Abstract
The current study examined behavioral measures and response-locked event-related brain potentials (ERPs) derived from a Go/No-Go task in a large (N = 328) sample of 5- to 7-year-olds in order to better understand the early development of response monitoring and the impact of child age and sex. In particular, the error-related negativity (ERN, defined on both error trials alone and the difference between error and correct trials, or ΔERN), correct response negativity (CRN), and error positivity (Pe) were examined. Overall, the ERN, CRN, and the Pe were spatially and temporally similar to those measured in adults and older children. Even within our narrow age range, older children were faster and more accurate; a more negative ΔERN and a more positive Pe were associated with: increasing age, increased accuracy, and faster reaction times on errors, suggesting these enhanced components reflected more efficient response monitoring of errors over development. Girls were slower and more accurate than boys, although both genders exhibited comparable ERPs. Younger children and girls were characterized by increased posterror slowing, although they did not demonstrate improved posterror accuracy. Posterror slowing was also related to a larger Pe and reduced posterror accuracy. Collectively, these data suggest that posterror slowing may be unrelated to cognitive control and may, like the Pe, reflect an orienting response to errors.
Keywords: children, event-related potential, error-related negativity, error positivity, age, sex differences
INTRODUCTION
Response monitoring involves the ability to detect errors and subsequently adjust behavior (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000). For instance, behavioral adjustments following errors are evident in slower reaction times (RT) and increased accuracy. The actual processing of correct and error responses has been studied extensively using response-locked event-related brain potentials (ERPs). At least three ERP components are relevant to response monitoring: the error-related negativity (ERN), the correct response negativity (CRN), and the error positivity (Pe). The current study examined these ERPs, as well as behavioral measures of response monitoring, in 5- to 7-year-old children in order to better understand its development and explore the impact of child age and sex.
The Error-Related Negativity
The ERN (Gehring, Coles, Meyer, & Donchin, 1990) or negativity error (NE; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991) is a negative deflection with a fronto-central maximum that peaks approximately 50 ms following an erroneous response (e.g., Davies, Segalowitz, & Gavin, 2004; Falkenstein et al., 2000; Gehring, Goss, Coles, Meyer, & Donchin, 1993). ERP studies using source localization techniques suggest that the ERN is generated in the medial frontal cortex, specifically the anterior cingulate cortex (ACC; Dehaene, Posner, & Tucker, 1994; Luu, Tucker, Derry-berry, Reed, & Poulsen, 2003; Mathewson, Dywan, & Segalowitz, 2005; van Veen & Carter, 2002). Work using functional magnetic resonance imaging (fMRI) also supports the involvement of the ACC in error detection (Kiehl, Liddle, & Hopfinger, 2000; Mathalon, Whitfield, & Ford, 2003; Menon, Adleman, White, Glover, & Reiss, 2001). Finally, intracerebral studies by Brázdil and colleagues have provided further evidence that the ERN is generated in the ACC (Brázdil, Roman, Daniel, & Rektor, 2005; Brázdil et al., 2002).
Preliminary data suggested that the ERN may not be reliably elicited before age 12 (Davies et al., 2004). However, Santesso, Segalowitz, and Schmidt (2006a) reported an ERN in 10-year-old children, and Wiersema, van der Meere, and Roeyers (2007) showed that the ERN could be elicited in children as young as 7–8 years old. Further, Kim, Iwaki, Imashioya, Uno, and Fugita (2007) demonstrated that, although the amplitude of the ERN elicited in 7- to 8-year-olds was smaller than that found in 9- to 11-year-old children, neither group significantly differed from young adults on ERN amplitude. Recently, our lab demonstrated the ERN in children as young as 5–7 years old (Torpey, Hajcak, & Klein, 2009). The disparate findings in children could be related to the difficulty of the paradigms used across studies. Davies et al. (2004), who demonstrated that there was substantial variability in the ERN elicited by children under 12 years old, used a letters version of the flanker paradigm, whereas Wiersema et al. (2007), Kim et al. (2007), and Torpey et al. (2009), who obtained reliable ERNs in younger children, used a simpler Go/No-Go design that employed geometric shapes.
There is also conflicting evidence regarding the association between gender and ERN amplitude in developmental studies: Santesso et al. (2006a, b) found no relationship between ERN amplitude and sex of the participant. On the other hand, Davies et al. (2004), who examined the ERN over the course of development, reported an interaction between age and sex: ERN amplitude was similar for the youngest children, but started to diverge in older boys and girls. One of the aims of the present study is to more thoroughly characterize the ERN in a large group of younger children, and to further examine the role of sex.
The Correct Response Negativity
On correct trials, there is a smaller ERN-like negative deflection, known as the CRN (Coles, Scheffers, & Holroyd, 2001; Falkenstein et al., 2000; Ford, 1999; Vidal, Burle, Bonnet, Grapperon, & Hasbroucq, 2003; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000), similar to the ERN in terms of its latency, morphology, and scalp topography (Roger, Bénar, Vidal, Hasbroucq, & Burle, 2010; Vidal et al., 2000). The functional significance of the CRN continues to be debated (Coles et al., 2001; Suchan, Jokisch, Skotara, & Daum, 2007; Vidal et al., 2003), although Burle et al. (2010) have recently suggested that the ERN and the CRN reflect the same cognitive process related to response monitoring, with the amplitude being larger for errors than correct responses.
However, there is some evidence that ERN and CRN amplitude are modulated by different factors. For example, Simon-Thomas and Knight (2005) demonstrated that increased cognitive and affective demands modulate the CRN but not the ERN. On the other hand, the ERN, but not the CRN, appears to be modulated by trial value—being larger when errors are worth more to the subject, and during performance evaluation (Chiu & Deldin, 2007; Endrass et al., 2010; Hajcak, Moser, Yeung, & Simons, 2005; Kim, Iwaki, Uno, & Fujita, 2005). Relatively few studies have examined the CRN in young children, but some suggest that the CRN is actually larger in children than in adults (Davies et al., 2004; although see Santesso et al., 2006a, whose results varied depending on the way in which they computed the CRN). Thus, it is possible that the CRN may be larger, and the ERN smaller, among younger participants—perhaps suggesting reduced differentiation between correct and error processing early in development. Sex differences in the amplitude of the CRN have not been reported.
The Error Positivity
Response-locked ERP studies have isolated a third component associated with response monitoring: a large positivity known as the error positivity (Pe), that appears within 200–500 ms following an erroneous response (Falkenstein et al., 2000; Santesso et al., 2006a). Although the exact function of the Pe is unknown (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005), there is evidence to suggest that the Pe is a P300-like response to error commission (Davies, Segalowitz, Dywan, & Pailing, 2001; Ridderinkhof, Ramautar, & Wijnen, 2009) and is independent of the ERN (Arbel & Donchin, 2009). Specifically, some source localization studies have demonstrated that, like the P300, the Pe is localized in more posterior regions than the ERN (Burgio-Murphy et al., 2007; Ullsperger & von Cramon, 2006) and there is evidence that it is differentially affected by a number of factors, such as awareness of error commission (Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001) and task demands (Mathewson et al., 2005). Finally, there is evidence that the Pe does not change over the course of development (Davies et al., 2004; Wiersema et al., 2007), and that there is no relationship between the Pe and child age and sex (Santesso & Segalowitz, 2008; Santesso et al., 2006a,b). Thus, the existing evidence suggests that the Pe is uniquely insensitive to both developmental changes and sex.
Behavioral Measures
Several behavioral measures have been examined in the context of response monitoring—including both the number of errors and correct responses, as well as RT measures on both trial types. Multiple studies have found that RTs on error trials are shorter than on correct trials (e.g., Davies et al., 2004; Gehring & Fencsik, 2001; Hajcak et al., 2005). Additionally, RT on correct trials that follow errors tend to be relatively long—a phenomenon known as posterror RT slowing (e.g., Davies et al., 2004; Rabbitt, 1966; Santesso et al., 2006a). Posterror slowing has been hypothesized to reflect enhanced control designed to increase accuracy on the subsequent trial (Holroyd, Yeung, Coles, & Cohen, 2005; Kerns et al., 2004; Yeung, Botvinick, & Cohen, 2004). Associations between behavioral measures and sex of participant have rarely been the focus of analyses; however, Kim et al. (2007) found nonsignificantly faster RTs for young boys compared to young girls.
The relationship between behavioral and ERP measures has been frequently studied in adults. In some studies, individuals who commit more errors exhibit a less negative ERN (Holroyd & Coles, 2002; Pailing & Segalowitz, 2004; Pieters et al., 2007; Santesso, Segalowitz, & Schmidt, 2005); however, others studies have not found a relationship between ERN and error rate (Falkenstein et al., 2000; Masaki, Falkenstein, Sturmer, Pinkpank, & Sommer, 2007; Weinberg, Olvet, & Hajcak, 2010). There is also evidence that a larger Pe amplitude may be related to increased accuracy (Falkenstein et al., 2000) and increased posterror slowing (Boksem, Tops, Wester, Meijman, & Lorist, 2006; Hajcak, McDonald, & Simons, 2003). However, other studies have found no relationships between ERN, CRN, or Pe amplitude and posterror slowing (Compton et al., 2008), or posterror accuracy (Compton et al., 2008).
Associations between behavioral and ERP measures have been less studied in children, and have yielded similarly conflicting results. Santesso et al. (2006b) found a reduced (i.e., less negative) ERN and a smaller (i.e., less positive) Pe in children who committed more errors. However, other studies have reported no relationship between ERN, CRN, or Pe amplitude and posterror slowing (Santesso et al., 2006a; Wiersema et al., 2007), or overall error rate (Santesso et al., 2006a). Although several studies have examined associations between ERP components and posterror accuracy in adults, there are no known studies that have examined these relationships in children.
The Present Study
Relative to studies in adults, far fewer studies have examined the ERN, CRN, and Pe among children. Among existing studies, most have examined relatively small numbers of participants, who tend to range in age from middle childhood to adolescents. The current study sought to characterize response-locked ERPs recorded during a simple Go/No-Go paradigm in a large (N = 328) sample of 5- to 7-year-olds. This sample is part of a prospective study, and the data examined here are from the first assessment of the ERN, and will set the stage for further developmental analyses when ERNs are obtained at age 9 and beyond. The purpose of this initial study was, therefore, to characterize both psychophysiological and behavioral aspects of response monitoring and their relation to one another among this relatively large sample of younger children. To this end, we examine the relationships between the ERN (defined on error trials alone, as well as the difference between the ERN and CRN, or ΔERN), CRN, and Pe, behavioral measures of error detection and subsequent performance adjustments, and child sex. Although the age range of the current sample is narrower than previous studies, exploratory analyses examined the impact of age on ERP and behavioral measures. The purpose is to better understand both psychophysiological and behavioral aspects of response monitoring and the impact of child sex using a Go/No-Go task in a large sample of younger children.
METHODS
Participants
Participants included 413 children (54.5% male, 45.5% female) from a suburban community. The mean age of the children in the current study was 6.14 years (SD = .42 years, range = 5.15–7.57 years). Participants were originally recruited via a commercial mailing list. Eligible families had a child with no significant medical conditions or developmental disabilities, and at least one English-speaking biological parent. The vast majority of children were Caucasian (87.4%), came from two-parent homes (95.4%), had at least one parent who was a college graduate (69.3%), and had mothers who worked outside the home part- or full-time (53.0%). These data were collected as part of a larger prospective study examining precursors to depression and anxiety in young children and represent the first ERP assessment of this sample. The purpose of this study is to provide initial data characterizing the ERPs associated with response monitoring and will set the stage for future developmental analyses. Associations between response monitoring and individual difference variables that may be related to the development of depression and anxiety will be reported in a subsequent paper.
Psychophysiological Assessment
Task
A Go/No-Go paradigm adapted from Kim et al. (2007) and Torpey et al. (2009) was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, CA). The stimuli were green equilateral triangles, 1.5 cm on each side, in four different orientations. There were a total of 240 trials, which were divided into 4 blocks of 60 trials each. In each block, 60% of the triangles were vertically aligned and pointed upward, 20% were vertically aligned and pointed downward, 10% were tilted slightly to the left, and 10% were tilted slightly to the right. All stimuli were presented on a black background.
Each trial started with the presentation of one of the four triangles for 1,200 ms in the middle of the monitor. Following this, a small gray fixation cross was displayed in the middle of the monitor for between 300 and 800 ms before the next trial commenced with the presentation of a new triangle.
Procedure
After the EEG sensors were attached, the children were taken into the recording chamber and the child was instructed to sit in a large chair facing a computer monitor in the chamber. A series of practice blocks were administered to ensure that the participant understood the various aspects of the task. First, each of the stimuli was presented on a card to the child. Participants were instructed to press a button with their thumb only when the vertically aligned upward-pointing triangle was displayed (Go stimulus) and not to respond when the other three types of triangles (No-Go stimuli) or the fixation cross were presented. Participants were then presented with eight triangles (two Go stimuli, six No-Go stimuli) and were given as much time as necessary to decide whether or not to press the button. Each Go trial ended when the participant pressed the response button. For the No-Go trials, an experimenter advanced to the next trial only when it was clear that the participant understood not to press the button when those stimuli were displayed.
The next practice block contained 20 trials. In addition to the triangles and fixation cross, participants also received feedback after each trial consisting of a “thumbs-up” or “thumbs-down” stimulus presented in the middle of the monitor, indicating whether their performance was correct or incorrect on the preceding trial. In addition to helping the participants learn to differentially respond to the triangle stimuli, the feedback stimulus also emphasized the importance of speedy response: if participants did not respond to Go stimuli within 1,300 ms, the thumbs-down feedback was presented.
The final practice block (30 trials) was identical to the task, as described above; however, there was no feedback to indicate the veracity or speed of the participant’s responses on a trial-by-trial basis.1 Following completion of this practice block, the children were told that the actual game was going to begin and that for each block, they would earn one point for correct responses on Go trials and for withholding responses on No-Go trials. They were told that if they earned enough points, they could win up to $5.00. Speed of response was emphasized to the children. At the end of each block, the number of points won by the participant was displayed in white numbers. Between each block, the experimenter reviewed how many points they earned and reminded the children of the task instructions. Additionally, the importance of response speed was re-emphasized before each block commenced. Following completion of the task, all children were told that they won the maximum number of points and received $5.00.
Psychophysiological Recording
Data were acquired using the Active Two system (Biosemi, Amsterdam, the Netherlands). A stretch Lycra cap was placed on the child’s head and 32 Ag/AgCl-tipped electrodes arranged according to the American Electroencephalographic Society labeling system (1994). A small amount of electrolyte (Signa Gel; Bio-Medical Instruments, Inc., Warren, MI) was applied to the child’s scalp at each electrode position. Additionally, flat electrodes were placed at supra and infra orbital sites of the right eye to monitor vertical eye movements and on the outer canthi of the left and right eyes to monitor horizontal eye movements; an electrode was also placed on the tip of the nose. All data were sampled at 512 Hz. Per BioSemi’s design, the ground electrode during acquisition was formed by the common mode sense active electrode and the driven right leg passive electrode.
Offline, all data processing was performed with Brain Vision Analyzer (Brain Products, Gilching, Germany). EEG data were re-referenced to the nose, and high- and low-pass filtered at 1 and 30 Hz, respectively. From the continuous EEG, 1,500 ms segments were extracted beginning 500 ms prior to correct and erroneous responses. ERP data were corrected for blinks and eye-movements using the method developed by Gratton, Coles, and Donchin (1983). Additional artifacts were rejected when any of the following criteria were met: a voltage step of more than 50 mV between data points, a voltage difference of 300 mV within a single trial, or a voltage difference of <.5 mV within 100 ms intervals. Data were also visually inspected for any remaining artifacts. ERP averages were then created separately for each trial type (correct and error) and were baseline corrected by subtracting from each data point the average activity in a window −500 to −300 ms prior to the response. Trials were not included in ERP averages if the RT occurred outside of a 200–1,300 ms window.
Consistent with previous studies, the ERN and CRN were evaluated along the midline (Fz, Cz, and Pz) and were defined as the average voltage in the window from 0 to 100 ms after the response. As is discussed by Pailing, Segalowitz, Dywan, and Davies (2002), the ERN can be calculated by either averaging only the error-trial waveform or by subtracting the correct-trial waveform from the error-trial waveform (ΔERN). Measurements of the ERN alone (i.e., averages that include only the error-trial waveform) include processes common to both errors and correct responses that impact the ERN. Specifically, brain activity on error trials might reflect a combination of error-specific activity and activity that occurs on all trials due to button presses. Subtracting correct from error trials (ΔERN), on the other hand, removes components common to both correct and error responses, and results in neural activity specific to errors. However, the primary disadvantages to using the difference wave are that it eliminates shared activity and it is not possible to determine whether relationships with other variables are due to associations with correct responses or errors, or both. Accordingly, we evaluated the CRN, ERN, and error-specific activity in the time-range of the ERN by subtracting the average voltage on correct trials from the average voltage on error trials (i.e., ΔERN). The Pe was also evaluated along the midline and was defined as the average voltage in the window 200–500 ms following the response on error trials. ERP and behavioral data points that were ±3 SD from the mean were excluded from subsequent analyses. Behavioral measures were analyzed using repeated measures analysis of variance (ANOVA). All ERP components were statistically evaluated using repeated measures ANOVA with the Greenhouse–Geisser epsilon correction (Jennings & Wood, 1976) applied to p-values to counteract heterogeneity of variance–covariance matrices associated with repeated measures. Correlational and simultaneous regression analyses were used to examine associations between the behavioral measures, ERP components, and demographic variables.
RESULTS
As studies have found that six or more error trials are needed for a stable ERN in children (Pontifex et al., 2010) and adults (Olvet & Hajcak, 2009), data from 85 out of 413 (20.58%) children were excluded from further analyses (69 due to committing 5 or fewer errors, and 16 subjects due to having 5 or fewer artifact-free error trials). Due to technical errors, behavioral data from seven participants were lost; however, the ERP data for these subjects are included in the analyses. The ERP data for one participant were lost due to technical error; however, the behavioral data are included in the aggregate analyses. The ERP data from one additional participant were excluded due to values that were different from the grand mean by multiple standard deviations; however, this participant’s behavioral data are included in the aggregate analyses. This left a total of 321 subjects included in the behavioral analyses and 326 subjects included in the ERP analyses.2
Behavioral Measures
Participants committed an average of 16.18 errors (SD = 7.72) and had an accuracy rate of 88.29% (SD = 6.81%). These numbers provide different information: the number of errors refers only to errors of commission, whereas the percentage of correct trials accounts for both correct responses to Go stimuli and correct withholding of responses to No-Go stimuli. Participants had an average of 132.79 (SD = 13.71) correct responses on Go trials and 10.16 (SD = 11.27) errors of omission. Post hoc paired sample t-tests con-firmed that RTs on error of commission trials (M = 507.54 ms, SD = 84.84 ms) were significantly faster than RTs on correct Go trials (M = 626.50 ms, SD = 70.07 ms; t(1,317) = −34.76, p < .001). Post hoc paired sample t-tests indicated that RTs on correct trials that followed errors of commission (M = 654.36 ms, SD = 114.96 ms) were significantly slower than all correct Go trials (t(1,318) = 6.18, p < .001).
Response-Locked ERPs
Error-Related Negativity (ERN) and Correct-Response Negativity (CRN)
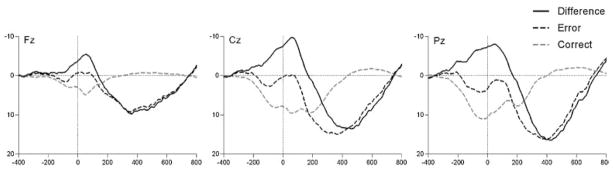
Response-locked ERPs for error and correct trials (as well as the error minus correct difference waveform) are presented in Figure 1 at Fz (left), Cz (middle), and Pz (right). At all midline electrode sites, the ERN was evident as a relative negative deflection approximately 50 ms after the commission of errors. The average ERP values for correct and error trials at the Fz, Cz, and Pz sites are presented in Table 1. Consistent with the impression from these data, a 3 (Region: Fz, Cz, and Pz) × 2 (Trial Type: Correct and Error) ANOVA confirmed that there was a significant main effect of Region, such that both error and correct trial averages were more negative at frontal-central sites, F(2, 628) = 99.78, p < .001. Consistent with the presence of the ERN on error trials, there was also a main effect of Trial Type, confirming that errors were associated with a greater negativity than correct trials, F(1,314) = 325.45, p < .001. Moreover, an interaction between Region and Trial Type confirmed that the difference between error and correct trials varied as a function of electrode site, F(2,628) = 56.60, p < .001. Post hoc paired sample t-tests indicated that the ERN was more negative than the CRN at all three electrode sites (t(1,314) = −11.44, p < .001 at Fz; t(1,314) = −19.47, p < .001 at Cz; and t(1,314) = −16.09, p < .001 at Pz). The difference between the error and correct trials (i.e., ΔERN) was larger at both Cz and Pz compared to Fz (t(1,314) = −12.13, p < .001; t(1,314) = −5.60, p < .001, respectively) and was significantly larger at Cz compared to Pz (t(1,314) = −4.16, p < .001), suggesting that the maximum difference between error and correct trials was at Cz. Consistent with this impression, the scalp topography of the error minus correct difference waveform in the time-range of the CRN/ERN suggests a maximum at Cz (Fig. 2, left).
FIGURE 1.
Response-locked ERP on error (dark dashed), correct (light dashed), and the error minus correct difference waveforem (solid) at Fz (left), Cz (middle), and Pz (right) midline electrode sites. Negative is plotted upward, and response onset occurred at 0 ms. The ERN is evident as a relative negative deflection approximately 50 ms after errors; the PE is the subsequent positivity peaking around 400 ms.
Table 1.
Mean (SD) of ERN and Pe Amplitude (μV) in Error Trials and Amplitude (μV) of Correct Trials at Midline Sites
| ERP Component | Electrode Site
|
||
|---|---|---|---|
| Fz | Cz | Pz | |
| ERN (N = 315) | −1.15 (7.13) | −.48 (8.12) | 1.09 (8.10) |
| Correct Trials Averaged (N = 315) | 3.91 (4.34) | 8.71 (5.44) | 8.85 (6.03) |
| Pe (Error Trials) (N = 308) | 6.23 (8.02) | 12.14 (9.31) | 13.28 (9.77) |
FIGURE 2.
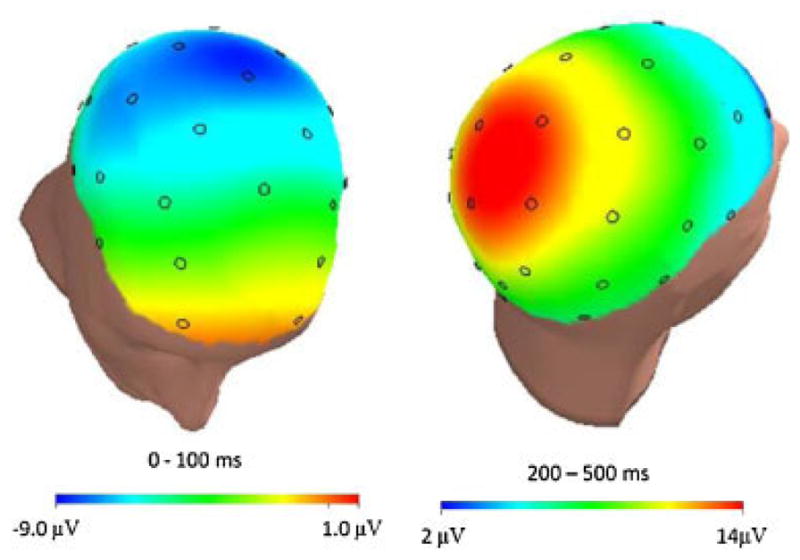
Scalp distribution of the difference between error and correct responses in the time-range of the ERN (i.e., 0–100 ms; left) and PE (i.e., 200–500 ms; right). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/dev]
Error Positivity (Pe)
In Figure 1, the Pe is evident as a large positivity following the ERN. The average Pe values at the Fz, Cz, and Pz sites are presented in Table 1. Consistent with the impression from these data, a 3 (Region: Fz, Cz, and Pz) × 2 (Trial Type: Correct and Error) ANOVA confirmed that there was a significant main effect of Region, in which both error and correct trial averages were more positive at central and posterior sites, F(2, 614) = 268.49, p < .001. Consistent with the presence of the Pe on error trials, there was also a main effect of Trial Type, confirming that errors were associated with a greater positivity than correct trials, F(1,307) = 375.42, p < .001. Moreover, there was an interaction between Region and Trial Type, such that the difference between error and correct trials varied as a function of electrode site, F(2,614) = 77.76, p < .001. Post hoc paired sample t-tests indicated that the Pe was more positive on error than correct trials at all three electrode sites (t(1,307) = 14.68, p < .001 at Fz; t(1,307) = 17.31, p < .001 at Cz; and t(1,307) = 20.29, p < .001 at Pz). The difference between the error and correct trials was larger at both Pz and Cz compared to Fz (t(1,307) = 10.25, p < .001, t(1,307) = 6.99, p < .001, respectively) and was larger at Pz compared to Cz, t(1,307) = 7.34, p < .001, suggesting that the maximum differentiation between errors and correct trials was at Pz for the Pe. The scalp distribution of the error minus correct waveform in the time-range of the Pe confirmed a parietal maximum (Fig. 2, right).
Correlations Between Behavioral Measures and ERP Components
Bivariate correlations were calculated between the behavioral measures and all ERP measures at the site of their maximum: ΔERN at Cz, ERN at Fz, CRN at Fz, and Pe at Pz. The Benjamini and Hochberg (1995) procedure was applied to each set of correlations between the ERP components and the nine behavioral measures to correct for multiple correlations.
Correlations Between ΔERN and Behavioral Measures
ΔERN was significantly correlated with most of the behavioral measures except for total correct Go trials and RT on correct Go trials following errors of commission (Tab. 2). More specifically, children with higher accuracy rates demonstrated a more negative ΔERN (r = −.23, p < .001). Similarly, correctly withholding responses on No-Go trials were also associated with a more negative ΔERN (r = −.20, p < .001), as were fewer total errors of commission and total errors of omission (rs = .15, p = .009 and .20, p = .001, respectively). Fewer total correct Go trials following errors of commission were associated with a more negative ΔERN (r = .13, p = .019). There were also associations with RTs, such that faster correct trial RT and RT on errors of commission had a smaller (i.e., less negative) ΔERN (rs = .14, p = .011 and .12, p = .035, respectively).
Because accuracy and speed typically improve with age, partial correlations were conducted in order to determine whether or not these associations remained significant when controlling for age. All of the associations remained significant, except for that between the ΔERN at Cz and Reaction Time on Correct Responses on Go Trials.
Correlations Between ERN and Behavioral Measures
The results of the analyses examining the correlations between the ERN and the behavioral measures are presented in Table 2. After correction for multiple tests, none of the correlations were significant.
Table 2.
Bivariate Correlations Between ERN and Pe Amplitude (μV) in Error Trials and Amplitude (μV) of Correct Trials at Midline Sites and Behavioral Measures With Outliers Removed
| ΔERN at Cz | ERN at Fz | CRN at Fz | Pe at Pz | |
|---|---|---|---|---|
| Total Correct Responses on Go Trials | −.07 (N = 308) | .01 (N = 308) | .10 (N = 311) | .15* (N = 307) |
| Reaction Time on Correct Responses on Go Trials | .14* (N = 312) | .02 (N = 312) | −.16* (N = 316) | −.20** (N = 311) |
| Total Correct No-Go Trials | −.20**(N = 314) | −.13 (N = 314) | .02 (N = 317) | .22*** (N = 313) |
| Total Errors of Commission | .15**(N = 308) | .10 (N = 308) | −.05 (N = 311) | −.25*** (N = 307) |
| Reaction Time on Errors of Commission | .12* (N = 313) | .01 (N = 313) | −.13 (N = 316) | −.21*** (N = 312) |
| Total Errors of Omission | .20** (N = 304) | .07 (N = 304) | −.09 (N = 307) | −.27*** (N = 303) |
| Total Correct Go Trials Following Errors of Commission | .13* (N = 311) | .08 (N = 311) | −.02 (N = 314) | −.18*** (N = 310) |
| Reaction Time on Correct Go Trials Following Errors of Commission | .11 (N = 313) | .06 (N = 313) | −.07 (N = 317) | −.13* (N = 312) |
| Total Accuracy | −.23** (N = 308) | −.14 (N = 307) | .06 (N = 310) | .27*** (N = 306) |
Correlations in bold remained significant following corrections for multiple tests and partial correlational analyses controlling for age.
p < .05.
p < .01.
p < .001.
Correlations Between CRN and Behavioral Measures
The results of the analyses examining the correlations between the CRN at Fz and the behavioral measures are presented in Table 2. After correction for multiple tests, only one correlation was significant: slower RT on correct trials was associated with a more negative CRN. Following a partial correlational analysis controlling for age, this association was no longer significant.
Correlations Between Pe and Behavioral Measures
The amplitude of Pe was significantly correlated with all behavioral measures (Tab. 2). Specifically, children with higher total accuracy demonstrated a more positive Pe (r = .27, p < .001). Similarly, higher rates of correct responses on Go and No-Go trials were also associated with a larger Pe (rs = .15, p = .01 and .22, p < .001, respectively), and more total errors of commission, total errors of omission, and total correct Go trials following errors of commission were all associated with a smaller Pe (rs = −.25, −.27, and −.18, all ps ≤ .001, respectively). Associations with RTs were also significant, such that children who responded faster on correct Go trials and on errors of commission demonstrating a smaller Pe (rs = −.20, p = .001, and −.21, p < .001, respectively). Additionally, faster RTs on correct Go trials following errors of commission were associated with a larger Pe (r = −.13, p = .020). However, after controlling for age, this last association was no longer significant.
Associations of Behavioral Measures With Child Sex and Age
Simultaneous regression analyses were used to explore the relationships of behavioral and ERP measures with child sex and age. The Benjamini and Hochberg (1995) procedure was applied to each regression equation to correct for multiple tests.
Table 3 depicts the results of the analyses examining RT on correct Go trials. Sex of child was associated with RT; specifically, girls were characterized by slower RTs on Go trials than boys. Child age was also significantly associated with RT: older children responded faster than younger children. The results of the analyses examining RT on errors of commission also appear in Table 3. Child sex and age were again associated with RT on errors of commission, such that girls responded more slowly than boys, and older children had faster RTs than younger children.
Table 3.
Simultaneous Regression Analyses Examining Associations Between Reaction Time on Correct Responses to Go Trials, Reaction Time on Errors of Commission, Reaction Time on Correct Go Trials Following Errors of Commission, Total Errors of Commission, Total Errors of Omission, Total Accuracy, ΔERN at Cz, ERN and CRN Amplitudes at Fz, and Pe Amplitude at Pz and Demographic Characteristics of the Sample
| Variables Entered | Reaction Time on Correct Responses to Go Trials (N = 319)
|
Reaction Time on Errors of Commission (N = 320)
|
Reaction Time on Correct Go Trials Following Errors of Commission (N = 320)
|
Total Correct Responses on Go Trials (N = 315)
|
Total Errors of Omission (N = 311)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | Standard Error | t | R2 | β | Standard Error | t | R2 | β | Standard Error | t | R2 | β | Standard Error | t | R2 | β | Standard Error | t | R2 | |
| Sex of child | .22 | 7.37 | 4.13*** | .21 | 9.00 | 3.99*** | .18 | 12.43 | 3.37** | −.06 | 1.35 | −1.12 | .15 | .90 | 2.73** | |||||
| Child age at assessment | −.30 | 8.44 | −5.76*** | .15 | −.30 | 10.31 | −5.77*** | .15 | −.26 | 14.26 | −4.88*** | .11 | .19 | 1.54 | 3.39** | .04 | −.28 | 1.03 | −5.25*** | .11 |
| Total Accuracy (N = 314)
|
ΔERN at Cz (N = 321)
|
ERN at Fz (N = 321)
|
CRN at Fz (N = 324)
|
Pe at Pz (N = 320)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | Standard Error | t | R2 | β | Standard Error | t | R2 | β | Standard Error | t | R2 | β | Standard Error | t | R2 | β | Standard Error | t | R2 |
| −.11 | 1.53 | −1.96 | −.02 | .92 | −.37 | −.05 | .81 | −.90 | −.04 | .50 | −.76 | .06 | 1.14 | 1.05 | |||||
| .21 | 1.77 | 3.74*** | .06 | −.16 | 1.05 | −2.90** | .03 | −.06 | .94 | −1.00 | .01 | .03 | .57 | .56 | .00 | .12 | 1.31 | 2.18 | .02 |
p < .01.
p < .001.
The results of analyses examining RT on correct Go trials following errors of commission are also presented in Table 3. Child sex and age were significantly associated with RT on trials following errors of commission, with girls and younger children responding more slowly than boys and older children, respectively.
Table 3 also depicts the results of analyses predicting total correct responses on Go trials. Age of child was associated with total correct responses on Go trials; specifically, older children had more correct responses on Go trials than younger children.
Additionally, Table 3 depicts the results of the analyses predicting total errors of omission, which were defined as responses occurring outside of the 200–1,300 ms window. Sex and age of child were associated with total errors of omission; specifically, girls had more errors of omission than boys and older children had fewer errors of omission than younger children.
Finally, the results of analyses examining total accuracy can be found in Table 3 as well. Child age was significantly associated with total accuracy, with older children being more accurate than younger children. Analyses examining total correct No-Go trials, total errors of commission, and total correct Go trials following errors of commission yielded no associations with child sex or age.
Associations of ERP Components With Child Sex and Age
Table 3 depicts the results of the analyses examining ΔERN amplitude. There was a significant effect of child age, indicating that older children demonstrated a larger (i.e., more negative) ΔERN than younger children. Table 3 also presents the results of analyses examining ERN amplitude. Neither child age nor sex was associated with ERN amplitude. Analyses examining CRN amplitude indicates there were no associations with child sex and age (Tab. 3). Analyses examining Pe amplitude indicate there were no associations with child sex and age (Tab. 3).
DISCUSSION
The current study used a simple Go/No-Go paradigm to examine ERP and behavioral measures of response monitoring in a sample of 328 5- to 7-year-old children. Consistent with our previous work using this task (Torpey et al., 2009), the ΔERN was both temporally and spatially similar to the ΔERN that has been described in the adult literature (e.g., Cavanagh & Allen, 2008; Johannes et al., 2001), peaking approximately 50 ms after errors with a central (i.e., Cz) maximum. Although the ΔERN difference was largest at Cz, both the CRN and ERN were most negative at Fz. This is consistent with other findings in adults (e.g., Brázdil et al., 2005; Hajcak & Simons, 2002; Holmes & Pizzagalli, 2008) and children (e.g., Kim et al., 2005).
There has been mixed evidence regarding whether or not the ERN is reliably more negative than the CRN among younger children (Davies et al., 2004; Kim et al., 2007; Santesso et al., 2006a; Wiersema et al., 2007) and only one published study to date has examined a sample this young (Torpey et al., 2009). The current results confirm the ERN is larger (i.e., more negative) than the CRN in young children if the task is simple enough.
Measurements of the ERN alone include processes common to both errors and correct responses that impact the ERN. Specifically, brain activity on error trials might reflect a combination of error-specific activity and activity that occurs on all trials due to button presses. Subtracting correct from error trials (i.e., ΔERN) is an attempt to isolate neural activity specific to errors. Despite the fairly narrow age range of the population, older children were characterized by a more negative ΔERN. These results cannot be directly compared to other studies that have examined the development of the ERN over the lifespan because they did not examine the ΔERN directly and conducted analyses on the ERN only (Davies et al., 2004; Kim et al., 2007; Santesso & Segalowitz, 2008; Santesso et al., 2006a; Wiersema et al., 2007). However, those studies demonstrated a similar relationship between the ERN and age, with older children exhibiting a more negative ERN. Although there were no source analyses conducted to confirm this, these results may be consistent with previous suggestions that these developmental changes are due to the later maturation of the ACC, from where the ERN is believed to originate (e.g., Brázdil et al., 2005; Mathalon et al., 2003; Mathewson et al., 2005). Notably, ΔERN was significantly associated with child age, such that older children were characterized by increased activity on error compared to correct trials; this association was not evident with either the ERN or the CRN alone. Like the ΔERN, we found that young children exhibit a Pe that is spatially and temporally similar to the Pe described in the adult literature (e.g., Falkenstein et al., 2000; Ullsperger & von Cramon, 2006), peaking between 200 and 500 ms after error commission and maximal at Pz—and like the ΔERN, the Pe increased with age in the current sample.
In addition to their association with age, both more negative ΔERN and a more positive Pe were related to better performance, as measured by greater overall accuracy, more total correct No-Go trials, and fewer errors of commission and omission. A more positive Pe was also associated with more correct responses on Go trials. In addition, a more negative ΔERN and a more positive Pe were both associated with faster RTs on error trials. Both the reinforcement learning and conflict monitoring models of the ERN predict that better performance ought to relate to a larger ERN (Holroyd & Coles, 2002; Yeung et al., 2004)—consistent with the current findings on the ΔERN.
However, the associations of the ΔERN with posterror accuracy do not support cognitive control hypotheses, which predict that a more negative ΔERN would be associated with increased posterror accuracy and RT (Holroyd et al., 2005; Kerns et al., 2004; Yeung et al., 2004). That is, an increased ERN ought to predict greater posterror compensatory behavior (e.g., Kerns et al., 2004). Nonetheless, ΔERN in the current study was associated with reduced posterror accuracy—and did not relate to posterror slowing. These data suggest that coupling between ΔERN and posterror compensatory behavioral adjustments may not be evident in younger children.
On the other hand, Pe was associated with increased posterror slowing and decreased posterror accuracy. Girls and younger children were characterized by increased posterror slowing, but not increased posterror accuracy. Further, the correlation between posterror slowing and posterror accuracy was negative (r = −.16, p = .006), which would indicate that more posterror slowing was associated with less accuracy on the subsequent trial. These findings are generally consistent with the view that both the Pe and posterror slowing may reflect an orienting response to errors (Notebaert et al., 2009; Ridderinkhof et al., 2009)—that is, both the Pe and posterror slowing may actually reflect an orienting response following relatively infrequent error commission, rather than a compensatory behavioral adjustment to improve performance following an error.
Associations between child sex and the behavioral measures suggested that girls were more cautious than boys. Specifically, boys had faster RTs than girls on both correct and error responses. This extends findings by Kim et al. (2007), who also demonstrated that boys had nonsignificantly faster RTs than girls, and that girls committed more errors of omission than boys. It is unclear whether these errors are absolute response omissions or if they are due to responses that were made outside of the 200–1,300 ms window, as there are no RTs available for errors of omission. Importantly, there were no significant differences between boys and girls in terms of total errors of commission, total correct responses on Go trials, total correct No-Go trials, or overall accuracy. Taken together, these findings may suggest that boys were able to complete the task with greater ease than girls. If, as was reported in a large meta-analysis by Else-Quest, Hyde, Goldsmith, and Van Hulle (2006), these differences in RTs are due to girls being higher in effortful control and lower in impulsivity, it would be expected that girls would demonstrate an enhanced ERN compared to boys but the data did not reflect this.
One of the primary limitations to the current analyses is that approximately one-quarter of the sample had to be excluded because these participants committed too few errors to generate a reliable ERN (Olvet & Hajcak, 2009; Pontifex et al., 2010). Although the age range of the participants in this study is relatively narrow, excluding so many children due to near-perfect performance suggests the difficulty of designing a task that is appropriately challenging for a young group that has such cognitive variability.
In conclusion, this study characterized the ERP components associated with response monitoring in a large community sample of 5- to 7-year-old children. Specifically, an ERN, CRN, and Pe were reliably elicited in these young children and were temporally and spatially similar to the ERN, CRN, and Pe that have been demonstrated in adults—even among this sample, the ERN was robustly more negative than the CRN. Moreover, the ΔERN and Pe were both related to better performance on the task—both in terms of low error rates and decreased RTs. Overall, children in the current study were faster on error than correct responses, and were characterized by significant posterror RT slowing. Even within our fairly narrow age range, older children were faster and more accurate, and had larger ΔERN and Pe amplitudes, and were characterized by decreased posterror slowing. ΔERN was unrelated to posterror slowing and was associated with reduced posterror accuracy; in fact, increased posterror slowing tended to predict worse posterror accuracy. Posterror slowing was predicted by a larger Pe—evidence that is consistent with the notion that both measures index an orienting response to errors. In the current study, girls had slower RTs overall and were characterized by more errors of omission, suggesting that they may have been more cautious than boys. However, we found no evidence of sex differences in ERP measures of response monitoring. We are currently collecting data from this sample at age 9 so that we can examine whether sex-related differences in response monitoring might emerge at later developmental periods (Davies et al., 2004), and to assess maturation-related changes in both behavioral and ERP measures of response monitoring.
Footnotes
If necessary due to poor performance, the children were shown the cards depicting the triangle stimuli and were instructed to tell the experimenter how they would respond to each stimulus.
Independent samples t-test indicated that children whose data were included in the final analyses were not significantly different from those children whose data were excluded in terms of child age. Chi square analyses confirmed that there were no significant differences between groups in the proportion of children with mothers who worked at least part-time outside of the home, who came from two-parent homes, and who had at least one parent with a college degree. There were also no statistically significant differences between the number of boys and girls or child race/ethnicity between the groups that were included and excluded from the final analyses.
References
- American Electroencephalographic Society. Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Arbel Y, Donchin E. Parsing the componential structure of posterror ERPs: A principal component analysis of ERPs following errors. Psychophysiology. 2009;46:1179–1189. doi: 10.1111/j.1469-8986.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Boksem MAS, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simple Go/NoGo task. Journal of Psychophysiology. 2005;19:244–255. [Google Scholar]
- Brázdil M, Roman R, Falkenstein M, Daniel P, Jurák P, Rektor I. Error processing—Evidence from intracerebral ERP recordings. Experimental Brain Research. 2002;146:460–466. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- Burgio-Murphy A, Klorman R, Shaywitz SW, Fletcher JM, Marchione KE, Holahan J, Shaywitz BA. Error-related event-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biological Psychology. 2007;75:75–86. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Allen JJB. Multiple aspects of the stress response under social evaluative threat: An electrophysiological investigation. Psychoneuroendocrinology. 2008;33:41–53. doi: 10.1016/j.psyneuen.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Chiu P, Deldin P. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Lin M, Vargas G, Carp J, Fineman SL, Quandt LC. Error detection and posterror behavior in depressed undergraduates. Emotion. 2008;8:58–67. doi: 10.1037/1528-3542.8.1.58. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Dywan J, Pailing PE. Error-negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biological Psychology. 2001;56:191–206. doi: 10.1016/s0301-0511(01)00080-1. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biological Psychology. 2010;84:257–263. doi: 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. Gender differences in temperament: A meta-analysis. Psychological Bulletin. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of cross-modal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: The broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Gehring WJ, Coles MGH, Meyer DE, Donchin E. The error-related negativity: An event-related brain potential accompanying errors. Psychophysiology. 1990;27:S34. [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. The Journal of Neuroscience. 2001;21:9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, Coles MGH, Cohen JD. A mechanism for error detection in speeded response time tasks. Journal of Experimental Psychology: General. 2005;134:163–191. doi: 10.1037/0096-3445.134.2.163. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. The epsilon-adjustment procedure for repeated-measures analysis of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, Dietrich DE. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging Section. 2001;108:101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulated conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Imashioya H, Uno H, Fujita T. Error-related negativity in a visual Go/No-Go task: Children vs. adults. Developmental Neuropsychology. 2007;31:181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-related negativity in children: Effect of an observer. Developmental Neuropsychology. 2005;28:871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Masaki H, Falkenstein M, Stürmer B, Pinkpank T, Sommer W. Does the error negativity reflect response conflict strength? Evidence from a Simon task. Psychophysiology. 2007;44:579–585. doi: 10.1111/j.1469-8986.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biological Psychology. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biological Psychology. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Van Opstal F, Gevers W, Fias W, Verguts T. Post-error slowing: An orienting account. Cognition. 2009;111:275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: On the functional significance of the Pe vis-á-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Pieters G, de Bruijn E, Mass Y, Hulstijn W, Vandereycken W, Peuskens J, et al. Action monitoring and perfectionism in anorexia nervosa. Brain and Cognition. 2007;63:42–50. doi: 10.1016/j.bandc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu CT, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ramautar JR, Wijnen JG. To PE or not to PE: A P3-like ERP component reflecting the processing of response errors. Psychophysiology. 2009;46:531–538. doi: 10.1111/j.1469-8986.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Roger C, Bénar CG, Vidal F, Hasbroucq T, Burle B. Rostral Cingulate Zone and correct response monitoring: ICA and source localization evidences for the unicity of correct- and error-negativities. NeuroImage. 2010;51:391–403. doi: 10.1016/j.neuroimage.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. Developmental differences in error-related ERPs in middle- to late-adolescent males. Developmental Psychology. 2008;44:205–217. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses in 10-year-old children and young adults. Developmental Science. 2006a;9:473–481. doi: 10.1111/j.1467-7687.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive disorder. Developmental Neuropsychology. 2006b;29:431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlated of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Simon-Thomas ER, Knight RT. Affective and cognitive modulation of performance monitoring: Behavioral and ERP evidence. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:362–372. doi: 10.3758/cabn.5.3.362. [DOI] [PubMed] [Google Scholar]
- Suchan B, Jokisch D, Skotara N, Daum I. Evaluation-related frontocentral negativity evoked by correct responses and errors. Behavioural Brain Research. 2007;183:206–212. doi: 10.1016/j.bbr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Klein DN. The impact of motivational influences on error-related brain activity in young children. Developmental Neuropsychology. 2009;34:749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. How does error correction differ from error signaling? An event-related potential study. Brain Research. 2006;1105:102–109. doi: 10.1016/j.brainres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vidal F, Burle B, Bonnet M, Grapperon J, Hasbroucq T. Error negativity on correct trials: A reexamination of available data. Biological Psychology. 2003;64:265–282. doi: 10.1016/s0301-0511(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’ specific to errors? Biological Psychology. 2000;51:109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet D, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, Roeyers H. Developmental changes in error monitoring: An event-related potential study. Neuropsychologia. 2007;45:1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]