Abstract
Although the use of renal replacement therapy (RRT) to support critically ill patients with AKI has become routine, many of the fundamental questions regarding optimal management of RRT remain. This review summarizes current evidence regarding the timing of initiation of renal replacement therapy, the selection of the specific modality of RRT, and prescription of intensity of therapy. While absolute indications for initiating RRT such as hyperkalemia and overt uremic symptoms are well recognized, the optimal timing of therapy in patients without these indications continues to be the subject of debate. There does not appear to be a difference in either mortality or recovery of kidney function associated with the various modalities of RRT. Finally, providing higher doses of RRT is not associated with improved clinical outcomes.
Keywords: Acute Kidney Injury, Hemodialysis, Hemofiltration, Continuous Renal Replacement Therapy, Critical Illness
Acute kidney injury (AKI) is one of the most common serious complications in critically ill patients. Severe AKI occurs in more than one of every twenty patients requiring intensive care unit care (1), and has been associated with mortality rates ranging from 50% to more than 70% (1–4). In the absence of any effective pharmacologic therapies for AKI, its management remains supportive, focused on optimizing fluid balance, maintaining nutrition, preventing or treating electrolyte and acid-base disturbances, adjusting the dosing of medications that are excreted by the kidney, and avoiding secondary hemodynamic and nephrotoxic renal injury. Although these conservative therapies provide the initial underpinning of AKI management, renal replacement therapy using one or more of the multiple modalities of dialysis and hemofiltration is often required. This review will summarize current evidence regarding the timing of initiation of renal replacement therapy, the selection of the specific modality of renal replacement therapy (RRT), and prescription of intensity of therapy.
Timing of initiation of renal replacement therapy
The question of when to initiate RRT in patients with AKI has been debated nearly as long as hemodialysis has been part of the armamentarium of clinical medicine. In 1960, in their seminal article on prophylactic dialysis in acute kidney injury, Paul Teschan and colleagues wrote:
“While there is increasing recognition of the value of earlier dialysis, the published consensus, and the practice in many centers at present, is still to apply dialysis to relatively ill rather than to relatively healthy patients. This is implied by the usually quoted indications for dialysis, namely, definite or progressive clinical uremic illness and/or progressive potassium intoxication, occurring despite careful suppressive therapy.” (5)
Emergent initiation of RRT in AKI in response to these standard indications - volume overload unresponsive to diuretic therapy; electrolyte and acid-base disturbances refractory to medical management, particularly severe hyperkalemia and metabolic acidosis; and overt uremic manifestations, such as pericarditis and encephalopathy – can be characterized as “rescue” therapy, in which initiation of treatment forestalls imminent death. More commonly, however, current practice is to initiate RRT preemptively, well before the development of these advanced complications, in patients with severe AKI in whom imminent recovery of kidney function is unlikely. The conundrum regarding the optimal timing for initiation of renal support in AKI derives, in large part, from uncertainty in predicting if and when kidney function will recover. In the absence of robust predictive markers, initiating therapy earlier increases the probability of exposing patients who might uneventfully recover kidney function if managed conservatively to the potential risks of RRT.
This tension between benefits of earlier and risks of unnecessary treatment has been central to the longstanding debate over the timing of therapy. In 1960, Teschan and colleagues opined:
“We would urge that dialyses applied to patients who might otherwise survive should not under any circumstances be considered to be superfluous. Rather, the judgment of whether to undertake dialysis should also be made in view of the possible risks of not employing this procedure. We would question both the wisdom and the safety of subjecting patients to several days of avoidable nausea, vomiting, drowsiness and thirst, which not only implies significant discomfort to the patient but may also impose considerable risk of aspiration, pneumonia and other unexpected ‘complications’” (5)
One of the primary factors that has changed over the ensuing half-century is our concept of what constitutes early as opposed to late therapy. At the time that Teschan and colleagues were pioneering the use of prophylactic dialysis, conventional management was to wait until severe uremic symptoms were present (5, 6). In contrast, as the technology for RRT has become safer and treatment has become more routine, practices that in prior decades would have been considered “early” therapy are now considered to represent “late” initiation of RRT. Despite increased safety, RRT remains associated with numerous risks – including catheter-related complications from insertion and infection; mechanical complications associated with the extracorporeal circuit, including the risk of severe blood loss; electrolyte disturbances and hemodynamic compromise associated with fluid and electrolyte shifts during treatment; and activation of humoral and cellular mediators by exposure to the extracorporeal circuit (7–9). Exposure of blood to bioincompatible surfaces in the extracorporeal circuit and recurrent episodes of dialysis-associated hypotension have been postulated to delay recovery of kidney function (7, 9–12). In addition, consideration must also be given to the financial implications of earlier initiation of treatment.
Although numerous studies over more than a half century have attempted to resolve the issue of optimal timing, the level of evidence guiding current practice remains weak, derived primarily from retrospective and observational cohort studies and small, underpowered prospective trials. A series of observational studies published in the 1960s and early 1970s compared outcomes of patients with AKI who were treated in the years immediately preceding and following adoption of strategies utilizing prophylactic initiation of dialysis (13–15). In each series, during the earlier periods, when dialysis was initiated “late” (blood urea nitrogen [BUN] >163–200 mg/dL), mortality rates were higher than subsequently, when dialysis was started earlier (BUN <93–150 mg/dL) (13–15). Subsequently, two small prospective studies compared more intensive strategies of dialysis management, with earlier initiation of therapy, to more “conventional” management (16, 17). In the first, 18 patients with post-traumatic AKI were assigned to either a more intensive regimen that maintained the pre-dialysis BUN <70 mg/dL and serum creatinine <5 mg/dL or to a less intensive strategy in which dialysis was not performed until the BUN approached 150 mg/dL, the creatinine reached 10 mg/dL or other indications for dialysis were present (16). Five of eight patients (64%) assigned to the more intensive regimen survived as compared to two of 10 patients (20%) assigned to the less intensive strategy (p=0.14). Major complications, including hemorrhage and sepsis were also less frequent with earlier and more intensive dialysis. In the subsequent study, 34 patients with severe AKI were randomized in a paired fashion when their serum creatinine reached 8 mg/dL to either an intensive regimen, designed to maintain the pre-dialysis BUN <60 mg/dL and serum creatinine <5 mg/dL or to delayed and less intensive regimen, in which the BUN was allowed to reach 100 mg/dL and the serum creatinine 9 mg/dL (17). The mean time from onset of AKI to initiation of dialysis was two days shorter (5±2 days versus 7±3 days) in the more intensive regimen. Mortality was slightly higher with the earlier and more intensive therapy (58.8% versus 47.1%), however this difference did not reach statistical significance (p=0.73). On the basis of these data, conventional teaching was that, in the absence of specific metabolic indications or symptoms, dialysis should be initiated when the BUN approached a level of approximately 100 mg/dL, but that no benefit was associated with earlier initiation of therapy.
The topic of timing of therapy then remained quiescent until the late 1990s, when Gettings and colleagues published a retrospective analysis of the timing of initiation of continuous renal replacement therapy (CRRT) in 100 consecutive patients with post-traumatic AKI (18). They observed that 39.0% of patients who were started on CRRT when their BUN was < 60 mg/dL (mean BUN 42.6±12.9 mg/dL) survived as compared to 20.3% of patients in whom CRRT was not begun until their BUN was > 60 mg/dL (mean BUN 94.5±28.3 mg/dL; p=0.041). Although this was not a randomized study, demographic factors and severity of illness at admission were comparable in the two groups, although rhabdomyolysis was more common in the early initiation group and multisystem organ failure in the late initiation group.
In the past decade, there have been multiple additional studies comparing “early” and “late” initiation of dialysis (19–32). The majority have been retrospective cohort studies or prospective observational studies and have used a wide variety of definitions for “early” and “late” dialysis, with only two small randomized controlled trials. In the first of these, Bouman and colleagues randomized 106 critically ill patients with AKI to early high-volume continuous venovenous hemodiafiltration (CVVHDF) (n=35), early low-volume CVVHDF (n=35) and late low-volume CVVHDF (n=36) (19). Hemodiafiltration was initiated in the two early-therapy groups within 12 hours of meeting study inclusion criteria, while in the late group it was withheld until metabolic or clinical criteria were met. There were no significant differences in survival among the three groups. Of note, of the 36 patients randomized to late-therapy six were never treated with RRT; four recovered kidney function and two died prior to meeting the criteria for late initiation of therapy. In the other randomized trial, 36 patients with AKI following coronary artery bypass surgery were randomized when their urine output was ≤30 mL/hour and their serum creatinine had increased by ≥0.5 mg/dL per day (20). In the early group, dialysis was started when the urine output remained <30 mL/hour for three consecutive hours while in the late group it was not started until the urine output fell to <20 mL/hour for at least 2 hours. Only 28 (14 in each group) actually received protocol treatment; the remaining 8 patients did not fulfill the criteria for initiation of therapy. Of the patients treated per-protocol, 12 patients in the early group (86%) were alive at two weeks as compared to only 2 patients (14%) in the late group (p<0.01).
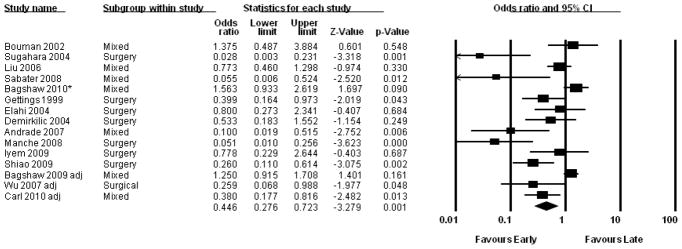
A recent systematic review and meta-analysis of studies comparing early and late initiation of renal support published between 1985 and July 2010 by Karvellas and colleagues included 15 unique studies, including the two randomized controlled trials described above (33) (Figure 1). They calculated an odds ratio for 28-day mortality of 0.45 (95% CI: 0.28 – 0.72) associated with early initiation of renal support, but noted that the methodologic quality of the included studies was low. In evaluating both the primary studies, as well as the pooled conclusions of this meta-analysis, it is important to recognize a critical methodologic flaw affecting the majority of studies evaluating timing of RRT. The vast majority of these studies restricted their analyses to patients who received RRT. However, patients who do not receive early RRT can follow several paths: in addition to “late” initiation of RRT, patients may die prior to initiation of dialysis or may survive and recover kidney function without ever requiring renal support. Limiting the comparison to patients treated “early” or “late” neglects the large number of patients who meet criteria for “early” treatment but are never dialyzed. Thus, rather than “early” versus “late” the question would be more appropriate framed as “early” versus “not-early” initiation of therapy.
Figure 1.
Forest plot of pooled odds ratios for mortality of studies comparing early to late initiation of renal replacement therapy published between 1985 and July 2010. Using a random effects model the calculated pooled odds ratio is 0.45 (95% confidence interval [CI]: 0.28 – 0.72). Reproduced from: Karvellas CJ, Farhat MR, Sajjad I, et al.: A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 15: R72, 2011, with permission.
The issue of severity of volume overload as an indication for initiation of renal support has garnered considerable attention and deserves special mention. Multiple studies have demonstrated that the severity of volume overload at initiation of RRT is a strong predictor of mortality (34–37). For example, in a pediatric cohort of CRRT patients, Sutherland and colleagues observed an increase in mortality from 29.4% in patients whose fluid gain was less than 10% of pre-morbid body weight as opposed to 65.6% in patients with ≥20% fluid overload at initiation of therapy (37). After adjusting for comorbidities, the presence of ≥20% fluid overload was associated with an odds ratio of dying of 8.5 (95% CI: 2.8 – 25.7). Similarly, Bouchard and colleagues observed an adjusted odds ratio for death of 2.1 (95% CI: 1.3 – 3.4) associated with the presence of >10% fluid overload at initiation of RRT in a cohort of 396 critically ill adult patients (36). This data needs to be interpreted with caution, as association does not imply causality. It is likely that many patients with more severe fluid overload required more aggressive volume resuscitation, potentially suggesting greater severity of their underlying critical illness. Although these analyses adjusted for severity of illness, residual confounding is a concern. While these data provide a strong caution regarding overly aggressive volume administration, the hypothesis that earlier initiation of renal support to prevent or reverse volume overload still needs to be tested in prospective clinical trials.
Modality of Renal Replacement Therapy
Over the past three decades, the use of various forms of continuous and prolonged intermittent RRT (PIRRT) in the management of critically ill patients with AKI has increased dramatically. These modalities are characterized by a “go slow” approach, prolonging the daily duration of therapy while reducing the rate of solute clearance and net ultrafiltration, based on the rationale that slower, gentler treatment will be better tolerated in hemodynamically compromised patients. Whether this approach is associated with better clinical outcomes, including improved survival and recovery of kidney function, remains a subject of debate.
Comparing outcomes between modalities is complicated. Patients treated with continuous or extended duration therapy are more likely to have greater severity of illness and be hemodynamically unstable. Comparing outcomes between CRRT or PIRRT and conventional intermittent hemodialysis in observational cohorts is therefore subject to selection bias. Not unexpectedly, observational studies have generally found higher unadjusted mortality when comparing CRRT to conventional IHD (38–44). Although statistical compensation for the inherent differences in patient characteristics can be provided by adjusting for differences in demographics, chronic comorbidities and severity of illness using multivariate and propensity score-adjusted analyses, such analyses have yielded varying conclusions ranging from improved survival (42), to no difference in outcome (39), to increased mortality (44) associated with CRRT.
Several randomized controlled trials comparing intermittent to continuous RRT have been performed (45–50), although many of these trials have been hampered by issues of patient selection and protocol adherence, excluding patients or having them cross between treatment arms because of hemodynamic instability. The largest of these trials, the Hemodiafe study, enrolled 360 patients across 21 ICUs in France (49). Patients were well matched with regard to severity of illness, with more than 85% of patients requiring vasopressor support and more than 95% being ventilator-dependent. Only 6 of the 184 patients (3%) randomized to intermittent therapy needed to cross-over to continuous therapy, although 31 of the 175 patients (18%) randomized to CRRT crossed over; 14 (8%) per-protocol to allow transfer out of the ICU and 17 (10%) predominantly because of bleeding complications associated with anticoagulation or difficulty maintaining circuit patency. No difference in survival at 2, 60 or 90 days (60-day survival 31.5% with IHD vs. 32.6% with CRRT, p=0.98) or recovery of kidney function was observed between groups. It should be noted, however, that the median treatment duration for each intermittent hemodialysis session was 5.2 hours, significantly longer than is typical in clinical practice.
Three systematic reviews and meta-analyses of modality of renal support in AKI have been published in the last five years, all of which found no differences in mortality or recovery of kidney function across modalities (51–53) (Figure 2). Analyses have suggested, however, that the cost of CRRT is higher than that of intermittent therapy (52) and that continuous therapy is more effective at attaining negative fluid balance (36).
Figure 2.
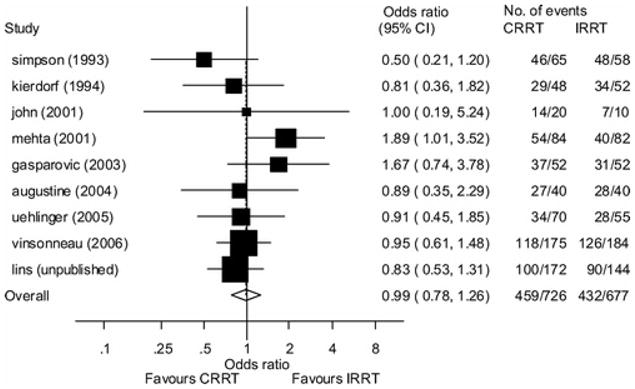
Forrest plot of pooled odds ratios for mortality from nine randomized trials comparing intermittent renal replacement therapy (IRRT) to continuous renal replacement therapy (CRRT). Using a random effects model the calculated pooled odds ratio is 0.99 (95% confidence interval [CI]: 0.78–1.26). Reproduced from: Bagshaw SM, Berthiaume LR, Delaney A and Bellomo R: Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med 36: 610–617, 2008, with permission
Based on these data, the recent Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for Acute Kidney Injury recommended that continuous and intermittent modalities of RRT be used as complementary therapies, with the suggestion to use CRRT preferentially for hemodynamically unstable patients (54). In patients with acute brain injury or increased intracranial pressure due to intracranial hemorrhage, fulminant liver failure or other causes, intermittent hemodialysis has been associated with greater decreases in cerebral perfusion than CRRT (55–59)
Only limited comparisons between PIRRT and either intermittent or continuous therapy are available. These comparisons have demonstrated similar hemodynamic stability and metabolic control (60–62) and comparable clinical outcomes (63) with prolonged intermittent hemodialysis as compared to CRRT. Peritoneal dialysis (PD) has long been used as a dialytic therapy in AKI; however only few studies have directly compared PD to other modalities of renal support. Although Phu and colleagues found substantially higher mortality associated with PD as compared to continuous venovenous hemofiltration (CVVH) (47% vs. 15%; p=0.005) in a 70-patient single center study, the interpretation of this study must be tempered by issues related to PD technique (use of rigid catheters, locally prepared acetate-buffered dialysate, manual exchanges and an open drainage system) (64). In addition, it is possible that the low-dose anticoagulation used during CVVH had an independent beneficial effect in the large proportion of patients (69%) with falciparum malaria-associated AKI (65). In contrast, Gabriel and colleagues have demonstrated biochemical and patient outcomes with high-volume peritoneal dialysis comparable to those seen with intermittent hemodialysis (66–68).
A final issue related to modality of therapy is the relative benefits of convective (hemofiltration) versus diffusive (hemodialysis) therapies. Convective therapies are generally thought to provide better clearance of solutes with molecular weights greater than 1000 Daltons (69, 70). It has therefore been suggested that convective therapies might provide an added benefit in patients with sepsis-associated AKI through enhanced removal of pro-inflammatory mediators (71). However, the cytokine clearances attainable with even high-volume CVVH are trivial in comparison to endogenous production, and cytokine removal by hemofiltration is non-selective and, resulting in removal of both pro- and anti-inflammatory mediators (72). In addition, the effects of convective solute flux as the result of internal filtration/back-filtration and protein concentration polarization along the membrane surface when high-flux membranes are employed may minimize the differences in solute clearance between convective and diffusive therapies (73). More importantly, no clinical trials have demonstrated better outcomes with hemofiltration as compared to hemodialysis.
Intensity of Renal Support in Acute Kidney Injury
Just as it has been hypothesized that prevention of severe metabolic derangements by earlier initiation of RRT in AKI might be beneficial, prevention or correction of severe metabolic derangements by providing more intensive RRT has also been proposed. Most studies evaluating the effect of more intensive RRT have quantified the dose of therapy in terms of the clearance of low molecular weight solutes, such as urea. It should be recognized, however, that modeling intensity of RRT based on solely on urea clearance provides an incomplete assessment of the adequacy of therapy, ignoring the clearance of higher molecular weight solutes and, even more importantly, the management of extracellular volume.
The dose of intermittent hemodialysis is dependent on both the intensity of therapy delivered with each individual treatment, usually quantified in terms of urea reduction ratio or the fractional clearance of urea (Kt/Vurea), and the frequency with which the treatments are provided. No prospective studies have evaluated the effect of dose per treatment on outcomes; the single prospective study of intensity of conventional intermittent hemodialysis assessed the effect of increasing the frequency of treatment from every other day to daily, while maintaining a constant dose per treatment (74) (Table 1). While this study reported a marked improvement in mortality with daily hemodialysis sessions (46% with alternate-day therapy vs. 28% with daily dialysis; p=0.01), the delivered Kt/Vurea was substantially lower in both treatment arms (0.94±0.11 in the alternate-day group and 0.92±0.16 in the daily dialysis group) than the target of 1.2 per treatment, potentially accounting for high rates of altered mental status, gastrointestinal bleeding and sepsis in the alternate day arm. Thus, rather than demonstrating a benefit to augmenting an adequate dose of therapy, this study demonstrated that a dose of therapy that is inadequate when delivered every-other day becomes sufficient when delivered on a daily schedule (75). In contradistinction, the Hanover Dialysis Outcome Study, which compared standard (daily) to intensified (more frequent) PIRRT found no differences in survival at either day 14 or day 28 (76).
Table 1.
Studies of Intensity of Renal Replacement Therapy in Acute Kidney Injury
| Study | N | Dose of RRT | Mortality | p-value | ||
|---|---|---|---|---|---|---|
| Less-Intensive Arm | More Intensive Arm | Less-Intensive Arm | More Intensive Arm | |||
| Conventional Intermittent Hemodialysis | ||||||
| Schiffl et al. (74) | 160 | Every-other day | Daily | 46%1 | 28%1 | 0.001 |
| Delivered Kt/V 0.94±0.11 | Delivered Kt/V 0.92±0.16 | |||||
| Prolonged Intermittent Hemodialysis | ||||||
| Faulhaber-Walter et al. (76) | 156 | Daily | 1–2 × per day | 44.4%2 | 38.7%2 | 0.47 |
| Target BUN: 56–70 mg/dL | Target BUN <42 mg/dL | |||||
| Continuous Renal Replacement Therapy | ||||||
| Ronco et al. (79) | 425 | CVVH: 20 ml/kg/hr | CVVH: 35 ml/kg/hr | 59%1 | 57%1 (35 mL/kg/hr) | <0.001 |
| CVVH: 45 ml/kg/hr | 58%1 (45 ml/kg/hr) | |||||
| Bouman et al. (19) | 106 | CVVH: 24–36 L/day | CVVH: 72–96 L/day | 25.7%2 | 28.2%2 | 0.80 |
| Saudan et al. (80) | 206 | CVVH | CVVHDF | 39%2 | 59%2 | 0.03 |
| QUF: 25±5 ml/kg/hr | QUF: 24±6 ml/kg/hr | |||||
| QD: 18±5 ml/kg/hr | ||||||
| Tolwani et al. (81) | 200 | CVVHDF: 20 ml/kg/hr | CVVHDF: 35 ml/kg/hr | 56%3 | 49%3 | 0.23 |
| Bellomo et al. (82) | 1,508 | CVVHDF: 25 ml/kg/hr | CVVHDF: 40 ml/kg/hr | 44.7%5 | 44.7%5 | 0.99 |
| Combined Modalities | ||||||
| Palevsky et al. (8) | 1,124 | IHD: 3x per week | IHD: 6x per week | 51.5%4 | 53.6%4 | 0.47 |
| Delivered Kt/V 1.32±0.37 | Delivered Kt/V 1.31±0.33 | |||||
| PIRRT: 3x per week | PIRRT: 6x per week | |||||
| CVVHDF: 20 ml/kg/hr | CVVHDF: 35 ml/kg/hr | |||||
mortality 15-days after discontinuation of study therapy;
28-day mortality;
30-day mortality;
60-day mortality;
90-day mortality;
RRT: renal replacement therapy; CVVH: continuous venovenous hemofiltration; CVVHDF: continuous venovenous hemodiafiltration; QUF: ultrafiltration rate; QD: dialysate flow rate; IHD: intermittent hemodialysis; PIRRT: prolonged intermittent renal replacement therapy
During continuous therapy, there is equilibration of low molecular weight solutes between the blood and dialysate and/or ultrafiltrate (77), although the degree of equilibration may be reduced by pre-filter administration of replacement fluids or by membrane fouling caused by clotting and by protein concentration polarization (78). The dose of CRRT has therefore been quantified based on effluent flow rates (the sum of the ultrafiltrate and dialysate) normalized to body weight. In a seminal study of 425 critically ill patients randomized to effluent flow rates of 20, 35 or 45 mL/kg per hour, Ronco and colleagues observed an increase in survival 15 days after discontinuation of CRRT from 41% in the lowest dose group to 57% and 58%, respectively, in the two higher dose groups (p<0.001) (79). However, subsequent small studies yielded conflicting results (19, 80, 81) and a definitive multi-center randomized controlled trial found no benefit to higher doses of CVVHDF (82). In this study, the Randomized Evaluation of Normal Versus Augmented Level (RENAL) Replacement Therapy study, 1,508 patients in 35 ICUs in Australia and New Zealand were randomly assigned to two doses (25 or 40 mL/kg per hour) of CVVHDF during ICU care. The mean duration of study therapy and overall duration of RRT were 6.3±8.7 days and 13.0±20.8 days, respectively, in the higher-intensity arm and 5.9±7.7 days and 11.5±18.0 days, respectively, in the less intensive arm, reflecting the use of non-protocol hemodialysis after ICU discharge. Survival to 90 days, was 55.3%, in both treatment arms (p=0.99).
In contrast to the studies that compared lower and higher doses of individual modalities of RRT, the Veterans Administration/National Institutes of health Acute Renal Failure Trial Network (ATN) study randomized 1,124 critically ill patients randomized patients to lower- or higher-intensity RRT using a strategy that allowed patients to shift between modalities as hemodynamic status changed over time (8). In the intensive arm, CVVHDF was provided with a total effluent flow of 35 mL/kg per hour and conventional and prolonged intermittent hemodialysis were provided 6-times per week (daily, except Sunday) with a target Kt/Vurea of 1.2–1.4 per treatment; in the less intensive arm, the dose of CVVHDF was 20 mL/kg per hour and conventional and prolonged intermittent hemodialysis were provided three-times per week schedule (every-other day, except Sunday), with the same target Kt/Vurea. Sixty-day all-cause mortality was 53.6% in the more-intensive arm as compared to 51.5% in the less-intensive arm (p=0.47).
Two systematic reviews have performed meta-analyses of the pooled the results from these trials (83, 84). Both found no significant benefit associated with more intensive RRT, although both observed significant statistical heterogeneity across the studies, associated, in one analysis (83), with year of publication and study quality as assessed by Jadad score.
While the published literature does not support the concept that more RRT is better, the data also suggest that there must be some floor below which mortality will increase, the precise level of which is not known. Based on these data, the KDIGO AKI guidelines recommend delivering an effluent volume of 20–25 mL/kg per hour for CRRT and a Kt/Vurea of 3.9 per week (the equivalent of 1.2–1.4 three-times per week) when using conventional or prolonged intermittent hemodialysis (54). Given the well known discrepancies between prescribed and delivered doses of RRT in the acute setting, prescribing a modestly higher dose of therapy may be necessary in order to actually deliver the desired target doses. In addition, the delivered dose of therapy should be closely monitored to assure that the targeted dose is actually achieved. Finally, it is important to recognize that the delivery of treatment must be individualized, and that higher doses of therapy may be required for extremely hypercatabolic patients or for control of severe hyperkalemia. However, when higher doses of therapy are employed, careful attention must be given to the effects on drug clearance and the potential need for enhanced monitoring of drug levels and modification of drug dosing. In addition, in patients receiving intermittent therapy, increased treatment frequency may be required to optimize volume management, even if additional solute clearance is not required.
Summary
Although the use of RRT to support critically ill patients with AKI has become routine, many of the fundamental questions regarding optimal management of RRT remain. While absolute indications for initiating RRT such as hyperkalemia and overt uremic symptoms are well recognized, the optimal timing of therapy in patients without these indications continues to be the subject of debate. The selection of modality does not appear to have a major impact on mortality or recovery of kidney function. Selection of modality of renal support should therefore be based on local expertise and logistic factors, with the emphasis on assuring that the treatment provided is the safest and most cost efficient for the particular health setting. Finally, reasonable minimal standards for the delivered dose of therapy appear to have been identified; local quality assurance and performance improvement process should be implemented to ensure that these are achieved. The mortality associated with severe AKI remains unacceptably high; however there is little evidence to suggest that this mortality will be substantially altered by improvements in the delivery of renal support. Rather, we must be realistic in our expectations of what dialysis and hemofiltration can accomplish and vigorously pursue other strategies to improve the care of these patients.
Footnotes
Conflict of Interest/Financial Disclosure:
Consultant/Medical Advisory Board: Sanofi-Aventis, Cytopherx Research Support: Spectral Diagnostics
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uchino S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12:538–543. doi: 10.1097/01.ccx.0000247448.94252.5a. [DOI] [PubMed] [Google Scholar]
- 2.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 3.Liano F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl. 1998;66:S16–24. [PubMed] [Google Scholar]
- 4.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155:1505–1511. [PubMed] [Google Scholar]
- 5.Teschan PE, Baxter CR, O’Brien TF, Freyhof JN, Hall WH. Prophylactic hemodialysis in the treatment of acute renal failure. Ann Intern Med. 1960;53:992–1016. doi: 10.7326/0003-4819-53-5-992. [DOI] [PubMed] [Google Scholar]
- 6.Teschan PE. Acute renal failure during the Korean War. Ren Fail. 1992;14:237–239. doi: 10.3109/08860229209106623. [DOI] [PubMed] [Google Scholar]
- 7.Palevsky PM, Baldwin I, Davenport A, Goldstein S, Paganini E. Renal replacement therapy and the kidney: minimizing the impact of renal replacement therapy on recovery of acute renal failure. Curr Opin Crit Care. 2005;11:548–554. doi: 10.1097/01.ccx.0000179936.21895.a3. [DOI] [PubMed] [Google Scholar]
- 8.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conger J. Does hemodialysis delay recovery from acute renal failure? Seminars in Dialysis. 1990;3:146–148. [Google Scholar]
- 10.Schulman G, Fogo A, Gung A, Badr K, Hakim R. Complement activation retards resolution of acute ischemic renal failure in the rat. Kidney Int. 1991;40:1069–1074. doi: 10.1038/ki.1991.316. [DOI] [PubMed] [Google Scholar]
- 11.Himmelfarb J, Ault KA, Holbrook D, Leeber DA, Hakim RM. Intradialytic granulocyte reactive oxygen species production: a prospective, crossover trial. J Am Soc Nephrol. 1993;4:178–186. doi: 10.1681/ASN.V42178. [DOI] [PubMed] [Google Scholar]
- 12.Hakim RM. Clinical implications of hemodialysis membrane biocompatibility. Kidney Int. 1993;44:484–494. doi: 10.1038/ki.1993.272. [DOI] [PubMed] [Google Scholar]
- 13.Parsons FM, Hobson SM, Blagg CR, Mc CB. Optimum time for dialysis in acute reversible renal failure. Description and value of an improved dialyser with large surface area. Lancet. 1961;1:129–134. doi: 10.1016/s0140-6736(61)91309-5. [DOI] [PubMed] [Google Scholar]
- 14.Fischer RP, Griffen WO, Jr, Reiser M, Clark DS. Early dialysis in the treatment of acute renal failure. Surg Gynecol Obstet. 1966;123:1019–1023. [PubMed] [Google Scholar]
- 15.Kleinknecht D, Jungers P, Chanard J, Barbanel C, Ganeval D. Uremic and non-uremic complications in acute renal failure: Evaluation of early and frequent dialysis on prognosis. Kidney Int. 1972;1:190–196. doi: 10.1038/ki.1972.26. [DOI] [PubMed] [Google Scholar]
- 16.Conger JD. A controlled evaluation of prophylactic dialysis in post-traumatic acute renal failure. J Trauma. 1975;15:1056–1063. doi: 10.1097/00005373-197512000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Gillum DM, Dixon BS, Yanover MJ, et al. The role of intensive dialysis in acute renal failure. Clin Nephrol. 1986;25:249–255. [PubMed] [Google Scholar]
- 18.Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med. 1999;25:805–813. doi: 10.1007/s001340050956. [DOI] [PubMed] [Google Scholar]
- 19.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30:2205–2211. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Sugahara S, Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodial Int. 2004;8:320–325. doi: 10.1111/j.1492-7535.2004.80404.x. [DOI] [PubMed] [Google Scholar]
- 21.Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ. Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur J Cardiothorac Surg. 2004;26:1027–1031. doi: 10.1016/j.ejcts.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Demirkilic U, Kuralay E, Yenicesu M, et al. Timing of replacement therapy for acute renal failure after cardiac surgery. J Card Surg. 2004;19:17–20. doi: 10.1111/j.0886-0440.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1:915–919. doi: 10.2215/CJN.01430406. [DOI] [PubMed] [Google Scholar]
- 24.Piccinni P, Dan M, Barbacini S, et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32:80–86. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 25.Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2:739–744. doi: 10.2215/CJN.00680207. [DOI] [PubMed] [Google Scholar]
- 26.Wu VC, Ko WJ, Chang HW, et al. Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: effect on postoperative outcomes. J Am Coll Surg. 2007;205:266–276. doi: 10.1016/j.jamcollsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Manche A, Casha A, Rychter J, Farrugia E, Debono M. Early dialysis in acute kidney injury after cardiac surgery. Interact Cardiovasc Thorac Surg. 2008;7:829–832. doi: 10.1510/icvts.2008.181909. [DOI] [PubMed] [Google Scholar]
- 28.Sabater J, Perez X, Albertos R, Gutierrez D, Labad X. Acute renal failure in septic shock. Should we consider different continuous renal replacement therapies on each RIFLE score stage? Intensive Care Med. 2009 [Google Scholar]
- 29.Bagshaw SM, Uchino S, Bellomo R, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24:129–140. doi: 10.1016/j.jcrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Iyem H, Tavli M, Akcicek F, Buket S. Importance of early dialysis for acute renal failure after an open-heart surgery. Hemodial Int. 2009;13:55–61. doi: 10.1111/j.1542-4758.2009.00347.x. [DOI] [PubMed] [Google Scholar]
- 31.Shiao CC, Wu VC, Li WY, et al. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care. 2009;13:R171. doi: 10.1186/cc8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carl DE, Grossman C, Behnke M, Sessler CN, Gehr TW. Effect of timing of dialysis on mortality in critically ill, septic patients with acute renal failure. Hemodial Int. 2010;14:11–17. doi: 10.1111/j.1542-4758.2009.00407.x. [DOI] [PubMed] [Google Scholar]
- 33.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 36.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 38.van Bommel E, Bouvy ND, So KL, et al. Acute dialytic support for the critically ill: intermittent hemodialysis versus continuous arteriovenous hemodiafiltration. Am J Nephrol. 1995;15:192–200. doi: 10.1159/000168832. [DOI] [PubMed] [Google Scholar]
- 39.Swartz RD, Messana JM, Orzol S, Port FK. Comparing continuous hemofiltration with hemodialysis in patients with severe acute renal failure. Am J Kidney Dis. 1999;34:424–432. doi: 10.1016/s0272-6386(99)70068-5. [DOI] [PubMed] [Google Scholar]
- 40.Guerin C, Girard R, Selli JM, Ayzac L. Intermittent versus continuous renal replacement therapy for acute renal failure in intensive care units: results from a multicenter prospective epidemiological survey. Intensive Care Med. 2002;28:1411–1418. doi: 10.1007/s00134-002-1433-0. [DOI] [PubMed] [Google Scholar]
- 41.Manns B, Doig CJ, Lee H, et al. Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med. 2003;31:449–455. doi: 10.1097/01.CCM.0000045182.90302.B3. [DOI] [PubMed] [Google Scholar]
- 42.Swartz RD, Bustami RT, Daley JM, Gillespie BW, Port FK. Estimating the impact of renal replacement therapy choice on outcome in severe acute renal failure. Clin Nephrol. 2005;63:335–345. doi: 10.5414/cnp63335. [DOI] [PubMed] [Google Scholar]
- 43.Jacka MJ, Ivancinova X, Gibney RT. Continuous renal replacement therapy improves renal recovery from acute renal failure. Can J Anaesth. 2005;52:327–332. doi: 10.1007/BF03016071. [DOI] [PubMed] [Google Scholar]
- 44.Cho KC, Himmelfarb J, Paganini E, et al. Survival by dialysis modality in critically ill patients with acute kidney injury. J Am Soc Nephrol. 2006;17:3132–3138. doi: 10.1681/ASN.2006030268. [DOI] [PubMed] [Google Scholar]
- 45.Mehta RL, McDonald B, Gabbai FB, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60:1154–1163. doi: 10.1046/j.1523-1755.2001.0600031154.x. [DOI] [PubMed] [Google Scholar]
- 46.Gasparovic V, Filipovic-Grcic I, Merkler M, Pisl Z. Continuous renal replacement therapy (CRRT) or intermittent hemodialysis (IHD)--what is the procedure of choice in critically ill patients? Ren Fail. 2003;25:855–862. doi: 10.1081/jdi-120024300. [DOI] [PubMed] [Google Scholar]
- 47.Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis. 2004;44:1000–1007. doi: 10.1053/j.ajkd.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Uehlinger DE, Jakob SM, Ferrari P, et al. Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrol Dial Transplant. 2005;20:1630–1637. doi: 10.1093/ndt/gfh880. [DOI] [PubMed] [Google Scholar]
- 49.Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 50.Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24:512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 51.Rabindranath K, Adams J, Macleod AM, Muirhead N. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev. 2007:CD003773. doi: 10.1002/14651858.CD003773.pub3. [DOI] [PubMed] [Google Scholar]
- 52.Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 53.Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med. 2008;36:610–617. doi: 10.1097/01.CCM.0B013E3181611F552. [DOI] [PubMed] [Google Scholar]
- 54.Group KDIGOKAKIW. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 55.Davenport A, Will EJ, Davison AM. Continuous vs. intermittent forms of haemofiltration and/or dialysis in the management of acute renal failure in patients with defective cerebral autoregulation at risk of cerebral oedema. Contrib Nephrol. 1991;93:225–233. doi: 10.1159/000420225. [DOI] [PubMed] [Google Scholar]
- 56.Davenport A, Will EJ, Davison AM. Early changes in intracranial pressure during haemofiltration treatment in patients with grade 4 hepatic encephalopathy and acute oliguric renal failure. Nephrol Dial Transplant. 1990;5:192–198. doi: 10.1093/ndt/5.3.192. [DOI] [PubMed] [Google Scholar]
- 57.Lin CM, Lin JW, Tsai JT, et al. Intracranial pressure fluctuation during hemodialysis in renal failure patients with intracranial hemorrhage. Acta Neurochir Suppl. 2008;101:141–144. doi: 10.1007/978-3-211-78205-7_24. [DOI] [PubMed] [Google Scholar]
- 58.Davenport A. Continuous renal replacement therapies in patients with liver disease. Semin Dial. 2009;22:169–172. doi: 10.1111/j.1525-139X.2008.00539.x. [DOI] [PubMed] [Google Scholar]
- 59.Davenport A. Continuous renal replacement therapies in patients with acute neurological injury. Semin Dial. 2009;22:165–168. doi: 10.1111/j.1525-139X.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- 60.Kielstein JT, Kretschmer U, Ernst T, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis. 2004;43:342–349. doi: 10.1053/j.ajkd.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 61.Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: a two-year single center report. Int J Artif Organs. 2004;27:371–379. doi: 10.1177/039139880402700505. [DOI] [PubMed] [Google Scholar]
- 62.Fieghen HE, Friedrich JO, Burns KE, et al. The hemodynamic tolerability and feasibility of sustained low efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 11:32. doi: 10.1186/1471-2369-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abe M, Okada K, Suzuki M, et al. Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artif Organs. 34:331–338. doi: 10.1111/j.1525-1594.2009.00861.x. [DOI] [PubMed] [Google Scholar]
- 64.Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347:895–902. doi: 10.1056/NEJMoa020074. [DOI] [PubMed] [Google Scholar]
- 65.Leitgeb AM, Blomqvist K, Cho-Ngwa F, et al. Low anticoagulant heparin disrupts Plasmodium falciparum rosettes in fresh clinical isolates. Am J Trop Med Hyg. 84:390–396. doi: 10.4269/ajtmh.2011.10-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabriel DP, Nascimento GV, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis for acute renal failure. Perit Dial Int. 2007;27:277–282. [PubMed] [Google Scholar]
- 67.Gabriel DP, Caramori JT, Martin LC, Barretti P, Balbi AL. Continuous peritoneal dialysis compared with daily hemodialysis in patients with acute kidney injury. Perit Dial Int. 2009;29 (Suppl 2):S62–71. [PubMed] [Google Scholar]
- 68.Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int Suppl. 2008:S87–93. doi: 10.1038/sj.ki.5002608. [DOI] [PubMed] [Google Scholar]
- 69.Brunet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis. 1999;34:486–492. doi: 10.1016/s0272-6386(99)70076-4. [DOI] [PubMed] [Google Scholar]
- 70.Troyanov S, Cardinal J, Geadah D, et al. Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003;18:961–966. doi: 10.1093/ndt/gfg055. [DOI] [PubMed] [Google Scholar]
- 71.Ronco C, Tetta C, Mariano F, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003;27:792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 72.Sieberth HG, Kierdorf HP. Is cytokine removal by continuous hemofiltration feasible? Kidney Int Suppl. 1999:S79–83. [PubMed] [Google Scholar]
- 73.Messer J, Mulcahy B, Fissell WH. Middle-molecule clearance in CRRT: in vitro convection, diffusion and dialyzer area. ASAIO J. 2009;55:224–226. doi: 10.1097/MAT.0b013e318194b26c. [DOI] [PubMed] [Google Scholar]
- 74.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–310. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 75.Bonventre JV. Daily hemodialysis--will treatment each day improve the outcome in patients with acute renal failure? N Engl J Med. 2002;346:362–364. doi: 10.1056/NEJM200201313460512. [DOI] [PubMed] [Google Scholar]
- 76.Faulhaber-Walter R, Hafer C, Jahr N, et al. The Hannover Dialysis Outcome study: comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant. 2009;24:2179–2186. doi: 10.1093/ndt/gfp035. [DOI] [PubMed] [Google Scholar]
- 77.Vijayan A, Palevsky PM. Dosing of renal replacement therapy in acute kidney injury. Am J Kidney Dis. 59:569–576. doi: 10.1053/j.ajkd.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macedo E, Claure-Del Granado R, Mehta RL. Effluent volume and dialysis dose in CRRT: time for reappraisal. Nat Rev Nephrol. 8:57–60. doi: 10.1038/nrneph.2011.172. [DOI] [PubMed] [Google Scholar]
- 79.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 80.Saudan P, Niederberger M, De Seigneux S, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 81.Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19:1233–1238. doi: 10.1681/ASN.2007111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 83.Jun M, Heerspink HJ, Ninomiya T, et al. Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 5:956–963. doi: 10.2215/CJN.09111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Wert R, Friedrich JO, Scales DC, Wald R, Adhikari NK. High-dose renal replacement therapy for acute kidney injury: Systematic review and meta-analysis. Crit Care Med. 38:1360–1369. doi: 10.1097/CCM.0b013e3181d9d912. [DOI] [PubMed] [Google Scholar]