Abstract
Presynaptic active zones are synaptic vesicle release sites that playessential roles in the function and pathology of mammalian neuromuscular junctions (NMJs). The molecular mechanisms of active zone organization utilize presynaptic voltage-dependent calcium channels (VDCCs) in NMJs as scaffolding proteins. VDCCs interact extracellularly with the muscle-derived synapse organizer, laminin β2, and interact intracellularly with active zone-specific proteins, such as Bassoon, CAST/Erc2/ELKS2alpha, ELKS, Piccolo, and RIMs. These molecular mechanisms are supported by studies in P/Q- and N-type VDCCs double-knockout mice, and they are consistent with the pathological conditions of Lambert-Eaton myasthenic syndrome and Pierson syndrome, which are caused by autoantibodies against VDCCs or by a laminin β2 mutation. During normal postnatal maturation, NMJs maintain the density of active zones, while NMJs triple their size. However, active zones become impaired during aging. Propitiously, muscle exercise ameliorates the active zone impairment in aged NMJs, which suggests the potential for therapeutic strategies.
Keywords: Bassoon, calcium channel, exercise, laminin, motor neuron, synapse
Introduction
Synaptic vesicles at adult mammalian NMJs accumulate near the electron-dense material of presynaptic active zones (Fig. 1). Synaptic transmission is initiated by Ca2+ influx through presynaptic P/Q-type VDCCs1, 2 and synaptic vesicle fusion at the active zones.3 The cytosolic regions of these active zones form large protein complexes, which are detected as electron-dense material extending from the presynaptic membrane toward the cytosolic region on transmission electron micrographs.4–6 The electron-dense materials align with postsynaptic junctional folds in adult NMJs.7–9 These electron dense materials of active zones distribute in a discrete pattern within one presynaptic terminal in mammalian NMJs,10, 6 similar to the salt crystals on a pretzel. The discrete distribution pattern of active zones is consistent with the distribution patterns of active zone-specific proteins that are detected using immunohistochemistry11, 12, 9 and the distribution patterns of intramembranous particles on the P-face of presynaptic membranes that are detected using freeze-fracture electron microscopy.13, 14 This discrete distribution pattern of active zones in mammalian NMJs is similar to Drosophila NMJs,15–17 but different from frog NMJs that contain elongated continuous active zones.18, 5 Furthermore, the discrete distribution pattern of active zones within one mammalian NMJ is similar to large synapses of the central nervous system, the calyx of Held, and large mossy fiber terminals in hippocampus, that show discrete distribution patterns of active zone specific proteins within a single presynaptic terminal.19–21 The presynaptic active zone of central nervous system has been reviewed recently elsewhere.22, 23
Figure 1.
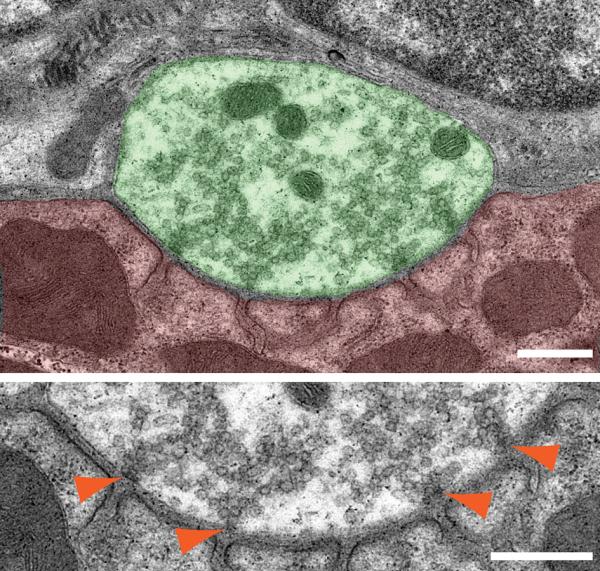
A transmission electron micrograph showing active zones in a mouse NMJ. The top micrograph shows an NMJ profile of diaphragm from a wild-type mouse at postnatal day 85. The motor nerve terminal is colored in green and muscle in red. Synaptic vesicles are preferentially accumulating to four active zones. The bottom micrograph shows a high-magnification view of the presynaptic membrane area of the same NMJ. Orange arrowheads indicate electron-dense materials of the active zones that are accumulating synaptic vesicles and are aligned with postsynaptic junctional folds. Scale bars: 500nm.
Impairments in active zone structure are known in two human neurological diseases, namely, Lambert-Eaton myasthenia syndrome (LEMS) and Pierson syndrome. LEMS patients exhibit a reduced number of NMJ active zones, reduced synaptic transmission, and weakened muscles due to autoantibodies against VDCCs and synaptic proteins.24, 14 Pierson syndrome patients exhibit a loss of NMJ active zones, impairments in synaptic transmission, and denervation of NMJs due to the lack of laminin β2 caused by a genetic mutation.25, 26 These clinical phenotypes of Pierson syndrome are identical to phenotypes of laminin β2 knockout mice,27which suggest that the synapse organizer laminin β2 plays a role in active zone organization in humans. Furthermore, these studies suggest essential roles of active zones and their electron-dense materials in synaptic transmission at NMJs.
Molecular mechanisms of NMJ active zone organization
What is known about the molecularmechanism to organize NMJ active zones? The synapse organizer, laminin β2, is an extracellular matrix proteinsecreted by muscles that specifically concentrates in the synaptic cleft of NMJs.28, 29 Laminin β2 knockout mice demonstrate a loss of active zones, impairment of presynaptic differentiation, and an attenuation of miniature endplate potential frequency and quantal content at NMJs.27, 30 These data suggest a role of laminin β2 in the organization of NMJ active zones. However, a specific receptor for this active zone organizer was previously unknown because the well-known laminin receptors, such as integrins and dystroglycans, do not distinguish between synaptic and non-synaptic laminins (with or without laminin β2).31–33 In a search for a specific receptor, laminin β2 was shown to bind directly and specifically to P/Q- and N-type VDCCs,11 both of which are concentrated at presynaptic terminals inmammalian NMJs.34, 1 These VDCC pore-forming subunits bind to synaptic laminins containing laminin β2 but not to non-synaptic laminins, which contain laminin β1.11 Furthermore, synaptic laminins bind to VDCCs that are highly concentrated at presynaptic terminals in NMJs (e.g., P/Q- and N-types) but not to other VDCCs (e.g., R- and L-type VDCCs (Cav1.2)).11 Therefore, these VDCCs are the first receptors that specifically bind synaptic laminins. The interaction between laminin β2 and VDCC leads to the clustering of VDCCs and presynaptic components in cultured motor neurons,11 which suggests presynaptic differentiation.
In vivo studies provide compelling support that extracellular interactions between laminins and VDCCs participate in the organization of NMJ active zones. The number of active zones decreases in P/Q-type VDCC knockout mice similar to laminin β2 knockout mice.11 In addition, active zone number decreases in wild-type mice when the interaction between VDCC and laminin β2 is blocked by infusing an inhibitor of the interaction.11 Moreover, P/Q- and N-type VDCC double knockout mice exhibit specific defects in the number of active zones, which is twice as severe as the defects in the single knockout mice of P/Q- or N-type VDCCs. However, the double knockout mouse displays normal axon projection, endplate recognition/innervation, and an accumulation of the synaptic vesicle-related proteins at presynaptic terminals without morphologically denervated endplates.12 These results suggest that the termination of axonal outgrowth and the accumulation of synaptic vesicle proteins at NMJs are based on a cell adhesion based signal or a retrograde signal from muscles to axons. However, active zone formation is based on a parallel or different signaling mechanisms utilizing VDCCs as a receptor for the active zone organizer.
The VDCCs that trigger synaptic vesicle release have been estimated to localize at or in close vicinity of active zones.35–39, 2 Adult mammalian NMJs rely on P/Q-type VDCCs for synaptic transmission.34, 40 However, the relative location of P/Q-type VDCCs and active zone proteins has not been analyzed in the immunohistochemistry studies of P/Q-type VDCCs in mammalian NMJs published by others.41–44 Three-dimensional reconstruction of immunohistochemistry signals revealed that P/Q-type VDCCs distribute in a discrete punctate pattern within the NMJs and co-localize preferentially with the active zone protein Bassoon.45 The punctate immunohistochemical-staining patterns of P/Q-type VDCCs and Bassoon were different from the diffuse distribution pattern of the synaptic vesicle-associated protein SV2, which suggests that these co-localization spots are discrete active zones within NMJs. This co-distribution pattern of P/Q-type VDCCs and Bassoon in NMJs is consistent with the direct binding of VDCCs and Bassoon (described below).12
Presynaptic VDCCscontribute to the tethering of thecytomatrix at the active zone (CAZ)45 to the presynaptic membrane and organize the NMJ active zones by directly binding to active zone-specific proteins (Fig. 2). The active zone proteins, Bassoon, CAST/Erc2/ELKS2alpha, ELKS, and RIMs, interact with VDCC β subunits,46, 47, 12, 48 which form a tight complex with the pore-forming α subunits of P/Q- and N-type VDCCs.49 The active zone proteins RIMs and Piccolo also interact with VDCC α subunits.50–52 These CAZ proteins are likely to form a large protein complex53–56 and become the electron-dense materials in the NMJ active zones. This view is further supported by the correlation between the loss of Bassoon immunohistochemical signals and the loss of active zone electron-dense materials in the NMJs of VDCC knockout mice.11, 12
Figure 2.
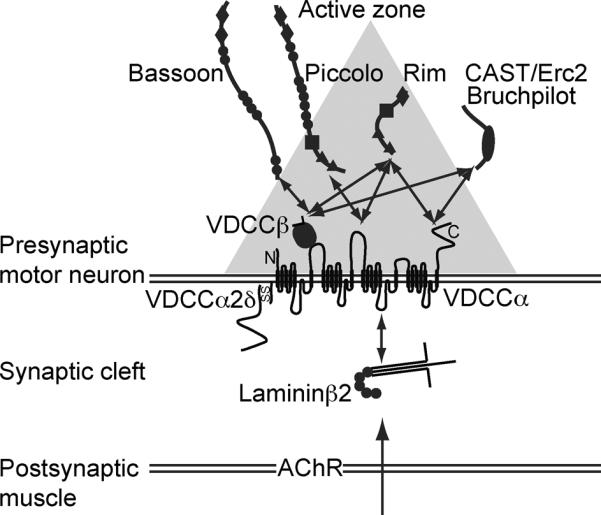
A diagram of the molecular mechanism of active zone organization in mammalian neuromuscular junctions. The diagram depicts interactions between the muscle derived active zone organizer laminin β2, presynaptic voltage-dependent calcium channel (VDCC) subunits, and active zone proteins. The gray triangle depicts the electron dense material of an active zone detected by electron microscopy. Horizontal double lines show pre- and post-synaptic membranes, and the space between these lines represents the synaptic cleft. The sizes of the proteins and the synaptic cleft are not in scale. Two headed arrows represent some of the reported interactions.
In summary, the muscle-derived active zone organizer laminin β2 anchors the VDCC subunits and active zone protein complex (Bassoon, CAST/ELKS/Erc family member, Piccolo, and RIMs) from the extracellular side to organize NMJ active zones. P/Q-type VDCCs function as scaffolding proteins that link the extracellular interaction to cytosolic active zone proteins.12, 48 The three-dimensional alignment of P/Q-type VDCCs and Bassoon in NMJs strongly supports this molecular mechanism to organize the active zones.57 The role of other NMJ organizers for active zone formation is less likely (Agrin, MuSK, and Lrp4) or not clear (collagens and amyloid precursor protein) at this point and awaits further study.58
Bassoon interaction modulates VDCC inactivation
P/Q-type VDCCs and Bassoon bind directly in a co-immunoprecipitation assay12, 45. Consistently, these two proteins preferentially co-localize in NMJs.57 Furthermore, VDCCs and other active zone-specific proteins form protein complexes in vitro and in vivo.12, 47, 48, 51, 52, 56, 57, 59, 60 These data raise the possibility of modifying VDCC function by the interacting active zone proteins. Indeed, Bassoon suppresses the inactivation property of P/Q-type VDCCs when the two proteins are co-expressed and analyzed using patch-clamp electrophysiology.45 A significant depolarizing shift in the voltage dependence of inactivation is observed, which is similar to the effect of active zone protein RIM1 on VDCCs.46, 47 These results suggested that Bassoon suppresses inactivation and prolongs channel opening of P/Q-type VDCCs that are opened by repetitive depolarization. Therefore, Ca2+ influx through P/Q-type VDCCs is augmented by Bassoon and may contribute to the efficiency of NMJ synaptic transmission. An alternative possibility is that Bassoon preferentially increases the open time of VDCCs positioned in active zones, while those VDCCs without Bassoon located outside the active zones would not. This might be a way to enhance calcium entry to the active zones, similar to the role of SNARE proteins.59 These hypotheses are consistent with the Bassoon-dependent increase in Ca2+ influx through L-type VDCCs in the inner hair cells of the auditory system.60 Other modifications of VDCC function by active zone specific proteins have been reported by other groups.46,60,47,61,52,62 The functional modification of VDCCs by Bassoon is likely to occur in addition to the previously reported role of Bassoon in synaptic vesicle tethering to the presynaptic membranes.60,63,64 In summary, active zone-specific proteins play a role in synaptic transmission by binding to presynaptic VDCC complexes, modifying channel function, and tethering synaptic vesicles.
The functional modification of VDCCs by active zone proteins does not seem to contribute to active zone formation. The synapse organizer laminin β2 induces presynaptic differentiation in cultured motor neurons despite P/Q- and N-type VDCC blockade by specific toxins, providing evidence for dispensability of Ca2+ influx into nerve terminals for active zone formation.11 This finding suggests that ion channel activity or the modification of channel function may not be required for the formation of NMJ active zones.
Active zone density
NMJ active zones distribute in a discrete pattern (like the salt crystals on a pretzel) within one presynaptic terminal of mammalian NMJs. Freeze-fracture electron microscopy has revealed active zones based on the intramembranous particles13,14 at a density of 2.4–2.7 active zones per μm2 in mouse and human adult NMJs.14,65,66 The density of active zones that are labeled by Bassoon immunohistochemistry is 2.3 active zones per μm2 in mouse NMJs.9 Importantly, the active zone densities match in two species using two different techniques, which suggests that the active zones of mammalian NMJs are maintained at 2.3–2.7 active zones per μm2.
The density of NMJ active zones is maintained at a constant level during postnatal maturation in mice. The density of active zones that are labeled by Bassoon immunohistochemistry remains constant at 2.3 active zones per μm2 while the synapse size and active zone numbers increase more than 3-fold during the first two months of postnatal maturation in mouse NMJs (Fig. 3).9 What is the significance to maintain a constant density of active zones while NMJs enlarge and change its morphology? A regulated distance between active zones ensures access to synaptic vesicles and Ca2+-buffering systems in the presynaptic terminal,37, 39 which may isolate each active zones as independent units and limit calcium-dependent short-term plasticity.59 It also aids the effective clearance of neurotransmitters in the synaptic cleft by maintaining the local neurotransmitter concentrations under a certain level.67 In addition, a constant active zone density allows postsynaptic membranes to maintain a constant density of neurotransmitter receptors during maturation, which secures the safety factor of NMJ neurotransmission.68 These points support the advantages of maintaining the active zone density for the efficacy of synaptic transmission at NMJs.
Figure 3.
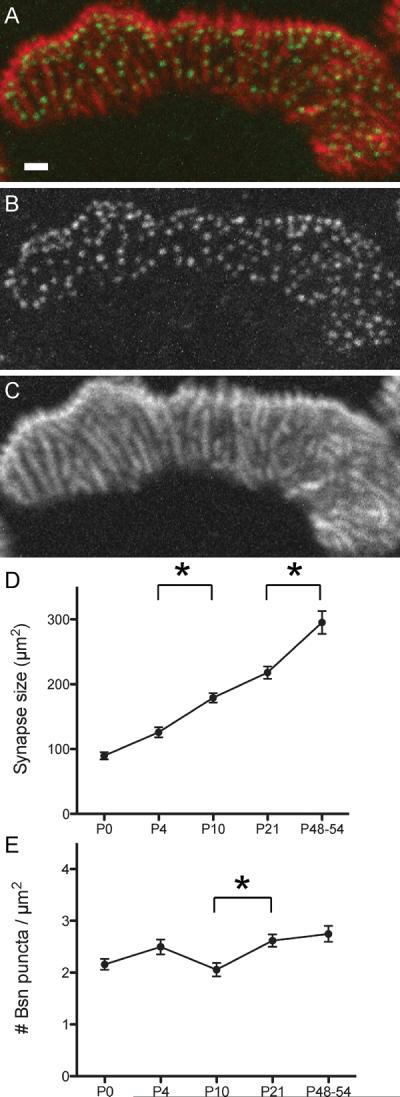
The constant density of active zones in postnatal mouse NMJs. A–C. The active zone-specific protein Bassoon forms puncta that are aligned with postsynaptic junctional folds in adult NMJs. A fluorescent immunohistochemical staining showing Bassoon (green in A, B) and acetylcholine receptors (red in A, C) in sternomastoid muscle of an adult mouse. Many Bassoon puncta are localized on top of the postsynaptic folds, which were visualized as bright lines of α-bungarotoxin staining. D. The synapse size (measured by the α-bungarotoxin stained area) shows about three-fold enlargement during the perinatal to adult stages. E. The density of the Bassoon puncta stays at a relatively constant level (2.3 puncta/μm2) during the postnatal development of NMJs. Asterisks indicates significant differences by one-way ANOVA analysis (P < 0.05). Modified from Ref. 9, © Wiley-Blackwell.
How do active zones maintain regulated distance between themselves in presynaptic terminals? Laminin α4 is an extracellular matrix molecule concentrated in the synaptic cleft of mouse NMJs, but it is excluded under the active zones and from junctional folds.8 Interestingly, laminin α4 knockout mice display normal numbers of NMJ active zones, but the active zones are not placed at the normal position facing the junctional folds.8 Therefore, laminin α4 plays a role in the specification of the active zone location in mouse NMJs. In the cytosolic side, cytoskeletal structures like presynaptic particle web, or cytoskeletal protein complexes that bind to VDCCs and active zone proteins may regulate the distance between the active zones.69,55,56 However, the molecular mechanism that control the number and spacing of active zones is further studied in Drosophila NMJs.70–74,58
Impairment of active zones in aged NMJs and recovery by exercise
Active zone density is maintained during the NMJ maturation; however, active zones are impaired in aged animals. Decreased Bassoon protein levels have been detected in the NMJs of aged mice and rats.9, 57 Would the reduced Bassoon protein level in aged NMJs affect NMJ function? Lack of Bassoon is known to cause an impairment of synaptic vesicle trafficking to presynaptic membranes in central nervous system and sensory neurons.60, 63, 64 As mentioned in the electrophysiological analyses of VDCC-Bassoon interaction, Ca2+ influx is decreased in VDCCs without Bassoon,57 which may weaken synaptic transmission. Furthermore, impaired NMJ synaptic transmission is known in human diseases and knockout mice that have decreased numbers of active zones.30, 2, 26 Taken together, the reduced Bassoon protein level in aged NMJs is likely to weaken synaptic transmission. This view is consistent with the attenuation of synaptic function in aged NMJs compared to young adult NMJs, including stronger synaptic depression during repeated stimulation,75 reduced end-plate potential amplitude (plateau level) after repetitive stimulation,76 and the reduced frequency of miniature end-plate potentials.77, 76 In summary, active zone protein loss may be a part of the molecular mechanism that causes impairment of aged NMJs.
This active zone impairment in aged NMJsis ameliorated by muscularexercise. Exercise intervention is beneficial for the nervous system because exercise upregulates neurotrophins78–81 and improves recovery after nerve injury.82 In addition, an effect of exercise on presynaptic active zones was demonstrated in our recent study.57 Anisometric strength exercise ameliorated the loss of Bassoon in the aged NMJs of two-year-old rats. The mean intensity of Bassoon immunohistochemical signal inthe NMJs ofexercised-aged rats was similar to young adult rats (Fig. 4). Importantly, the improvement in this active zone protein in aged NMJs after exercise is consistent with improvements in NMJ function after endurance training in aged mice revealed using electrophysiology.83 Furthermore, the beneficial effects of exercise in theattenuation or reversal of age-related changes in NMJs have been observed in human and rodents by several groups as well.84, 85 In summary, the exercise-induced preservation of active zone proteins in aged NMJs is likely to exert a positive effect on NMJ function.
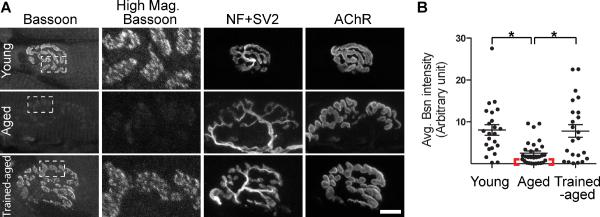
Figure 4.
Exercise ameliorates the decrease in active zone protein level observed in aged NMJs. A. NMJs of aged rats (aged, two-year-old rats) exhibit lower Bassoon signal intensity compared to young adult rats (young, postnatal day 56). Aged rats that underwent isometric strength training of muscles for two months (trained-aged) show higher Bassoon immunohistochemical signal intensity compared to the NMJs of untrained aged rats (aged). Highly magnified Bassoon staining of the area marked by white dotted boxes are shown in the second column from the left (High Mag. Bassoon). Nerve and endplate morphology is shown using anti-neurofilament and -SV2 antibodies (NF+SV2) and α-bungarotoxin for acetylcholine receptors (AChR). Scale bar: 10 μm. B. Average signal intensity of Bassoon is significantly higher in NMJs of young rats and trained-aged rats compared to those of untrained-aged rats (scattered plot shows each NMJ data, and whiskers show mean ± standard error in arbitrary intensity units). The red bracket indicates a subgroup of aged NMJs with a minuscule level of Bassoon signal. Young rats and trained-aged rats do not show significant difference. Modified from Ref. 57.
Summary
Recent findings related to NMJ active zones are summarized in this review, including the molecular mechanisms of formation, density, impairmentsduring aging, and recovery by exercise. The trans-synaptic signaling between the active zone organizer laminin β2 secreted by muscle, its specific receptor presynaptic VDCCs, and active zone-specific proteins play an important role in active zone organization. The interactions of VDCCs and active zone proteins modulate VDCC function, but may cause presynaptic impairment in aged NMJs due to the loss of active zone proteins. However, this loss of active zone proteins in aged NMJsis ameliorated by muscular exercise.
The impaired condition of aged NMJs and pathological conditions of Lambert-Eaton myasthenic and Pierson syndromesreiterate the importance of active zones in NMJ function. Furthermore, impairmentsin active zones may underlie the etiological mechanisms of other neuromuscular diseases. Propitious improvement ofactive zone protein levels in aged NMJs after muscle exercise creates anovel therapyfor themaintenanceand amelioration of NMJ functions under pathological conditions. However, the molecular mechanisms of these beneficial effects are not well known, which makes pharmacological intervention difficult. Patient conditions may not permit effective exercise interventions, due to restricted mobility. Therefore, further investigation to elucidate the organizational mechanismsin NMJ active zones is required for the development ofnovel therapeutic strategies.
Acknowledgements
The author thanks J. Chen, Y. Mori, J. A. Stanford, and T. Numata for their contributions to the findings described in this review, R. Barohn for comments. The work in my laboratory is supported by grants from the Whitehall foundation, NIH-NCRR (RR024214),and NICHD (HD002528).
Footnotes
Conflicts of interest The author declares no conflicts of interest.
References
- 1.Rosato Siri MD, Uchitel OD. Calcium channels coupled to neurotransmitter release at neonatal rat neuromuscular junctions. J Physiol. 1999;514(Pt 2):533–540. doi: 10.1111/j.1469-7793.1999.533ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbano FJ, et al. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci USA. 2003;100:3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heuser JE, et al. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couteaux R, Pecot-Dechavassine M. Synaptic vesicles and pouches at the level of “active zones” of the neuromuscular junction. C R Acad Sci Hebd Seances Acad Sci D. 1970;271:2346–2349. [PubMed] [Google Scholar]
- 5.Harlow ML, et al. The architecture of active zone material at the frog's neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- 6.Nagwaney S, et al. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J Comp Neurol. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirokawa N, Heuser JE. Internal and external differentiations of the postsynaptic membrane at the neuromuscular junction. Journal of Neurocytology. 1982;11:487–510. doi: 10.1007/BF01257990. [DOI] [PubMed] [Google Scholar]
- 8.Patton BL, et al. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4:597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520:434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowley KL, et al. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. Journal of neurophysiology. 2007;98:478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- 11.Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432:580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin beta2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci. 2011;31:512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellisman MH, et al. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976;68:752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukunaga H, et al. Paucity and disorganization of presynaptic membrane active zones in the lambert-eaton myasthenic syndrome. Muscle & Nerve. 1982;5:686–697. [Google Scholar]
- 15.Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. Journal of neurobiology. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meinertzhagen IA, et al. Regulated spacing of synapses and presynaptic active zones at larval neuromuscular junctions in different genotypes of the flies Drosophila and Sarcophaga. J Comp Neurol. 1998;393:482–492. doi: 10.1002/(sici)1096-9861(19980420)393:4<482::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Liu KS, et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- 18.Ko CP. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. J Neurocytol. 1985;14:487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- 19.Satzler K, et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci. 2002;22:10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dondzillo A, et al. Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins Bassoon and Piccolo in the rat calyx of Held before and after the onset of hearing. J Comp Neurol. 2010;518:1008–1029. doi: 10.1002/cne.22260. [DOI] [PubMed] [Google Scholar]
- 21.Bednarek E, Caroni P. beta-Adducin Is Required for Stable Assembly of New Synapses and Improved Memory upon Environmental Enrichment. Neuron. 2011;69:1132–1146. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Schneggenburger R, Han Y, Kochubey O. Ca(2+) channels and transmitter release at the active zone. Cell calcium. 2012;52:199–207. doi: 10.1016/j.ceca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Sudhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert EH, Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci. 1971;183:183–199. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- 25.Zenker M, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Human molecular genetics. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 26.Maselli RA, et al. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. J Med Genet. 2009;46:203–208. doi: 10.1136/jmg.2008.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noakes PG, et al. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 28.Sanes JR, Hall ZW. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. J Cell Biol. 1979;83:357–370. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter DD, et al. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- 30.Knight D, et al. Functional analysis of neurotransmission at beta2-laminin deficient terminals. J Physiol. 2003;546:789–800. doi: 10.1113/jphysiol.2002.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki N, Yokoyama F, Nomizu M. Functional sites in the laminin alpha chains. Connective tissue research. 2005;46:142–152. doi: 10.1080/03008200591008527. [DOI] [PubMed] [Google Scholar]
- 33.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 34.Uchitel OD, et al. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pumplin DW, Reese TS, Llinas R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci USA. 1981;78:7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 37.Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 38.Haydon PG, Henderson E, Stanley EF. Localization of individual calcium channels at the release face of a presynaptic nerve terminal. Neuron. 1994;13:1275–1280. doi: 10.1016/0896-6273(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 39.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 40.Protti DA, et al. Calcium channel blockers and transmitter release at the normal human neuromuscular junction. Neurology. 1996;46:1391–1396. doi: 10.1212/wnl.46.5.1391. [DOI] [PubMed] [Google Scholar]
- 41.Day NC, et al. Differential localization of voltage-dependent calcium channel alpha1 subunits at the human and rat neuromuscular junction. J Neurosci. 1997;17:6226–6235. doi: 10.1523/JNEUROSCI.17-16-06226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Laet A, et al. Immunohistochemical localization of voltage-activated calcium channels in the rat oesophagus. Neurogastroenterology and motility. 2002;14:173–181. doi: 10.1046/j.1365-2982.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 44.Santafé MM, et al. Changes in the neuromuscular synapse induced by an antibody against gangliosides. Annals of Neurology. 2005;57:396–407. doi: 10.1002/ana.20403. [DOI] [PubMed] [Google Scholar]
- 45.Dresbach T, et al. The presynaptic cytomatrix of brain synapses. Cell Mol Life Sci. 2001;58:94–116. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiyonaka S, et al. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat Neurosci. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uriu Y, et al. Rab3-interacting molecule gamma isoforms lacking the Rab3-binding domain induce long lasting currents but block neurotransmitter vesicle anchoring in voltage-dependent P/Q-type Ca2+ channels. J Biol Chem. 2010;285:21750–21767. doi: 10.1074/jbc.M110.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Billings SE, Clarke GL, Nishimune H. ELKS1 and Ca2+ channel subunit beta4 interact and colocalize at cerebellar synapses. Neuroreport. 2012;23:49–54. doi: 10.1097/WNR.0b013e32834e7deb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annual review of cell and developmental biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 50.Shibasaki T, et al. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 51.Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ Signals in Insulin Granule Exocytosis. Diabetes. 2004;53:S59–62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- 52.Kaeser PS, et al. RIM genes differentially contribute to organizing presynaptic release sites. Proc Natl Acad Sci USA. 2012;109:11830–11835. doi: 10.1073/pnas.1209318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takao-Rikitsu E, et al. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J Cell Biol. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, et al. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and Rim converge on the N-terminal domain of Munc13-1. J Neurosci. 2009;29:12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlson SS, Valdez G, Sanes JR. Presynaptic calcium channels and alpha3-integrins are complexed with synaptic cleft laminins, cytoskeletal elements and active zone components. J Neurochem. 2010;115:654–666. doi: 10.1111/j.1471-4159.2010.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller CS, et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci USA. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimune H, et al. Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PLoS ONE. 2012;7:e38029. doi: 10.1371/journal.pone.0038029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimune H. Molecular mechanism of active zone organization at vertebrate neuromuscular junctions. Molecular neurobiology. 2012;45:1–16. doi: 10.1007/s12035-011-8216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catterall WA, Few AP. Calcium Channel Regulation and Presynaptic Plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Frank T, et al. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han Y, et al. RIM determines Ca(2)+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiyonaka S, et al. Physical and functional interaction of the active zone protein CAST/ERC2 and the beta-subunit of the voltage-dependent Ca2+ channel. J Biochem. 2012;152:149–159. doi: 10.1093/jb/mvs054. [DOI] [PubMed] [Google Scholar]
- 63.Hallermann S, et al. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee K, et al. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc Natl Acad Sci USA. 2010;107:6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukunaga H, et al. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci USA. 1983;80:7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukuoka T, et al. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann Neurol. 1987;22:193–199. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- 67.Massoulie J, Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annual review of neuroscience. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- 68.Kelly SS. The effect of age on neuromuscular transmission. J Physiol. 1978;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips GR, et al. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- 70.Dickman DK, et al. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 71.Pielage J, Fetter RD, Davis GW. A postsynaptic Spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J. Cell Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graf ER, et al. Rab3 Dynamically Controls Protein Composition at Active Zones. Neuron. 2009;64:663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wairkar YP, et al. Unc-51 controls active zone density and protein composition by downregulating ERK signaling. J Neurosci. 2009;29:517–528. doi: 10.1523/JNEUROSCI.3848-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pielage J, et al. Hts/Adducin Controls Synaptic Elaboration and Elimination. Neuron. 2011;69:1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith DO. Acetylcholine storage, release and leakage at the neuromuscular junction of mature adult and aged rats. J Physiol. 1984;347:161–176. doi: 10.1113/jphysiol.1984.sp015059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banker BQ, Kelly SS, Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gutmann E, Hanzlikova V, Vysokocil F. Age changes in cross striated muscle of the rat. J Physiol. 1971;216:331–343. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dupont-Versteegden EE, et al. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- 79.Cote MP, et al. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCullough MJ, et al. Glial cell line-derived neurotrophic factor protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 2011;174:234–244. doi: 10.1016/j.neuroscience.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schaser AJ, et al. The effect of age and tongue exercise on BDNF and TrkB in the hypoglossal nucleus of rats. Behav Brain Res. 2012;226:235–241. doi: 10.1016/j.bbr.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Udina E, Puigdemasa A, Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve. 2011;43:500–509. doi: 10.1002/mus.21912. [DOI] [PubMed] [Google Scholar]
- 83.Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- 84.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 85.Valdez G, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]