Abstract
Peripheral neuropathy is a common complication of many of the systemic amyloidoses. Although the cause of neuropathy is not entirely clear, it is likely related to amyloid deposition within the nerve. This may lead to focal, multifocal, or diffuse neuropathies involving sensory, motor and/or autonomic fibers. The presenting symptoms depend on the distribution of nerves affected. One of the most common phenotypes is sensorimotor polyneuropathy, which is characterized by symptoms of neuropathic pain, numbness, and in advanced cases weakness. Symptoms begin in the feet and ultimately progress to the proximal legs and hands. The most common focal neuropathy is a median neuropathy at the wrist, or clinically known as carpal tunnel syndrome. Carpal tunnel symptoms may include pain and sensory disturbances in the lateral palm and fingers; hand weakness may ensue if the focal neuropathy is severe. Autonomic neuropathy may affect a variety of organ systems such as the cardiovascular, gastrointestinal, and genitourinary systems. Symptoms may be non-specific making the diagnosis of autonomic neuropathy more difficult to identify. However, it is important to recognize and distinguish autonomic neuropathy from diseases of the end-organs themselves. This chapter reviews the inherited and acquired amyloidoses that affect the peripheral nervous system including familial amyloid polyneuropathy, and primary, secondary and senile amyloidosis. We emphasize the clinical presentation of the neurologic aspects of these diseases, physical examination findings, appropriate diagnostic evaluation, treatment and prognosis.
Keywords: amyloid, neuropathy, autonomic, genetic
The amyloidoses are a heterogeneous group of disorders that may present with a diverse spectrum of clinical manifestations. The disorders are characterized by tissue deposition of insoluble, misassembled fibril proteins that ultimately lead to the disruption of normal tissue structure and function (1). Up to the present time, 30 proteins have been identified as main amyloid fibril components (2). Depending on the etiology, amyloid deposits can affect a variety of organ systems most commonly the kidneys, liver and heart. Amyloid can also affect the peripheral motor, sensory and autonomic nerves. The degree of nervous system involvement is variable and may begin at different time points in the course of disease. Occasionally neuropathy is the initial manifestation of the disease. In this case, arriving at an accurate diagnosis is particularly crucial so that patients may undergo the appropriate testing to find other affected organ systems. The discovery of which, may lead to life-saving interventions such as a liver transplant. In addition, documenting the degree of neuropathy gauges disease progression, guides treatment decisions, and determines response to therapy in the clinical and research settings. This chapter will review the various types of inherited and acquired amyloidoses that affect the peripheral nervous system.
INHERITED FORMS OF AMYLOID NEUROPATHY
The term Familial Amyloid Polyneuropathy (FAP) refers to a group of hereditary amyloidoses which typically have prominent clinical manifestations involving the peripheral sensorimotor and/or autonomic nervous system. FAP can be further classified according to the type of amyloid protein that causes the disease process. These include transthyretin, apoprotein A1, and gelsolin. Of these three, transthyretin amyloidosis is the most common (see Case Illustration 1). FAP was first described in 1952 by Andrade in individuals living in northern Portugal, where the condition was found to be prevalent (3). Later, the disease was observed in large groups of individuals in Japan and Sweden (4,5). The identified abnormal amyloid fibril was found to be the result of a substitution of methionine for valine at position 30 of the transthyretin gene (TTR, Val30Met) (6). Since that time a number of other mutations in the TTR gene have been described, however Val30Met remains the most common pathogenic point mutation that causes FAP worldwide (7).
Case Illustration 1. Familial Amyloid Polyneuropathy.
A 64 year-old man of Italian ancestry, with known familial amyloid polyneuropathy, presented for evaluation. The patient was diagnosed by genetic testing 4 years previously while asymptomatic, after his older brother, who had longstanding disabling neuropathy was finally diagnosed with FAP. His sister, who is the oldest sibling, was also found to have the same mutation (Val30Met), and amyloid on fat pad biopsy. However she has remained relatively asymptomatic. The patient began taking diflunisal, but symptoms worsened and he underwent liver transplant two years after diagnosis. He worried that his neuropathy was progressing after surgery. He reported numbness in the feet and imbalance, and fatigue with walking. He was dizzy especially in the morning. He had early satiety, poor appetite and difficulty maintaining his weight. He had sexual dysfunction and decreased sweating.
Examination revealed dry skin especially on the fingers and toes. There was distal hair loss and a slight bluish color in the feet despite normal color and temperature. Mental status and cranial nerves were normal. There was distal muscle atrophy, especially in the legs. Strength was full to confrontation but the patient had difficulty arising on his toes and heels. His reflexes were normal except at the ankles, where they were absent. Vibration sensation was absent at the toes, moderately decreased at the ankle, and mildly so at the knees and fingers. Temperature and sharp sensation was decreased to the knee bilaterally. Proprioception was relatively preserved. His gait was steady but slightly wide-based and cautious.
Diagnostic testing, including nerve conduction studies and electromyography and an autonomic reflex screen, was performed to document the severity of the neuropathy. Nerve conductions revealed reduced motor responses and absent sensory responses in the lower extremity (see figure 1–2). Autonomic reflex screen confirmed significant autonomic neuropathy involving sympathetic, cardiovagal, and sudomotor function (figures 3–6).
Comment
This case describes a family with relatively late onset of FAP. Although all three siblings possess the same mutation, there is marked variability in neuropathy severity. As is often the case, diagnosis was significantly delayed in the first member of the family to seek medical attention for neuropathy. This patient demonstrates typical signs and symptoms of sensory, motor and autonomic neuropathy.
Transthyretin Gene-Related Familial Amyloid Polyneuropathy
Genetics and Epidemiology
The TTR gene is located on chromosome 18 (8). More than 100 mutations in the TTR protein have been reported to be associated with amyloidosis (9). Most of the mutations are the product of a single nucleotide substitution in the TTR gene. Other mutations are the result of two nucleotide substitutions in a codon and the deletion of a 3-base codon (Val122) (10). Transthyretin amyloidosis is inherited in an autosomal dominant manner, thus the majority of affected individuals possess one mutant and one wild-type allele leading to an expression of both variant and normal TTR proteins, although rare compound heterozygotes have been described (11).
The prevalence of TTR mutations is high in Portugal, Sweden, and Japan. The estimated gene frequency of Val30Met TTR mutations is 1:538 in northern Portugal; whereas it is much lower, 1: 1,000,000 to 1: 100,000 in the United States (12). Patients with TTR Val30Met amyloidosis can exhibit great clinical heterogeneity with respect to disease severity and age of symptom onset; this can be seen within and between different ethnic groups (13). For example, individuals living in endemic areas in Japan and Portugal will typically have early disease onset (30–40 years); whereas individuals of Japanese ancestry living in non-endemic areas will typically have a much older onset of disease (14,15). Individuals of Swedish ancestry also have a later age of onset (16). However, late age of onset has been described in Portugal and earlier age of onset has been described in Sweden. The disease in France is heterogeneous (13). The degree of penetrance also exhibits a great deal of variability. The TTR Val30Met mutation produces disease in 80% of Portugese carriers, whereas it only produces disease in 5%–10% of Swedish carriers (16,17). In one Japanese kindred there were asymptomatic individuals as old as 81 years who had high levels of variant TTR amyloid protein; this was similar to those who had neuropathy symptoms (18). Differences in genetic phenotypes and age of onset were even seen among monozygotic twins (19). We have observed that in some families men are more severely affected than women with the same mutation; other authors have reported gender differences in age of onset or prevalence of disease (15,16). The variations in disease onset and differing phenotypes are thought to be the result of both genetic and non-genetic modifying factors (20).
Types of Neuropathy
Patients with FAP may experience different patterns of neuropathy including focal neuropathies, sensorimotor polyneuropathy, autonomic neuropathy or combinations of the three. The median nerve at the wrist is a common and early site of involvement in FAP. Patients experience symptoms of carpal tunnel syndrome: paresthesias in the thumb and digits 2 and 3, pain in the wrist which may extend to the elbow, and in more severe cases, weakness in grip.
[Callout] Patients with familial amyloid polyneuropathy (FAP) may experience different patterns of neuropathy including focal neuropathies, sensorimotor polyneuropathy, autonomic neuropathy or combinations of the three. The median nerve at the wrist is a common and early site of involvement in FAP. Patients experience symptoms of carpal tunnel syndrome.
Although carpal tunnel syndrome is common in the general population and is a nonspecific manifestation of FAP, when it does occur in patients with FAP the lesions tend to be more severe than in patients with idiopathic carpal tunnel syndrome (21). This is due to endoneurial amyloid deposits that accompany the nerve entrapment under the carpal tunnel ligament (7). Focal nerve lesions other than median neuropathy at the wrist are rare. There have been case reports of FAP patients presenting with vocal cord paresis and lower extremity pain at onset of the disease (22).
TTR related FAP commonly affects the peripheral motor and sensory nerves in a length dependent manner leading to a sensorimotor polyneuropathy. The fibers that tend to be affected first are the small myelinated and unmyelinated fibers (23–25). At this stage, patients may complain of discomfort in their feet. Symptoms include numbness, pins and needles sensation, burning that is often worse at night and is associated with allodynia. Some patients may not experience pain as a symptom. In a predominantly small fiber neuropathy, neurologic examination may show decreased pin prick in the feet and impaired thermal sensibility, but with preserved light touch sensation, proprioception, motor strength and reflexes. Over the following months to years, the neurologic deficit worsens and begins to affect larger sensory and motor nerve fibers. Loss of large fibers results in muscle weakness and more severe sensory loss. Neurologic examination reveals weakness in the feet and hands, decreased or absent reflexes particularly at the ankle, and diminished vibration sense and proprioception in a distal distribution.
[Callout] Neurologic examination reveals weakness in the feet and hands, decreased or absent reflexes particularly at the ankle, and diminished vibration sense and proprioception in a distal distribution.
Symptoms then gradually extend to the proximal lower extremity, trunk and upper extremities. Walking may become increasing difficulty due to both proprioceptive deficits as well as weakness. Patients are also susceptible to joint deformities, like Charcot joints, and plantar ulcers due to painless trauma to the feet. As the disease progresses, some patients may experience life threatening autonomic dysfunction and generalized malaise, weight loss and muscle wasting (7).
Dysfunction of the autonomic nervous system is commonly seen in individuals with early onset FAP, and less commonly in those with late onset FAP. Cardiovascular, genitourinary, and gastrointestinal systems are frequently affected. Patients may experience orthostatic hypotension due to cardiovascular dysautonomia and complain of sensations of light headedness, dizziness, fatigue, and blurry vision when standing. Some patients remain asymptomatic despite significant orthostatic hypotension which may be due to compensatory changes in central auto-regulation that occur in response to slowly progressive peripheral autonomic dysfunction. Gastrointestinal symptoms of autonomic dysfunction can include postprandial diarrhea alternating with constipation which can be severe. Postprandial vomiting due to gastroparesis may lead to progressive weight loss and dehydration worsening postural hypotension. Genitourinary symptoms may include urinary retention or incontinence. Sexual dysfunction also occurs. In men, erectile dysfunction is sometimes an early feature that may precede the sensory symptoms of neuropathy (7,22).
[Callout] Dysfunction of the autonomic nervous system is commonly seen in individuals with early onset FAP, and less commonly in those with late onset FAP. Cardiovascular, genitourinary, and gastrointestinal systems are frequently affected.
Central Nervous System Involvement
Although potential sources of TTR protein include the choroid plexus epithelium and some leptomeningeal cells, central nervous system manifestations of amyloidosis is atypical (26). However there are cases of occulo-leptomeningeal amyloidosis, dementia, seizures, stroke-like episodes, subarachnoid hemorrhage, ataxia, myelopathy, hydrocephalus, and deafness with or without peripheral neuropathy that have been reported in the literature (27–32).
Pathophysiology
Transthyretin (TTR) exists as a tetrameric plasma transport protein. Each monomer consists of a single polypeptide chain of 127 amino acid residues that is approximately 14,000 Da in size; the whole structure is approximately 55,000 Da. The tetrameric structure has surface receptors for retinol-binding protein/vitamin A as well as binding sites for thyroxine (33). The misfolding of the protein leads to inappropriate aggregation and accumulation in a variety of organ systems. In tissue cultures, non-native oligomers have also been shown to be cytotoxic (34). Most of the plasma TTR protein is produced in the liver; however some of it is produced in the retinal pigment epithelium of the eye and the choroid plexus of the brain (26,35).
[Callout] Transthyretin (TTR) exists as a tetrameric plasma transport protein. The misfolding of the protein leads to inappropriate aggregation and accumulation in a variety of organ systems. In tissue cultures, non-native oligomers have also been shown to be cytotoxic. Most of the plasma TTR protein is produced in the liver;
Recently, studies have also reported TTR synthesis in neurons and peripheral nerve Schwann cells (36,37). Amyloid deposits within nerve lesions are unevenly distributed and often appear within fascicles. As the amyloid fibrils accumulate, they cause damage to the nerve by mechanical compression, direct blood vessel invasion and possibly through toxic effects of the amyloid fibrils. Electron microscopy studies of Schwann cells have demonstrated cytoplasmic degenerative changes and destruction of the basal lamina when these cells come into contact with amyloid fibrils (7). Peripheral nerve specimens from individuals with TTR FAP revealed amyloid deposits located in the endoneurium, as well as in endoneurial, perineurial and epineurial blood vessels (36,37).
Diagnosis
The diagnosis of FAP should be considered in patients with sensorimotor polyneuropathy, with or without autonomic features, along with a family history of neuropathy. The diagnosis should also be considered in patients without family history, but with an idiopathic progressive axonal polyneuropathy, particularly when there is associated autonomic dysfunction, cardiac findings, or severe carpal tunnel syndrome. One can proceed directly to DNA testing if a familial cause of polyneuropathy is evident.
[Callout] The diagnosis of FAP should be considered in patients with sensorimotor polyneuropathy, with or without autonomic features, along with a family history of neuropathy. One can proceed directly to DNA testing if a familial cause of polyneuropathy is evident.
However if the diagnosis is in question, or if there is a lack of family history as in sporadic cases, an evaluation to exclude more common causes of neuropathy is appropriate. It is important to determine whether the patient has other risk factors for neuropathy such as alcohol abuse, poor nutritional status, diabetes mellitus, autoimmune disease, malignancy, or dysfunction of the kidney, liver, or thyroid. Diagnostic blood tests should include at a minimum: complete metabolic panel, liver function tests, hemoglobin A1C, vitamin B12 level, and thyroid function tests. Urine and serum immunofixation should also be obtained, as a monoclonal protein is found in approximately 90% of patients with AL amyloidosis (38)and is also common in other disorders associated with neuropathy such as multiple myeloma. Additional tests often ordered in the evaluation of neuropathy, depending on the clinical scenario, include: glucose tolerance test, antinuclear antibody, rheumatoid factor, erythrocyte sedimentation rate, rapid plasma regain, antibodies for Sjogren’s syndrome (SS-A and SS-B), serology for infectious causes (HIV, Lyme, Hepatitis C), heavy metals screen, angiotensin converting enzyme level, anti GM-1, antineutrophil cytoplasmic antibodies (c-ANCA, p-ANCA), cryoglobulins, complement levels, anti-myelin-associated antibodies, screening for celiac disease, paraneoplastic antibody screen, and other genetic testing (e.g. mutations associated with Charcot-Marie-Tooth).
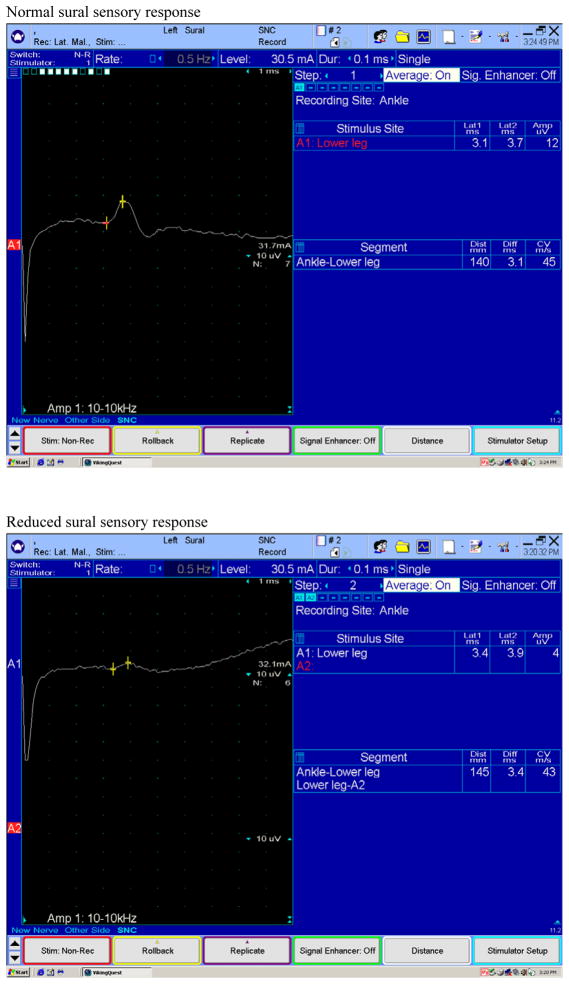
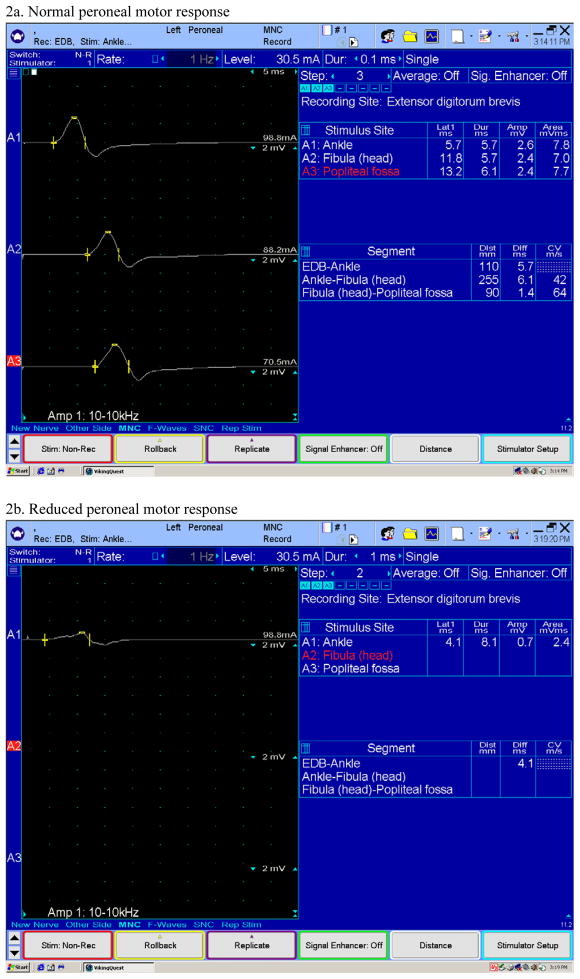
Electrophysiologic tests are an important part of the diagnostic evaluation. These may include nerve conduction studies (NCS), electromyography (EMG), autonomic function testing (AFT), and quantitative sudomotor axon reflex testing (QSART). EMG and NCS are useful in demonstrating large fiber neuropathy in sufficiently advanced cases, whereas tests like AFT and QSART is useful in demonstrating small fiber involvement that is typically involved early in the course of the disease. NCS typically shows an axonal polyneuropathy, involving sensory more than motor fibers, often with a superimposed median neuropathy at the wrist (i.e. carpal tunnel syndrome). Sensory nerve conduction amplitudes can be absent or reduced (see figure 1). Motor nerve conduction amplitudes can be reduced or normal, with normal to mildly slowed conduction velocities (see figure 2). EMG may show spontaneous activity in the form of fibrillation potentials and positive sharp waves signifying axonal injury and active denervation. Volitional motor unit recruitment may reveal large, neurogenic motor unit potentials and reduced motor unit recruitment consistent with chronic denervation and reinnervation (38,39). In patients with normal nerve conduction studies, quantitative sensory testing and sympathetic skin tests can provide data supportive of small fiber involvement. Quantitative autonomic tests (see figures 3–6), including quantitative sudomotor axon reflex testing (QSART), heart rate response to deep breathing, Valsalva maneuver, and tilt table studies, may be more useful and are also important to document the degree of autonomic nervous system dysfunction (40). In addition to electrophysiologic testing, skin biopsy may also be a useful tool to document small fiber involvement early in the disease. Histopathologic examination may reveal reduced intraepidermal nerve fiber densities and occasionally amyloid deposits (41). It should be emphasized that these diagnostic tests are used in general for the assessment of small fiber neuropathy. However their utility specifically in FAP remains unclear and they may not be available at all centers. Nerve biopsy may be considered for cases in which the diagnosis remains elusive after less invasive tests are non-diagnostic. Nerve biopsies reveal amyloid deposits in the endoneurial and epineurial connective tissue, along with deposits in the endoneurial and epineurial blood vessel walls. There is a decreased density of all types of nerve fibers with small myelinated and umyelinated fibers being affected the most. Teased nerve fiber preparations reveal axonal degeneration. With Congo red staining, amyloid exhibits an apple-green birefringence under polarized light and appears red when viewed under a light microscope (38,42). Amyloid deposits may also be seen in biopsy specimens of muscle, abdominal fat or salivary glands (7). Specific types of amyloid may also be identified using mass spectrometric (MS)-based proteomic analysis of biopsy specimens (43).
Figure 1.
Examples of normal and reduced sural sensory responses obtained with surface electrodes by stimulating the nerve proximally in the calf and recording distally in the ankle. Reduced sural sensory responses are seen in many different neuropathies, including those due to amyloid.
Figure 2.
Examples of normal and reduced peroneal motor responses obtained with surface electrodes by recording the extensor digitorum brevis muscle in the dorsum of the foot. In the reduced response (Fig. 2b) only a distal stimulation is performed because the response is very small. In the normal response (Fig. 2a) the peroneal nerve was stimulated at three different sites in the leg. Reduced motor responses may be seen in moderate to severe neuropathy.
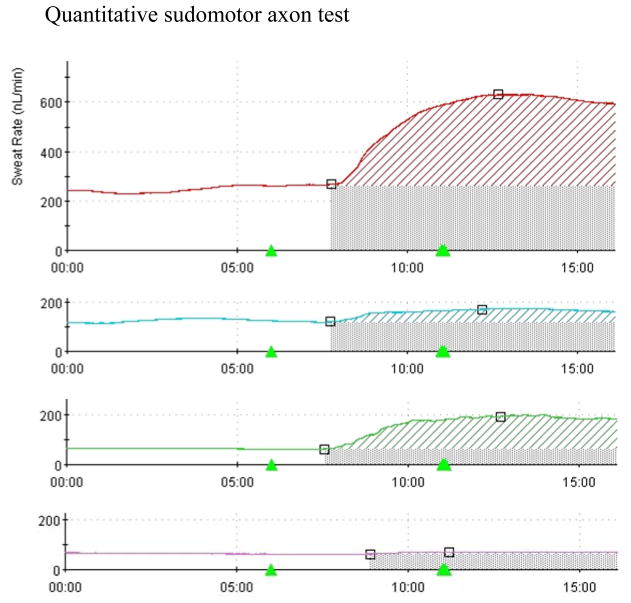
Figure 3.
Quantitative sudomotor axon test demonstrating normal sweat volume in the forearm (top trace), diminished sweating in the proximal and distal leg (middle traces), and near absent sweating in the foot. This is consistent with a length dependent neuropathic process.
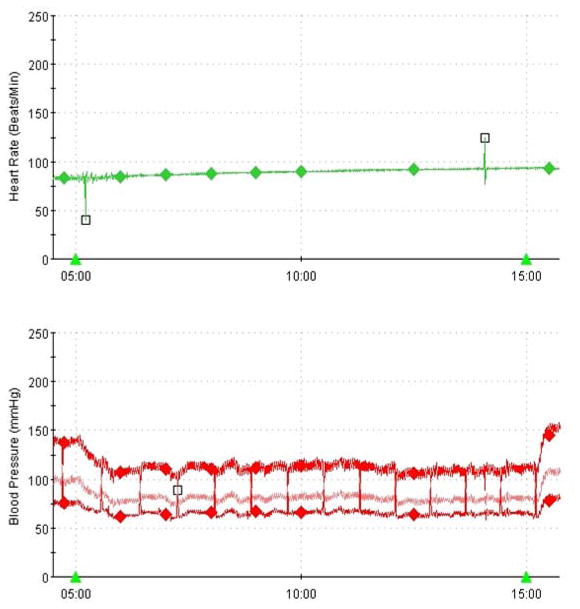
Figure 6.
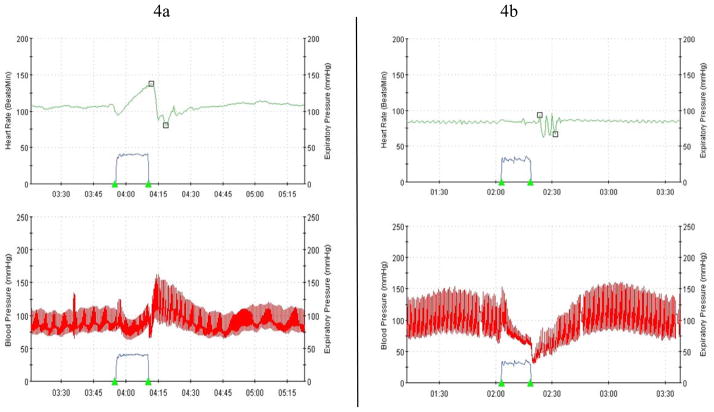
Hemodynamic responses to upright tilt in a patient with autonomic neuropathy demonstrate orthostatic hypotension and lack of compensatory tachycardia.
Treatment
Treatment of FAP can be divided into disease-modifying and symptomatic therapies. Symptomatic therapy for FAP focuses on relief of neuropathic pain and amelioration of the symptoms of autonomic neuropathy. Treatment recommendations for neuropathic pain are based on studies done in more prevalent diseases such as diabetic neuropathy and post-herpetic neuralgia. Four main classes of agents are used: anticonvulsants, antidepressants, topical treatments, and nonspecific analgesics. Among the anticonvulsants, gabapentin and pregabalin have the strongest evidence for efficacy in neuropathic pain. They are typically well tolerated and have the benefit of renal excretion without hepatic metabolism (44,45). Two classes of antidepressants are commonly used for the treatment of neuropathic pain, the tricyclics (TCAs) and the serotonin-norepinephrine reuptake inhibitors (SNRI) (46,47). The TCAs nortriptyline and amitriptyline are probably the most commonly prescribed. Sedation is among the most common side effects, which can be used to advantage if the patient’s symptoms are worst at night. Caution must be used when prescribing TCAs to patients with significant autonomic neuropathy because they may worsen orthostatic hypotension and urinary retention. Cognitive side effects may also occur, particularly in the elderly. Duloxetine is the best studied SNRI for the treatment of neuropathic pain; venlafaxine and milnacipran are also available (46,47). A topical patch containing high dose capsaicin was recently approved by the Food and Drug Administration (FDA) for the treatment of post-herpetic neuralgia and has also shown efficacy in other types of neuropathic pain (48). Drawbacks include expense, and the time and specialized expertise needed to apply the patch in a doctor’s office. Topical lidocaine preparations are also commonly used (49). Nonsteroidal anti-inflammatory drugs and acetaminophen are typically ineffective in the management of neuropathic pain. Opioids may be appropriate for moderate to severe neuropathic pain with appropriate monitoring for the development of aberrant use behaviors.
Pharmacologic therapies have aimed at reducing the amount of amyloidogenic fibrils by stabilizing the original tetrameric form of the TTR molecule. These therapeutic molecules have been created after in vitro studies have shown that the binding of L-thyroxine to the TTR tetramer stabilizes its structure and function and inhibits amyloid fibril formation. Thus small molecule thyroxine mimetics have been developed to treat FAP (50). Diflunisal and tafamidis are two drugs that have been studied in worldwide clinical trials for the treatment of FAP but are not currently approved by the FDA.
[Callout] Diflunisal and tafamidis are two drugs that have been studied in worldwide clinical trials for the treatment of FAP but are not currently approved by the FDA.
Results from a randomized, controlled trial of diflunisal, a non-steroidal anti-inflammatory drug, are expected to be published shortly (51). Tafamidis is a benzoxazole derivative that binds highly selectively to TTR in human plasma. A phase II/III randomized, double-blind, placebo-controlled trial enrolled more than 100 patients heterozygous for the Val30Met TTR mutation. The trial showed that 60% of patients treated with tafamidis, 20mg once a day, slowed progression of both autonomic and peripheral neuropathies versus 38% of the placebo group. In addition, improved quality of life and improved nutritional status, as reflected in an increase in modified body mass index, was seen in the treatment group (52). Tafamidis has recently been granted marketing authorization by the European Commission for the treatment of symptomatic peripheral neuropathy to delay neurological impairment.
Liver transplantation is the first line treatment of Val30Met FAP and should be considered early in the disease course.
[Callout] Liver transplantation is the first line treatment of Val30Met FAP and should be considered early in the disease course.
However, anecdotal experience suggests that liver transplantation may be less effective for other mutations and in patients over the age of 50. Furthermore, the invasive nature of the procedure requires careful consideration in all patients. The goal of liver transplantation is to limit the production of mutant TTR. It is assumed that since the liver produces most of the TTR protein in serum, liver transplantation should stop the production of mutant TTR. However since livers from FAP patients are otherwise healthy, and FAP typically takes decades to develop, “domino” transplants have been performed, during which the liver from the patient with FAP is transplanted into another patient. Although this procedure has been justified on the basis of organ scarcity, recent studies have found that amyloidosis may develop more quickly in the recipient than had been expected (53). According FAP World Transplant Registry over 1900 liver transplants were performed between 1990 and 2010 mostly in patients with Val30Met TTR mutations (54). A recent Japanese study of patients with Val30Met FAP demonstrated an estimated probability of survival at 10 years of 100% in the transplant group compared to 56.1% for the non-transplant group (55). Five year survival rates are up to 92% in one Swedish study. Pure sensory symptoms remain stable in over 90% of patients after liver transplantation although objective measures of improvement are not significant (56). Although liver transplantation may halt the production of mutant TTR protein in the serum, it has no effect on CNS symptoms or ocular complications that are caused by persistent production of mutant TTR by the choroid plexus or retinal epithelial cells. There may also be progression of cardiac amyloidosis and worsening of peripheral neuropathy despite liver transplantation. The cardiac amyloidosis is thought to be due continued deposition of wild type TTR on pre-existing amyloid deposits; worsening cardiac dysfunction is the main factor affecting prognosis after liver transplantation. A similar mechanism may lead to progression of neuropathy, although another mechanism is continued production of mutant TTR from the choroid plexus which may deposit in the nerve’s endoneurial space through the subarachnoid space (7).
New treatments for TTR amyloidosis are under development. Suppression of hepatic transthyretin has been achieved through antisense oligonucleotides that degrade TTR messenger RNA which suppresses gene expression (57). Similar strategies using small interfering RNAs and specific cleavage ribozymes are also being studied (58,59).
Apoprotein A1-related Familial Amyloid Polyneuropathy
Sixteen mutations of the APOA1 gene are associated with hereditary apolipoprotein A1 amyloidosis (60). The plasma protein, which is 28 kDa in size, is synthesized by the small intestine and liver. Although the Gly26Arg mutation can cause a length-dependent polyneuropathy, it is not the prominent feature of the disease overall; organ systems more commonly involved are renal, hepatic and gastrointestinal tract. Progressive renal failure can also lead to worsening peripheral neuropathy. There are no specific treatments for apolipoprotein A1 FAP. One patient with a Gly26Arg mutation experienced a reduction in variant protein by 50% and improvement in neuropathy symptoms following hepatorenal transplant, which may have been in part from improved renal function (61). Thus, although liver transplants have been performed for apolipoprotein A1 amyloidosis, it is not clear that it is beneficial.
Gelsolin-Related Familial Amyloid Polyneuropathy
Hereditary gelsolin amyloidosis, also known as familial amyloidosis of Finnish type, was first described in Finland in 1969 (62). Gelsolin is a calcium dependent protein that binds actin and regulates its filament assembly and disassembly. The Finnish type is caused by a single mutation in the type 2 metal ion-binding site which impairs calcium binding resulting in increased cleavage of gelsolin into the amyloid precursor (63). The gelsolin gene is located on chromosome 9 and is inherited in an autosomal dominant fashion. Gelsolin amyloidosis is characterized by a triad of cranial neuropathies, corneal lattice dystrophy and cutis laxa. First symptoms of corneal lattice dystrophy can start around the age of 25–30. This is followed by slowly progressive cranial neuropathies most commonly affecting the upper branch of the facial nerve. Typical patients will have reduced facial expressions due to bilateral facial paresis. Other cranial nerves frequently involved are the hypoglossal, glossopharyngeal, and vagus nerves. A sensory neuropathy affecting the lower extremities usually occurs after 40–50 years of age (7). Involvement of the autonomic nervous system can occur, but symptoms are usually mild (64). Central nervous system involvement is usually due to gelsolin related amyloid angiopathy causing vascular malformations in the brain and spinal cord (65). Other systemic manifestations can include cardiac, renal and pharyngeal abnormalities but life threatening cardiac or renal complications are rare. Treatment consists of good ophthalmological care and plastic surgery to correct facial laxity. No treatment exists to suppress gelsolin related amyloid formation.
ACQUIRED FORMS OF AMYLOID NEUROPATHY
Primary Systemic AL amyloidosis
AL amyloidosis is caused by plasma cell dyscrasias or B-cell lymphoproliferative disorders. The abnormal populations of cells produce amyloid fibrils made up of either heavy or light chain immunoglobulin fragments which are then deposited into various organs including the heart, liver, kidney, and gastrointestinal tract (66). Peripheral neuropathy occurs in 17% of patients with AL amyloidosis, making AL amyloidosis the most common type of acquired amyloid polyneuropathy (see Case Illustration 2).
[Callout] Peripheral neuropathy occurs in 17% of patients with AL amyloidosis, making AL amyloidosis the most common type of acquired amyloid polyneuropathy.
Case Illustration 2. AL Amyloidosis Associated Neuropathy.
A 69 year-old man presented for neurologic evaluation. Two years previously he had been diagnosed with primary amyloidosis based on biopsy of a lung nodule discovered on a routine chest x-ray. Shortly after the diagnosis was made he developed tingling in the toes. This progressed to painful numbness and weakness in the feet and then the proximal legs and hands. He had been unable to walk independently for about one year. Review of systems revealed that he often experienced haziness of vision upon standing and had fainted twice. He also had transient difficulty urinating after a catheterization during a hospitalization, but this resolved. Neurologic examination revealed normal mental status and cranial nerves. There was diffuse atrophy especially in the posterior shoulders, hands and thighs. Muscle bulk could not be assessed in the distal lower extremities due to edema. He had weakness in all four limbs in a predominantly distal distribution. He was areflexic. Sensation was absent to all modalities in the lower extremities. Vibration sense was also absent at the fingertips. With help, he was able to arise from his wheelchair and take a few steps with a walker. The patient began treatment with bortezomib for the primary amyloidosis, was given gabapentin for neuropathic pain and referred to physical therapy.
Comment
This case demonstrates the severe and debilitating neuropathy that may accompany primary amyloidosis. Motor and sensory deficits are present, and the symptoms lightheadedness and urinary retention could suggest autonomic involvement.
Up to 65% of patients with peripheral neuropathy also have autonomic nervous system involvement (67). Sensorimotor axonal polyneuropathy and carpal tunnel syndrome are the most common types of neuropathy associated with AL amyloidosis. However multiple upper limb mononeuropathies, mononeuropathy multiplex, lumbosacral radiculoplexopathy and chronic inflammatory demyelinating polyneuropathy have also been reported (68–70). Symptoms typically begin with painful paresthesias in the feet signifying small fiber involvement. As the disease progresses it can affect larger nerve fibers and patients may complain of numbness and motor weakness. Autonomic symptoms can include nausea, vomiting, early satiety, bloating, constipation, diarrhea, postural lightheadedness and erectile dysfunction (71). Diagnostic testing can include electromyography/nerve conduction studies, autonomic function tests, and skin biopsy. Demonstration of free light chains in serum is an important diagnostic element. Mass spectrometry to document the type of amyloid and genetic testing to exclude FAP may also be helpful. However, the definitive diagnosis of AL amyloidosis is based on histological tissue examination that demonstrates amyloid deposition by Congo red staining or by the presence of lambda or kappa light chains by immunohistochemical staining (66). Treatment options include chemotherapy (e.g. proteasome inhibitors, drugs in the thalidomide class, lower dose alkylating agents) and autologous peripheral blood stem cell transplantation. Peripheral neuropathy often persists even in treated patients, although some recovery based on electrophysiologic tests and histopathological evidence of AL amyloid regression may occur (66).
[Callout] Peripheral neuropathy often persists even in treated patients, although some recovery based on electrophysiologic tests and histopathological evidence of AL amyloid regression may occur.
AA and β2-Microglobulin Amyloidosis
AA amyloidosis can occur in the setting of chronic infectious or inflammatory diseases such as tuberculosis and rheumatoid arthritis. The AA amyloid fibrils are fragments of a circulating acute phase reactant called SAA protein. The main organ that is affected in AA amyloidosis is the kidney; the liver, spleen and gastrointestinal tract are involved to a lesser degree (72). AA amyloidosis rarely affects the nervous system; however there have been case reports of autonomic neuropathy (73,74).
β2-microglobulin associated amyloidosis is potentially a serious complication seen in long-term hemodialysis patients. The β2M protein is a part of the MHC class I molecule that is present in all nucleated cells. The protein is normally broken down in the renal tubules, and can accumulate with chronic renal failure. Carpal tunnel syndrome and joint pains are early clinical manifestations of β2M amyloidosis. As the disease progresses, it can cause severe erosive changes to the joints and vertebral bodies leading to disabling arthropathies of large joints and spondylolisthesis (72). β2-microglobulin associated amyloidosis has become uncommon as dialysis techniques have improved. Recently, a hereditary amyloidosis due to a variant β2-microglobulin (Asp76Asn) has been described; which is associated with a predominantly autonomic neuropathy (75).
Age-Related Senile Systemic Amyloidosis
Senile systemic amyloidosis (SSA) is caused by deposition of wild-type transthyretin protein. Unlike the TTR-related amyloidosis seen in familial amyloid polyneuropathy, the amyloid fibrils in SSA maintain their primary structure. It is a prevalent disorder affecting up to 25% of people over the age of 80. Most of the amyloid deposition occurs in the heart leading to cardiomyopathy and atrial fibrillation. However, deposition of the TTR protein can be found in other organ systems such as the liver, kidney, gastrointestinal tract, aorta and connective tissues. Carpal tunnel syndrome is a common clinical manifestation which can precede cardiac manifestations of the disease (76). We, as well as others, have observed patients with SSA and sensorimotor polyneuropathy (77).
CONCLUSION
The systemic amyloidoses are a diverse group of disorders that can lead to multi-organ dysfunction through the deposition of abnormal amyloid fibrils. Mutations in the TTR gene lead to the most common form of inherited amyloidosis, whereas AL amyloidosis is the most common acquired form. Peripheral nervous system involvement is common and may present as a length dependent sensorimotor polyneuropathy, focal neuropathy, multi-focal neuropathy, or autonomic neuropathy. When peripheral neuropathy is the presenting manifestation of amyloidosis diagnosis is often delayed, since significant time may be spent pursuing more common causes of neuropathy such as diabetes or autoimmune disorders. These delays are a source of considerable frustration to patients and family members, particularly if a genetic disorder is ultimately diagnosed. Greater recognition of these conditions is needed for earlier diagnosis and optimization of currently available treatment, as well as a better understanding of the pathophysiology of the disease and research leading to new treatments.
Figure 4.
(a) Normal hemodynamic responses to standardized Valsalva maneuver (forced exhalation to a pressure of 40 mmHg for 15 seconds). Blood pressure (red trace) falls due to decreased venous return, but soon begins to recover due to autonomically mediated compensatory responses including increased heart rate (top trace). (b) In the patient with autonomic neuropathy, heart rate does not increase during Valsalva maneuver and blood pressure drops precipitously.
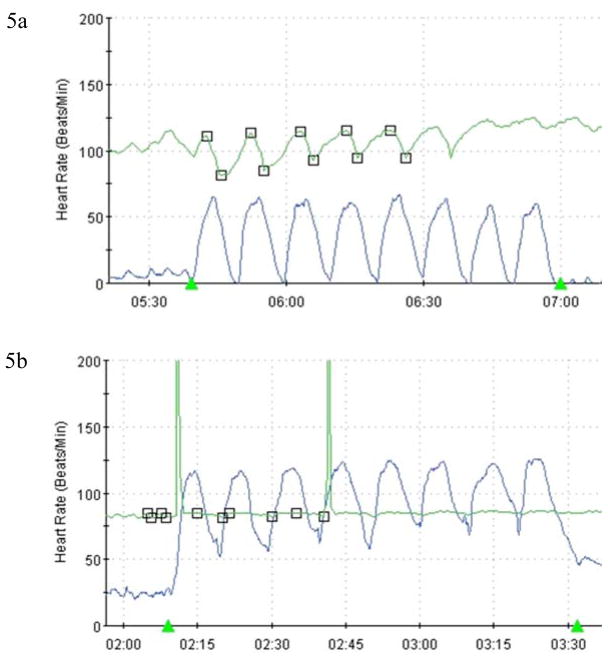
Figure 5.
(a) Normal heart rate variability (green trace) in response to paced deep breathing (blue trace). This reflex is mediated by the vagus nerve. (b) This figure demonstrates near absent heart rate variability in a patient with autonomic neuropathy.
TABLE.
| Type of Amyloidosis | Transmission | Clinical Features | Age of Onset | Treatment |
|---|---|---|---|---|
| TTR related-FAP | Autosomal Dominant | Sensorimotor PN, autonomic neuropathy, CTS, cardiomyopathy, vitreous deposits | 3rd to 4th decade for early onset 6th to 8th decade for late onset |
Liver transplant Tafamidis Diflunisal |
| ApoA1 related-FAP | Autosomal Dominant | Sensorimotor PN, kidneys, liver, gastrointestinal tract affected | 4th to 5th decade | Transplantation of affected organs |
| Gelsolin related-FAP | Autosomal Dominant | Cranial neuropathies, CTS, cutis laxa, corneal lattice dystrophy | 3rd to 4th decade | Plastic surgery for facial deformities |
| Primary AL | Acquired | CTS, sensorimotor PN, cardiomyopathy, liver and kidney involvement, macroglossia | 5th to 6th decade | Treatment of malignancy |
| Reactive AA | Acquired | Kidney, liver, spleen and gastrointestinal tract affected, autonomic neuropathy (rare) | Varies | Treat the primary inflammatory/infectious disease |
| B2-microglobulin | Acquired | CTS, spondyloarthropathy | 7–10 years after starting hemodialysis | Renal transplantation NSAIDs for joint pains |
| Senile systemic Wild-type TTR | Acquired | CTS, cardiomyopathy, atrial fibrillation | 7th decade | Symptomatic treatment |
Acknowledgments
Funding: Dr. Robinson-Papp receives funding from the National Institute of Neurologic Disorders and Stroke (NINDS; K23NS066789).
References
- 1.Kyle RA, Kelly JJ, Dyck PJ. Amyloidosis and neuropathy. In: Dyck PH, Thomas PK, editors. Peripheral Neuropathy. 4. Philadelphia: Elsevier Saunders; 2005. pp. 2427–2451. [Google Scholar]
- 2.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010 Sep;17(3–4):101–4. doi: 10.3109/13506129.2010.526812. Epub 2010 Nov 2. [DOI] [PubMed] [Google Scholar]
- 3.Andrade C. A peculiar form of peripheral neuropathy. Familial atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- 4.Araki S, Mawatari S, Ohta M, et al. Polyneuritic amyloidosis in a Japanese family. Arch Neurol. 1968;18:593–602. doi: 10.1001/archneur.1968.00470360015001. [DOI] [PubMed] [Google Scholar]
- 5.Andersson R. Familial amyloidosis with polyneuropathy. A clinical study based on patients living in northern Sweden. Acta Med Scand (Suppl) 1976;590:1–64. [PubMed] [Google Scholar]
- 6.Costa PP, Figueira A, Bravo F. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci USA. 1978;75:4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plante-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurology. 2011;10:1086–97. doi: 10.1016/S1474-4422(11)70246-0. [DOI] [PubMed] [Google Scholar]
- 8.Wallace MR, Naylor SL, Kluve-Beckerman B, Long GL, Mc-Donald L, Shows TB, et al. Localization of the human prealbumin gene to chromosome 18. Biochem Biophys Res Commun. 1985;129:753–758. doi: 10.1016/0006-291x(85)91956-4. [DOI] [PubMed] [Google Scholar]
- 9.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 10.Uemichi T, Liepnieks JJ, Waits RP, Benson MD. A trinucleotidedeletion in the transthyretin gene (V122) in a kindredwith familial amyloidotic polyneuropathy. Neurology. 1997;48:1667–1670. doi: 10.1212/wnl.48.6.1667. [DOI] [PubMed] [Google Scholar]
- 11.Benson M, Kincaid J. The molecular biology and clinical features of Amyloid neuropathy. Muscle Nerve. 2007;36:411–423. doi: 10.1002/mus.20821. [DOI] [PubMed] [Google Scholar]
- 12.Hund E, Linke RP, Willig F, et al. Transthyretin associated neuropathic amyloidosis: pathogenesis and treatment. Neurology. 2001;56:431–435. doi: 10.1212/wnl.56.4.431. [DOI] [PubMed] [Google Scholar]
- 13.Planté-Bordeneuve V, Carayol J, Ferreira A, Adams D, Clerget-Darpoux F, Misrahi M, et al. Genetic study of transthyretin amyloid neuropathies: carrier risks among French and Portuguese families. J Med Genet. 2003;40:e120. doi: 10.1136/jmg.40.11.e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misu K, Hattori N, Nagamatsu M. Late-onset familial amyloid polyneuropathy type I (transthyretin Met30- associated familial amyloid polyneuropathy) unrelated to endemic focus in Japan. Clinicopathological and genetic features. Brain. 1999;122:1951–1962. doi: 10.1093/brain/122.10.1951. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda S, Nakazato M, Ando Y, et al. Familial transthyretin-type amyloid polyneuropathy in Japan: clinical and genetic heterogeneity. Neurology. 2002;58(7):1001–1007. doi: 10.1212/wnl.58.7.1001. [DOI] [PubMed] [Google Scholar]
- 16.Sousa A, Andersson R, Drugge U, et al. Familial amyloidotic polyneuropathy in Sweden: geographical distribution, age of onset, and prevalence. Hum Hered. 1993;43:288–294. doi: 10.1159/000154146. [DOI] [PubMed] [Google Scholar]
- 17.Sousa A, Coelho T, Lobato L, et al. Genetic epidemiology of familial amyloidotic polyneuropathy (FAP)-type I in Povoa do Varzim and Vila do Conde (North of Portugal) Am J Med Genet. 1995;60:512–521. doi: 10.1002/ajmg.1320600606. [DOI] [PubMed] [Google Scholar]
- 18.Araki S, Ando Y. Transthyretin-related familial amyloidotic polyneuropathy—progress in Kumamoto, Japan (1967–2010) Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:694–97. doi: 10.2183/pjab.86.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda S, Takei Y, Tokuda T, et al. Clinical and pathological findings of non-Val30Met TTR type familial amyloid polyneuropathy in Japan. Amyloid. 2003;10 (suppl 1):39–47. [PubMed] [Google Scholar]
- 20.Soares ML, Coelho T, Sousa A, Holmgren G, Saraiva MJ, Kastner DL, Buxbaum JN. Haplotypes and DNA sequence variation within and surrounding the transthyretin gene: genotype-phenotype correlations in familial amyloid polyneuropathy (V30M) in Portugal and Sweden. Eur J Hum Genet. 2004 Mar;12(3):225–37. doi: 10.1038/sj.ejhg.5201095. [DOI] [PubMed] [Google Scholar]
- 21.Koike H, Morozumi S, Kawagashira Y, et al. The significance of carpal tunnel syndrome in transthyretin Val30Met familial amyloid polyneuropathy. Amyloid. 2009;16:142–48. doi: 10.1080/13506120903094074. [DOI] [PubMed] [Google Scholar]
- 22.Plante-Bordeneuve V, Ferreira A, Lalu T, et al. Diagnostic pitfalls in sporadic transthyretin familial amyloid polyneuropathy (TTR-FAP) Neurology. 2007;69:693–98. doi: 10.1212/01.wnl.0000267338.45673.f4. [DOI] [PubMed] [Google Scholar]
- 23.Dyck PJ, Lambert EH. Dissociated sensation in amyloidosis. Compound action potential, quantitative histologic and teased-fibre, and electron microscopic studies of sural nerve biopsies. Arch Neurol. 1969;20:490–507. doi: 10.1001/archneur.1969.00480110054005. [DOI] [PubMed] [Google Scholar]
- 24.Thomas PK, King RH. Peripheral nerve changes in amyloid neuropathy. Brain. 1974;97:395–406. doi: 10.1093/brain/97.1.395. [DOI] [PubMed] [Google Scholar]
- 25.Said G, Ropert A, Faux N. Length dependent degeneration of fibres in Portuguese amyloid polyneuropathy. A clinicopathological study. Neurology. 1984;34:1025–32. doi: 10.1212/wnl.34.8.1025. [DOI] [PubMed] [Google Scholar]
- 26.Soprano DR, Herbert J, Soprano KJ, Schon EA, Goodman DS. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985;260:11793–11798. [PubMed] [Google Scholar]
- 27.Brett M, Persey MR, Reilly MM, et al. Transthyretin Leu12Pro is associated with systemic, neuropathic and leptomeningeal amyloidosis. Brain. 1999;122:183–190. [Google Scholar]
- 28.Uemichi T, Uitti RJ, Koeppen AH, et al. Oculoleptomeningeal amyloidosis associated with a new transthyretin variant Ser64. Arch Neurol. 1999;56:1152–1155. doi: 10.1001/archneur.56.9.1152. [DOI] [PubMed] [Google Scholar]
- 29.Ellie E, Camou F, Vital A, et al. Recurrent subarachnoid hemorrhage associated with a new transthyretin variant (Gly53Glu) Neurology. 2001;57:135–137. doi: 10.1212/wnl.57.1.135. [DOI] [PubMed] [Google Scholar]
- 30.Blevins G, Maccaulay R, Harder S, et al. Oculoleptomeningeal amyloidosis in a large kindred with a new transtryretin variant Tyr69His. Neurology. 2003;60:1625–1630. doi: 10.1212/01.wnl.0000065901.18353.ab. [DOI] [PubMed] [Google Scholar]
- 31.Goren H, Steinberg MC, Farboody GH. Familial oculoleptomeningeal amyloidosis. Brain. 1980;103:473–95. doi: 10.1093/brain/103.3.473. [DOI] [PubMed] [Google Scholar]
- 32.Uitti RJ, Donat JR, Rozdilsky B, et al. Familial oculoleptomeningeal amyloidosis: report of a new family with unusual features. Arch Neurol. 1988;45:1118–22. doi: 10.1001/archneur.1988.00520340072015. [DOI] [PubMed] [Google Scholar]
- 33.Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995 May 19;268(5213):1039–41. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- 34.Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):2817–22. doi: 10.1073/pnas.0400062101. Epub 2004 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickson PW, Aldred AR, Marley PD, Guo-Fen T, Howlett GJ, Schreiber G. High prealbumin and transferrin mRNA levels in the choroid plexus of rat brain. Biochem Biophys ResCommun. 1985;127:890–895. doi: 10.1016/s0006-291x(85)80027-9. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Masliah E, Reixach N, Buxbaum JN. Neuronal production of transthyretin in human and murine Alzheimer’s disease: is it protective? J Neurosci. 2011 Aug 31;31(35):12483–90. doi: 10.1523/JNEUROSCI.2417-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami T, Ohsawa Y, Zhenghua L, Yamamura K, Sunada Y. The transthyretin gene is expressed in Schwann cells of peripheral nerves. Brain Res. 2010 Aug 12;1348:222–5. doi: 10.1016/j.brainres.2010.06.017. Epub 2010 Jun 12. [DOI] [PubMed] [Google Scholar]
- 38.Simmons Z, Specht C. The Neuromuscular Manifestations of Amyloidosis. Journal of Clinical Neuromuscular Disease. 2010;11(3):145–157. doi: 10.1097/CND.0b013e3181d05994. [DOI] [PubMed] [Google Scholar]
- 39.Kelly JJ. The electrodiagnostic findings in peripheral neuropathy associated with monoclonal gammopathy. Muscle Nerve. 1983;6:504–509. doi: 10.1002/mus.880060706. [DOI] [PubMed] [Google Scholar]
- 40.Kim DH, Zeldenrust SR, Low PA, et al. Quantitative sensation and autonomic test abnormalities in transthyretin amyloidosis polyneuropathy. Muscle Nerve. 2009;40:363–70. doi: 10.1002/mus.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang N, Lee M, Chao C, et al. Clinical presentations and skin denervation in amyloid neuropathy due to transthyretin Ala97Ser. Neurology. 2010;75(6):532–538. doi: 10.1212/WNL.0b013e3181ec7fda. [DOI] [PubMed] [Google Scholar]
- 42.Li K, Kyle RA, Dyck PJ. Immunohistochemical characterization of amyloid proteins in sural nerves and clinical associations in amyloid neuropathy. Am J Pathol. 1992;141:217–226. [PMC free article] [PubMed] [Google Scholar]
- 43.Klein CJ, Vrana JA, Theis JD, Dyck PJ, Dyck PJ, Spinner RJ, et al. Mass spectrometric-based proteomic analysis of amyloid neuropathy type in nerve tissue. Arch Neurol. 2011;68(2):195–9. doi: 10.1001/archneurol.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: A randomized controlled trial. Neurology. 2004;63(11):2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 45.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: A randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Shlay JC, Chaloner K, Max MB, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: A randomized controlled trial. Terry Beirn community programs for clinical research on AIDS. JAMA. 1998;280(18):1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- 47.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 48.Simpson DM, Brown S, Tobias J NGX-4010 C107 Study Group. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 49.Estanislao L, Carter K, McArthur J, Olney R, Simpson D Lidoderm-HIV Neuropathy Group. A randomized controlled trial of 5% lidocaine gel for HIV-associated distal symmetric polyneuropathy. J Acquir Immune Defic Syndr. 2004;37(5):1584–1586. doi: 10.1097/00126334-200412150-00010. [DOI] [PubMed] [Google Scholar]
- 50.Sekijima Y, Dendle M, Kelly J. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13(4):236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 51.Berk J, Dyck P, Obici L. The diflunisal trial: update on study drug tolerance and disease progression. Amyloid. 2011;(Suppl 1):191–2. doi: 10.3109/13506129.2011.574354073. [DOI] [PubMed] [Google Scholar]
- 52.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology. 2012 Aug 21;79(8):785–92. doi: 10.1212/WNL.0b013e3182661eb1. Epub 2012 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lladó L, Baliellas C, Casasnovas C, Ferrer I, Fabregat J, Ramos E, et al. Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl. 2010 Dec;16(12):1386–92. doi: 10.1002/lt.22174. [DOI] [PubMed] [Google Scholar]
- 54.Herlenius G, Wilczek HE, Larsson M, et al. Ten years of international experience with liver transplantation for familial amyloidotic polyneuropathy: results from the Familial Amyloidotic Polyneuropathy World Transplant Registry. Transplantation. 2004;77:64–71. doi: 10.1097/01.TP.0000092307.98347.CB. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita T, Ando Y, Okamoto S, et al. Long-term survival after liver transplantation in patients with familial amyloid polyneuropathy. Neurology. 2012;78(9):637–43. doi: 10.1212/WNL.0b013e318248df18. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto S, Wilczek HE, Nowak G, et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant. 2007;7:2597–604. doi: 10.1111/j.1600-6143.2007.01969.x. [DOI] [PubMed] [Google Scholar]
- 57.Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, Sloop KW. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006 May;33(5):609–18. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]
- 58.Kurosawa T, Igarashi S, Nishizawa M, et al. Selective silencing of a mutant transthyretin allele by small interfering RNAs. Biochem Biophys Res Commun. 2005;337:1012–18. doi: 10.1016/j.bbrc.2005.09.142. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka K, Yamada T, Ohyagi Y, et al. Suppression of transthyretin expression by ribozymes: a possible therapy for familial amyloidotic polyneuropathy. J Neurol Sci. 2001;183:79–84. doi: 10.1016/s0022-510x(00)00481-0. [DOI] [PubMed] [Google Scholar]
- 60.Eriksson M, Schonland S, Yumlu S, et al. Hereditary apolipoprotein A1-associated amyloidosis in surgical pathologyspecimens: identification of three novel mutations in the APOA1 gene. J Mol Diagn. 2009;11:257–62. doi: 10.2353/jmoldx.2009.080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Testro AG, Brennan SO, Macdonell RA, et al. Hereditary amyloidosis with progressive peripheral neuropathy associated with apolipoprotein AI Gly26Arg: outcome of hepatorenal transplantation. Liver Transpl. 2007;13:1028–31. doi: 10.1002/lt.21176. [DOI] [PubMed] [Google Scholar]
- 62.Meretoja J. Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes and various internal symptoms: a previously unrecognized heritable syndrome. Ann Clin Res. 1969;1:314–24. [PubMed] [Google Scholar]
- 63.Maury CP. Immunohistochemical localization of amyloid in Finnish hereditary amyloidosis with antibodies to gelsolin peptides. Lab Invest. 1991;64:400–04. [PubMed] [Google Scholar]
- 64.Chastan N, Baert-Desurmont S, Saugier-Veber P, et al. Cardiac conduction alterations in a French family with amyloidosis of the Finnish type with the Asp187Tyr mutation in the GSN gene. Muscle Nerve. 2006;33:113–19. doi: 10.1002/mus.20448. [DOI] [PubMed] [Google Scholar]
- 65.Kiuru S, Salonen O, Haltia M. Gelsolin-related spinal and cerebral amyloid angiopathy. Ann Neurol. 1999;45:305–11. doi: 10.1002/1531-8249(199903)45:3<305::aid-ana5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 66.Matsuda M, Gono T, Morita H, Katoh N, Kodaira M, Ikeda S. Peripheral nerve involvement in primary systemic AL amyloidosis: a clinical and electrophysiological study. European Journal of Neurology. 2010 doi: 10.1111/j.1468-1331.2010.03215.x. [DOI] [PubMed] [Google Scholar]
- 67.Gertz MA, Lacy MQ, Dispenzieri A. Amyloidosis. Hematol Oncol Clin North Am. 1999;13:1211–1233. doi: 10.1016/s0889-8588(05)70122-2. [DOI] [PubMed] [Google Scholar]
- 68.Adams D, Lozeron P, et al. Varied pattern of inaugural light-chain (AL) amyloid polyneuropathy: a monocentric study of 24 patients. Amyloid. 2011;(Suppl 1):93–5. doi: 10.3109/13506129.2011.574354036. [DOI] [PubMed] [Google Scholar]
- 69.Luigetti M, Papacci M, Bartoletti S, et al. AL amyloid neuropathy mimicking a chronic inflammatory demyelinating polyneuropathy. Amyloid. 2012;19(1):53–5. doi: 10.3109/13506129.2011.650247. [DOI] [PubMed] [Google Scholar]
- 70.Tracy J, Dyck P, Dyck J. Primary Amyloidosis Presenting as Upper Limb Multiple Mononeuropathies. Muscle Nerve. 2010;41(5):710–5. doi: 10.1002/mus.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim DK, Zeldenrust SR, Low PA, Dyck PJ. Quantitative sensation and autonomic test abnormalities in transthyretin amyloidosis polyneuropathy. Muscle & Nerve. 2009;40:363–70. doi: 10.1002/mus.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perfetto F, Moggi-Pignone A, Livi R, et al. Systemic amyloidosis: a challenge for the rheumatologist. Nature Reviews Rheumatology. 2010;6:417–429. doi: 10.1038/nrrheum.2010.84. [DOI] [PubMed] [Google Scholar]
- 73.Nordborg C, Kristensson K, Olsson Y, Sourander P. Involvement of the autonomous nervous system in primary and secondary amyloidosis. Acta Neurol Scand. 1973;49:31–8. doi: 10.1111/j.1600-0404.1973.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 74.Tsunoda I, Awano H, Kayama H. Idiopathic AA amyloidosis manifested by autonomic neuropathy vestibulocholeopathy and lattice corneal dystrophy. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:635–637. doi: 10.1136/jnnp.57.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valleix S, Gillmore JD, Bridoux F, Mangione PP, Dogan A, Nedelec B, et al. Hereditary Systemic Amyloidosis Due to Asp76Asn Variant β2-Microglobulin. N Engl J Med. 2012 Jun;366:2276–2283. doi: 10.1056/NEJMoa1201356. [DOI] [PubMed] [Google Scholar]
- 76.Sekijima Y, Uchiyama S, Tojo K, et al. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Human Pathology. 2011;42:1785–1791. doi: 10.1016/j.humpath.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Wang AK, Gorevic PD. Neurologic Improvement after Cervical Decompression in Wild Type Transthyretin Amyloidosis. In: Dyck PJ, Dyck PJB, Engelstad HT, Low PA, Amrani KK, Spinner RJ, Klein CJ, editors. Companion to Peripheral Neuropathy. Saunders Elsevier; 2010. pp. 145–149. [Google Scholar]