Abstract
Tumor cells often utilize developmental processes in order to progress towards advanced disease. The E-box transcription factor TWIST1 is essential to epithelial-mesenchymal transition and cell migration in the developing neural crest. In melanoma, which derives from the neural crest cell lineage, enhanced TWIST1 expression has been linked to worse clinical prognosis. However, mechanisms underlying TWIST1 expression and whether aberrant TWIST1 levels promote steps in melanoma progression remain unknown. Here, we report that elevated TWIST1 mRNA/protein expression is dependent on ERK1/2 signaling, which is hyperactive in the majority of melanomas. We show that TWIST1 protein levels are especially high in melanoma cell lines generated from invasive, pre-metastatic stage tumors. Furthermore, TWIST1 expression is required and sufficient to promote invasion through Matrigel and spheroid outgrowth in three-dimensional dermal-mimetic conditions. Alterations to spheroid outgrowth were not as a result of altered cell death, cell cycle profile, or paradigm EMT protein changes. Importantly, we identify matrix metalloproteinase-1 (MMP-1) as a novel downstream target of TWIST1. We have determined that TWIST1 acts, in a dose-dependent manner, as a mediator between hyperactive ERK1/2 signaling and regulation of MMP-1 transcription. Together, these studies mechanistically demonstrate a previously unrecognized interplay between ERK1/2, TWIST1, and MMP-1 which is likely significant in the progression of melanoma towards metastasis.
Keywords: TWIST1, ERK, MMP-1, invasion, melanoma
Introduction
The process of invasion allows tumor cells to traverse the boundaries of the basement membrane and move through the stromal extracellular matrix (ECM) in order to intravasate and ultimately colonize distant sites (1). Often, tumor cells will utilize core developmental processes in order to progress towards advanced or metastatic disease. One such process is the epithelial-mesenchymal transition (EMT). EMT prompts morphologic and cytoskeletal changes in precursor cells which allow for the migratory reorganization of embryonic germ layers and is required for mesoderm formation during gastrulation (2). EMT or EMT-like processes are thought to be a critical step in the metastatic cascade by regulating motility and/or invasion, resistance to anoikis, and stem cell-like properties (3).
One important EMT regulator during development is the basic helix-loop-helix transcription factor, TWIST1 (2–5). It was originally discovered as a gene crucial for proper gastrulation and mesoderm formation in Drosophila (4). In mammals, TWIST1 expression during a precise time frame in embryogenesis allows for the migration and differentiation of several mesodermal and neural crest cell lineages (5, 6). Many of the phenotypes attributed to TWIST1 occur as a result of its binding to E-box consensus sites in gene promoters, ultimately leading to transcriptional activation or repression (4, 7).
TWIST1 is overexpressed in many primary tumors including colon, breast, prostate, and gastric carcinomas (8–11). In agreement with its role in embryonic cell migration, TWIST1 overexpression has been linked to increased tumor cell migration, invasion, and metastasis (7, 11–13). These actions of TWIST1 have been correlated with changes in classical EMT targets such as E-cadherin and N-cadherin (7, 11, 12); however, the extent to which TWIST1 regulates non-EMT targets is not fully understood.
Recently, TWIST1 was found to be highly up-regulated in the vast majority of melanoma tumors and cell lines, and was correlative to worse patient survival (8, 14). Melanoma is an aggressive skin cancer which arises from neural crest-derived melanocytes (15, 16). Invasion plays a critical role in melanoma progression. If cells are mainly confined to expansion within the epidermis (radial growth phase, RGP), melanoma is easily cured through surgical intervention (15, 16). If undiagnosed and properties of invasion begin to emerge, cells escape the basement membrane and expand through the deeper dermal layers. This conversion to vertical growth phase (VGP) is the direct precursor to metastasis (15). The depth of melanoma invasion and tumor thickness are used as predictors of poor clinical prognosis (17, 18); however, the mechanisms underlying melanoma invasion from the epidermis into the dermis remain poorly characterized. Up-regulation of the RAS-RAF-MEK-ERK1/2 signaling pathway may be critically important in this process. Hyperactivation of this pathway is common in multiple cancer types but especially in melanoma, where mutations in N-RAS (15–20%) or B-RAF (40–60%) are prevalent (15, 16, 19). Additionally, mutant B-RAF, especially B-RAFV600E, is required for enhanced growth and invasion of melanoma cells (20). Many of the factors influencing increased melanoma invasion downstream of RAS-RAF-MEK-ERK1/2 are unknown.
Since TWIST1 plays important roles in the developing and highly migratory neural crest, and since tumor cells often aberrantly regulate developmental pathways, we sought to determine the role of TWIST1 in melanoma invasive growth. In this study, we have found that TWIST1 promotes invasion in 3D dermal-mimetic assays and reveal an ERK1/2-TWIST1-MMP-1 pathway which likely will have a major impact on invasion and metastasis.
Materials and Methods
siRNA Transfection
WM793 and WM115 cells were transfected for 4 hours with chemically synthesized siRNAs (Dharmacon, Lafayette, CO) at a final concentration of 25nM using Oligofectamine (Invitrogen). Transfections were harvested at 72 hours. siRNA sequences are listed in Supplementary Table S1.
Quantitative RT-PCR
RNA was extracted from cells using PerfectPure RNA Cultured Cell Kit (5Prime, Gaithersburg, MD) as per the manufacturer’s instructions. Conversion to cDNA was achieved through the iScript cDNA Synthesis Kit (Biorad). Quantitative RT-PCR was carried out using iQ SYBR Green Supermix (Biorad), 0.4µM oligonucleotide primers, and 0.1µg cDNA. Primer sets can be found in Supplementary Table S1. Relative fold change in mRNA levels were calculated after normalization to β-Actin using the comparative Ct method (21).
Statistical Analysis
Statistical analysis was performed using a two-tailed Student’s t test calculated with Excel (Microsoft). A p value < 0.05 was considered statistically significant.
Additional methods
Detailed methods for cell culture, patient samples, lentiviral and adenoviral construction/transduction, invasion/migration assays, spheroid outgrowth asssays, live/dead staining, EdU incorporation assays, dual-luciferase assays, ChIP, biotinylated oligonucleotide pulldown assays, inhibitors, and western blot analysis are available in Supplementary Materials and Methods.
Results
TWIST1 is up-regulated in melanoma cell lines, particularly in VGP, downstream of oncogenic B-RAF and is positively regulated by active ERK1/2 signaling
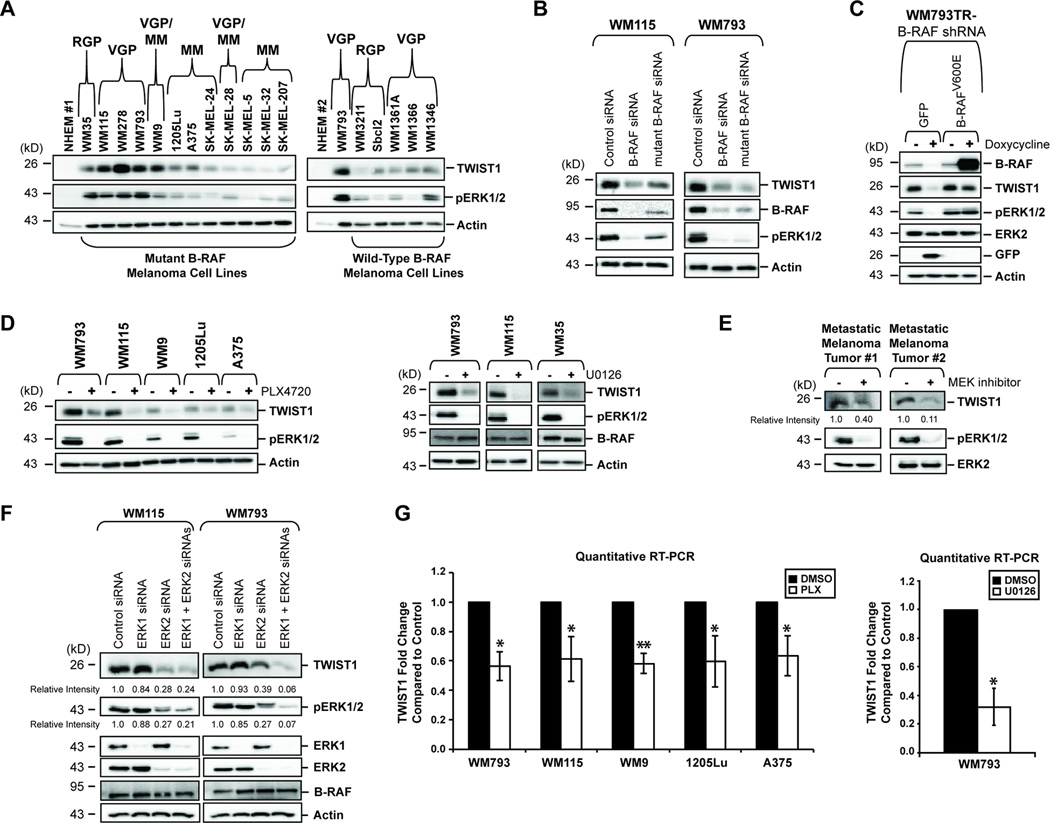
We explored the TWIST1 expression profile across an extensive panel of melanoma cell lines representing various tumor stages and genotypes. Our results show that TWIST1 protein is up-regulated in all melanoma cell lines tested compared to neonatal human epidermal melanocytes (NHEM) (Fig. 1A). Furthermore, TWIST1 protein is especially high in invasive VGP tumors compared to RGP and metastatic tumors of similar genotype, which may denote a selective requirement of TWIST1 in early steps of metastasis.
Figure 1.
TWIST1 is up-regulated in melanoma cell lines, particularly in VGP, downstream of oncogenic B-RAF and is positively regulated by active ERK1/2 signaling. (A) Western blot analysis of melanoma cell lines,organized by genotype and tumor stage, or neonatal human epidermal melanocytes (NHEM) was performed to confirm up-regulation of TWIST1 protein in melanoma cells. WM793 cells appear in both panels for comparison; RGP (Radial Growth Phase), VGP (Vertical Growth Phase), MM (Metastatic Melanoma). (B) WM115 or WM793 cells were transfected with a control siRNA or siRNA directed against total or mutant B-RAF. After 72 hours, lysates were harvested and western blots performed. (C) WM793TR (TR= Tet Repressor) cells were transduced with an inducible B-RAF shRNA, and either an inducible GFP or an shRNA-resistant B-RAFV600E gene cassette. After 7 days of doxycycline treatment, lysates were harvested for western blot. (D) Western blot analysis was conducted on lysates from mutant B-RAF cells treated with PLX4720 (1µM) or U0126 (10µM) for 24 hours. (E) Metastatic melanoma tumor #1 and #2 were placed in short-term culture after surgical resection and treated for 24 hours with U0126 (20µM) and AZD6244 (3.3µM), respectively. Lysates were generated and western blot analysis performed. (F) WM115 cells and WM793 cells were transfected with control siRNA, ERK1 siRNA and/or ERK2 siRNA. After 72 hours, lysates were harvested and western blots performed. (G) Quantitative RT-PCR was performed from WM793 and 1205Lu cells (treated with 1µM PLX4720, 24 hours), from WM115, WM9, and A375 cells (treated with 1µM PLX4032, 24 hours), and from WM793 cells treated with U0126 (10µM, 24 hours). Columns, average of three independent experiments; bars, SD; *p<0.05, **p<0.01.
Interestingly, protein expression of TWIST1 in mutant B-RAF melanoma cell lines is elevated compared to similar stage wild-type B-RAF cell lines and correlates relatively well with the levels of phosphorylated ERK1/2 (Fig. 1A). Further, we found that siRNA-mediated depletion of either mutant B-RAF or mutant and wild-type B-RAF greatly reduced TWIST1 protein levels in WM793 (B-RAFV600E) and WM115 (B-RAFV600D) cells (Fig. 1B). To eliminate the possibility of off-target effects, a rescue experiment was conducted in which inducible B-RAF knock-down cell lines were engineered to express a GFP control or to re-express a shRNA-resistant form of B-RAFV600E . Upon rescue of B-RAFV600E expression, with concomitant re-establishment of phosphorylated ERK1/2, TWIST1 protein levels were restored (Fig. 1C), This was further confirmed by the inhibition of RAF signaling by PLX4720 (a non-clinical analog of PLX4032/vemurafinib), and by the inhibition of active ERK1/2 signaling by the MEK inhibitor, U0126 (Fig. 1D).
To extend our studies further, we utilized two independent metastatic melanoma samples surgically-resected from patients. Both tumors were genotyped: tumor #1 harbors wild-type N-RAS and wild-type B-RAF, while tumor #2 harbors wild-type N-RAS and B-RAFV600E (data not shown). After short-term culture and transient MEK inhibition, both tumor samples evidenced down-regulation of TWIST1 protein concurrent with reduction in phosphorylated-ERK1/2 (Fig. 1E).
Individual knock-down of ERK1 and ERK2 in mutant B-RAF cell lines revealed that both isoforms contribute to regulation of TWIST1 (Fig. 1F). The amount of TWIST1 ablation correlated closely with the total amount of phosphorylated-ERK1/2 remaining after knockdown. Despite links with the ERK1/2 pathway, inhibition of RSK (22) or STAT3 (23), did not result in TWIST1 protein changes (Supplementary Fig. S1A & B). In addition, AKT does not appear to contribute to TWIST1 regulation (Supplementary Fig. S1C) (13). Thus, the molecule(s) that acts as a bridge between ERK1/2 and TWIST1 still remains elusive and requires future analysis.
TWIST1 regulation by active ERK1/2 signaling occurs at the transcript level, as TWIST1 mRNA is down-regulated upon treatment with PLX4720 or PLX4032 and is further confirmed by the use of U0126 (*p<0.05, **p<0.01) (Fig. 1G). In addition, while TWIST1 stability may be regulated post-translationally in other cell types (24), the combination of cycloheximide and U0126 did not hasten TWIST1 protein degradation in mutant B-RAF melanoma cells (Supplementary Fig. S1D). Therefore, we conclude that TWIST1 mRNA is positively regulated downstream of mutant B-RAF via activation of ERK1/2 signaling.
TWIST1 is required and sufficient for melanoma cell invasion through Matrigel
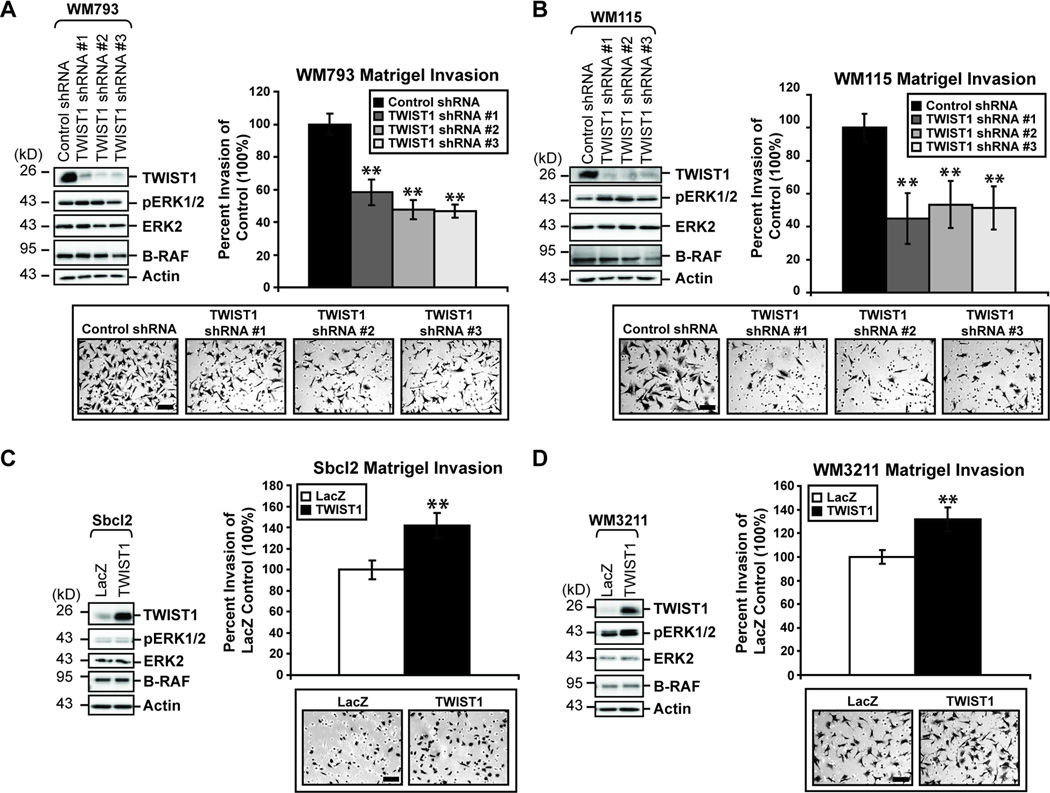
The role of TWIST1 expression in the biology of advanced melanoma cells is currently unknown. Oncogenic RAF/MEK/ERK signaling has been shown to be critical for the invasive capabilities of melanoma cells (20, 25, 26). In addition, studies in breast cancer have implicated TWIST1 in the early invasive steps of the metastatic cascade (7). To assess the role of the ERK target, TWIST1, in the ability of melanoma cells to invade in a Matrigel transwell assay, we utilized the VGP cell lines WM793 and WM115. Reduced expression of TWIST1 protein via three independent shRNAs in WM793 and WM115 cells significantly reduced the invasive capacity of those cells by 40–50% (**p<0.01) (Fig. 2A & B). Conversely, we overexpressed TWIST1 in poorly invasive, RGP cell lines which displayed low endogenous TWIST1 expression. TWIST1 overexpression in Sbcl2 and WM3211 cells significantly increased the ability of cells to invade through Matrigel by approximately 40% and 30%, respectively (**p<0.01) (Fig. 2C & D).
Figure 2.
TWIST1 is required and sufficient for melanoma cell invasion through Matrigel. (A) WM793 and (B) WM115 cells constitutively expressing control or one of three independent TWIST1 shRNAs were utilized. Cells were allowed to invade through Matrigel-coated chambers towards an attractant of full serum media. Counts taken (in triplicate fields of view) from the control shRNA (average set at 100% invasion) were used to calculate percent invasion for all other treatments. Columns, average of three independent experiments; bars, SD; scale, 100µm; **p<0.01. (C) WM3211 and (D) Sbcl2 cells overexpressing either LacZ (control) or TWIST1 were assessed for invasion through Matrigel-coated boyden chambers, as above. Columns, average of three independent experiments; bars, SD; scale, 100µm; **p<0.01.
High TWIST1 expression promotes melanoma spheroid outgrowth
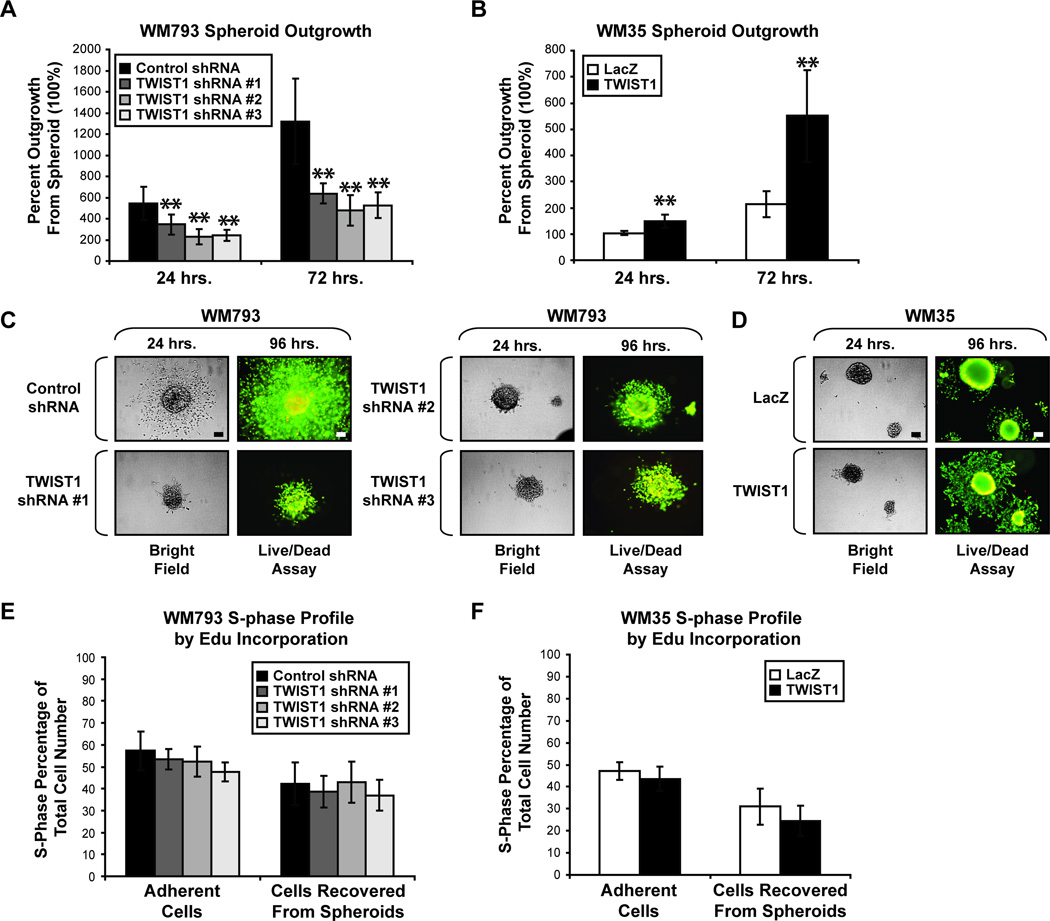
Many melanoma cell lines have the ability to aggregate into spheroids when grown in non-adherent conditions. In comparison to individual cells, these spheroids more closely represent melanoma tumor architecture. The ability of melanoma cells to expand outward from an initial spheroid into surrounding 3D collagen, mimicking the in vivo collagen-rich dermal layer, is a mark of invasive capacity (27, 28). Since differences in gene expression profiles and phenotypic outcomes can arise between cells grown in 2D versus 3D culture (29, 30), we sought to determine whether high TWIST1 expression is sufficient for invasion in 3D dermal-mimetic conditions. The WM793 cell line is invasive and displays robust spheroid outgrowth in this context (27). Therefore, WM793 spheroids constitutively expressing control or one of three TWIST1 shRNAs were implanted into 3D collagen and monitored for several days. Strikingly, WM793 cells depleted for TWIST1 displayed significant defects in the ability to begin and sustain spheroid outgrowth compared to control cells (**p<0.01) (Fig. 3A). To determine whether TWIST1 expression in a RGP cell line would promote outgrowth, WM35 cells constitutively overexpressing either LacZ or TWIST1 were analyzed. Indeed, significant increases in the spheroid outgrowth of WM35 cells which constitutively overexpress TWIST1 were observed (**p<0.01) (Fig. 3B).
Figure 3.
High TWIST1 expression allows for melanoma spheroid outgrowth. (A) Spheroids of WM793 cells constitutively expressing control or TWIST1 shRNAs were implanted into 3D collagen. Bright field images were taken at 24 hour increments imaging the same spheroids at each time point. Percent outgrowth was calculated by comparing the surface area of the spheroid (100%) to that of the total outgrowth at each time point. Columns, average of three independent experiments; bars, SD; **p<0.01. (B) WM35 constitutively overexpressing LacZ or TWIST1 was also assessed for spheroid outgrowth, as above. Columns, average of three independent experiments; bars, SD; **p <0.01. (C & D) At 96 hours, embedded spheroids were stained with both calcein AM (green = live cells) and ethidium bromide (red = dead cells) to determine apoptosis. One representative field of view is shown; scale, 100µm. (E & F) Alternatively, at 96 hours, embedded spheroids were subjected to EdU incorporation for 7 hours. Also, adherent cells grown in standard tissue culture dishes were subjected to EdU incorporation assay. Columns, average of three independent experiments; bars, SD.
Spheroid outgrowth may be affected by changes in cell death and/or cell cycle progression. Previous studies showed a TWIST1 involvement in the p53/p21Cip1 pathway, and that reduction of TWIST1 results in increased G1 arrest and senescence (8, 31). Therefore, we examined the effect of TWIST1 alteration on cell death and proliferation. At ninety-six hours post-collagen embedding, spheroids were co-stained with calcein AM and ethidium bromide to visualize live and dead cells, respectively. No qualitative differences were apparent between any of the spheroids with altered TWIST1 and their control counterparts (Fig. 3C & D, Supplementary Fig. S2). Similarly, at ninety-six hours, embedded spheroids were subjected to an EdU incorporation assay to evaluate potential differences in S-phase profile. We also analyzed the S-phase profile of adherent cells grown in standard tissue culture dishes. No significant differences were found between control and TWIST1-altered cells in either condition (Fig. 3E & F). From these data, we conclude that the TWIST1-regulated differences in spheroid outgrowth do not result from alterations in cell death or the ability of cells to enter S-phase.
TWIST1 regulates MMP-1 expression in melanoma cell lines
In several cancer types, TWIST1 overexpression has been linked to increased tumor invasion and metastasis correlating with features of EMT, a process commonly seen in cell migration during embryonic development (7, 11, 12). Hallmarks of EMT include loss of cell-cell adhesions and cell polarity through the suppression of epithelial markers (i.e. E-cadherin) and simultaneous acquisition of mesenchymal protein expression (i.e. N-cadherin, β-catenin, and Vimentin). Interestingly, the melanoma cell lines utilized in our studies with altered TWIST1 expression did not display changes in their morphology (data not shown) or EMT protein expression (Supplementary Fig. S3). As a result, an alternative target(s) that is independent of classical EMT must exist in melanoma cells that functions to mediate the effect of TWIST1 expression on altered invasion patterns.
Matrix metalloproteinases (MMPs) are key players in the ability of cancer cells to proteolytically degrade the basement membrane and ECM in order to invade and metastasize. We therefore examined whether TWIST1 might alter the expression of MMPs. Quantitative RT-PCR analysis of several MMPs implicated in melanoma progression was performed (data not shown). MMP-1 (collagenase-1) was chosen for further analysis as it showed considerable up-regulation at the mRNA level after TWIST1 overexpression (**p<0.01, Fig. 4A & C). MMP-1 is capable of degrading several types of collagen, which is a major component of the dermis in vivo and in the synthetic extracellular matrices utilized in our in vitro invasion studies in Fig. 2 & 3 (32, 33). In addition, MMP-1 has a well-established role in promoting melanoma cell invasion and metastasis (see Discussion).
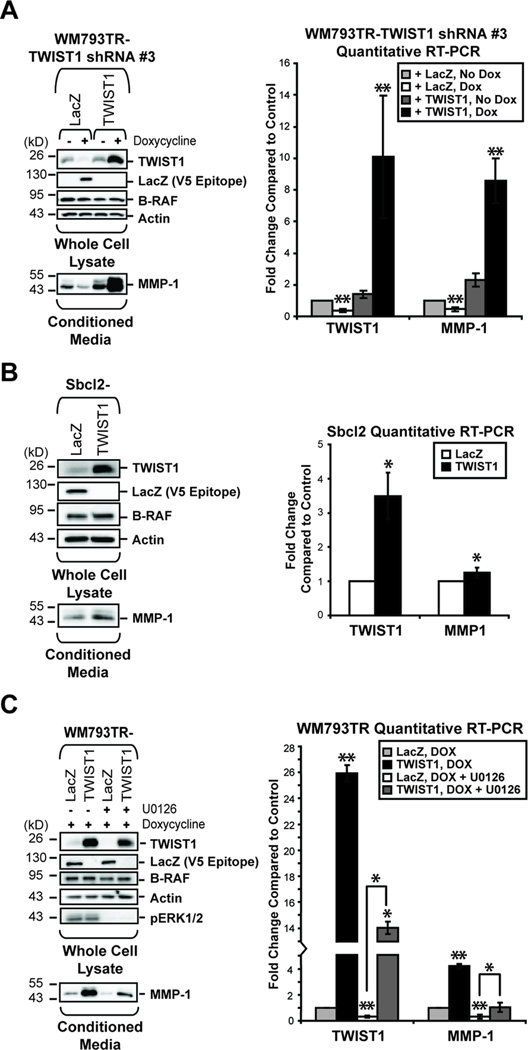
Figure 4.
TWIST1 regulates MMP-1 expression in melanoma cell lines. (A) WM793TR cells were engineered to inducibly express TWIST1 shRNA #3 and either a LacZ gene cassette or a shRNA-resistant TWIST1 gene. Cells were left untreated or induced with doxycycline for 96 hours. After treatment, western blots/qRT-PCR were performed. In addition, induction was performed for the final 24 hours in serum-free media for detection of secreted MMP-1. Columns, average of three independent experiments; bars, SD; **p<0.01. (B) Sbcl2 cells overexpressing either LacZ or TWIST1 were harvested at approximately 70% confluency and analyzed as above. Columns, average of three independent experiments; bars, SD; *p<0.05. (C) WM793TR cells were induced with doxycycline to overexpress either LacZ or TWIST1. At the same time, those cells were either treated with DMSO or 10µM U0126. Lysates and mRNA were harvested and analyzed as above. Columns, average of three independent experiments; bars, SD; *p<0.05; **p<0.01.
In WM793TR cells, TWIST1 shRNA #3, targeting the 3’ untranslated region of TWIST1, elicited a significant decrease in MMP-1 mRNA and secreted protein (**p<0.01) (Fig. 4A). Importantly, re-expression of ectopic (and shRNA-resistant) TWIST1 rescued levels of MMP-1 mRNA and secreted protein in these cells (**p<0.01) (Fig. 4A). Therefore, the effect of TWIST1 on MMP-1 expression is specific and cannot be explained by off-target effects. In addition, overexpression of TWIST1 in the non-invasive cell line Sbcl2 resulted in a more modest but significant increase in MMP-1 mRNA and protein (*p<0.05) (Fig. 4B).
Previous studies found that MMP-1 is regulated downstream of the active RAS-RAF-MEK-ERK1/2 pathway in melanoma (34, 35). We hypothesized that TWIST1 may be a link between activated ERK1/2 and MMP-1 up-regulation. Therefore, we assessed the ability for overexpressed TWIST1 to compensate for the loss of active ERK1/2 signaling after treatment with the MEK inhibitor, U0126. Similar to Fig. 4A, TWIST1 overexpression can rescue decreased MMP-1 mRNA and protein expression caused by ERK1/2 pathway inhibition (*p<0.05, **p<0.01) (Fig. 4C). Taken together, these results demonstrate that TWIST1 is a positive regulator of MMP-1 and is a mediator of ERK1/2 signaling to MMP-1 in melanoma.
TWIST1 alters MMP-1 promoter activity in a dose-dependent manner and directly binds the MMP-1 promoter
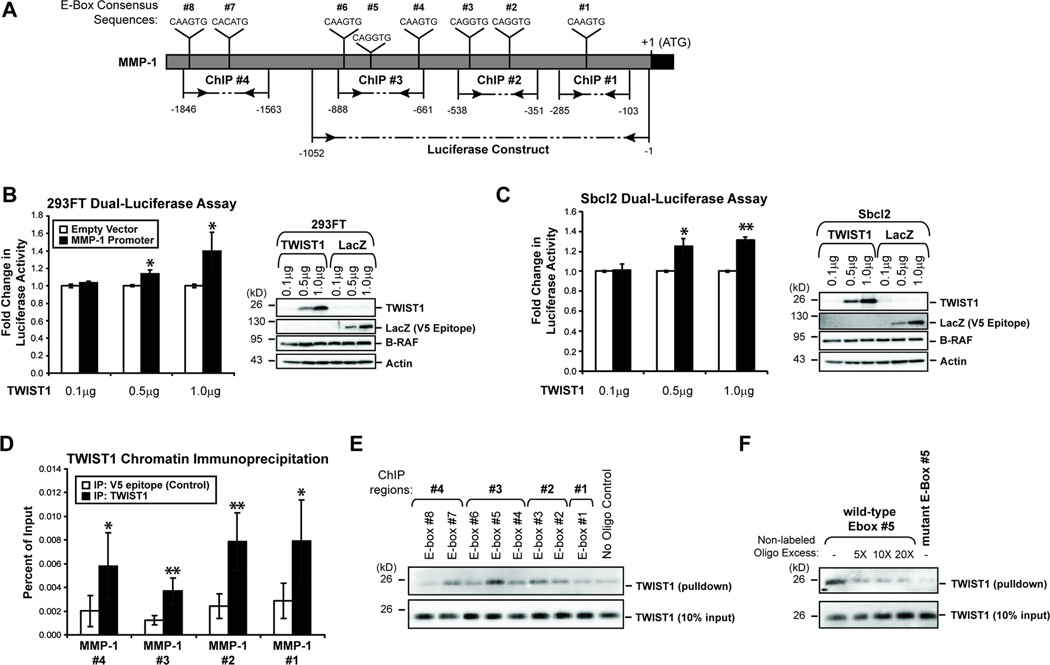
TWIST1 is a transcription factor capable of directly binding E-box consensus sites (5’-CANNTG-3’) to exert transcriptional effects (4, 7). Direct human target genes of TWIST1 remain poorly defined. Their identification is especially intriguing in melanoma given our finding that TWIST1 alteration does not affect EMT genes as it does in other cancer types (7, 11). Several potential binding sites for TWIST1 are present in the MMP-1 proximal promoter (Fig. 5A). We utilized a 1052bp fragment of the MMP-1 promoter (Fig. 5A) and increasing concentrations of either LacZ or TWIST1 overexpression in dual-luciferase reporter assays in wild-type B-RAF 293FT cells and Sbcl2 cells. Corresponding to the MMP-1 mRNA/protein expression changes downstream of TWIST1, we found that TWIST1 overexpression increases MMP-1 promoter activity in a dose-dependent manner (*p<0.05, **p<0.01) (Fig. 5B & C).
Figure 5.
TWIST1 alters MMP-1 promoter activity in a dose-dependent manner and directly binds the MMP-1 promoter. (A) A schematic of the MMP-1 promoter highlighting the regions utilized in the dual-luciferase assay and ChIP assay, as well as the E-box consensus sequences (CANNTG) present. (B) A dual-luciferase assay was performed to determine whether the MMP-1 promoter activity would change as a result of increasing TWIST1 expression in 293FT cells. Fold change was calculated by dividing the normalized luciferase activities of TWIST1-expressing 293FT cells by the normalized luciferase activity of LacZ-expressing cells. A western blot confirmed differential expression of TWIST1 and LacZ. Columns, average of three independent experiments; bars, SD; *p<0.05. (C) A dual-luciferase assay was performed in Sbcl2 melanoma cells as above. Columns, average of three independent experiments; bars, SD; *p<0.05, **p<0.01. (D) In parental WM793 cells, ChIP followed by quantitative PCR was performed to determine if TWIST1 binds directly to the MMP-1 promoter. V5 epitope immunoprecipitation was performed as a negative control for enrichment. Columns, average of six independent experiments; bars, SD; *p<0.05, **p<0.01. (E) A biotinylated oligonucleotide pulldown assay was performed with whole cell protein lysates from parental WM793 cells. A pulldown without oligonucleotide was performed as a negative control. Following the pulldown, western blots were conducted to determine TWIST1 enrichment relative to 10% input. (F) Oligonucleotide pulldowns were repeated for MMP-1 E-box #5 without non-labeled competitor, or in the presence of 5-, 10-, and 20-fold excess of non-labeled E-box #5 competitor. Also, a biotinylated mutant E-box #5 oligonucleotide (4 mutant nucleotides) was utilized to determine the specificity of the interaction.
To determine whether TWIST1 was able to bind directly to the MMP-1 promoter, we performed ChIP of endogenous TWIST1 in parental WM793 cells followed by quantitative RT-PCR of four MMP-1 promoter regions (Fig. 5A). Immunoprecipitation of a V5 epitope, which is not present in parental WM793 cells, was utilized as a negative control. As expected, TWIST1 immunoprecipitation was unable to significantly enrich the β-actin locus compared to control (data not shown). Notably, all four MMP-1 promoter regions were significantly enriched by TWIST1 immunoprecipitation (*p<0.05; **p<0.01) (Fig. 5D).
To further confirm direct binding of TWIST1 to E-boxes in the MMP-1 promoter, we performed a biotinylated oligonucleotide pulldown assay. In this assay, each of eight E-boxes in the MMP-1 promoter was assessed individually for their ability to pulldown TWIST1 protein (Fig. 5A). A reaction without biotinylated oligonucleotide was utilized as a negative control. Several sites were strong in their ability to enrich TWIST1 protein compared to other sites, especially E-box #5 (Fig. 5E). TWIST1 binding to E-box #5 was reduced through the addition of increasing amounts of non-labeled competitor oligonucleotide (Fig. 5F). The binding of TWIST1 to this site was also ablated by the mutation of four key residues within E-box #5. These results indicate that TWIST1 not only regulates MMP-1 promoter activity in a dose-dependent manner but also TWIST1 directly binds to regulatory regions in the MMP-1 promoter.
MMP-1 is able to rescue defects in spheroid outgrowth resulting from TWIST1 ablation
Previous studies have established that changes in MMP-1 expression in melanoma cells lead to alterations in collagen degradation abilities, invasion in vitro, and metastasis in vivo (33, 36–39). We sought to determine whether forced expression of MMP-1 could rescue defects in melanoma spheroid outgrowth resulting from TWIST1 ablation.
For these studies, we utilized WM793TR cells lines which inducibly express either control shRNA or TWIST1 shRNA #3. We then engineered each of these cell lines to overexpress either a LacZ control or MMP-1 protein upon doxycycline addition (Fig. 6A). WM793TR spheroids, generated in the presence or absence of doxycycline, were implanted into 3D collagen and doxycycline treatments maintained. WM793TR spheroids that were grown in the absence of doxycycline displayed spheroid growth typical of a VGP cell line with no significant differences apparent between the variants (Fig. 6B & C, left; Supplementary Fig. S4). The induction of TWIST1 shRNA #3 + LacZ in WM793TR cells caused significant defects in spheroid outgrowth at all time points examined, as was previously established in Figure 3 (*p<0.05, **p<0.01) (Fig. 6B & C, right; Supplementary Fig. S4). Importantly, WM793TR-TWIST1 shRNA #3 cells engineered with a rescue of MMP-1 expression showed a significant increase in spheroid outgrowth area as compared to those cells with TWIST1 depletion alone (*p<0.001). From these studies, we can gather that MMP-1 is a major effector utilized by TWIST1 to alter invasion in melanoma.
Figure 6.
MMP-1 is able to rescue defects in spheroid outgrowth resulting from TWIST1 ablation. (A) WM793TR cells were engineered to express either control shRNA or TWIST1 shRNA #3 as well as a LacZ gene cassette or a MMP-1 gene cassette upon doxycycline addition. Cells were left untreated (basal TWIST1 & MMP-1 expression) or induced with doxycycline for 96 hours prior to spheroid formation. After treatment, lysates were harvested and western blots were performed. In addition, induction was performed for the final 24 hours in serum-free media for detection of secreted MMP-1. (B) WM793TR cells described above (+/− doxycycline) were allowed to form spheroids which were then implanted into 3D collagen. Bright field images were taken at 24 hour increments with careful consideration to image the same spheroids at each time point. Percent outgrowth was calculated by comparing the surface area of the spheroid (set at 100%) to that of the total outgrowth at each time point. Columns, average of three independent experiments; bars, SE; *p<0.05, **p<0.01, ***p<0.001. (C) At 96 hours, embedded spheroids were stained with both calcein AM (green = live cells) and ethidium bromide (red = dead cells) to determine apoptosis. One representative field of view is shown; scale, 100µm.
Discussion
Our results provide evidence that active ERK1/2 signaling promotes up-regulation of TWIST1, which in turn positively regulates the known invasion protein, MMP-1 (Fig. 7). Mutations that lead to hyperactivation of the RAS-RAF-MEK-ERK1/2 pathway occur in 30% of all malignancies and are considered in many contexts to be an early initiators of neoplasia (40). Melanoma is an optimal model system to study the downstream effects of hyperactive ERK1/2 signaling, since it occurs in approximately 90% of these patients and leads to evasion of apoptosis and increased tumor growth and invasion (19). The mechanisms stemming from constitutively active ERK1/2 signaling leading to increased tumor invasion have yet to be fully established. In this study, we present clear evidence that RAS-RAF-MEK-ERK1/2 signaling positively regulates TWIST1. Through pharmacologic inhibition, siRNA/shRNA ablation, and protein rescue experiments, we demonstrate that active ERK1/2 signaling tightly controls TWIST1 protein expression through alterations in transcript levels in mutant B-RAF cells. These data are consistent with microarray data previously published by Shields et al (41). We have also found changes in TWIST1 in four mutant N-RAS melanoma cell lines (Supplementary Fig. S5); however due to variability in the timing of down-regulation and effects on transcript levels (data not shown), we have not further examined the mechanism in this sub-group. Thus, we cannot rule out ERK1/2-dependent, post-transcriptional regulation of TWIST1 in these cells.
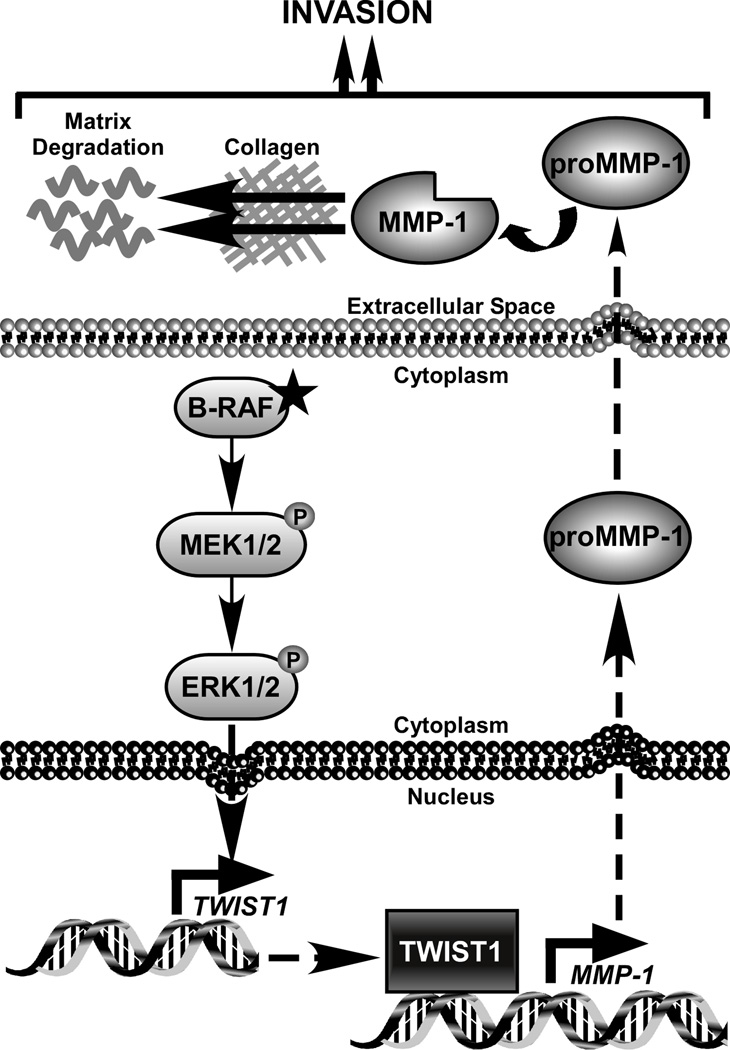
Figure 7.
Working model of an ERK1/2-TWIST1-MMP-1 cascade in melanoma. In our model, active ERK1/2 signaling leads to increased TWIST1 transcription. The factors responsible for TWIST1 regulation downstream of ERK1/2 require further investigation. Through our studies, we find that TWIST1 is able to alter the invasive capability of melanoma cells in multiple systems. While TWIST1 is likely to regulate multiple factors which impact invasion, we establish MMP-1 as a novel downstream effector of TWIST1. We demonstrate that accumulated TWIST1 protein is able to bind the MMP-1 promoter and lead to increased transcription and secreted MMP-1 protein. It is well established that upon secretion of pro-MMP-1 and its cleavage into an active form, MMP-1 promotes cancer cell invasion associated with its ability to degrade collagen in the ECM and activate protease activated receptor 1.
Melanoma is also a model for the invasive process, as the highly metastatic nature of this tumor type leads to chemotherapy resistance and its classification as the deadliest form of skin cancer. Aberrant invasion is one of the main processes by which cancer cells evade tissue boundaries to achieve colonization at metastatic sites. To that end, many cancer cells will exploit developmental mechanisms of motility and migration. The TWIST1 protein has been well studied in development as a main molecule responsible for cell migration from the neural crest during embryogenesis through EMT mechanisms. Prior to this study, TWIST1 was known to be up-regulated in melanoma and associated with poor prognosis (8, 14); however, the mechanism and role of elevated TWIST1 expression in melanoma has remained unexplored. Here, we report that TWIST1 positively regulates melanoma cell invasion. The promotion of invasion by TWIST1 was observed in two 3D invasion assays, one representing invasion through a basement membrane and the other mimicking the dermal microenvironment following invasion through the basement membrane. Despite previous reports of TWIST1 interaction with the p53 pathway (8, 31), cell cycle or cell death changes were not involved in the invasion phenotypes that we observed.
Interestingly, TWIST1 expression appears to be the highest in VGP melanoma cell lines, which are derived from tumors that have invaded vertically into the dermis and can eventually lead to metastasis. Within our mutant B-RAF cell line panel, all metastatic melanoma cells overexpress TWIST1 compared to melanocytes, but have reduced expression compared to mutant B-RAF VGP cell lines. This finding appears to contrast with previous studies where TWIST1 immunohistochemical staining was more prominent in metastatic melanoma tissue cores compared to primary melanomas (14). One potential explanation for this disparity is that the tissues in the microarray utilized in this study were not separated by genotype. In our cell line panel, TWIST1 was more highly expressed in mutant B-RAF cell lines in comparison to similar stage wild-type B-RAF cell lines. In the absence of organization by genotype, the prominence of TWIST1 expression in mutant B-RAF VGP tumors might have been obscured. In support of our data, previous work by Weinberg and colleagues describes the requirement for TWIST1 in early metastatic steps of invasion and intravasation and not in later steps (7). For these studies, they utilized four cell lines derived from the same tumor with differing metastatic potential and found that TWIST1 RNA/protein expression is higher in the cell lines which can only invade and intravasate, which is in agreement with our data. We hypothesize that once melanoma invasion and intravasation is accomplished, the selective pressure for TWIST1 expression is removed and therefore maintenance of high TWIST1 expression is no longer necessary.
We further provide a mechanistic basis for the effects of TWIST1 on melanoma invasion. Surprisingly, TWIST1 did not alter EMT-associated proteins as has been well documented in other cancer types (7, 11, 12). Instead, we provide considerable evidence that TWIST1 positively regulates MMP-1 mRNA leading to substantial changes in secreted protein levels. Through luciferase reporter assays, ChIP, and in vitro oligonucleotide pulldown assays, TWIST1 regulation of MMP-1 was found to be dose-dependent and likely occurs through direct contact at multiple E-box sites within the MMP-1 proximal promoter. The expression of various MMPs has been linked to tumor invasion in a number of systems through matrix-degrading activities (33, 38). Specifically, MMP-1 (collagenase-1) plays a vital and substantial role in melanoma cell invasion most likely as a result of the collagen-rich dermal environment surrounding these cells (33). Melanoma cells that are invasive express much higher levels of MMP-1 in comparison to non-invasive melanomas (36). Furthermore, depletion of MMP-1 expression in invasive melanoma cells results in decreased invasion through chambers coated with type I collagen, type IV collagen, or Matrigel (33, 39). Also, forced expression of MMP-1 in RGP melanoma cells increased collagen degradation in vitro, and tumor growth and metastasis in vivo (39). We demonstrate that cells depleted of TWIST1, but with forced MMP-1 expression, display a restored ability to invade into 3D collagen. These effects are likely ECM protein-specific, as changes in TWIST1 expression significantly alters the ability of melanoma cells to invade through and migrate towards collagen type 1 in boyden chamber assays, but does not significantly affect migration towards other ECM components such as fibronectin and gelatin (Supplementary Fig. S6). Therefore, MMP-1 is likely to be a major mechanism that TWIST1 utilizes to increase invasion of melanoma cells through the collagen-rich dermis. Of note, TWIST1 was recently found to be critical in the formation of invadopodia, which degrade the ECM through protease action, in mammary tumor models (42). In light of our studies, it is intriguing to postulate that TWIST1 might increase both the formation activity of invadopodia through up-regulation of MMP-1.
MMP-1 was recently highlighted as target of RAS-RAF-MEK-ERK1/2 signaling in melanoma (34, 35). Here, we uncover a novel mediator of this signaling with evidence of an ERK1/2-TWIST1-MMP-1 signaling axis. B-RAF mutations are common in pre-neoplastic nevi and, therefore, are considered by many to represent an initiation step in melanoma progression (43). While the majority of common melanocytic nevi harbor mutant B-RAF, only 23% of those display phosphorylated ERK1/2. However, 54% of B-RAF-mutant atypical nevi, which are those that represent an increased risk of developing into melanoma, have active ERK1/2 (44). One mechanism atypical nevi may utilize to progress into melanoma might be through removal of negative checks in the ERK1/2 pathway leading to up-regulation of TWIST1 and subsequent increases in MMP-1. Indeed in the clinic, melanoma patients with higher MMP-1 serum levels had significantly shorter time to progression as compared to those patients with lower MMP-1 serum levels (45). MMP inhibitors failed in clinical trials for melanoma and other cancers largely due to the enrollment of patients with extremely advanced disease (46). Due to the limited availability of treatment options for metastatic melanoma patients, it might be of value to explore the use of MMP-1 inhibitors as prophylactics in the pre-metastatic stages of melanoma disease.
Supplementary Material
Acknowledgements
We thank Dr. Meenhard Herlyn for WM melanoma cell lines and Dr. Dave Solit for SK-MEL-32 and SK-MEL-207. We thank Gideon Bollag (Plexxikon Inc., Berkeley, CA) for PLX4720/PLX4032. We thank Dr. Yongping Shao, Curtis Kugel, and Kaitlyn Le for reagents, and Dr. Ethan Abel and Dr. Yongping Shao for manuscript review.
This work is funded by the ACS John W. Thatcher, Jr. Postdoctoral Fellowship in Melanoma Research (PF-11-240-01-DDC), and NIH RO1s CA125103 and GM67893. Dr. Ethan Abel and Kevin Basile were supported, in part, by fellowships from the Joanna M. Nicolay Melanoma Research Foundation. The TJU KCC core facilities are supported by the NCI Support Grant 1P30CA56036.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare they have no competing financial relationships.
References
- 1.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 2.Vernon AE, LaBonne C. Tumor metastasis: a new twist on epithelial-mesenchymal transitions. Curr Biol. 2004;14:R719–R721. doi: 10.1016/j.cub.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Castanon I, Baylies MK. A Twist in fate: evolutionary comparison of Twist structure and function. Gene. 2002;287:11–22. doi: 10.1016/s0378-1119(01)00893-9. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke MP, Tam PP. Twist functions in mouse development. Int J Dev Biol. 2002;46:401–413. [PubMed] [Google Scholar]
- 6.Fuchtbauer EM. Expression of M-twist during postimplantation development of the mouse. Dev Dyn. 1995;204:316–322. doi: 10.1002/aja.1002040309. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–5162. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 10.Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, et al. Twist is a potential oncogene that inhibits apoptosis. Genes Dev. 1999;13:2207–2217. doi: 10.1101/gad.13.17.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Zhang X, Gang H, Li X, Li Z, Wang T, et al. Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Commun. 2007;358:925–930. doi: 10.1016/j.bbrc.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 13.Koefinger P, Wels C, Joshi S, Damm S, Steinbauer E, Beham-Schmid C, et al. The cadherin switch in melanoma instigated by HGF is mediated through epithelial-mesenchymal transition regulators. Pigment Cell Melanoma Res. 2011;24:382–385. doi: 10.1111/j.1755-148X.2010.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270–5282. doi: 10.1158/0008-5472.CAN-04-0731. [DOI] [PubMed] [Google Scholar]
- 15.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 16.Russo AE, Torrisi E, Bevelacqua Y, Perrotta R, Libra M, McCubrey JA, et al. Melanoma: molecular pathogenesis and emerging target therapies (Review) Int J Oncol. 2009;34:1481–1489. doi: 10.3892/ijo_00000277. [DOI] [PubMed] [Google Scholar]
- 17.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elder DE, Gimotty PA, Guerry D. Cutaneous melanoma: estimating survival and recurrence risk based on histopathologic features. Dermatol Ther. 2005;18:369–385. doi: 10.1111/j.1529-8019.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas NE. BRAF somatic mutations in malignant melanoma and melanocytic naevi. Melanoma Res. 2006;16:97–103. doi: 10.1097/01.cmr.0000215035.38436.87. [DOI] [PubMed] [Google Scholar]
- 20.Sumimoto H, Miyagishi M, Miyoshi H, Yamagata S, Shimizu A, Taira K, et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene. 2004;23:6031–6039. doi: 10.1038/sj.onc.1207812. [DOI] [PubMed] [Google Scholar]
- 21.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, et al. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 23.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, et al. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong J, Zhou J, Fu J, He T, Qin J, Wang L, et al. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71:3980–3990. doi: 10.1158/0008-5472.CAN-10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell. 2003;4:67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 27.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–2233. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–1144. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 29.Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med. 2010;16:1450–1455. doi: 10.1038/nm.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiota M, Izumi H, Onitsuka T, Miyamoto N, Kashiwagi E, Kidani A, et al. Twist and p53 reciprocally regulate target genes via direct interaction. Oncogene. 2008;27:5543–5553. doi: 10.1038/onc.2008.176. [DOI] [PubMed] [Google Scholar]
- 32.Benbow U, Schoenermark MP, Mitchell TI, Rutter JL, Shimokawa K, Nagase H, et al. A novel host/tumor cell interaction activates matrix metalloproteinase 1 and mediates invasion through type I collagen. J Biol Chem. 1999;274:25371–25378. doi: 10.1074/jbc.274.36.25371. [DOI] [PubMed] [Google Scholar]
- 33.Durko M, Navab R, Shibata HR, Brodt P. Suppression of basement membrane type IV collagen degradation and cell invasion in human melanoma cells expressing an antisense RNA for MMP-1. Biochim Biophys Acta. 1997;1356:271–280. doi: 10.1016/s0167-4889(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 34.Huntington JT, Shields JM, Der CJ, Wyatt CA, Benbow U, Slingluff CL, Jr, et al. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells: role of BRAF mutation and fibroblast growth factor signaling. J Biol Chem. 2004;279:33168–33176. doi: 10.1074/jbc.M405102200. [DOI] [PubMed] [Google Scholar]
- 35.Ryu B, Moriarty WF, Stine MJ, DeLuca A, Kim DS, Meeker AK, et al. Global analysis of BRAFV600E target genes in human melanocytes identifies matrix metalloproteinase-1 as a critical mediator of melanoma growth. J Invest Dermatol. 2011;131:1579–1583. doi: 10.1038/jid.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Airola K, Karonen T, Vaalamo M, Lehti K, Lohi J, Kariniemi AL, et al. Expression of collagenases-1 and -3 and their inhibitors TIMP-1 and -3 correlates with the level of invasion in malignant melanomas. Br J Cancer. 1999;80:733–743. doi: 10.1038/sj.bjc.6690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackburn JS, Rhodes CH, Coon CI, Brinckerhoff CE. RNA interference inhibition of matrix metalloproteinase-1 prevents melanoma metastasis by reducing tumor collagenase activity and angiogenesis. Cancer Res. 2007;67:10849–10858. doi: 10.1158/0008-5472.CAN-07-1791. [DOI] [PubMed] [Google Scholar]
- 38.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackburn JS, Liu I, Coon CI, Brinckerhoff CE. A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene. 2009;28:4237–4248. doi: 10.1038/onc.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantwell-Dorris ER, O'Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10:385–394. doi: 10.1158/1535-7163.MCT-10-0799. [DOI] [PubMed] [Google Scholar]
- 41.Shields JM, Thomas NE, Cregger M, Berger AJ, Leslie M, Torrice C, et al. Lack of extracellular signal-regulated kinase mitogen-activated protein kinase signaling shows a new type of melanoma. Cancer Res. 2007;67:1502–1512. doi: 10.1158/0008-5472.CAN-06-3311. [DOI] [PubMed] [Google Scholar]
- 42.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 44.Uribe P, Andrade L, Gonzalez S. Lack of association between BRAF mutation and MAPK ERK activation in melanocytic nevi. J Invest Dermatol. 2006;126:161–166. doi: 10.1038/sj.jid.5700011. [DOI] [PubMed] [Google Scholar]
- 45.Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kahari VM, Pyrhonen S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res. 2005;11:5158–5166. doi: 10.1158/1078-0432.CCR-04-2478. [DOI] [PubMed] [Google Scholar]
- 46.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.