Abstract
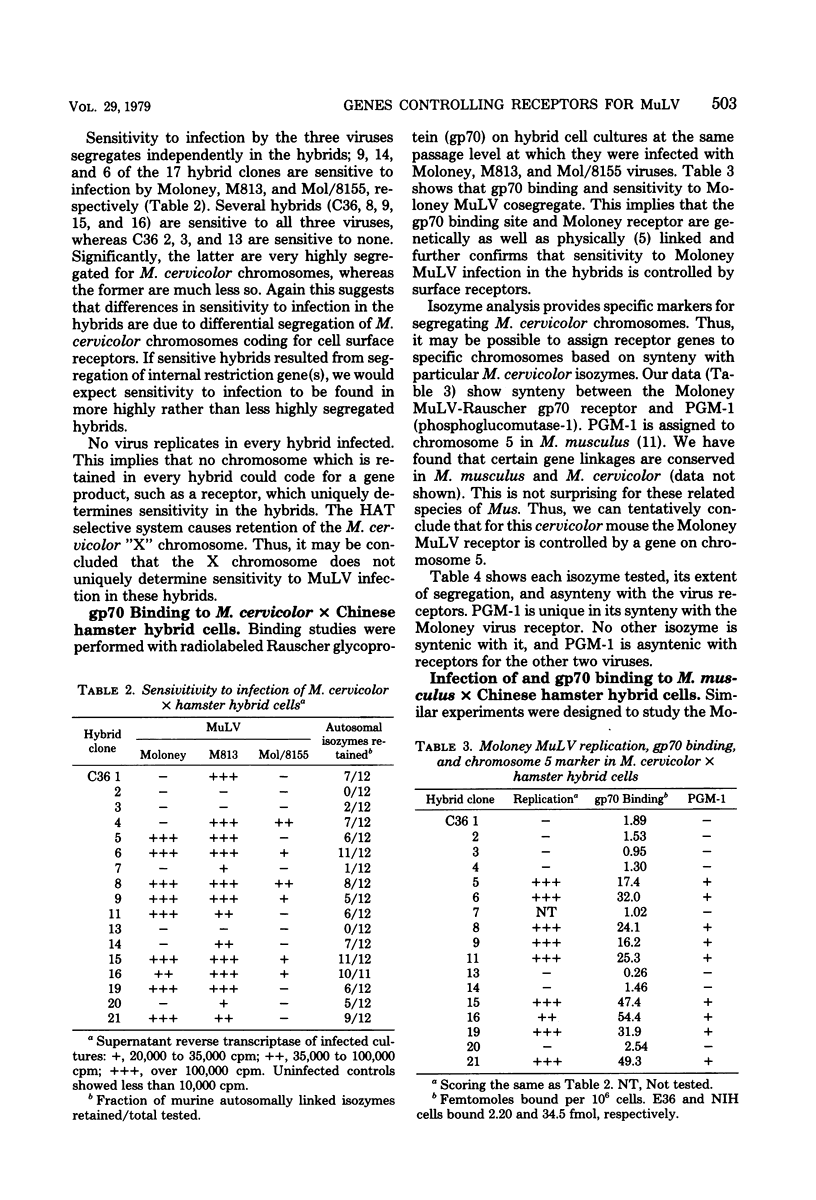
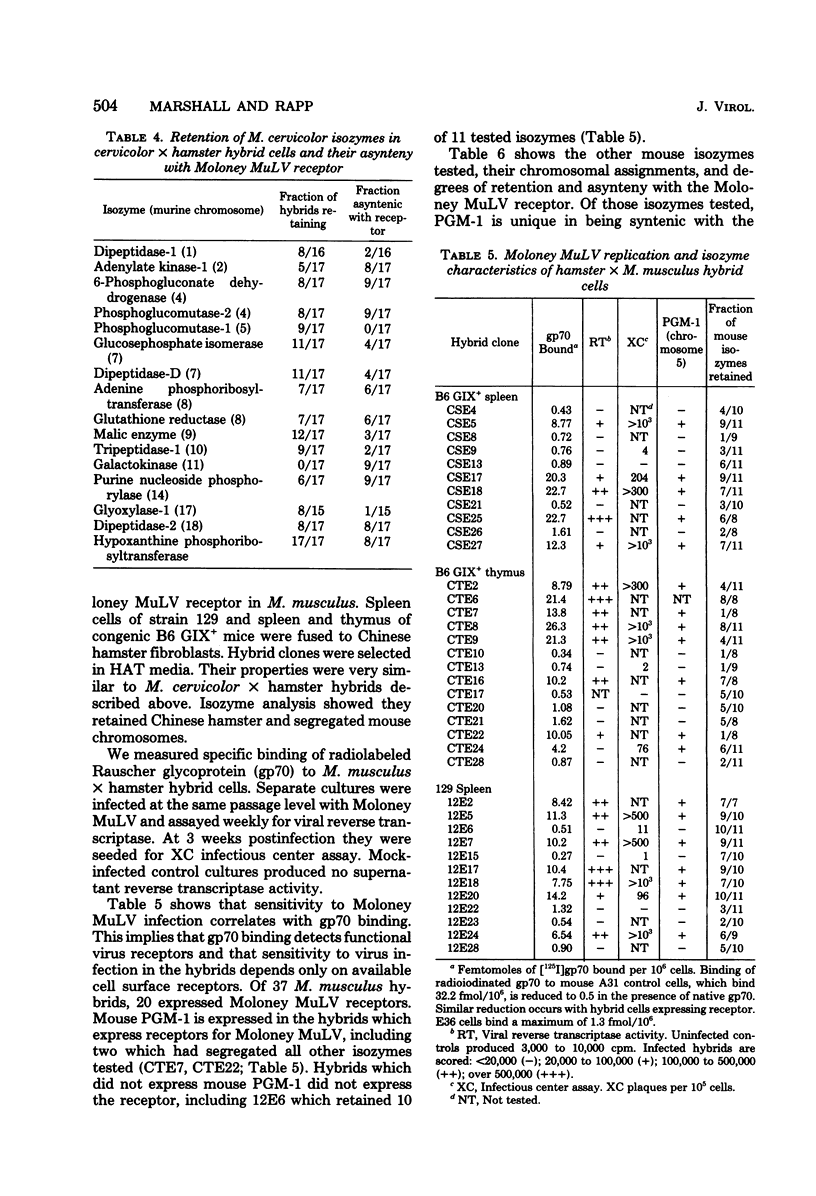
Gene loci controlling cell surface receptors for murine leukemia virus were studied by using murine X Chinese hamster hybrid cells. Hybrids which exclusively segregate murine chromosomes were made by fusing Mus cervicolor and Mus musculus lymphocytes to hamster fibroblasts. Sensitivity to Moloney murine leukemia virus infecotion and specific binding of the envelope glycoprotein of Rauscher murine leukemia virus (gp70) cosegregate and isozyme analysis show an association with chromosome 5 in both species. With the possible exception of one clone, no evidence was found for a proviral integration site independent of chromosome 5. Evidence is presented for additional unlinked ectropic and xenotropic receptors independent of chromosome 5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Todaro G. J. A distinct class of inducible murine type-C viruses that replicates in the rabbit SIRC cell line. Proc Natl Acad Sci U S A. 1974 Mar;71(3):602–606. doi: 10.1073/pnas.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977 Mar;21(3):965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco J. E., Rapp U. R., Todaro G. J. Cell surface receptors for ecotropic MuLV: detection and tissue distributions of free receptors in vivo. Int J Cancer. 1978 Mar 15;21(3):356–360. doi: 10.1002/ijc.2910210317. [DOI] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Dev V. G., Miller D. A., Miller O. J., Marshall J. T., Jr, Hsu T. C. Quinacrine fluorescence of Mus cervicolor chromosomes. Bright centrometric heterochromatin. Exp Cell Res. 1973 Jun;79(2):475–479. doi: 10.1016/0014-4827(73)90475-8. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Binding characteristics of Rauscher leukemia virus envelope glycoprotein gp71 to murine lymphoid cells. J Virol. 1977 Dec;24(3):729–735. doi: 10.1128/jvi.24.3.729-735.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Oie H., Lalley P., Moss W. W., Minna J. D. Identification of mouse chromosomes required for murine leukemia virus replication. Cell. 1977 Aug;11(4):949–956. doi: 10.1016/0092-8674(77)90306-3. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Roderick T. H. Linkage analyses using biochemical variants in mice. 3. Linkage relationships of eleven biochemical markers. Biochem Genet. 1970 Apr;4(2):339–350. doi: 10.1007/BF00485782. [DOI] [PubMed] [Google Scholar]
- Ishimoto A., Hartley J. W., Rowe W. P. Detection and quantitation of phenotypically mixed viruses: mixing of ecotropic and xenotropic murine leukemia viruses. Virology. 1977 Sep;81(2):263–269. doi: 10.1016/0042-6822(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Shander M. H., Halpern M. S. A structural protein complex in Moloney leukemia virus. Virology. 1977 Jan;76(1):437–439. doi: 10.1016/0042-6822(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Lemons R. S., Nash W. G., O'Brien S. J., Benveniste R. E., Sherr C. J. A gene (Bevi) on human chromosome 6 is an integration site for baboon type C DNA provirus in human cells. Cell. 1978 Aug;14(4):995–1005. doi: 10.1016/0092-8674(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Lemons R. S., O'Brien S. J., Sherr C. J. A new genetic locus, Bevi, on human chromosome 6 which controls the replication of baboon type C virus in human cells. Cell. 1977 Sep;12(1):251–262. doi: 10.1016/0092-8674(77)90203-3. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lieber M., Sherr C., Potter M., Todaro G. Isolation of type-C viruses from the Asian feral mouse Mus musculus molossinus. Int J Cancer. 1975 Feb 15;15(2):211–220. doi: 10.1002/ijc.2910150206. [DOI] [PubMed] [Google Scholar]
- McClintock P. R., Ihle J. N., Joseph D. R. Expression of AKR murine leukemia virus gp71-like and BALB(X) gp-71-like antigens in normal mouse tissues in the absence of overt virus expression. J Exp Med. 1977 Aug 1;146(2):422–434. doi: 10.1084/jem.146.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. A review of enzyme polymorphism, linkage and electrophoretic conditions for mouse and somatic cell hybrids in starch gels. J Histochem Cytochem. 1973 Dec;21(12):1066–1081. doi: 10.1177/21.12.1066. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Lalley P. A., Russell E. K., Minna J. D., DeLarco J., Todaro G. J., Francke U. Mouse chromosome 5 codes for ecotropic murine leukaemia virus cell-surface receptor. Nature. 1978 Jul 6;274(5666):60–62. doi: 10.1038/274060a0. [DOI] [PubMed] [Google Scholar]
- Stockert E., Boyse E. A., Obata Y., Ikeda H., Sarkar N. H., Hoffman H. A. New mutant and congenic mouse stocks expressing the murine leukemia virus-associated thymocyte surface antigen GIX. J Exp Med. 1975 Aug 1;142(2):512–517. doi: 10.1084/jem.142.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T., Jaenisch R. Oncornavirus gene expression during embryonal development of the mouse. Virology. 1977 Feb;76(2):886–890. doi: 10.1016/0042-6822(77)90271-9. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Tevethia S. S., Melnick J. L. Isolation of an RD-114 related type-C virus from feline sarcoma virus-transformed baboon cells. Intervirology. 1973;1(5):399–404. doi: 10.1159/000148868. [DOI] [PubMed] [Google Scholar]



