 2008;197:618–621. doi: 10.1086/526500. [DOI] [PubMed] [Google Scholar]
- 46.Ramaprakash H, Hogaboam CM. Intranasal CpG therapy attenuated experimental fungal asthma in a TLR9-dependent and -independent manner. Int Arch Allergy Immunol. 2010;152:98–112. doi: 10.1159/000265531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez-Ortiz ZG, et al. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun. 2008;76:2123–2129. doi: 10.1128/IAI.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tam JM, et al. Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr Biol (Camb) 2012;4:220–227. doi: 10.1039/c2ib00089j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuart LM, et al. A systems biology analysis of the Drosophila phagosome. Nature. 2007;445:95–101. doi: 10.1038/nature05380. [DOI] [PubMed] [Google Scholar]
- 51.Cebrian I, et al. Sec22b regulates phagosomal maturation and antigen cross presentation by dendritic cells. Cell. 2011;147:1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]