Abstract
The Th2 cytokines interleukins-4 and -13 (IL4; IL13) are acknowledged regulators of lymphocyte proliferation and activation. They have also been well-studied in the regulation of various myeloid-derived populations in tumor biology. It has become clear however, that both that cytokines can have direct effects on epithelial tumor cells expressing appropriate receptors. Changes in tumor proliferation, survival and metastatic capability have all been ascribed to IL4 and/or IL13 action. Here, we evaluate the evidence to support direct tumor-promoting roles of these cytokines. We also identify the questions that should be addressed before proceeding with therapeutic approaches based on neutralization of IL4 or IL13 pathways.
Keywords: type I receptor, type II receptor, proliferation, survival, apoptosis, metastasis, chemotherapy
Introduction
Cancer cell survival, proliferation, and metastasis are influenced by multiple factors including cytokines in the tumor microenvironment interacting with cells and regulating complex signaling pathways. Two such cytokines are the Th2 molecules, interleukins-4 (IL4) and -13 (IL13). IL4 and IL13 are structurally similar multifunctional peptides that can affect multiple cell types. IL4 is a well-characterized regulator of proliferation and immunoglobulin class-switching in B cells, and IL13 stimulates changes in epithelial and smooth muscle cell functions leading to hypersensitivity reactions (1-3). These immuno-regulating and effector cytokines are produced by macrophages, dendritic cells, mast cells, NKT, NK cells, basophils, eosinophils, and T lymphocytes (4). As depicted in figure 1A, there are 4 different receptor subunits associated with these cytokines, which can combine in different ways to result in various functional units. When IL4 binds IL4Rα with high affinity, resultant heterodimerization with either the gamma common chain (γc) forms the type I IL4R, or with IL13 receptor alpha 1 (IL13Rα1) forms the type II IL4R (1, 3). The type II IL4R can also be generated by binding of IL13 to IL13Rα1 and subsequent heterodimerization with IL4Rα (2, 3). The type I IL4R complex is present on lymphoid T and NK cells, basophils, mast cells and most mouse B cells, while type II IL4R is found on non-lymphoid and tumor cells (2, 4). Although both cytokines can thus signal through the same receptor complex (type II IL4R), they do not appear to always activate identical signaling pathways (2, 5). Another receptor that is expressed and activated on tumor cells is IL13Rα2. IL13 binds IL13Rα2, which is distinct from IL13Rα1 and present in 2 forms. A soluble form resulting from either alternative splicing or proteolytic cleavage has no signaling ability and has been coined the decoy receptor (2), while a larger membrane-spanning form results in activation of downstream effectors.
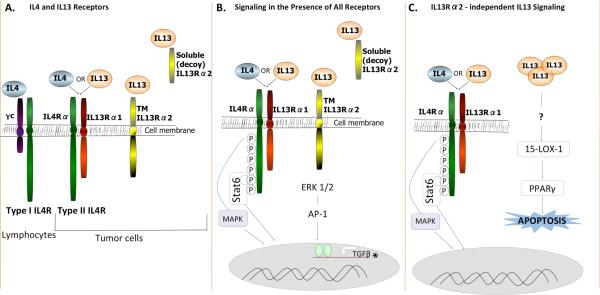
Figure 1.
(A) Receptors for IL4 and IL13 are comprised of four different subunits, IL4Rα (green), gamma chain (γc; purple), IL13Rα1 (red), and IL13Rα2 (yellow). IL4 and IL13 both bind and signal through type II IL4R. Type II IL4R and IL13Rα2 are present on tumor cells. (B) In the presence of al signaling receptors, IL4 and IL13 can both bind type II IL4R and phosphorylate Stat6 leading to increased proliferation and apoptotic resistance. JNK/MAPK and p38 pathways are also activated downstream of IL4, and possibly IL13 signaling through type II IL4R. IL13 can also bind soluble or transmembrane IL13Rα2, potentially leading to induction of TGFβ expression, downstream of ERK 1/2 and AP-1. * Note this pathway has thus far only been delineated in other cell types, but is speculated to occur in epithelial cancer cells. (C) In the absence of IL13Rα2, IL13 may have enhanced signaling through type II IL4R and/or signaling resulting in expression of 15-LOX-1.
High levels of Th2 cytokines are observed in the tumor microenvironment and peripheral blood of patients with prostate, bladder, and breast cancer (6-8). Several reports indicate that the type II IL4R (IL4Rα and IL13Rα1 chains) is upregulated and activated in various epithelial tumor types including malignant glioma, ovarian, lung, breast, pancreas, and colon carcinomas (9, 10). However, the expression and activation of the type I IL4R (IL4Rα and γC chains) remains to be examined in these tumor types. IL13Rα2 is expressed in colon, pancreatic, ovarian tumors and malignant glioma (11-14). In summary then, both ligands and receptors are present in many tumor types and their interaction may have important consequences. Here, we focus on the direct effects of IL4 and IL13 on epithelial tumor cells. Other pro-tumorigenic functions, including roles as activators of tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs) are described elsewhere (15). Since IL4 in particular is an important regulator of lymphocyte expansion and function (2), it is possible that some adaptive anti-tumor functions can also be related to this cytokine system. While this is largely outside the scope of the present article, it is an important issue when considering therapeutic interventions and will be touched on in the final section.
IL4 and the type II IL4 receptor
The IL4/IL4Rα interaction on epithelial cancer cells supports tumor growth in vivo in part by mediating resistance to apoptosis. Epithelial cancers are often cross-resistant to both intrinsic and extrinsic apoptotic pathways as they have a common downstream effector protein, caspase 3. While TRAIL and other death ligands activate the extrinsic pathway of apoptosis, chemotherapeutic agents typically activate the intrinsic pathway. To examine the ability of IL4 to protect against death ligand induced apoptosis, Todaro et al. treated subcutaneous human colon and breast tumors in mice with an IL4 neutralizing antibody and TRAIL. Treatment with the antibody alone modestly decreased the growth of breast and colon tumors, but strongly sensitized tumors to co-treatment with TRAIL. Reduction in tumor growth was attributed to the downregulation of anti-apoptotic proteins PED, cFLIP, Bcl-xL and Bcl-2 in response to IL4 blockade (9). This study and others have utilized the species specificity of IL4/IL4Rα binding to target the autocrine production of human IL4 in mouse xenograft models. However, distinguishing the actions of autocrine versus paracrine IL4 is less relevant in patients where both act similarly. Still, xenograft models have been instrumental in demonstrating that IL4 enhances the survival of epithelial cancer cells.
In order to establish IL4 as a survival factor capable of inducing chemoresistance, Todaro et al. implanted human colorectal cancer spheroids containing colorectal cancer stem cells into the flanks of nude mice. Mice bearing colorectal tumors were treated with an anti-human IL4 neutralizing antibody or IL4 double ‘mutein’ (IL4DM), an antagonist of human IL4Rα, to interfere with the IL4/IL4Rα interaction (16). A modest decrease in tumor size compared to controls was seen with either treatment alone. However, efficacy of chemotherapy with oxaliplatin and/or 5-fluorouracil (5-FU) was significantly enhanced when animals were co-treated with either the anti-IL4 antibody or IL4DM. Further, these anti-tumor effects were sustained following cessation of treatment. Increased tumor cell death was again attributed to the downregulation of anti-apoptotic proteins PED, cFLIP, Bcl-xL, Bcl-2 (16), and survivin expression (9) following IL4 blockade. With the pro-survival functions of autocrine IL4 translating to chemoresistance in epithelial tumors in vivo, it follows that anti-IL4/IL4Rα treatments in combination with standard chemotherapeutics could have a synergistic effect in reducing tumor growth in patients. Since IL4 levels are often elevated in the tumor microenvironment (8-10), anti-IL4/IL4Rα treatment may be even more potent in patients as total IL4, both autocrine and paracrine, would be neutralized.
In addition to enhancing cancer cell survival for tumor growth, IL4 has been shown to directly induce the proliferation rate of colon, breast, head and neck, ovarian, and prostate cancer cells in vitro (4, 17, 18). The majority of these studies attributed IL4-induced proliferation to the activation of downstream Stat6 signaling, however the up-regulation of survivin may also play a role. Survivin, a well-known inhibitor of apoptosis, can act as a mitotic regulator that directly influences cell proliferation by controlling cell cycle entry (19). In a recent study, IL4-induced proliferation of human prostate cancer cells correlated with increased expression of survivin in vitro. This finding translated to increased tumor cell proliferation in vivo, overall tumor progression, and increased mouse survival (18). Significantly, survivin mRNA levels did not differ between control and IL4 stimulated prostate cancer cells, indicating that survivin up-regulation was not controlled by a transcriptional mechanism, but rather by differences in mRNA translation (18). In contrast to proliferation-promoting roles, early in vitro models defined a possible growth inhibitory role for IL-4 that was also shown in vivo using tumor cells genetically modified to secrete IL-4 (20). However, it is important to note that in such experiments cancer cell IL-4 expression was driven by viral promoters resulting in consistent and copious overexpression that may not be physiologically relevant. Importantly, clinical trials in which exogenous IL-4 was tested as an anti-tumor agent were not successful (20). Since we now know that IL-4 levels are often already elevated in the tumor microenvironment of cancer patients (6-8), the lack of effect of additional IL4 is perhaps not unsurprising.
While the above studies examined the role of autocrine IL4 in the growth of established epithelial tumors, the IL4/IL4Rα interaction may also contribute to tumor formation. Khaled et al. recently published that IL4 and IL13-induced Stat6 signaling is crucial for normal mammary gland development in vivo (21). It is plausible that epithelial tumor cells could exploit normal developmental pathways to promote tumor formation. Consistent with the studies in established tumors, IL4 has been shown to promote fibrosarcoma tumor formation in vivo by enhancing tumor cell survival (22). However, the relative contribution of tumor versus host-derived IL4 or the receptor was not examined in this study. This is significant because several cell types may contribute to IL4/IL4Rα induced proliferation and survival during epithelial tumor development. For example, as reviewed elsewhere (15) tumor-promoting macrophages can be activated by IL4.
In an attempt to distinguish the contribution of epithelial versus host IL4Rα signaling in tumor development, Koller et al. utilized two mouse models: (i) implantation of wild type murine colon cancer cells or shRNA-mediated IL4Rα knockdown (IL4Rα KD) clones into the ceca of C57BL/6 mice; and (ii) a carcinogen- induced model of colitis associated colon cancer in IL4Rα knockout (IL4Rα-/-) mice (4). Results obtained from the implantation model indicated that tumor cell IL4Rα was important for inducing proliferation but not survival in vivo In the carcinogen model, IL4Rα-/- mice had significantly smaller tumors compared to wild type mice. The reduction in tumor size correlated with a trend in decreased proliferation and a significant increase in apoptosis, suggesting that cell death was mediated by host cell IL4Rα(4). However, since both host and tumor cells were deficient of IL4Rα in the carcinogen-induced model, it is impossible to be certain of the relative contribution of each. These results established a role for IL4Rα in promoting colon cancer development, yet they appear to contradict previous studies demonstrating a strong pro-survival function of the IL4/IL4Rα interaction in epithelial tumor cells (9, 16, 22, 23). It is important to note that active survival response pathways in developing versus established tumors may differ. Other variables include colon cancer stem cell content as well as the presence and function of IL13. It is also possible that in the colitis associated colon cancer model, increased production of IL13 by inflammatory cells in the tumor microenvironment may promote tumor development as IL13 can also bind and activate IL4Rα. The contribution of each cytokine remains to be distinguished, and this may be particularly important in models where immune infiltrates are prevalent.
IL13 and its receptors
Numerous studies have reported the expression and role of IL4 in epithelial cancers, however few have also focused on the implications of IL13 and IL13R expression. This gap in knowledge may be due to the complexity of the IL13 system. IL13 can signal through type II IL4R (IL4Rα and IL13Rα1) or through IL13Rα2, once believed to serve only as a decoy receptor. Elevated levels of IL13 were detected in primary breast cancer tumor tissue (24) and in the peripheral blood of 131 other cancer patients (pancreatic, esophageal and gastric) when compared to healthy controls (25). Recent studies by Formentini, et al. and Barderas, et al. suggested possible roles for IL13 and its receptors IL13Rα1 and IL13Rα2 in colorectal cancer (11, 26). In tumor tissue specimens, expression of IL13Rα1 did not influence survival and IL13 expression did not influence patient lymph node metastasis. Surprisingly, low IL13 expression correlated with worse overall survival compared with high IL13 expression (26). However, high expression of IL13Rα2 in colon cancer patients is a predictor of later stages of disease and poor outcome (11). Similarly, in highly invasive Glioblastoma Multiforme (GBM), IL13Rα2 is over-expressed whereas it is absent in normal brain tissue (13). Finally, IL13Rα2 has been reported to be a biomarker of disease in ovarian cancer and targeting this receptor with a specific immunotoxin in mice resulted in decreased tumor burden and extended host survival (14). While these expression findings suggest a possible contribution of IL13Rα2 to tumor metastasis and survival, they do not establish a causal role for IL13 in cancer cell growth or metastasis.
To establish functional relationships between IL13 and tumor behavior, it is helpful to understand the various signaling pathways associated with receptor activation. The dominant signaling pathway activated by IL13 binding to IL13Rα1 in the context of the type II IL4R, is the Stat6 pathway (figure 1B) (2). This is the same pathway activated by IL4 binding to the type II IL4R, although signaling kinetics appear to be different (3). In many of the studies discussed previously, IL4Rα was targeted to abolish IL4 binding and resultant signaling. This does not affect IL13 binding to IL13Rα1 but in the absence of IL4Rα, signaling is eliminated. Several studies identifying the role of IL4R in tumorigenesis have assumed the response is equal regardless of which cytokine, IL4 or IL13, is activating the signaling cascade. This however may be inaccurate (3) and further studies isolating the signaling pathways activated downstream of each cytokine are required. Additional mediators in tumorigenesis may include AP-1 dependent induction of TGFβ expression after IL13 binding to IL13Rα2, as shown in macrophages (figure 1B) (27). Another mediator is 15-lipoxygenase-1 (15-LOX-1), which conversely occurs only in the absence of IL13Rα2 (figure 1C) (28).
A correlation between IL13Rα2 expression and metastasis of tumor cells has been observed in multiple epithelial cancers. In an orthotopic model of human ovarian cancer, IL13Rα2-expressing tumors metastasized to lymph nodes faster than IL13Rα2-negative tumors and resulted in a higher rate of animal mortality (14). Exogenous IL13 treatment further increased mortality in mice carrying IL13Rα2-expressing tumors (14), linking IL13/IL13Rα2 to increased aggressiveness of tumor cells. Similarly, over-expression of IL13Rα2 increased invasion and metastasis, and decreased survival in a mouse model of human pancreatic cancer (12). Recent studies in a mouse model of human colorectal cancer indicated that genetic knockdown of IL13Rα2 in tumor cells reduced liver homing ability, resulting in increased survival of mice compared to those injected with IL13Rα2 expressing cells (11). Additionally, in mouse models of GBM, tumor cells that survive IL13Rα2 targeted therapy, presumably due to low receptor expression, were less tumorigenic than untreated control cells (13). Possible molecular mediators of this pro-metastastic function of IL13 include the transcription factor AP-1 and its target TGFβ. Treatment of IL13Rα2-expressing tumor cells with exogenous IL13 activated ERK1/2 followed by AP-1, in vitro, with expression confirmed in vivo (14). AP-1 is at least one of the transcription factors downstream of IL13Rα2 signaling leading to expression of TGFβ in monocytic leukemia and macrophages as a result of IL13 treatment (27). Therefore, we speculate this pathway may also be important in epithelial tumor cells.
Another important mechanism for promotion of metastasis is regulation of apoptosis. Interestingly, IL13 can be both pro- and anti-apoptotic depending on receptor presence and/or signaling pathways activated. As described previously, signaling through type II IL4R is strongly associated with increased expression of anti-apoptotic proteins. The IL13-induced pro-apoptotic pathway is activated by 15-LOX-1 catalyzing the oxidation of arachidonic and linoleic acids, leading to activation of PPARγ, whose ligands can then activate cell apoptosis. According to Hsi, et al., IL13-dependent production of 15-LOX-1 occurs only in the absence of IL13Rα2. In their model, decreased in vivo growth of GBM due to increased apoptosis was associated with IL13 (28). Some have postulated this IL13-induced apoptosis signaling pathway occurs through IL4R, however there have not been any studies to either support or dispute this contention.
These multiple studies established contradictory roles for IL13 in promoting and combating cancer progression that is seemingly dependent on receptor expression. For example, when all receptors are expressed, IL13 can either (i) bind to IL13Rα1, recruit IL4Rα and signal through this heterodimer to phosphorylate stat6 and promote proliferation and/or apoptotic resistance; (ii) bind to soluble IL13Rα2 decoy receptor and result in no signaling; or (iii) bind transmembrane monomeric IL13Rα2 and induce TGFβ resulting in increased metastasis (figure 1B, C). Additionally, in mice genetically deficient for IL4Rα, where any IL13 signaling must be through IL13Rα2, Ko et al showed increased development of precancerous lesions in an intestinal carcinogen model (29). These mice also had increased levels of TGFβ. It is important to note that induction of AP-1 by IL13 signaling through IL13Rα2 can also activate other factors including matrix metalloproteinases, which are active in tumor growth, invasion and metastasis (14). In contrast, in the absence of IL13Rα2 expression, IL13 signaling through Type II IL4R is possibly increased and the IL13-induced apoptotic pathway is activated, although, it is not yet clear if this IL13-induced apoptotic pathway is downstream of Type II IL4R. Overall then, It is clear that IL13Rα2 targeted therapy may be just as important as IL4Rα targeted therapy.
Implications and Future Directions
As indicated by the foregoing discussion, there is now significant evidence supporting IL4/IL13 and their receptors as valid therapeutic targets in many different cancers. Even without considering the biological pathways involved, the high level expression of IL4Rα (also known as CD124) or IL13Rα2 on several types of tumor cells has allowed tumor cell-selective delivery of various toxins or lytic peptides resulting in reduced tumor load (30). So, is it plausible to target the IL4R or IL13Rα2 signaling pathways in patients as anti-cancer therapy? There are several key concerns to address prior to the use of anti-IL4 or -13 receptor treatments. (i) IL4Rα inhibiting treatments would inhibit signaling through both type I and II IL4Rs. To our knowledge there are no comprehensive studies evaluating the expression of Type I IL4R in epithelial cancers. Type I IL4R should be ruled out as a potential player in tumor cell behavior, but is clearly a critical regulator of immune cell behavior. When given systemically, agents that target IL4Rα will not only affect cancer cells, but also tumor-associated macrophages and other myeloid cell types as well as lymphocytes. It is this last group of cells that may be of concern. It is known that there are polymorphisms in the gene for IL4RA that result in enhanced receptor activity. Small genetic studies have suggested that colorectal cancer patients with these polymorphisms actually have a better prognosis (31). This is likely due to more effective B and T cell function, although actual experimental evidence is lacking. Hence therapies that specifically target cancer cells via the type II IL4R, but avoid the lymphocyte-expressed type I receptor may be most beneficial. (ii) Targeting of one receptor type may impact the activity or ligand-binding of other related receptors. One of most exciting recent studies in the IL4/IL13 field defines the crystal structures of the various receptors and shows IL4 and IL13 having different potencies through the same receptor in tumor cells (3). IL4 and IL13 have differing affinities for the various receptor subunits, which can make one or other receptor the more likely target when all are available. However, this will be changed if one subunit is no longer able to bind ligand. For example, in the absence of IL13Rα2, will there be increased binding of the type II IL4R by IL13 that would have a different outcome to when that receptor is bound by IL4? We are currently undertaking studies using single and dual receptor knockdown in tumor cells expressing these receptors at various levels to answer these complex, yet pertinent questions. (iii) Efficacy may be different in primary and metastatic tumors. Many studies have found particularly strong evidence for pro-survival functions of IL4 and IL13. This might suggest that metastatic colonization and resistance to chemotherapy, both dependent on survival programming, are key roles for IL4 and IL13 signaling. In mouse models, we have preliminary evidence that growth of primary tumors is unaffected whereas metastatic burden is strongly reduced when IL4Rα is ablated on mammary tumor cells (Venmar and Fingleton, unpublished data). Further analysis of the different mechanisms involved will be necessary before making recommendations about which patient populations would most benefit from IL4/IL13 pathway-directed therapies.
In conclusion, IL4, IL13 and their receptors appear to have significant functions on tumor cell biology that is distinct from their roles in regulation of immune cells. Although complex and still in need of further elucidation, the signaling pathways activated by these Th2 cytokines may offer novel means to target cancer-specific behaviors.
Acknowledgments
Financial Support: R01 CA157781 to BF; KTV is supported by T32GM008554; MAH is supported by T32CA119925.
Footnotes
Conflicts of interest: No authors have any conflicts.
References
- 1.Ito T, Suzuki S, Kanaji S, Shiraishi H, Ohta S, Arima K, et al. Distinct structural requirements for interleukin-4 (IL-4) and IL-13 binding to the shared IL-13 receptor facilitate cellular tuning of cytokine responsiveness. J Biol Chem. 2009;284:24289–96. doi: 10.1074/jbc.M109.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–72. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koller FL, Hwang DG, Dozier EA, Fingleton B. Epithelial interleukin-4 receptor expression promotes colon tumor growth. Carcinogenesis. 2010;31:1010–7. doi: 10.1093/carcin/bgq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, et al. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsasser-Beile U, Kolble N, Grussenmeyer T, Schultze-Seemann W, Wetterauer U, Gallati H, et al. Th1 and Th2 cytokine response patterns in leukocyte cultures of patients with urinary bladder, renal cell and prostate carcinomas. Tumour Biol. 1998;19:470–6. doi: 10.1159/000030039. [DOI] [PubMed] [Google Scholar]
- 7.Wise GJ, Marella VK, Talluri G, Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000;164:722–5. doi: 10.1097/00005392-200009010-00024. [DOI] [PubMed] [Google Scholar]
- 8.Camp BJ, Dyhrman ST, Memoli VA, Mott LA, Barth RJ., Jr In situ cytokine production by breast cancer tumor-infiltrating lymphocytes. Ann Surg Oncol. 1996;3:176–84. doi: 10.1007/BF02305798. [DOI] [PubMed] [Google Scholar]
- 9.Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ. 2008;15:762–72. doi: 10.1038/sj.cdd.4402305. [DOI] [PubMed] [Google Scholar]
- 10.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–8. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barderas R, Bartolome RA, Fernandez-Acenero MJ, Torres S, Casal JI. High Expression of IL-13 Receptor alpha2 in Colorectal Cancer Is Associated with Invasion, Liver Metastasis, and Poor Prognosis. Cancer Res. 2012;72:2780–90. doi: 10.1158/0008-5472.CAN-11-4090. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–85. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen V, Conyers JM, Zhu D, Gibo DM, Dorsey JF, Debinski W, et al. IL-13Ralpha2-Targeted Therapy Escapees: Biologic and Therapeutic Implications. Transl Oncol. 2011;4:390–400. doi: 10.1593/tlo.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisawa T, Joshi BH, Puri RK. IL-13 regulates cancer invasion and metastasis through IL-13Ralpha2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J Cancer. 2012;131:344–56. doi: 10.1002/ijc.26366. [DOI] [PubMed] [Google Scholar]
- 15.Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle. 2010;9:4824–35. doi: 10.4161/cc.9.24.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang WJ, Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine. 2008;42:39–47. doi: 10.1016/j.cyto.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Roca H, Craig MJ, Ying C, Varsos ZS, Czarnieski P, Alva AS, et al. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin up-regulation. J Cell Biochem. 2012;113:1569–80. doi: 10.1002/jcb.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Chen L, Qin Z. Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol. 2009;6:415–22. doi: 10.1038/cmi.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khaled WT, Read EK, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, et al. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development. 2007;134:2739–50. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Jiang J, Wang Z, Zhang J, Xiao M, Wang C, et al. Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis. Cancer Res. 2008;68:8687–94. doi: 10.1158/0008-5472.CAN-08-0449. [DOI] [PubMed] [Google Scholar]
- 23.Roca H, Craig MJ, Ying C, Varsos ZS, Czarnieski P, Alva AS, et al. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin upregulation. J Cell Biochem. 2011 doi: 10.1002/jcb.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srabovici N, Mujagic Z, Mujanovic-Mustedanagic J, Muminovic Z, Softic A, Begic L. Interleukin 13 expression in the primary breast cancer tumour tissue. Biochem Med (Zagreb) 2011;21:131–8. doi: 10.11613/bm.2011.021. [DOI] [PubMed] [Google Scholar]
- 25.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formentini A, Braun P, Fricke H, Link KH, Henne-Bruns D, Kornmann M. Expression of interleukin-4 and interleukin-13 and their receptors in colorectal cancer. Int J Colorectal Dis. 2012 doi: 10.1007/s00384-012-1456-0. [DOI] [PubMed] [Google Scholar]
- 27.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 28.Hsi LC, Kundu S, Palomo J, Xu B, Ficco R, Vogelbaum MA, et al. Silencing IL-13Ralpha2 promotes glioblastoma cell death via endogenous signaling. Mol Cancer Ther. 2011;10:1149–60. doi: 10.1158/1535-7163.MCT-10-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko CW, Cuthbert RJ, Orsi NM, Brooke DA, Perry SL, Markham AF, et al. Lack of interleukin-4 receptor alpha chain-dependent signalling promotes azoxymethane-induced colorectal aberrant crypt focus formation in Balb/c mice. J Pathol. 2008;214:603–9. doi: 10.1002/path.2316. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima H, Terabe M, Berzofsky JA, Husain SR, Puri RK. A novel combination immunotherapy for cancer by IL-13Ralpha2-targeted DNA vaccine and immunotoxin in murine tumor models. J Immunol. 2011;187:4935–46. doi: 10.4049/jimmunol.1102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkening S, Tavelin B, Canzian F, Enquist K, Palmqvist R, Altieri A, et al. Interleukin promoter polymorphisms and prognosis in colorectal cancer. Carcinogenesis. 2008;29:1202–6. doi: 10.1093/carcin/bgn101. [DOI] [PubMed] [Google Scholar]