Abstract
BRCA1 and BRCA2 dysfunction, frequently seen in high-grade serous ovarian carcinomas, often results from germline mutations, somatic mutations, and promoter methylation. Identification of tumors with BRCA defects has therapeutic and prognostic implications. Identifying germline BRCA mutations is also important given the increased risk for hereditary breast and ovarian carcinoma. Our goal was to assess if immunohistochemistry for BRCA1 is an effective method for detection of BRCA1 dysfunction in molecularly characterized high-grade ovarian serous carcinoma. We identified 43 high-grade ovarian serous carcinomas with known events in BRCA1 and BRCA2 included in The Cancer Genome Atlas Project (TCGA). BRCA1 stain was first assessed without knowledge of BRCA status, and a semiquantitative assessment for intensity and amount of staining was performed. The stains were re-evaluated and divided into 3 categories (retained, loss, and equivocal) based on correlation with genotyping data. Presence of retained BRCA staining was considered normal, while the other patterns, including equivocal staining or loss of staining, were considered abnormal. Two pathologists, blinded to BRCA status, then scored 2 sets of validation cases selected based on available molecular data—one with only germline mutation status available (n=31) and one with comprehensive genomic data (n=39). The pathologists agreed 88% of the time in the training set and 91% in the validation sets. In the training set, abnormal BRCA staining was seen in 24 cases, of which 21 (87%) showed BRCA1 genetic abnormalities, one showed BRCA2 mutations, and 2 showed no BRCA abnormalities. Abnormal BRCA1 staining was noted in all 5 cases with BRCA1 germline mutations, in 3 (60%) of 5 with BRCA1 somatic mutations, and in 13 (93%) of 14 with BRCA1 promoter methylation. The 2 validation sets included 70 additional patients, and all cases with germline BRCA1 mutations (n=11) showed abnormal BRCA1 staining. Tumors with BRCA1 promoter methylation also showed abnormal staining in 6 (86%) of 7 cases. In the entire study, no cases with BRCA1 germline mutation showed intact immunostaining (negative predictive value= 100%). This study shows BRCA1 immunohistochemistry is well correlated with molecular events in ovarian carcinoma. Considering the high negative predictive value for germline mutations, BRCA1 immunohistochemistry appears to be an effective approach to stratify patients for germline genetic testing and to detect other mechanisms of BRCA1 dysfunction in high-grade serous ovarian carcinomas.
Keywords: BRCA1, BRCA1 immunohistochemistry, ovarian carcinoma, protein expression, serous carcinoma
Introduction
Patients with ovarian cancer usually present at advanced stages and have high rates of morbidity and mortality. High-grade serous carcinoma accounts for the majority of these cases (1). There are many risk factors for ovarian high-grade serous carcinoma, including BRCA1 and BRCA2 dysfunction. BRCA dysfunction frequently results from germline mutations, as seen in hereditary breast and ovarian cancer syndrome. Germline mutations in BRCA1 and BRCA2 are detected in 14.5% of serous ovarian carcinomas (2). Other mechanisms of BRCA dysfunction include somatic mutations and promoter methylation. Methylation of the BRCA1 promoter has been found in 11% of ovarian cancers (2), and somatic mutations of BRCA1/BRCA2 have been found in 6% of high-grade ovarian cancers (2). BRCA dysfunction appears to be mostly confined to high-grade serous carcinomas of the ovary and does not appear to be associated with increased risk for other histologic subtypes of ovarian cancer, including clear cell, mucinous, and endometrioid carcinomas.
The presence of BRCA germline mutations in ovarian carcinomas has been shown to have prognostic and therapeutic significance, including increased sensitivity to platinum-based chemotherapy and susceptibility to PARP (polyADP-ribose polymerase) inhibition. BRCA1 and BRCA2 are critical components of double-strand DNA break repair by the conservative mechanism of homologous recombination (HR). Loss of BRCA function results in DNA repair by alternative non-conservative mechanisms, which are prone to error, leading to genomic instability and increased cancer susceptibility. BRCA defective cancers are therefore highly susceptible to DNA damaging chemotherapeutic agents such as platinum analogs (3, 4).
PARP is important for the repair of DNA single-strand breaks. Inhibition of PARP leads to accumulation of single-strand breaks that are converted to double-strand breaks during replication; these are normally repaired by the HR mechanism. In the absence of intact HR (as with BRCA dysfunction), there is accumulation of irreparable double-strand DNA breaks, resulting in increased sensitivity to PARP inhibitors (3).
In addition to implications for therapy, defects in BRCA function also have prognostic significance in ovarian carcinomas. The presence of BRCA germline mutations has been shown to correlate with improved clinical outcomes; this may be attributable to younger patient age, ability to achieve optimal debulking, and/or increased sensitivity to platinum agents (3–5). Detection of BRCA germline mutations has important implications for patients and their family members; they may benefit from genetic counseling regarding their increased risk for breast and ovarian carcinoma. It also appears that somatic mutations in BRCA1 and BRCA2 may confer improved clinical outcomes, similar to those seen with BRCA germline mutations (5).
For the reasons outlined above, the identification of ovarian cancers with defective BRCA is clinically relevant and therapeutically important. However, BRCA mutation analysis is cumbersome, expensive, and may be impractical as a screening method for detection in all patients. Moreover, patients with BRCA promoter methylation and other mechanisms of loss are not identified using current approaches.
Immunohistochemistry (IHC) is an easy and inexpensive technique that is available to most pathologists, and has been incorporated as a routine test for screening for other hereditary syndromes, like Lynch syndrome in colorectal cancer, and more recently, endometrial cancer (6–9). A large study showed that screening with IHC for DNA mismatch repair proteins, followed by confirmatory tests such as mutation analysis or hypermethylation studies, is the most cost-effective method for detection of Lynch syndrome patients (10).
We similarly hypothesize that IHC for BRCA1 could serve as a screening test for detection of BRCA1 dysfunction. This would provide an efficient and low-cost initial screen to select patients for targeted therapies, specifically PARP inhibition. This methodology will also narrow the pool of patients who may benefit from screening for hereditary cancer syndromes and should provide prognostically relevant information.
Materials and Methods
After Institutional Review Board (IRB) approval, we identified a training set of 43 high-grade ovarian serous carcinomas included in The Cancer Genome Atlas (TCGA), selected for known events in BRCA1 and BRCA2.
Genetic subgroups represented included the following:
BRCA1 germline mutation (n=5)
BRCA1 somatic mutation (n=5)
BRCA1 promoter methylation (n=14)
BRCA2 germline mutation (n=5)
BRCA2 somatic mutation (n=3)
BRCA unaffected (n=11)
The IHC staining method for BRCA1 was optimized using the antibody in a variety of different conditions until one was chosen based on its ability to discriminate different staining patterns in a small group of ovarian cancers with known BRCA genotype. One whole section from each of these 43 high-grade serous ovarian carcinomas was evaluated with a commercially available monoclonal antibody against BRCA1 (Ab-1) clone MS110 (mAb) from Calbiochem (Catalogue number OP92). Whole sections (and not TMAs) were used. Heat retrieval was performed with steaming with EDTA pH 8 for 30 minutes. This was followed by incubation of primary antibody for 30 minutes at room temperature (dilution 1:100), followed by incubation with labeled polymer from Envision TM+ System HRP (Dako) for 30 minutes at room temperature. DAB (3,3'-diaminobenzidine) was used as counterstain.
BRCA1 staining was first assessed by a single pathologist (RAS) without knowledge of BRCA status. On initial review, a semiquantitative assessment for intensity and amount of staining was performed. The intensity of staining was recorded relative to that seen in internal positive control. The stains were then re-evaluated with knowledge of the BRCA status, and a cutoff separating tumors into distinct groups based on genotype was selected. In other words, semiquantitative assessment was used to develop categorical criteria for scoring cases as BRCA1 loss, BRCA1 retained, or BRCA1 equivocal.
The staining pattern was recorded as BRCA1 loss, BRCA1 retained, or BRCA1 equivocal as follows:
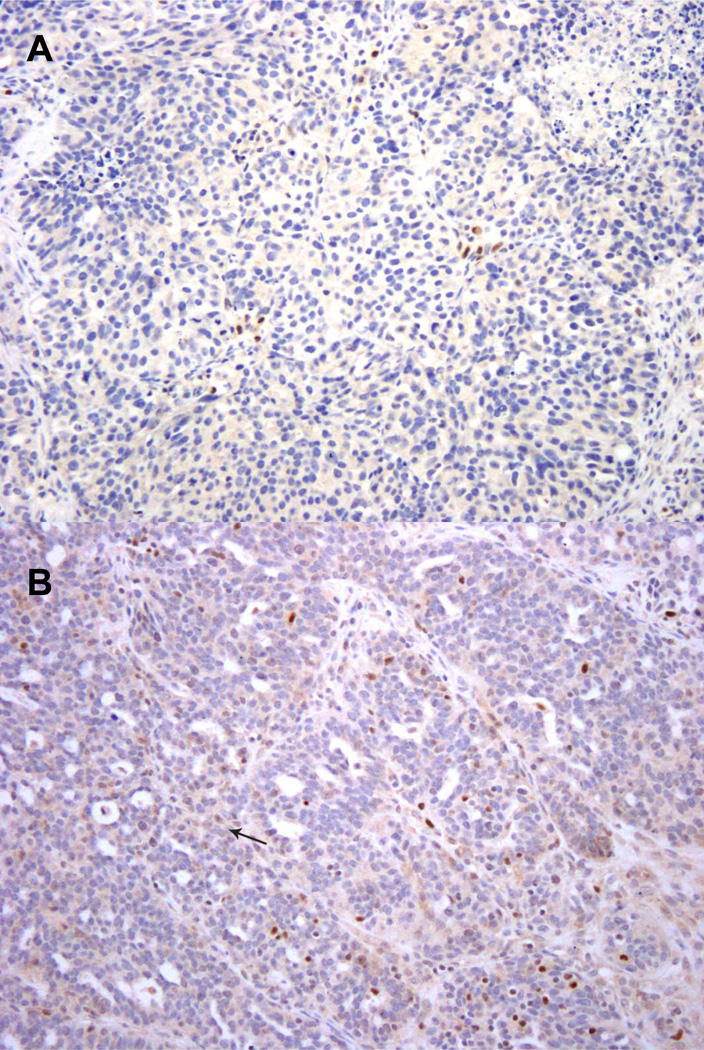
Figure 1.
A. High-grade serous carcinoma lacking nuclear expression of BRCA1. There is a positive internal control. This is interpreted as “BRCA1 loss.”
B. High-grade serous carcinoma with very rare BRCA1 positive tumor cell nuclei (example indicated by arrow). The weak staining intensity of positive tumor cell nuclei contrasts with the strong staining seen in tumor infiltrating lymphocytes. This is interpreted as “BRCA1 loss.”
Figure 2.
A. High-grade serous carcinoma with numerous tumor cell nuclei that express BRCA1. This is interpreted as retained expression of BRCA1.
B. High-grade serous carcinoma with moderate intensity staining in 5–10% of tumor nuclei in a selected intermediate power field. There is a moderately intense internal control. This is interpreted as retained expression of BRCA1.
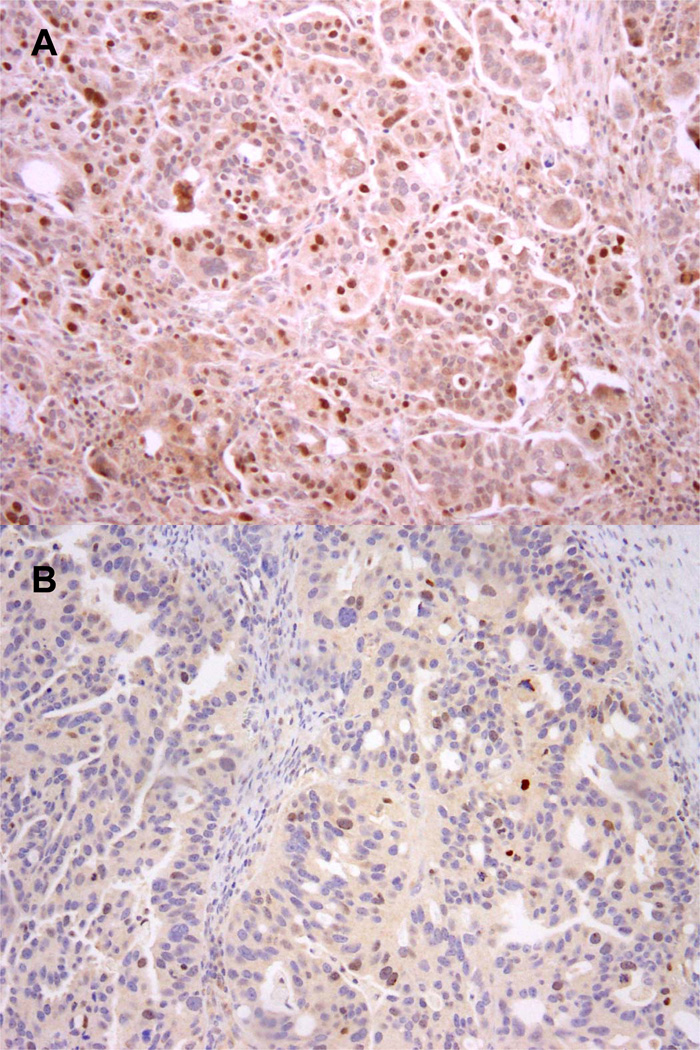
Figure 3.
A. High-grade serous carcinoma with weak staining in 5–10% of tumor cell nuclei, in presence of moderate to strong internal positive control. The thick arrow indicates a positive internal control with moderately intense staining. The thin arrows indicate examples of weakly stained tumor cell nuclei. The density of weakly stained nuclei is higher than in Figure 1B. This is interpreted as an equivocal result
B. High-grade serous carcinoma lacking nuclear expression of BRCA1. There is no positive internal control. This is interpreted as an equivocal result.
C. High-grade serous carcinoma with equivocal staining results. One pathologist scored this case as showing intact BRCA1 expression whereas the other pathologist scored the case as equivocal. Reasons to explain this discrepancy include differences in estimating the percentage of positive tumor cell nuclei and difficulties distinguishing tumor infiltrating lymphocytes from tumor cells. Compared to Figure 2B, staining intensity of tumor cell nuclei is very weak. Cases with this type of discrepancy were captured as “equivocal” for purposes of the study. The tumor had BRCA1 promoter methylation.
The same set of slides was then reviewed by a second pathologist (KG) using the above defined scoring criteria, but without knowledge of the BRCA genotype status. The 2 pathologists, using the above criteria while blinded to BRCA status, then scored 2 sets of validation cases selected based on available molecular data (BRCA1 and BRCA2 germline mutation status only or comprehensive BRCA1/2 genomic status).
Simple associations were performed and tested for statistical significance using Fisher’s Exact Test, with a two-sided P < 0.05 considered significant. Cases with loss of staining and equivocal staining were finally combined into an “abnormal BRCA staining” set when correlation between staining and BRCA1 molecular abnormalities was further evaluated in the validation sets.
TCGA genomic data for the training set were obtained from various sources, but all data are available on the MSKCC Computational Biology Center Cancer Genomics Portals at http://www.cbioportal.org/public-portal/. DNA and RNA were co-isolated from ovarian tumors using standard methods. RNA was hybridized to one or more gene expression platforms, and expression values were normalized as previously described (2). Median-centered gene expression values for BRCA1 were extracted from microarray data. DNA was bisulfite modified and hybridized to the Illumina Infinium HumanMethylation27 arrays to identify regions of promoter methylation. DNA methylation beta values reflect the proportion of methylated to unmethylated probes. Four BRCA1 probes were averaged to obtain a mean value for BRCA1 methylation. To identify which samples had BRCA1 promoter methylation, K-means clustering (assuming K=2) on the two-dimensional space of DNA methylation and expression data was used to separate the epigenetically silenced group and non-epigenetically silenced group of samples (2). This process allowed for the selection of cases that had evidence of BRCA1 promoter methylation based on both DNA methylation and RNA expression.
Results
Training set
Interobserver concordance in interpretation of stains
In the initial training set of 43 TCGA cases, interpretation of the BRCA1 IHC stain was concordant between both pathologists in 88% of cases. There were no cases interpreted as positive (loss of BRCA1 immunostaining) vs negative (no loss) by the 2 pathologists. There was disagreement in 5 (12%) of 43 cases, all of which involved an equivocal call by one of the pathologists; the interpretation was equivocal versus positive (n=3) or equivocal versus negative (n=2) (Figure 3C). For the 5 discrepant cases, the slides were re-reviewed by both pathologists and a consensus agreement was reached.
Staining characteristics
Twenty (47%) of 43 cases had loss of BRCA1 expression by IHC, 19 (44%) had intact BRCA1 expression, and 4 (9%) showed equivocal staining for BRCA1. Cases scored as BRCA1 loss (n=20) showed either complete absence of staining (n=6) or weak staining in <5% of tumor cells (n=14). Cases scored as intact BRCA1 (n=19) showed moderate to strong staining in >10% of tumor nuclei (n=17) or moderate intensity staining in 5% of tumor nuclei with a moderately intense internal control (n=2). In most cases, positive staining was noted in approximately 20–30% of tumor cells only. Diffuse strong staining of all tumor cells was not observed in any tumor. Cases scored as equivocal (n=4) showed weak staining in 5–10% of tumor cell nuclei in the presence of moderate to strong internal positive control (n=2), intense staining in 1% tumor nuclei (n=1), or lack of staining in tumor and surrounding normal tissue (n=1). Correlation between BRCA1 IHC and BRCA genotype is shown in Table 1.
Table 1.
Correlation between loss of BRCA1 IHC and BRCA1 genomic events for the training set (n=43)
|
BRCA1 germline mutations (n=5) |
BRCA1 somatic mutations (n=5) |
BRCA1 promoter methylation (n=14) |
BRCA2 mutations (n=8) |
BRCA unaffected (n=11) |
|
|---|---|---|---|---|---|
| BRCA1 IHC loss (n=20) | 4 (80%) | 3 (60%) | 12 (86%) | 1 (12%) | 0 |
| BRCA1 IHC equivocal (n=4) | 1 (20%) | 0 | 1 (7%) | 0 | 2 (18%) |
| BRCA1 IHC intact (n=19) | 0 | 2 (40%) | 1 (7%) | 7 (88%) | 9 (82%) |
Results stratified by BRCA1 IHC staining pattern
Among the 20 cases with BRCA1 IHC loss, 19 showed BRCA1 genetic abnormalities (promoter methylation=12, germline mutation=4, somatic mutation=3), and one had a BRCA2 germline mutation. Of the 4 cases that showed equivocal BRCA1 staining, one showed BRCA1 promoter methylation, one had a BRCA1 germline mutation, and 2 were BRCA unaffected. Of the 19 cases with retained BRCA1 IHC staining, 9 were BRCA unaffected, 7 showed BRCA2 abnormalities (3 somatic and 4 germline mutations), 2 showed BRCA1 somatic mutations, and one showed BRCA1 promoter methylation.
Results stratified by genotype
The test results were correlated with genomic results by considering all BRCA1 events together: promoter methylation, germline mutation, and somatic mutation. Loss of BRCA1 immunostaining is abnormal and was considered a positive test result. Only loss of BRCA1 immunostaining and retention of BRCA1 staining were considered in the metrics presented. Equivocal cases were excluded for this component of the analysis, as it is unclear to which group they belong. Considering all genetic and epigenetic abnormalities, the positive predictive value (PPV) of BRCA1 IHC was 95% (19 of 20), and the negative predictive value (NPV) was 84% (16 of 19). Sensitivity and specificity for cases in the training set were 86% and 94%, respectively. If only cases with retained BRCA1 staining were considered “normal” and cases with loss of BRCA1 staining and equivocal staining were considered abnormal, then the PPV, NPV, sensitivity, and specificity were 87%, 87%, 87%, and 84%, respectively. The NPV for BRCA1 germline mutation, specifically, was 100%.
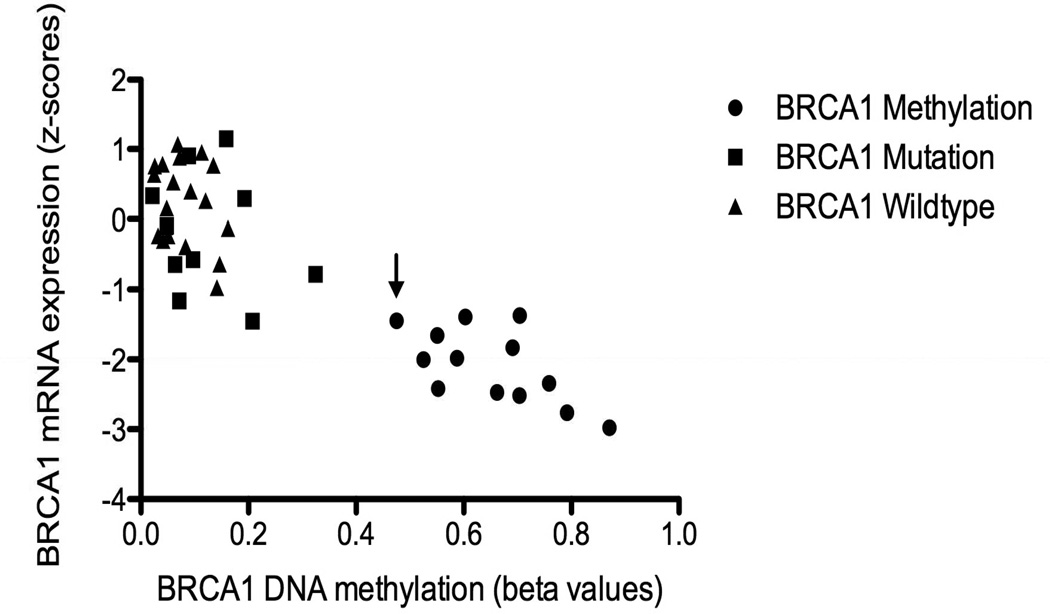
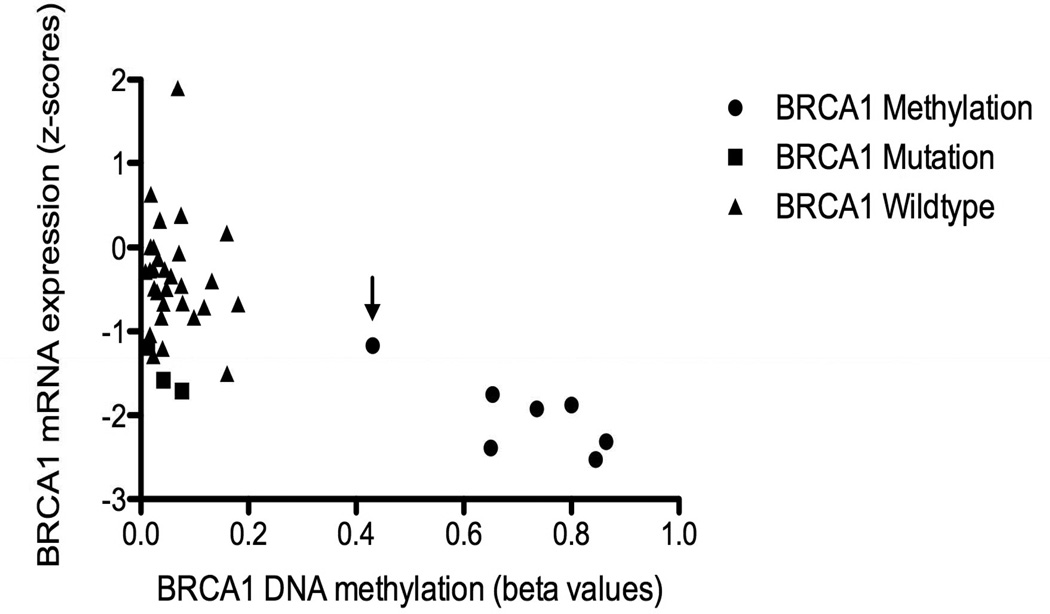
Interestingly, most of the BRCA1 somatic mutations are insertions or deletions. However, one of the 2 BRCA1 somatic mutations with retained immunostaining involved a missense mutation of uncertain functional significance at C47W that does not appear in the BIC database (Breast Cancer Information Core Database, http://research.nhgri.nih.gov/projects/bic/). The other case contained a nonsense mutation at the very end of the protein, R1835*, which may result in a sufficiently long protein that is potentially protected from nonsense mediated decay. The one case of BRCA1 methylation with retained immunostaining had the lowest level of promoter methylation of all the designated methylated cases. It is therefore possible that low level methylation, in contrast to higher levels of methylation, results in detectable BRCA1 IHC staining. This pattern is, apparently, uncommon (Figure 4).
Figure 4.
BRCA1 gene expression and promoter methylation values in the training set (n=43). Arrow indicates methylated case with retained immunostaining.
Validation sets
We used 2 sets of high-grade serous ovarian carcinomas for validation purposes. One set was composed of 31 high-grade serous ovarian carcinomas with known BRCA1 and BRCA2 germline mutation status from patients who had clinical genetic testing at MSKCC. The status of somatic mutation or promoter methylation was not known for these cases. The second set consisted of 39 separate TCGA high-grade serous ovarian carcinomas for which comprehensive genomic status, including BRCA1 methylation, somatic and germline mutations, was known.
Interobserver concordance in interpretation of stains
The 2 pathologists, without knowledge of each other’s interpretation or the molecular data, independently assessed BRCA1 IHC on these cases. In the first validation set, agreement between pathologists was reached in almost all cases (29 of 31, 93.5%). In the 2 cases with differences in interpretations, one was scored as equivocal versus negative, while the second was interpreted as equivocal versus positive. In the second validation set, agreement between pathologists was reached in 35 (90%) of 39 cases. All 4 cases with discrepancies were interpreted as retained versus equivocal by the 2 pathologists.
Results stratified by genotype
In the first validation set (Table 2), there were 11 cases with germline BRCA1 mutations; 7 showed loss of BRCA1 staining (64%) and 4 showed equivocal staining (36%). There were no cases with retained BRCA1 staining in this group. There was one case with a BRCA2 germline mutation that showed retained BRCA1 staining. There were 19 cases lacking BRCA1/2 germline mutations for which somatic mutation and methylation status were not known, and all showed intact BRCA1 staining. Whether equivocal cases are excluded or included as abnormal, the sensitivity, specificity, PPV, and NPV for BRCA1 IHC are all 100% when considering germline BRCA1 mutations. In the second validation set (Table 3), there were 7 cases with BRCA1 promoter methylation; of these, the staining was interpreted as lost in 4 cases, equivocal in 2 cases, and retained in one case. Two additional cases showed loss of BRCA1 staining: one with a BRCA1 somatic mutation and one without a known mutation. Seven showed equivocal staining, including the 2 methylated cases, one BRCA1 somatic mutation, and 4 without known mutation. Twenty-six cases showed retained staining, including one case with a BRCA1 somatic mutation and one with BRCA1 methylation; the remaining 24 cases included 5 cases with BRCA2 mutations and 19 cases with no known BRCA genomic events. If the equivocal cases are again excluded, and considering all genetic and epigenetic abnormalities, the sensitivity is 71%, the PPV is 83%, the specificity is 96%, and the NPV is 92% for BRCA1 IHC. If only cases with retained BRCA1 staining were considered “normal” and cases with loss of BRCA1 staining and equivocal staining were considered abnormal, then the PPV, NPV, sensitivity, and specificity are 62%, 92%, 80%, and 83%, respectively. The NPV for BRCA1 germline mutation, specifically, was 100%.
Table 2.
Correlation between loss of BRCA1 IHC and BRCA1 germline mutations for validation set 1 (n=31)
|
BRCA1 germline mutations (n=11) |
BRCA2 germline mutations (n=1) |
BRCA unaffected / unknown (n=19) |
|
|---|---|---|---|
| BRCA1 IHC loss (n=7) | 7 (64%) | 0 | 0 |
| BRCA1 IHC equivocal (n=4) | 4 (36%) | 0 | 0 |
| BRCA1 IHC intact (n=15) | 0 | 1 (100%) | 19 (100%) |
Table 3.
Correlation between loss of BRCA1 IHC and BRCA1 genomic events for validation set 2 (n=39)
|
BRCA1 somatic mutations (n=3) |
BRCA1 promoter methylation (n=7) |
BRCA2 mutations (n=5) |
BRCA unaffected (n=24) |
|
|---|---|---|---|---|
| BRCA1 IHC loss (n=6) | 1 (33%) | 4 (57%) | 0 | 1 (4%) |
| BRCA1 IHC equivocal (n=7) | 1 (33%) | 2 (29%) | 0 | 4 (17%) |
| BRCA1 IHC intact (n=26) | 1 (33%) | 1 (14%) | 5 (100%) | 19 (79%) |
Interestingly, of the 3 cases with BRCA1 somatic mutation, the one case with retained immunostaining had a nonsense mutation, whereas the other 2 cases had a frameshift mutation. The one case of BRCA1 methylation with retained immunostaining had much lower levels of DNA promoter methylation than all of the other designated methylated cases (Figure 5).
Figure 5.
BRCA1 gene expression and promoter methylation values in validation set 2 (n=39). Arrow indicates methylated case with retained immunostaining.
Combined training and validation sets
When data were combined from the training and both validation sets, 40 (89%) of 45 cases with known BRCA1 events showed abnormal BRCA1 staining (Table 4). If the equivocal cases are again excluded, the sensitivity is 86%, the PPV is 94%, the specificity is 97%, and the NPV is 92% for BRCA1 IHC. If only cases with retained BRCA1 staining were considered “normal” and cases with loss of BRCA1 staining and equivocal staining were considered abnormal, then the PPV, NPV, sensitivity, and specificity are 83%, 92%, 89%, and 88%, respectively. Overall, of 16 cases with BRCA1 germline mutation, no cases showed intact BRCA1 IHC staining, indicating a 100% NPV for this molecular defect compared to all other results. A summary of all performance metrics is provided in Table 5.
Table 4.
Correlation between loss of BRCA1 IHC and BRCA1 genomic events for the training and validation Sets combined (n=113)
|
BRCA1 germline mutations (n=16) |
BRCA1 somatic mutations (n=8) |
BRCA1 promoter methylation (n=21) |
BRCA2 mutations (n=14) |
BRCA unaffected (n=54) |
|
|---|---|---|---|---|---|
| BRCA1 IHC loss (n=33) | 11 (69%) | 4 (50%) | 16 (76%) | 1 (7%) | 1 (2%) |
| BRCA1 IHC equivocal (n=15) | 5 (31%) | 1 (12%) | 3 (14%) | 0 | 6 (11%) |
| BRCA1 IHC intact (n=65) | 0 | 3 (38%) | 2 (10%) | 13 (93%) | 47 (87%) |
Table 5.
Summary of performance metrics for BRCA1 immunostaining
| Case set | PPV | NPV | Sensitivity | Specificity |
|---|---|---|---|---|
| Training set, equivocal cases excluded (n=40) | 0.95 | 0.84 | 0.86 | 0.94 |
| Training set, equivocal cases included* (n=43) | 0.87 | 0.87 | 0.87 | 0.84 |
| Validation set 1, equivocal cases excluded (n=27) | 1 | 1 | 1 | 1 |
| Validation set 1, equivocal cases included* (n=31) | 1 | 1 | 1 | 1 |
| Validation set 2, equivocal cases excluded (n=32) | 0.83 | 0.92 | 0.71 | 0.96 |
| Validation set 2, equivocal cases included* (n=39) | 0.62 | 0.92 | 0.80 | 0.83 |
| All data combined, equivocal cases excluded (n=99) | 0.94 | 0.92 | 0.86 | 0.97 |
| All data combined, equivocal cases included* (n=113) | 0.83 | 0.92 | 0.89 | 0.88 |
Note: These data account for test performance in the detection of any genetic or epigenetic abnormality involving BRCA1. BRCA2 results are combined with cases lacking BRCA1 mutation or promoter methylation for the purposes of this calculation. NPV for BRCA1 germline mutation, specifically, was 100%.
equivocal cases are considered abnormal
Discussion
Detection of BRCA dysfunction in ovarian carcinomas has prognostic and therapeutic significance. Immunohistochemistry for BRCA1 is an inexpensive and easy initial screen to detect these patients. Our study shows that BRCA1 IHC is an effective method to detect BRCA1 dysfunction in ovarian carcinoma. Based on our data, BRCA1 immunostaining has a negative predictive value of 100% for BRCA1 germline mutations (all staining intact cases lack BRCA1 germline mutations). This result is clinically relevant since at present only BRCA germline genetic testing is routinely offered to patients considered at high risk for hereditary breast and ovarian cancer syndromes. BRCA1 immunostaining could also potentially be used to screen for BRCA1 genetic defects other than germline mutation if the clinical significance of these defects becomes better understood and new agents are developed for targeted therapy (Table 4). Thus, BRCA1 IHC provides a robust initial screen for triaging patients for BRCA1 germline mutation testing and will also detect other relevant genetic defects in BRCA1.
Nearly all cases with intact staining lacked a BRCA abnormality, while almost all cases with staining loss were found in cases with BRCA1 germline mutation, somatic mutation or promoter methylation, but there were exceptions. Of 33 cases with BRCA1 IHC loss, one was found to have a BRCA2 mutation and one lacked a detectable abnormality in the BRCA1 gene. Of 66 cases with intact BRCA1 staining, there were 3 with a BRCA1 somatic mutation and 2 with BRCA1 promoter methylation. BRCA1 IHC appeared to be the least sensitive for tumors with BRCA1 somatic mutations, although the data are limited by a small sample size. The reason for this is not clear, but since it appears that haplo-insufficiency of BRCA may be sufficient to initiate breast tumorigenesis, it is plausible that it is not essential for tumors to demonstrate complete loss of BRCA1 staining in the presence of somatic BRCA1 mutation (11). Furthermore, the type of mutation type may also be responsible for the observed staining pattern. Work by Kashima et al (12) indicates that antibodies against the N-terminus of BRCA1 fail to detect tumors with BRCA1 mutations upstream of exon 11. The antibody used for the current work, likewise, recognizes only the N-terminus of BRCA1 and one case with a somatic BRCA1 mutation indeed had intact BRCA1 staining. Both cases with BRCA1 promoter methylation and retained immunostaining had lower levels of promoter methylation, and each had equal or higher mRNA expression compared to other methylated cases, indicating that BRCA1 IHC does not recognize all cases with BRCA1 promoter methylation, especially when methylation levels are low. The reason for loss of BRCA1 IHC staining in one case of BRCA2 mutation is not clear, although it is possible that an undetected BRCA1 alteration may exist or other unknown molecular function could be relevant.
The prevalence of somatic mutations and BRCA1 promoter methylation could not be determined in the first validation set. This set was selected from a cohort that had undergone genetic testing and was therefore preselected—all cases were known to be either mutated or wild type in the germline. Somatic mutations in BRCA1 occur at a relatively low frequency, and in fact, the 8 BRCA1 somatically mutated cases included in the entire study set of 113 (7%) over-represents this subgroup based on an expected prevalence of approximately 3%. It was nevertheless unexpected that there were no IHC abnormal cases lacking germline mutations in this set.
The BRCA1 antibody has some limitations and can be difficult to interpret. The stain cannot be assessed at scanning or low magnification, but instead it requires assessment of the entire tumor at relatively high magnification. The internal control may show only patchy and weak staining, although it is often moderately intense and diffuse. Comparison of the staining intensity between tumor and non-neoplastic elements must be taken into consideration for the purposes of interpretation. It is also important to note that even tumors with retained staining do not typically show strong and diffuse staining; rather, staining is often focal and patchy with moderate to strong intensity and limited in distribution to less than 30% of tumor cells. Interpretation does become easier and more consistent with experience, however. In our study, the 2 pathologists reached agreement in 38 of 43 cases in the training set and in 64 of 70 cases in the validations sets. In all discrepant cases, one reviewer had diagnosed an equivocal staining pattern, such that none of the discrepancies involved a negative versus positive result. We found that many of the cases with equivocal staining showed BRCA1 genetic abnormalities. As we gain more experience with the antibody, we may be able to further segregate these cases. Currently, we advocate that these cases should be treated like cases with BRCA1 IHC loss so as to not miss cases of potential clinical significance.
Several groups have previously published the results of BRCA1 IHC in ovarian cancer. As long ago as 2000, Byrne and colleagues reported on the sensitivity and specificity of BRCA1 IHC in a small group of ovarian carcinomas and matched controls (13). The sensitivity, specificity, positive and negative predictive values were 100%. Recently, investigators have described tight associations between the loss of BRCA1 expression and relatively improved survival, although genetic analyses were not performed (14–16). There is also a number of papers describing loss of BRCA1 expression in the setting of BRCA1 gene mutation (12, 17, 18). Kashima described loss of expression in 8 cases with BRCA1 germline mutation (12), while Skytte (17) reported a sensitivity of 80%, a specificity of 93%, and an estimated PPV of 73% based on having studied 15 “BRCA1 cancers.” In comparison, our study included significantly larger numbers of carcinomas lacking germline mutations and a detailed analysis of genetic abnormalities other than germline mutations. Furthermore, the methodology we employed provided robust results based on having tested the validity of the IHC scoring system derived in the test set using 2 additional validation cohorts. In addition, we have presented information regarding interobserver variability in the interpretation of the stains and recognized an equivocally stained group of cases, all of which may be useful for adaptation of the methodology for clinical practice.
This is therefore a comprehensive study of a BRCA1 IHC antibody in ovarian serous carcinomas. Going forward, the utility of this antibody would be primarily as a screening test to select patients for genetic counseling and testing, although it is acknowledged that we cannot, at present, use morphologic (19) or IHC algorithms to detect patients at highest risk of having a BRCA2 germline mutation. BRCA1 IHC may also be useful to detect ovarian cancer patients with BRCA dysfunction who might benefit from PARP inhibitors. PARP inhibitors have been shown to be effective in serous carcinomas with germline BRCA mutations, and it has been suggested that they may also be beneficial to patients with sporadic ovarian cancers that have defective HR due to other mechanisms, including promoter methylation and somatic mutations (20–22). Studies correlating BRCA1 staining patterns and response to PARP inhibitors would therefore be helpful to determine utility of IHC for this purpose. Considering the mutual exclusivity between BRCA1 methylation events and mutation of BRCA1 or BRCA2 in the germline or tumor (2), a dedicated BRCA1 methylation assay may help to further stratify patients without intact BRCA1 IHC for genetic counseling or testing.
Conclusions
These data suggest that BRCA1 IHC is an effective method to screen for BRCA1 alterations in patients with high-grade serous ovarian cancer. The overwhelming majority of cases with BRCA dysfunction and only a few of the BRCA unaffected cases showed loss of staining with a high NPV. Immunohistochemistry for BRCA1 may be a useful initial screening test to select patients for targeted therapy and to detect patients at risk for hereditary breast and ovarian cancer syndromes. These preliminary data indicate that BRCA1 IHC is a clinically useful test.
Acknowledgments
Source of Funding: Support for this work was provided in part by a National Institutes of Health grant (3 U24 CA143840-02 S1, MSKCC Center for Translational Cancer Genomic Analysis), DoD Award W81XWH-10-1-0222, Stand Up to Cancer/ American Association for Cancer Research Dream Team Translational Cancer Research Grant (Grant No. SU2C-AACR- DT0209), Entertainment Industry Foundation Revlon Run/Walk for Women, and the Eileen Genet Ovarian Cancer Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest to report.
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–3561. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 5.Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg K, Leitao MM, Jr, Kauff ND, et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol. 2009;33:925–933. doi: 10.1097/PAS.0b013e318197a046. [DOI] [PubMed] [Google Scholar]
- 8.Modica I, Soslow RA, Black D, Tornos C, Kauff N, Shia J. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol. 2007;31:744–751. doi: 10.1097/01.pas.0000213428.61374.06. [DOI] [PubMed] [Google Scholar]
- 9.Kwon JS, Scott JL, Gilks CB, et al. Testing women with endometrial cancer to detect Lynch syndrome. J Clin Oncol. 2011;29:2247–2252. doi: 10.1200/JCO.2010.32.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resnick K, Straughn JM, Jr, Backes F, et al. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstet Gynecol. 2009;114:530–536. doi: 10.1097/AOG.0b013e3181b11ecc. [DOI] [PubMed] [Google Scholar]
- 11.King TA, Li W, Brogi E, et al. Heterogenic loss of the wild-type BRCA allele in human breast tumorigenesis. Ann Surg Oncol. 2007;14:2510–2518. doi: 10.1245/s10434-007-9372-1. [DOI] [PubMed] [Google Scholar]
- 12.Kashima K, Oite T, Aoki Y, et al. Screening of BRCA1 mutation using immunohistochemical staining with C-terminal and N-terminal antibodies in familial ovarian cancers. Jpn J Cancer Res. 2000;91:399–409. doi: 10.1111/j.1349-7006.2000.tb00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrne TJ, Reece MT, Adams LA, et al. An antibody assay predictive of BRCA1 mutations in ovarian tumors and normal tissue. Oncol Rep. 2000;7:949–953. doi: 10.3892/or.7.5.949. [DOI] [PubMed] [Google Scholar]
- 14.Carser JE, Quinn JE, Michie CO, et al. BRCA1 is both a prognostic and predictive biomarker of response to chemotherapy in sporadic epithelial ovarian cancer. Gynecol Oncol. 2011;123:492–498. doi: 10.1016/j.ygyno.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Radosa MP, Häfner N, Camara O, et al. Loss of BRCA1 protein expression as indicator of the BRCAness phenotype is associated with favorable overall survival after complete resection of sporadic ovarian cancer. Int J Gynecol Cancer. 2011;21:1399–1406. doi: 10.1097/IGC.0b013e318227c990. [DOI] [PubMed] [Google Scholar]
- 16.Weberpals JI, Tu D, Squire JA, et al. Breast cancer 1 (BRCA1) protein expression as a prognostic marker in sporadic epithelial ovarian carcinoma: an NCIC CTG OV.16 correlative study. Ann Oncol. 2011;22:2403–2410. doi: 10.1093/annonc/mdq770. [DOI] [PubMed] [Google Scholar]
- 17.Skytte AB, Waldstrøm M, Rasmussen AA, et al. Identification of BRCA1-deficient ovarian cancers. Acta Obstet Gynecol Scand. 2011;90:593–599. doi: 10.1111/j.1600-0412.2011.01121.x. [DOI] [PubMed] [Google Scholar]
- 18.Vaz FH, Machado PM, Brandão RD, et al. Familial breast/ovarian cancer and BRCA1/2 genetic screening: the role of immunohistochemistry as an additional method in the selection of patients. J Histochem Cytochem. 2007;55:1105–1113. doi: 10.1369/jhc.7A7209.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soslow RA, Han G, Park KJ, Garg K, Olvera N, Spriggs DR, Kauff ND, Levine DA. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012;25:625–636. doi: 10.1038/modpathol.2011.183. [DOI] [PubMed] [Google Scholar]
- 20.Drew Y, Mulligan EA, Vong WT, et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011;103:334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 21.Dedes KJ, Wilkerson PM, Wetterskog D, et al. Synthetic lethality of PARP inhibition in cancers lacking BRCA1 and BRCA2 mutations. Cell Cycle. 2011;10:1192–1199. doi: 10.4161/cc.10.8.15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibragimova I, Cairns P. Assays for hypermethylation of the BRCA1 gene promoter in tumor cells to predict sensitivity to PARP-inhibitor therapy. Methods Mol Biol. 2011;780:277–291. doi: 10.1007/978-1-61779-270-0_17. [DOI] [PMC free article] [PubMed] [Google Scholar]