Abstract
Clinical Research Coordinators (CRCs) are a vital component of the clinical research enterprise providing a pivotal role in human subject protection through the numerous activities and responsibilities assigned to them. In 2006, the National Institutes of Health’s National Center for Research resources (NCRR) implemented the Clinical and Translational Science Awards program (CTSA) to advance biomedical research. As a part of this endeavor, many workgroups were formed among the Consortium to support translational research. The Research Coordinator Taskforce was created as part of the Regulatory Knowledge group of the Clinical Research Innovation Key Function Committee, and focuses on enhancing CTSA capabilities to provide support and training for CRCs. In the spring of 2008, this taskforce conducted two surveys of the then 24 CTSA Consortium members to better understand the current expectations and responsibilities of research coordinators in addition to the mechanism for providing education, training, and support in order for CRCs to successfully meet the study responsibilities placed upon them. The results of these surveys are summarized in this article and provide context to the recommendations of the Research Coordinator Taskforce for institutional considerations, approaches, and best practices for providing education, training, and support the expanding role of CRCs in fulfilling their responsibilities delegated to them by investigators. Clin Trans Sci 2012; Volume 5: 470–475
Keywords: clinical research coordinator, training and support, CTSA
Introduction
Clinical Research Coordinators (CRCs) are an essential component of the clinical research enterprise within an Academic Health Center (AHC), serving a pivotal role in human subject protection through the numerous activities and responsibilities assigned to them, and serving as the liaison between the investigators and the human research subjects, clinical care providers, regulatory bodies, sponsors, and numerous others involved in the research process. It is important to recognize that while CRCs assume many responsibilities of the study which are delegated to them by the clinical investigators; it is the investigator, according to FDA regulations, who ultimately bears the responsibility for the conduct of the research study. 1 Indeed, many of the responsibilities needed to conduct a study may be delegated to the research coordinator. Given that the investigator is ultimately responsible for the conduct of the study, it is imperative that the investigators together with the AHC ensure that the research coordinators receive adequate training and support to carry out each of the tasks assigned to them. Provision of adequate training and support to the research coordinator is critical to the overall goal of human subject protection at a given institution.
In this report we provide an overview of the multifaceted, expanding role of the CRCs, highlight issues surrounding inadequate support and training that AHCs should consider, present data on the current training and support and general job satisfaction of CRCs at Clinical and Translational Science Awards (CTSA) centers, and finally discuss approaches to support the retention of CRCs through certification programs, promotion of career paths, and networking opportunities for CRCs within an institution.
Background
NIH/AHC partnership and the CTSA Research Coordinator Taskforce
In 2006, the National Institutes of Health’s National Center for Research Resources (NCRR) launched The CTSA program to support a national consortium of medical research institutions designed to transform how biomedical research is conducted. The goals of the CTSA are to speed the translation of laboratory discoveries into treatments for patients, to engage communities in clinical research efforts, and to train a new generation of clinical and translational researchers. 2
The CTSA consortium began in 2006 with 12 AHCs and since expanded to 60 institutions. The objectives of this initiative include enhancing interinstitutional research collaboration, identifying and fostering best practices, policies, procedures, and other measures, in order to advance and support clinical and translational research. As part of the CTSA Consortium’s effort to enhance translational research, a variety of working groups and taskforces were created. The Research Coordinator Taskforce is part of the Regulatory Knowledge group of the Clinical Research Innovation Key Function Committee, and focuses on enhancing CTSA AHC capabilities to provide support and training for CRCs. In the spring of 2008 the Research Coordinator Taskforce conducted two surveys of the then 24 CTSA Consortium members to better understand the current expectations and responsibilities of the research coordinators in addition to the mechanism for providing education, training, and support in order for CRCs to successfully meet the study tasks placed upon them. The results of these surveys are summarized in this paper and provide context to the recommendations of the Research Coordinator Taskforce for institutional considerations, approaches, and best practices for providing education, training, and support to CRCs in fulfilling their responsibilities delegated to them by investigators.
The expanding role of the clinical research coordinator (CRC)
The role of the CRC expanded well beyond the original concept of clinical management of the research subject ( Table 1 ), where primary tasks were to recruit patients, obtain informed consent, manage research studies, and collect the data. Protocols are progressively more complex, and regulations intended to support research subject safety and study data integrity have continued to evolve. As a result, the CRC’s role is more sophisticated and responsibilities expanded to include legal and regulatory compliance, budget preparation and financial management, quality assurance, database management, and investigational product accountability. The continual layering of additional responsibilities onto the role of the CRC provides increasing burdens that could adversely affect their primary role of research subject management. These additional responsibilities have the potential to cause gaps in research subject protections causing risks not only to the research subjects, but also to principal investigators and research institutions.
Table 1.
Typical “Core” and “Additional” responsibilities of the CRC.
| Core CRC Responsibilities | Additional CRC Responsibilities |
|---|---|
| · Adherence to an IRB approved protocol | · Submissions to regulatory authorities (e.g., IRB, FDA, etc.) |
| · Participation in the proper consenting of study subjects | · Regulatory documentation development and management |
| · Support of the safety of clinical research subjects | · Completion of case report forms (paper & electronic data capture) |
| · Coordination of clinical treatment, study visits, and follow‐up care | · Coordination of pre study, initiation, and monitoring visits |
| · Subject screening, recruitment, and enrollment | · Collection, processing, and shipping of laboratory specimens |
| · Maintenance of study source documents | · Maintenance of drug accountability documentation |
| · Proper reporting of adverse events | · Study budget preparation |
| · Management of study finances including resolving study subject billing issues | |
| · Acting as liaison for research subject, investigator, IRB, sponsor, and healthcare professionals |
AHCs and investigators need to be aware that many of the tasks assumed by today’s CRCs are, in the eyes of the regulatory authorities, the responsibility of the principal investigator. Indeed, the FDA defines an investigator as a person responsible for the conduct of a clinical drug or device study and outlines investigator responsibilities to include:
-
•
Assurance of local IRB review and approval and ongoing communication with the IRB
-
•
Compliance with protocol
-
•
Control of investigational product
-
•
Informed consent of trial subjects
-
•
Protect the rights, safety and welfare of clinical trial subjects
-
•
Safety reporting to the sponsor and local IRB
-
•
Progress and final reports to the sponsor and local IRB
-
•
Record maintenance and retention
-
•
Management of site‐based finances
As seen in Table 1 , each of the above investigator responsibilities is typically assigned to the research coordinator. Thus, given the responsibilities and importance of the CRC role in clinical research management and human subjects’ protections, and the current trends of increasing administrative burdens and responsibilities of CRCs, it is important for research institutions to consider transforming approaches to research management in order to decrease burdens and enhance effectiveness of research teams. The following report provides some ideas offered by the CTSA CRC Taskforce to that end.
Methods
In order to assess the question of what education and training programs and support are available to CRCs, the Taskforce conducted two surveys in 2008 of the then 24 CTSA awardees: (1) The institutional‐directed survey was designed to evaluate the institutional management and development of CRCs and (2) The individual survey directed to the CRCs at each CTSA institution was designed to evaluate education and background, responsibilities, training and support, and overall satisfaction with their career. Both surveys were programmed into a web‐based application and distributed during a 3‐month period from June through August 2008. The Institutional Survey was sent to a representative from each of the 24 CTSA awardees at that time, and the individual CRC survey was distributed via the representative to all known CRCs at the site.
Responses were anonymous and as such, this study was deemed exempt from IRB review in accordance with 45 CFR 46. 101 (B) (2). The results of the survey questions were categorized by topic area and are presented in targeted sections in the manuscript. The data were then summarized using descriptive statistics and are presented in figures and tables.
Results
Twenty‐two of the 24 CTSA sites responded to the institutionally directed survey and 1,597 coordinators responded to the CRC‐directed survey.
Demography
The majority of the AHC surveyed had a similar sized CRC workforce ranging from 13 to 1500 CRCs, with a mean and a median number of 385 and 350 coordinators, respectively. Approximately 37% of the CRCs reported less than 3 years of experience ( Figure 1 ), with 16% having 1 year or less experience. Results indicate that almost one half of the CRC workforce at AHCs had greater than 5 years of experience.
Figure 1.
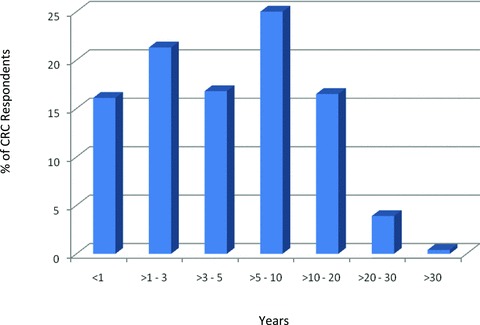
Years as a research coordinator.
The Institutional Survey suggests that approximately 39% of the CRC work force holds an RN degree with 50% of those RN’s holding a Master’s level degree or higher. Data from the individual survey closely mirror these results with approximately 33% of the responding CRCs holding an RN degree and 37% of the RNs with a Masters or doctoral degree.
Hiring practices
Most open CRC positions (approximately 60%) take an average of 3–6 months to fill with almost all positions being filled within 9 months. The majority of CRCs are hired and managed by individual principal investigator or departments with only about 20% of institutions reporting that they have or will be implementing a central CRC recruitment program.
Most of the academic centers surveyed hire study coordinators from internal sources with 40% hired into new positions within the institution where they are currently working and 24% from another academic center. The following pie charts ( Figure 2 ) show the most typical sources of newly hired CRCs and the typical careers to which CRCs move when leaving a CRC position. Figure 2B illustrates that approximately half of CRCs leaving a position move to positions either outside of academia or leave research altogether.
Figure 2.
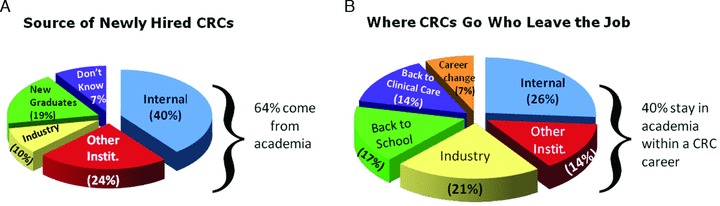
CRC hiring practices and career changes.
These data show the conventional assumption that CRCs mainly leave academia for more lucrative positions in the pharmaceutical industry is an oversimplification: 21% leave for industry positions however, 10% actually come from industry, suggesting a boomerang effect. Approximately one‐third (31%) of CRCs either go back to clinical care or return to school.
Job responsibilities
The majority (80%) of the coordinators surveyed were scheduled to work 40‐hour weeks, however, 42% of these coordinators reported working in excess of their scheduled 40 hours per week, with 21%, 16%, and 5% working between 40–45 hours per week, 46–50 hours per week, and greater than 50 hours per week, respectively.
The total number of studies supported by the 1,574 respondents was 9,842, with an individual coordinator supporting from 1 to 85 studies, for a mean of 7.6 studies per coordinator. The total number of investigators supported by the 1,574 CRC respondents was 5,262, ranging from 1 to 50 investigators per coordinator, for a mean of 3.7 investigators per coordinator. Of those CRCs who supported multiple PIs, approximately 46% found managing multiple investigators difficult and 62% reported that their PIs expected them to spend more time on their studies than they were allotted.
When queried about responsibilities for their studies, greater than 75% of respondents reported the following ( Figure 3 ): managing study files/regulatory files, adverse event reporting, informed consent, managing subject visits, responding to data queries, conducting study visits, patient scheduling, subject recruitment, IRB submission and study training, case report form (CFF) completion, and study document development.
Figure 3.
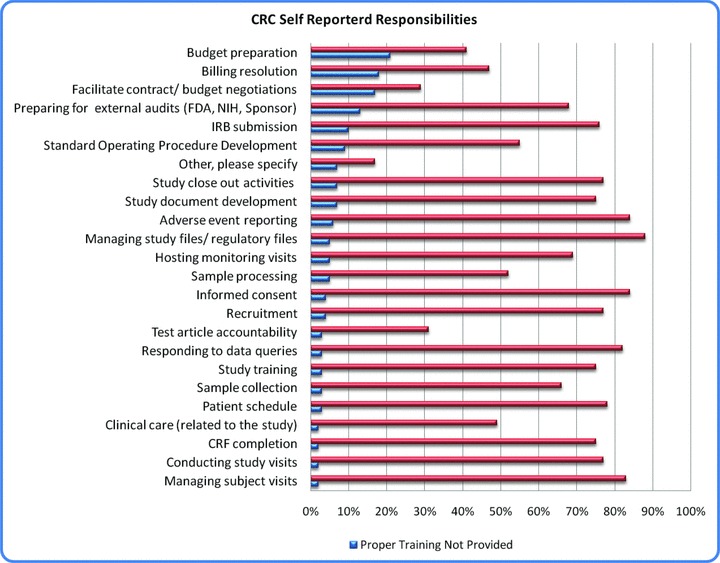
CRC self‐reported responsibilities.
Coordinators were asked to indicate which tasks requested of their position were believed to be outside of their job responsibilities. Overall, 68% of coordinators specified that tasks were assigned to them appropriately. The CRC survey respondents indicated that the three most common tasks for which they did not have experience and training to manage were billing, budget preparation, and contract negotiation.
Training and education
The Institutional Survey indicates that 100% of the 22 responding CTSA academic centers currently provide training for the newly hired CRC and 90% provide continuing education for the CRC. The type, length and frequency of training, both orientation and continuing education, vary widely from one institution to another (other, i.e., informal mentoring program, “brown bag” seminars, educational classes; see Figure 4 ).
Figure 4.
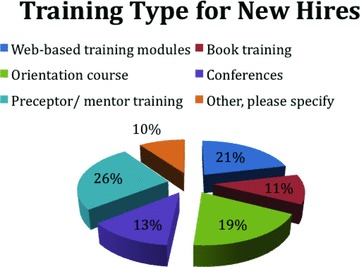
Types of training provided for newly hired CRCs.
Based on survey results, 45% of CRCs reported they received appropriate training for all the tasks they were required to do. However, gaps in initial and ongoing training were also reported. The majority of institutions (61%) with formalized education offer on‐line, full‐day/or longer in‐house education and written training programs.
CRC satisfaction
Greater than 75% of CRCs responding to the survey described their work as both professionally and personally fulfilling, and 85% believe their job is an important aspect of the overall mission of their institution (See Table 2 ). However, 41% of CRCs reported that there is no opportunity for career advancement or development, and 51% indicated that they do not receive a fair salary for what they do. Sixty‐two percent (62%) of CRCs reported that their investigators expect them to work more time on their studies than the CRCs are allotted. Being overworked was reported as one of the top negative aspects about their job and 15% of respondents listed burnout as a reason for leaving their job.
Table 2.
CRC job satisfaction findings (% responses).
| Top positives about the CRC job | |
| Patient/Subject interaction | 29% |
| Multitasking/diversity/variety | 18% |
| Contribution to medical advancement | 16% |
| Flexibility and autonomy | 15% |
| Top negatives about the CRC job | |
| Overworked and inadequate pay | 20% |
| Paperwork, budgets, billing | 14% |
| Top motivations for leaving the job | |
| Better salary | 23% |
| Career advancement | 17% |
| Burnout | 15% |
The most commonly reported day to day work‐related issues that hinder CRC responsibilities were logistical in nature and included lack of storage and/or office space, inadequate or malfunctioning equipment (computers, fax machines), location of an office not near a clinic requiring commuting time, and excessive noise. CRCs reported a need for additional support with clerical work (answering phones, copying, faxing, filing, and paying bills) and recruitment of study participants.
Concerning receiving recognition for their work, 66% of the CRC’s noted that when they do a good job, they receive praise and recognition and 72% indicated that their ideas and opinions were valued. Sixty‐one percent (61%) indicated that information and knowledge is shared openly in their work environment.
Lack of career advancement was reported as one of the top reasons for leaving the position of CRC. Sixty‐three percent (63%) of surveyed institutions do not have programs for CRC development or recognition, though half of those are planning such programs. The data from the survey of individual coordinators supports the importance of institutions creating a program for CRC development and recognition.
Discussion
The continual layering of additional responsibilities onto the role of the CRC provides increasing burdens that could adversely affect their primary role of managing their research subjects. Institutions need to consider that overburdening the CRCs has the potential to cause gaps in research subject protections resulting in risks not only to the research subjects but also to principal investigators and research institutions, ultimately leaving an institution vulnerable to severe regulatory and financial consequences. Data from the CTSA Research Coordinator survey suggest that nearly 50% of coordinators are not able to complete their tasks within their allotted 40 hours per week and individual coordinators are responsible for supporting an average of 3.7 investigators and 7.6 studies. Additionally, 62% of coordinators report that their PIs expect them to spend more time on their studies than they are allotted. Proper support, education, and recognition of the role of the CRC are critical to for a successful and compliant clinical research infrastructure within an AHC. As such, the CTSA Research Coordinator Taskforce has examined the expanded CRC role and offers critical points for AHCs to consider when identifying how an AHC can best provide support and training to its CRCs. Approaches to programs that ensure adequate training and support of the coordinator, recognition of the CRC as a profession and models for networking coordinators at a site are further described later.
Training and education
Anecdotal reports reveal that historically, many CRCs transitioned into their position as an adjunct to a clinical or administrative position. Preparatory and ongoing training for the CRC was “on the job” and “learn from your mistakes.” The past decade saw an increased focus on defining the scope, role, and responsibilities of the CRC and a recognition of how pivotal the CRC is to the overall success of study conduct and outcomes. Concurrent with this evolution was the need to provide appropriate job training to arm the CRC with the tools to be successful in the execution of their job responsibilities. Numerous training programs for clinical research staff, including CRCs, blossomed in the private sector. Although these external training programs provided valuable information, they were not inclusive of all responsibilities assigned to the CRC role. The cost of external programs may be a driving force for some academic institutions to establish their own internal CRC training programs.
The majority of institutions surveyed do provide training programs for CRCs. To bolster such efforts, the Taskforce recommends that institutions conduct a gap analysis of their training programs to determine areas of weakness or additional needs in CRC training. This effort should include a focus on CRC core competencies and career development. The focus on core competencies should include an evaluation of current CRC job descriptions and career ladders. Such efforts are important to both advancing the skills and capabilities of CRCs and to CRC retention.
Rethinking the investigator‐CRC “business unit”
The historical model of a principal investigator and CRC as a self‐contained business unit for managing a clinical research study was spawned in an era of less complex studies and fewer regulatory requirements. As research complexity, oversight needs, and regulatory requirements have increased, the ever‐expanding role of the CRC was a predictable outcome. In “Pulling the Plug” Jim Collins 3 suggests that while great companies have a lot on their “to do” list, they should consider what to stop doing. In other words “What is it that we are doing that diverts us from making progress?” Part of incorporating that concept into transforming approaches to research management includes doing away with tasks that do not add value, and streamlining or off‐loading tasks that divert CRCs from their primary role in research subject management. When considering all the tasks delegated to the CRC and the number of projects assigned to an individual coordinator, it becomes evident that one person or one position should not be responsible for managing all aspects of the project. The challenge to AHCs in designing such a transformation in research coordination and management is how to do this in a cost effective manner.
One approach is to expand the definition of the “business unit” from individual investigator–coordinator teams to department or division enterprises. Such an approach supports resource sharing of personnel focused on specialized administrative tasks such as maintenance of regulatory files, protocol submission to IRBs and ancillary review bodies, and resolution of billing discrepancies. Such shared subspecialization provides a cost effective means of off‐loading administrative tasks that divert CRCs from their main role in managing research subjects and protocol requirements. Part of expanding the notion of “business unit” to an entire department or division includes identifying those necessary administrative processes that are common to all clinical research studies so that such processes can be standardized across entire departments thus alleviating individual investigator‐CRC teams from “reinventing the wheel” when implementing such processes. Such focused and thoughtful standardization provide a means to enhance the ability of teams to cross‐cover for each other and to share resources, and should also shorten orientation and training time for newly hired CRCs and study research staff.
Professional development of CRCs
Historically, CRCs work for individual investigators and typically lack an institutional identity or institutional recognition that they are part of an important research profession. In order to further develop CRCs in their profession and give them an identity and voice in evolving and enhancing clinical research management practices, some academic centers have developed an institutional approach to networking and organizing their CRCs. Through a research coordinator society or network, an institution can enhance the quality of clinical research programs by enriching the training and education of clinical research personnel.
Additionally, institutions may consider providing support to cover the cost of CRC professional certification. Two well‐known professional organizations offer such certification, ACRP (Association of Clinical Research Professionals) and SoCRA (Society of Clinical Research Professionals). Both organizations offer for‐fee membership and certification and require continuing education or contact hours to maintain the professional certification. However, the cost associated with these professional societies is often an important barrier to seeking such certification by the CRCs. When considering means of supporting the professional development of the CRC, whether through establishment of formal CRC networks or offering partial or full subsidy for professional CRC certification, the AHC may consider the advantages of such programs. Through professional development, CRCs gain knowledge and expertise that serve not only to advance their careers, but to enhance their performance and skills, and ultimately will help improve the practice of clinical research at an institution.
Concluding Remarks
The expanded role and resulting increased burden placed on today’s CRCs can have a direct impact on the safety of human research subjects and resulting consequences to the investigator and ultimately the AHC if adequate support and training are not provided to allow the CRC to carry out these tasks successfully. Ultimately, the responsibility for the conduct and oversight of a clinical study lies with the investigator. As detailed in recent FDA Warning letters sent to clinical investigators, common findings cited include:
“ You failed to personally conduct or supervise the clinical investigation [21 CFR 312.60] While you may delegate certain study tasks to individuals qualified to perform them, as a clinical investigator you may not delegate your general responsibilities. Our investigation indicates that your supervision of personnel to whom you delegated study tasks was not adequate to ensure that the clinical trial was conducted according to the signed investigator statement, the investigational plan, and applicable regulations, and that these trials were conducted in a manner that protects the rights, safety, and welfare of human subjects.”
We note that your failure to adequately supervise this study led to significant problems with the conduct of the study as mentioned below, which included enrollment of subjects who did not meet eligibility criteria, and failure to follow safety monitoring procedures….” 4
Additional warning letter to a clinical investigator note:
“Specifically, you failed to adequately supervise the study staff to whom you delegated tasks. Many, if not all, of the other violations listed in this letter are traceable to your failure to adequately supervise staff and the conduct of the investigation.” 5
In rare cases are the research coordinators themselves cited on the FDA warning letters.
These excerpts from recent FDA warning letters to clinical investigators strengthen the argument for investigators and AHCs to work together to ensure that their CRC staff are well trained and supported. The CTSA Research Coordinator Taskforce remains committed to working toward these goals to assist AHCs in providing best practice approaches for CRC education, training, and support to help ensure robust protection of human research subjects and to mitigate regulatory risks.
Acknowledgment
This project has been funded in whole or in part with Federal funds from the National Center for Research Resources (NCRR), National Institutes of Health (NIH), through the Clinical and Translational Science Awards Program (CTSA), part of the Roadmap Initiative, Re‐Engineering the Clinical Research Enterprise. The manuscript was approved by the CTSA Consortium Publications Committee.
On behalf of the CTSA Research Coordinator Taskforce
References
- 1. Title21, Code of Federal Regulations–21 CFR–Part 312 and 814.
- 2. The Clinical and Translational Science Awards (CTSA) Consortium website. Available at https://www.ctsacentral.org/ Accessed May 25, 2012.
- 3. Pulling the Plug–Jim Collins–March 1997. http://www.jimcollins.com/article_topics/articles/pulling‐the‐plug.html. Accessed May 25, 2012.
- 4. FDA Clinical Investigator Warning Letter Ref: 11‐HFD‐45‐02‐02. Available at: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2011/ucm244485.htm. Accessed May 25, 2012.
- 5. FDA Clinical Investigator Warning Letter Ref: 11‐HFD‐45‐02‐04. Available at: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2011/ucm248622.htm. Accessed May 25, 2012.


