Abstract
NGF plays a pivotal role in regulating sympathetic neuron survival and target field innervation during development. Here we show that a member of the TNF superfamily, GITR and its ligand GITRL are co–expressed in mouse sympathetic neurons when their axons are innervating their targets under the influence of target–derived NGF. In culture, GITRL enhances NGF–promoted neurite growth from neonatal sympathetic neurons, and preventing GITR–GITRL interaction in these neurons or GITR knockdown inhibits NGF–promoted neurite growth without affecting neuronal survival. GITR−/− neonates have reduced sympathetic innervation density in vivo compared with GITR+/+ littermates. GITR activation is required for the phosphorylation of ERK1/ERK2 by NGF that is necessary for neurite growth. Our results reveal a completely unsuspected signalling loop in developing sympathetic neurons that is crucial for NGF–dependent axon growth and target innervation.
The growth and guidance of axons to their targets and the terminal arborization of axons within their targets is controlled by numerous locally acting and diffusible signalling molecules. These molecules are derived from a wide variety of cells and bind to receptors on advancing axons to influence growth rate, direction and branching1, 2. Sympathetic neurons of the developing superior cervical ganglion (SCG) are an extensively studied, experimentally tractable model for analysing the molecular basis of axonal growth and target innervation as well as other aspects of neuronal development. Several secreted factors have been implicated in promoting the growth sympathetic axons, including nerve growth factor (NGF), neurotrophin-3 (NT–3), artemin and hepatocyte growth factor (HGF)3. Of these, the most extensively studied and best understood is NGF which is needed for the innervation and terminal sprouting of sympathetic axons within many distal targets4, 5. In addition, target–derived NGF has a well–established role in promoting and regulating sympathetic neuron survival during development6.
To identify other candidate signalling molecules involved in sympathetic neuron development, we carried out a PCR screen for the expression of known families of receptors in the developing SCG. Among the receptor transcripts expressed at high levels was that for the glucocorticoid–induced tumour necrosis factor-receptor-related protein (GITR). GITR, like the p75 neurotrophin receptor, is a member of the TNFR superfamily, but unlike p75NTR lacks a cytoplasmic death domain. GITR and its specific ligand GITRL are expressed in many tissues, but have only been functionally characterized in the immune system where they play key roles in regulating innate and acquired immune responses7. Depending on the cellular context, GITR activates or inhibits multiple intracellular signalling pathways of which the two most well described are NF–κB8, 9 and MAP kinase signalling10.
Here we present data examining the functional significance of GITR expression in developing sympathetic neurons and show that GITRL–GITR signalling acting in a co–stimulatory manner is crucial during the immediate postnatal period for NGF–dependent axonal growth, branching and target innervation.
RESULTS
Co-expression of GITR and GITRL in sympathetic neurons
Quantification of GITR mRNA levels in the SCG at intervals throughout the period of target field innervation and naturally occurring neuron death revealed a significant increase from E15 to reach a peak at P1 followed by a decline to P5 (Fig. 1a). To explore the possibility of local GITR signalling in the SCG, we also quantified the level of GITR ligand (GITRL) mRNA over the same age range, and found that this displayed a very similar developmental profile to that of GITR mRNA (Fig. 1b). Thus, transcripts for both receptor and ligand are expressed in the developing SCG with highest levels shortly after the onset of naturally occurring neuronal death in the SCG when sympathetic axons have begun to innervate and branch extensively in their targets4, 11, 12. To determine which kinds of cells in the SCG express GITR and GITRL, immunocytochemistry was used to localize these proteins in dissociated SCG cultures established from P1 mice. Triple–labelled preparations in which neurons were positively identified with anti–β–III tubulin antibodies and cell nuclei were labelled with the fluorescent nuclear marker DAPI revealed that both GITR and GITRL were expressed in all neurons. Non–neuronal cells displayed little or no immunoreactivity for either ligand or receptor (Fig. 1c). Likewise, immunohistochemistry revealed that GITR and GITRL co–localise with the neuronal markers TrkA and β–III tubulin in sections of P0 SCG (Figs 1d, 1e).
Figure 1. GITR and GITRL expression in developing SGC neurons.
Levels of (a) GITR mRNA and (b) GITRL mRNA relative to GAPDH mRNA (arbitrary units) in E15, E18, P1 and P5 SCG. Means and standard errors of data from 3 separate sets ganglia at each age are shown (** P < 0.001, statistical comparison with E15 data, ANOVA with Fisher's post hoc). (c) Photomicrographs of the same fields of P1 SCG dissociated cultures triple labelled for either GITR, β-III tubulin and DAPI or GITRL, β-III tubulin and DAPI. Scale bars = 20 μm. (d,e) Immunohistochemistry showing that GITRL (d) and GITR (e) are co-localised with the neuronal markers β-III tubulin and TrkA in sections of P0 SCG. Scale bar = 50μm.
GITRL–GITR signalling is required for NGF-promoted growth
The co–expression of GITR and GITRL in neonatal SCG neurons raised the possibility of GITRL–GITR signalling within or between these neurons. To investigate the potential significance of GITRL–GITR signalling, we used three approaches to inhibit GITRL–GITR signalling in cultured P1 SCG neurons: expression of antisense GITR RNA in the neurons to knockdown GITR, treatment of the neurons with a GITR–IgG fusion protein that binds GITRL and prevents it from interacting with GITR extracellularly and comparative studies of neurons obtained from wild type and GITR–deficient mice. The neurons were grown at low density in defined, serum–free medium containing NGF to sustain their survival. Antisense GITR RNA was expressed in SCG neurons by ballistic transfection with gold particles coated with an antisense GITR pcDNA3.1 expression plasmid 2 hours after plating. Neurons transfected with this plasmid, but not control–transfected neurons (transfected with a pcDNA3.1 plasmid contained a random nucleotide insert), exhibited a pronounced decrease in GITR immunoreactivity 24 hours after plating (Fig. 2a). Sholl analysis, which provides a graphic illustration of neurite length and branching with distance from the cell body, showed that antisense GITR (Fig. 2b) and GITR–IgG (Fig. 2c) caused very substantial decreases in NGF–promoted neurite growth compared with control–transfected and control–IgG treated neurons, respectively. Furthermore, antisense GITR caused an 83% reduction in neurite length and a 78% reduction in branching and GITR–IgG caused a 42% reduction in neurite length and a 31% reduction in branching respectively (data not shown). Neither antisense GITR nor GITR–IgG significantly affected NGF–promoted survival (Fig. 2d).
Figure 2. Effect of inhibiting GITR signalling on NGF-promoted neurite growth.
(a,b) P1 SCG neurons were transfected with YFP and either antisense GITR or a control (Cont) plasmid. Neurons were then grown in 10 ng/ml NGF, and 24 hours later images of YFP-expressing neurons were acquired for analysis. (a) Expression of antisense GITR reduces GITR immunoreactivity in transfected neurons (white arrow) versus non-transfected neurons. Scale bar = 10 μm. (b) The Sholl plots for antisense GITR expressing and control neurons are shown. (c) The effect of sequestering GITRL with a soluble GITR-IgG. P1 SCG neurons were plated in medium containing 10 ng/ml NGF with 1 μg/ml GITR-IgG or 1 μg/ml control-IgG (anti-β-III tubulin). 24 hours later and images of neurons were digitally acquired for analysis. The Sholl plots for GITR-IgG-treated and control neurons are shown. (d) Percentage survival of neurons in experimental series (b,c). (e) Percentage survival of SCG neurons cultured from GITR+/+, GITR+/− and GITR−/− P1 mice and branch point number (f), total neurite length (g) and Sholl analysis (h) of their neurite arbors after 24 hours incubation with 10 ng/ml NGF. The mean ± s.e.m. of at least 150 neurons in each condition from at least 3 independent experiments are shown for each data set (** P < 0.001, statistical comparison with control, ANOVA with Fisher's post hoc). (i) Line drawings of representative examples of the neurite arbors of SCG neurons of P1 GITR+/+, GITR+/− and GITR−/− pups cultured for 24 hours with NGF. (Scale bar = 50 μm).
To investigate the effect of genetic deletion of GITR on neurite growth, GITR+/− mice were crossed to generate GITR+/+, GITR+/− and GITR−/− pups. Separate SCG cultures were established from each pup, and neuronal survival and neurite arbor size were quantified after 24 hours incubation with NGF. GITR deletion had no significant effect on the number of neurons surviving with NGF (Fig. 2e), but caused very substantial decreases in neurite length (Fig. 2f), branching (Fig. 2g) and Sholl profiles (Fig. 2h) with a clear gene dosage effect in heterozygous mice. Representative SCG neurons of GITR+/+, GITR+/− and GITR−/− P1 pups cultured for 24 hours with NGF are shown (Fig. 2i). These findings demonstrate that NGF–promoted neurite growth from neonatal SCG neurons, but not survival of these neurons, is crucially dependent on GITRL–GITR interaction.
Although the GITR knockdown and GITR deletion experiments cannot distinguish between paracine and autocrine GITRL–GITR signalling in these cultures, the GITR–IgG experiments suggest that GITRL–GITR signalling operates via an autocrine route. In these latter experiments, GITRL–GITR interaction was blocked at the level of individual neurons because the neurons were plated at such low density as to eliminate any direct contact between the neurite arbors of the neurons. The immunohistochemical staining pattern of GITRL in vivo is also consistent with autocrine GITRL–GITR signalling in sympathetic neurons. Whereas GITRL and GITR are clearly co–expressed in neurons of the SCG (Figs 1d,e), GITRL immunoreactivity was undetectable in intermediate and distal SCG targets such as cranial arteries, nasal mucosa and iris (not shown).
Activation of GITR increases NGF–promoted neurite growth
To determine if NGF–dependent neurite growth is maximally stimulated by endogenous GITRL–GITR signalling in SCG neurons, we studied the effect of enhancing GITR activation in these neurons. This was done by either overexpressing GITRL or by activating GITR with a soluble GITRL–IgG fusion protein. We demonstrate that GITRL–IgG is a potent co-stimulator of T lymphocyte proliferation in the presence of sub–optimal concentrations of anti–CD3, but is ineffective on lymphocytes from GITR−/− mice (Supplementary Fig. 1). Sholl analysis showed that GITRL overexpression (Fig. 3a) and GITRL–IgG treatment (Fig. 3b) promoted significant increases in the size and complexity of the neurite arbors of SCG neurons grown with NGF without significantly affecting survival (data not shown). GITRL–IgG failed to enhance neurite growth from SCG neurons of GITR−/− mice (Supplementary Fig. 2), confirming that GITRL–IgG acts specifically via GITR in neurons. Our finding that GITR overexpression did not enhance neurite growth from NGF–supported SCG neurons (data not shown) implies that the endogenous level of GITR expression is not limiting for GITRL–GITR signalling in these neurons. Rather, it appears that the endogenous level of GITRL synthesis or subsequent events that lead to its interaction with GITR, governs the degree of GITRL–GITR signalling in SCG neurons.
Figure 3. Effect of enhancing GITRL-GITR signalling on neurite growth from SCG neurons grown with and without NGF.

(a) The effect of overexpressing GITRL on NGF-promoted neurite growth. Two hours after plating, P1 SCG neurons were transfected with a YFP expression plasmid together with either a plasmid expressing GITRL or an empty control plasmid (Cont). The Sholl plots for GITRL-overexpressing and control neurons are shown after 24 hours incubation with 10 ng/ml NGF. GITRL overexpression caused a 90% increase in neurite length and a 40% increase in the number of branch points (significant in both cases P < 0.001). (b) The effect of activating GITR with a soluble GITRL-IgG agonist on NGF-promoted neurite growth. P1 SCG neurons were plated in medium containing 10 ng/ml NGF with either 1 μg/ml GITRL-IgG or 1 μg/ml control-IgG (anti-β-III tubulin). The Sholl plots for GITRL-IgG-treated and control neurons are shown after 24 hours incubation. GITRL-IgG caused a 37% increase in neurite length and a 24% increase in the number of branch points (significant in both cases P < 0.001). (c) The effect of GITRL-IgG on neurite growth from P1 SCG neurons grown without NGF. The neurons were incubated with either 1 μg/ml GITRL-IgG or 1 μg/ml control-IgG (anti-β-III tubulin) in medium containing the caspase inhibitor Boc-D-FMK. The Sholl profiles after 24 hours incubation are shown. The mean ± s.e.m. of at least 150 neurons in each condition from at least three independent experiments are shown for each data set.
To investigate if GITRL–GITR signalling is capable of promoting neurite growth independently of NGF, we cultured SCG neurons with the broad–spectrum, irreversible caspase inhibitor Boc–D–FMK to prevent the neurons from dying in the absence of NGF and treated them with GITRL–IgG. The smaller neurite arbors of these NGF-deprived neurons were not significantly affected by GITRL–IgG (Fig. 3c), suggesting that enhanced GITRL–GITR signalling cannot promote neurite growth alone. GITRL–IgG did not affect the number of neurons surviving with or without NGF (data not shown), confirming that GITRL–GITR signalling does not affect sympathetic neuron survival.
To determine if the effects of GITR signaling are specific for NGF–promoted neurite growth from SCG neurons, we carried out additional experiments on these and other neurons from P1 mice. Neither GITR–IgG nor GITRL–IgG affected hepatocyte growth factor-promoted neurite growth from SCG neurons (data not shown). In contrast to SCG neurons, the sensory neurons of the trigeminal and nodose ganglia express only low levels of GITR, and overexpression of GITRL or antisense GITR had no effect on NGF–promoted and BDNF–promoted neurite growth, respectively (data not shown).
GITRL-GITR influences growth over a developmental window
To determine if GITRL–GITR signalling facilitates neurite growth throughout the period of development when SCG neurons respond to NGF or whether its influence is restricted to a particular stage of development, we studied the effects of GITRL overexpression and GITR–IgG on neurite growth from SCG neurons cultured with NGF at several developmental ages. These experiments revealed that in P1 and P3 cultures, GITRL overexpression significantly increased and GITR–IgG significantly decreased NGF–promoted neurite growth. However, neither GITRL overexpression (Fig. 4a) nor GITR–IgG (Fig. 4b) affected the extent of NGF–promoted neurite growth in E18 and P5 cultures To see if GITRL responsiveness could be conferred at these latter ages, we co–transfected E18 and P5 SCG neurons with GITR and GITRL plasmids, however we found no differences in the extent of neurite outgrowth from these neurons compared with control transfected neurons (data not shown). This suggests that the developmental window of the effects of GITR signaling on NGF–promoted neurite growth is not simply governed by developmental changes in the expression of receptor and ligand.
Figure 4. GITRL-GITR influences NGF-promoted neurite growth during a window of post-natal development.
(a) Sholl plots showing the effect of overexpressing GITRL on NGF-promoted neurite growth from SCG neurons cultured at intervals throughout development. Two hours after plating, the neurons were transfected with a YFP expression plasmid together with either a plasmid expressing GITRL or an empty control plasmid. The cultures were then incubated with 10 ng/ml NGF, and 24 hours after plating, images of YFP-expressing neurons were digitally acquired for neurite arbor analysis. (b) Sholl plots showing the effects of GITR-IgG on NGF-dependent neurite growth from SCG neurons cultured at the same stages of development. After 24 hours incubation with 10 ng/ml NGF and either 1 μg/ml GITRL-IgG or 1 μg/ml control-IgG (anti-β-III tubulin), the neurons with labelled with calcein-AM and images were digitally acquired for neurite arbor analysis. In all experiments, Sholl analysis was carried out on at least 150 neurons in each condition. The results are derived from the grouped data of at least three independent experiments of each kind at each age.
Together these results show that GITRL–GITR signalling exerts its effects on neurite growth during a narrow window of development in the immediate postnatal period. Thus, GITRL–GITR signalling affects neurite growth and branching during the period of development when SCG axons are growing and branching within their targets under the influence of target–derived NGF. Because the neurites in short–term SCG cultures, such as those used in our study, are exclusively axons rather than dendrites13, it is reasonable to assume that GITRL–GITR signalling regulates axonal growth and branching during this period of development.
GITR is required for sympathetic innervation in vivo
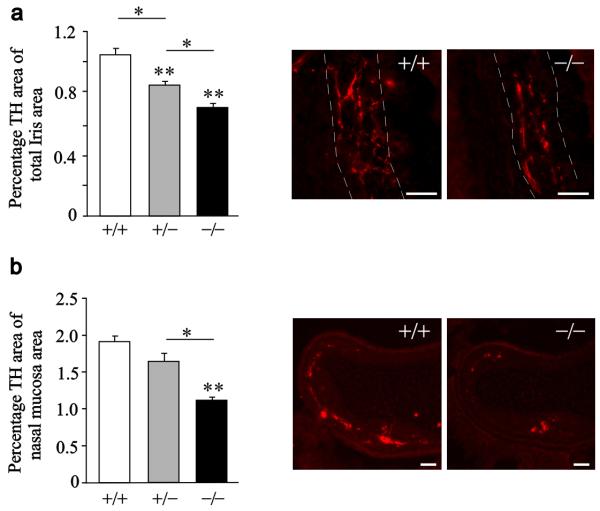
To determine if our in vitro observations are physiologically relevant for sympathetic innervation in vivo, we compared sympathetic innervation density in GITR null mice and their wild type littermates. The iris and the nasal mucosa normally receive a dense sympathetic innervation from the SCG by axons that can be easily identified and quantified by immunostaining for tyrosine hydroxylase (TH). At P1, there were marked, highly significant reductions in sympathetic innervation density in both targets in GITR−/− mice and clear gene dosage effects in GITR+/− mice (Figs. 5a and 5b). To determine if this in vivo defect in sympathetic innervation was still evident beyond the period of development when GITRL–GITR signalling is required for NGF–promoted sympathetic axon growth in vitro, we compared sympathetic innervation density in the nasal mucosa of GITR−/− mice and wild type littermates at P10. This analysis (Supplementary Fig. 3a) revealed a very similar reduction in the TH immunofluorescence in GITR−/− mice compared with GITR+/+ mice (41% reduction) to that observed in P1 mice (42% reduction), indicating that the innervation deficit evident at P1 does not recover with age.
Figure 5. Sympathetic innervation density in GITR+/+, GITR+/− and GITR−/− mice.
Bar charts showing the percentage area of sectioned irises (a) and nasal mucosa (b) stained for TH in P1 GITR+/+, GITR+/− and GITR−/− mice (** P < 0.001, statistical comparison with GITR+/+, ANOVA with Fisher's post hoc, n = 6 per genotype for iris and n=3 per genotype for nasal mucosa). The photomicrographs show typical TH-stained sections of irises (a) and nasal mucosas (b) of GITR+/+ and GITR−/− mice (scale bar = 50 μm). The data represent the mean ± s.e.m.
To exclude the possibility that decreased TH immunofluorescence in the sympathetic targets of GITR−/− mice was not simply due to down regulation of TH expression in the innervating neurons, we quantified TH mRNA levels and TH immunofluorescence in the SCG of these mice and wild type littermates. These studies revealed no significant differences in the levels of either TH mRNA or TH immunofluorescence intensity in the ganglia of all three genotypes (Supplementary Figs. 3b and 3c), supporting the idea that the reduction in TH immunofluorescence in the sympathetic targets of GITR null mice is due to a reduction in the number of sympathetic axons in these targets.
Reduced sympathetic innervation density could not only result from defective axonal growth and branching but from a decrease in the size of the innervating population of neurons. To investigate if excessive loss of SCG neurons in GITR null mice contributes to the innervation defect, we counted the number of neurons in the SCG of GITR+/+, GITR+/− and GITR−/− mice at P1 and found no significant differences (Supplementary Fig. 3d). Taken together, these results demonstrate that GITRL–GITR signalling plays a crucial role in the establishment of sympathetic innervation but is not required for the survival of developing sympathetic neuron in vivo. These in vivo findings are entirely consistent with our demonstration that GITRL–GITR signalling is selectively required for the effects of NGF on axon growth but not survival of neonatal SCG neurons in vitro
Our in vitro studies suggest a selective requirement for GITRL–GITR signalling in NGF–promoted axonal growth from sympathetic but not sensory neurons. To ascertain if a similar selectivity operates in vivo, we quantified the innervation density of the irises of P1 GITR+/+ and GITR−/− mice by CGRP–positive axons from NGF-dependent sensory neurons of the trigeminal ganglion. Quantification of CGRP immunoreactivity revealed no significant differences in intensity between GITR+/+ and GITR−/−mice (Supplementary Fig. 3e), demonstrating that sensory innervation is established independently of GITRL–GITR signalling.
GITR facilitates NGF–promoted activation of ERK1 and ERK2
To elucidate the molecular mechanism underlying the selective influence of GITRL–GITR on NGF–promoted axonal growth, we explored potential common links in intracellular signalling. Among these is MAP kinase signalling which has been shown to contribute to NGF–promoted neurite growth in PC12 cells and sympathetic neurons14-16 Because GITR acts as a co–accessory molecule in facilitating CD–3–induced activation of the MAP kinases ERK1 and ERK2 in T–lymphocytes10, we asked whether GITR plays a similar role in NGF-induced ERK1/ERK2 activation in SCG neurons. NGF induced a robust activation of ERK1/ERK2 in P1 SCG neurons that peaked between 45 and 60 minutes after NGF treatment (Fig. 6a). To determine if endogenous GITRL–GITR signalling is required for NGF–induced ERK1/ERK2 activation, we compared NGF–induced ERK activation in SCG neurons obtained from P1 GITR null mice and their wild type littermates. Compared with wild type SCG neurons, ERK1/ERK2 activation was reduced in neurons GITR+/− mice and almost eliminated in neurons from GITR−/− mice (Fig. 6b). This suggests that GITRL–GITR signalling facilitates NGF–induced ERK1/ERK2 activation in neonatal sympathetic neurons. To assess the importance of ERK1/ERK2 activation in NGF–promoted axonal growth in our in vitro paradigm, we studied the effect of U0126, a selective pharmacological blocker of MEK1/MEK2, the kinases that phosphorylate ERK1/ERK2. This compound caused a substantial decrease in the size and complexity of the neurite arbors of P1 SCG neurons grown with NGF (Fig. 6c) without affecting the survival of these neurons (data not shown). These results suggest that facilitation of NGF–induced ERK1/ERK2 activation by GITR is central to the mechanism underlying the effects of endogenous GITRL–GITR signalling on NGF–induced axonal growth.
Figure 6. GITR−/− mice display a reduced ERK phosphorylation in response to NGF.
(a) Western blots showing phospho ERK1/ERK2 and total ERK1/ERK2 in P1 SCG neurons grown for 12 hours in medium containing Boc-D-FMK before treatment with 10 ng/ml NGF for times ranging from 5 to 180 minutes (0′ represents cultures not treated with NGF). (b) Western blots showing phospho ERK1/ERK2 and total ERK1/ERK2 in SCG neurons from P1 GITR+/+, GITR+/− and GITR−/− mice grown for 12 hours in medium containing Boc-D-FMK before 45 minutes exposure to 10 ng/ml NGF These experiment were repeated three times with consistent results. (c) Cultures of P1 SCG neurons were pretreated with either 10 μM U0126 (Calbiochem) or the inactive control compound U0124 (Calbiochem) for 2 hours before addition of 10 ng/ml NGF 24 hours later, the neurons with labelled with calcein-AM and images were digitally acquired for neurite arbor analysis. The Sholl plots for U0126-treated and U0124-treated (control) neurons are shown. U0126 caused a 44% decrease in neurite length and a 38% decrease in the number of branch points (significant in both cases P < 0.01). The results are derived from the grouped data of three independent experiments.
GITR enhances axonal growth by increasing ERK activation
To further investigate the MAP kinase link between GITRL–GITR signalling and NGF–promoted axon growth, we examined the role of ERK1/ERK2 activation in mediating the increase in NGF–promoted axonal growth produced by GITRL–IgG. To determine if GITRL IgG treatment increases ERK1/ERK2 activation in SCG neurons and whether this depends on the presence of NGF, we incubated P1 SCG neurons with and without NGF for 12 hours before exposing the neurons to GITRL–IgG for different times and studying ERK1/ERK2 phosphorylation by Western blotting. The initial period of culture prior to GITRL–IgG treatment permitted phospho–ERK1/ERK2 levels to fall to basal levels so that any subsequent increase could be clearly observed. Treatment of NGF–supplemented neurons with GITRL–IgG caused a clear increase in ERK1/ERK2 activation that was maximal by 30 minutes (Fig. 7a). Neurons that were incubated without NGF and sustained with caspase inhibitors prior to GITRL–IgG treatment did not have detectable phospho-ERK1/ERK2 at any time point examined (Fig. 7b). These results show that GITRL–IgG increases NGF–promoted ERK1/ERK2 activation but is incapable of activating ERK1/ERK2 in the absence of NGF.
Figure 7. GITRL IgG mediated Erk activation is required for the increase in axonal growth in the presence of NGF.
(a,b) Western blots showing phospho ERK1/ERK2 and total ERK1/ERK2 in P1 SCG neurons grown for 12h in medium containing Boc-D-FMK either with 10 ng/ml NGF (a) or without NGF (b) before being treated with 1μg/ml GITRL-IgG for times ranging from 5 to 45 minutes (0′ represents cultures not treated with GITRL-IgG). These experiment were repeated three times with consistent results. Branching (c), length (d) and Sholl analysis (e, f) of the neurite arbors of P1 SCG neurons grown for 4 hours in 10 ng/ml NGF followed by 1 hour pre-treatment with either 10 μm U0126 (Calbiochem) or the inactive control compound (U0124: Calbiochem) before 24 hours incubation with either 1μg/ml GITRL-IgG or 1μg/ml control-IgG (anti-β-III tubulin). Data from at least 150 neurons in each condition from three independent experiments are shown (** P < 0.001, statistical comparison with control, one-way ANOVA with Fisher's post hoc).
To investigate if the enhanced ERK1/ERK2 activation brought about by GITRL–IgG in NGF–supplemented cultures is responsible for the increase in NGF–promoted axonal growth, we examined whether U0126 could prevent this increase in growth. Because U0126 reduces NGF–promoted growth if added before or at the time neurons are exposed to NGF (Fig. 6c), we first grew the neurons with NGF for 4 hours to allow phospho–ERK1/ERK2 levels to return to a low level before treating with U0126 and GITRL–IgG so that we could selectively study the effect of GITRL–IgG-enhanced ERK1/ERK2 activation on neurite growth. In these experiments, the enhanced axonal growth and branching produced by GITRL–IgG was completely abolished by U0126 (Figs. 7c-f). These results demonstrate that the enhanced ERK1/ERK2 activation brought about by stimulating GITR with exogenous ligand is responsible for the increase in NGF–promoted axonal growth.
Taken together, the above findings indicate that GITR acts as a co-accessory molecule in NGF–induced ERK activation in neonatal sympathetic neurons in much the same way it does in CD3–stimulated T cells, and that this underlies the selective effect of GITRL–GITR signalling on axonal growth. GITR ligation in T–cells also causes robust activation of NF–κB8. While NF-κB has been implicated in promoting neurite growth from sensory neurons17, 18, we have found using a sensitive NF–κB reporter that GITR ligation does not activate NF-κB in SCG neurons (data not shown), suggesting that GITR does not enhance NGF–promoted neurite growth in these neurons by activating NF–κB.
DISCUSSION
We have shown that GITRL–GITR interaction in developing sympathetic neurons is crucial for NGF–promoted axonal growth and target innervation in the immediate postnatal period when sympathetic axons are ramifying in their distal targets under the influence of target–derived NGF. Inhibiting GITRL–GITR interaction in cultured neonatal sympathetic neurons by antisense knockdown of GITR, targeted deletion of GITR or blocking interaction between GITRL and GITR with a soluble GITR–IgG decoy substantially reduces the ability of NGF to promote neurite growth from these neurons without affecting their survival. Furthermore, the highly significant reduction in sympathetic innervation density in GITR-deficient neonates and the clear gene dosage effect in heterozygous mice demonstrate the importance of GITR signalling for the proper establishment of sympathetic innervation in vivo. These findings not only reveal the existence of a novel and unsuspected mechanism regulating NGF–dependent target innervation but provide the first evidence that GITRL–GITR interactions have important functions outside the immune system.
The co–expression GITR and GITRL in sympathetic neurons and our demonstration that blocking GITRL–GITR interaction at the level of individual neurons with GITR–IgG inhibits NGF–promoted neurite growth indicates that GITRL–GITR signalling operates via an autocrine mechanism in developing sympathetic neurons. Autocrine signalling has previously been demonstrated in sympathetic neuroblasts. Studies using function–blocking anti–HGF antibodies in low density cultures of mouse SCG neuroblasts have shown that a HGF/Met autocrine loop promotes the differentiation of these neuroblasts into neurons19. HGF also enhances neurite growth from mouse SCG neurons later in development either alone or in the presence of NGF, but at no stage during fetal or postnatal development or in the adult does anti–HGF significantly reduce neurite arbor size in low density cultures15, 19, suggesting that HGF may play a role in regulating the growth of SCG axons independently of NGF throughout life. Although anti–HGF has been reported to reduce neurite network complexity in dense cultures of postnatal rat SCG neurons20, because individual neurite arbors were not analyzed, it is unclear whether this was due to changes in arbor size or neurite fasciculation. Although we suggest that GITRL–GITR signalling is most likely to operate by an autocrine route in sparse neuronal cultures, this does not preclude the operation of paracrine GITRL-GITR signalling in vivo influencing axonal growth in addition to the autocrine GITRL–GITR rheostat. Indeed, our experiments with GITRL overexpression and GITRL–IgG have shown that NGF–stimulated sympathetic neurons have the capacity to respond to enhanced GITR activation with increased neurite growth. However, we have not detected GITRL immunoreactively in the intermediate and distal targets of SCG neurons at the stage when GITR signalling affects sympathetic innervation density.
The effect of GITRL on sympathetic axon growth displays several distinctive features that distinguish it from the effects of other factors on sympathetic axon growth. Whereas HGF, MSP, GDNF, NT-3 and NGF promote the growth of sympathetic axons in vitro at particular stages of development, they also promote sympathetic neuron survival to varying extents and are each able to exert these effects independently of the presence of other factors15, 19, 21-23 (and unpublished data). In contrast, GITRL–GITR interaction only influences neurite growth in the presence of NGF and has no effect on the survival of sympathetic neurons in vitro either in the presence or absence of NGF. Likewise, the number of SCG neurons in vivo is not reduced in GITR−/− mice.
GITRL–GITR signalling appears to exert its selective effect on NGF–promoted axonal growth from neonatal SCG neurons by facilitating a key step in the NGF signalling cascade, ERK1/ERK2 activation, that is selectively required for NGF–promoted axonal growth but not survival. Whereas NGF–promoted ERK1/ERK2 phosphorylation is attenuated in neurons from GITR+/− mice, it is almost eliminated in neurons from GITR−/− mice. Although GITRL IgG enhances NGF–promoted ERK1/ERK2 phosphorylation, GITRL–IgG does not activate ERK1/ERK2 in the absence of NGF. This effect of GITR signalling on NGF–promoted ERK1/ERK2 activation mirrors the co–accessory role of GITR in CD3–stimulated T–lymphocytes. CD3 promotes ERK1/ERK2 activation in T–cells that in turn mediates a robust proliferative response10. Co-stimulation of T–cells with anti–CD3 and GITRL enhances ERK1/ERK2 activation and increases proliferation10, but activation of GITR in the absence of anti-CD3 neither increases ERK1/ERK2 activation (C. Riccardi, unpublished observations) nor induces any proliferative response (Supplementary Fig. 1). In addition to GITR, several other members of the TNFR superfamily, including CD30, CD40 and RANK, have been shown to act as co-accessory molecules in facilitating ERK1/ERK2 phosphorylation in the immune system24, 25. Multiple members of the TNFR superfamily are also expressed in the SCG with different developmental time courses (unpublished observations). In future work it will be interesting to ascertain whether any of these TNFR members play a similar role to GITR in axonal growth at stages before and after the period of development GITR activation is crucial for NGF-promoted axonal growth.
NGF was discovered over half a century ago by its ability to promote axonal growth, and is the paradigmatic target-derived neurotrophic factor on which the neurotrophic theory is based26. The discovery of a novel signalling loop that is essential for the ability of NGF to promote axonal growth has revealed a fascinating and completely unexpected regulatory element in target field innervation in the developing sympathetic nervous system. Preliminary expression studies have revealed that sympathetic neurons are not the only neurons to express GITR and GITRL. In future work it will be important to ascertain how extensively GITRL–GITR signalling regulates the growth of neural processes in the nervous system.
METHODS
Real-time PCR quantification of mRNA levels
Total RNA was isolated with the RNeasy Mini extraction kit (Qiagen, Germany) from dissected SCG. The RNA was reverse transcribed for 1 hr at 37°C with StrataScript RT (Stratagene) in a 40 μl reaction containing the manufacturer's buffer with 5 mM dNTPs (Stratagene) and 10 μM random hexamers (Amersham). 3 μl aliquots of the RT reactions were amplified in a 25 μl reaction volume using the Brilliant QPCR core reagent kit (Stratagene). Each reaction mixture consisted of 1xPCR buffer, 3 mM MgCl2, 300 pmol primers, 0.4 mM dNTPs, 1 unit of Taq, 1x reference dye and 1 unit of SYBR green (Molecular Probes). The respective forward and reverse primers for real-time PCR were: GITR, 5′–aggtcagccgagtgtagttg–3′ and 5′–gcagcagcgagtgttgttg-3′; GITRL, 5′-tggctctgttactgatgctg-3′ and 5′-agtgaggtttgggagatgtc-3′; TH, 5′-aaggaaagtgtcagagttg-3′ and 5′-accctgcttgtattggaa-3′; GAPDH, 5′-tcccactcttccaccttc-3′ and 5′-ctgtagccgtattcattgtc-3′. The PCR was performed with the Mx3000P (Stratagene) for 45 cycles of 95°C for 30 sec, 54°C (for GITR and TH), 51°C (for GITRL and GAPDH) for 1 min, 72°C for 30 sec. A melting curve was obtained to confirm that the SYBR green signal corresponded to a unique and specific amplicon. Standard curves were generated for every real-time PCR run by using serial three-fold dilutions of a reverse transcribed RNA extract from an E13 whole mouse embryo.
Neuron cultures
Dissociated cultures of SCG neurons were set up from E18, P1, P3 and P5 CD-1 mice or from P1 GITR+/+, GITR+/− and GITR−/− C57/BL6 pups and grown in defined medium on a poly-ornithin/laminin substratum as previously described27, 28. The results obtained in cultures established from both mouse strains are comparable because the SCG of C57-BL6 and CD-1 mice express similar levels of GITR and show identical responses to GITR–IgG and GITRL–IgG (data not shown). All animal work was approved by our institutional animal use committee and by the Home Office.
Gold microcarrier preparation and ballistic transfection of cultured neurons was carried out using a hand-held gene gun (BioRad, CA USA) as previously described17. Neuronal survival was quantified as previously described17, 18. The neurite arbors of transfected neurons were visualized by expression of YFP. In non-transfection experiments, neurite arbors were labelled with the fluorescent dye calcein-AM (Invitrogen) at the end of the experiment. For every condition in each experiment, images of at least 50 neurons were digitally acquired by confocal microscopy, and analysed to obtain total neurite length, number of branch points and Sholl profiles 29 as previously described 17, 18. Pair-wise comparisons were made using the student T-test. For multiple comparisons ANOVA was performed followed by Fishers post-hoc test.
Immunocytochemistry
Cultures were fixed in ice-cold methanol, washed with phosphate-buffered-saline (PBS) and blocked with 5% BSA in PBS. The cells were incubated overnight with primary antibody in 1% BSA at 4°C. The primary antibodies were: β-III tubulin (Promega, 1:1000), GITR (Chemicon, 1:200), GITRL (R&D systems, 1:100) and YFP (Chemicon, 1:1000). After washing, the cells were incubated with the appropriate secondary antibody (Alexa-Fluor, Invitrogen, 1:500) and were counterstained with DAPI (Chemicon).
Quantification of neuron number in the SCG.
Estimates of the numbers of neurons in the SCG of P1 GITR+/+, GITR+/− and GITR−/− pups was carried out by stereological analysis of serial section as previously described31.
Quantification of the sympathetic innervation of SCG targets
The eyes and nose of P1 GITR+/+, GITR+/− and GITR−/− littermates were fixed in 4% paraformaldehyde for 24 h and were cryoprotected in 30% sucrose before being frozen. 15 μm serial sections were cut at right angles to the visual axis. The sections were mounted onto poly-lysine-coated slides (BDH), blocked with 10% normal goat serum containing 0.1% tritonX-100 in 10 mM PBS for 1 h at room temperature, and then incubated for 18 hr at 4°C with a rabbit anti-TH polyclonal antibody (Chemicon) diluted 1:200 in PBS with 1% normal goat serum. The sections were washed three times in PBS before being incubated with goat anti-rabbit secondary antibody (Alexa-Fluor, Invitrogen, 1:500). All sections were counterstained with DAPI (Chemicon). For the iris, four images were digitally acquired from sections passing through the iris, each one from a different quadrant of the iris. For the nasal mucosa, every third section beginning from the tip of the nose and extending caudally was imaged. The outline of the iris and nasal mucosa in these images was traced using Adobe Photoshop 7. The total iris and nasal mucosa area and the area containing TH-positive fibres were estimated by automated pixel counts, and the ratio TH-positive area to total iris area was calculated as a percentage. Six eyes and three nose for each genotype were sectioned and analyzed before the genotypes were ascertained so as to avoid any sampling bias.
Western blotting
Western blotting was carried out using the Bio-Rad TransBlot (Bio-Rad, CA-USA) as previously described18, using the following primary antibodies: anti-phospho-Erk1/2 (1:1,000; Cell signalling), anti-total Erk (1:1,000; Cell signalling) or anti-β-III tubulin (1:10,000; Promega) antibodies, which were detected with the appropriate peroxidase-linked secondary antibodies (1:2,000; Promega, UK) and ECL-plus (Amersham).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by a grant from the Wellcome Trust. We are grateful to Dr. Avi Ashkenazi (Genentech Inc., South San Francisco, CA, USA) for GITRL IgG and GITR IgG and to Prof. Tim Cowen (UCL, London, UK) for advise on studying iris innervation.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
REFERENCES
- 1.Chilton JK. Molecular mechanisms of axon guidance. Dev Biol. 2006;292:13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 2.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 4.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuruvilla R, et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 7.Krausz LT, et al. GITR-GITRL system, a novel player in shock and inflammation. Thescientificworldjournal. 2007;7:533–566. doi: 10.1100/tsw.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esparza EM, Arch RH. Glucocorticoid-induced TNF receptor, a costimulatory receptor on naive and activated T cells, uses TNF receptor-associated factor 2 in a novel fashion as an inhibitor of NF-kappa B activation. Journal of Immunology. 2005;174:7875–7882. doi: 10.4049/jimmunol.174.12.7875. [DOI] [PubMed] [Google Scholar]
- 9.Esparza EM, Lindsten T, Stockhausen JM, Arch RH. Tumor necrosis factor receptor (TNFR)-associated factor 5 is a critical intermediate of costimulatory signaling pathways triggered by glucocorticoid-induced TNFR in T cells. Journal of Biological Chemistry. 2006;281:8559–8564. doi: 10.1074/jbc.M512915200. [DOI] [PubMed] [Google Scholar]
- 10.Ronchetti S, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 11.Wright LL, Cunningham TJ, Smolen AJ. Developmental neuronal death in the rat superior cervical sympathetic ganglion: cell counts and ultrastructure. J. Neurocytol. 1983;12:727–738. doi: 10.1007/BF01258147. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt S, Piñón LGP, Ernfors P, Davies AM. Sympathetic neuron survival and TrkA expression in NT3-deficient mouse embryos. EMBO J. 1997;16:3115–3123. doi: 10.1093/emboj/16.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 14.Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27:265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- 15.Thompson J, Dolcet X, Hilton M, Tolcos M, Davies AM. HGF promotes survival and growth of maturing sympathetic neurons by PI-3 kinase- and MAP kinase-dependent mechanisms. Mol Cell Neurosci. 2004;27:441–452. doi: 10.1016/j.mcn.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Goold RG, Gordon-Weeks PR. The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Molecular & Cellular Neurosciences. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez H, Hale V, Dolcet X, Davies A. NF-kB signalling regulates the growth of neural processes in the developing peripheral and central nervous systems. Development. 2005;132:1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher D, et al. Nuclear factor-kappaB activation via tyrosine phosphorylation of inhibitor kappaB-alpha is crucial for ciliary neurotrophic factor-promoted neurite growth from developing neurons. Journal of Neuroscience. 2007;27:9664–9669. doi: 10.1523/JNEUROSCI.0608-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maina F, et al. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron. 1998;20:835–846. doi: 10.1016/s0896-6273(00)80466-3. [DOI] [PubMed] [Google Scholar]
- 20.Yang XM, et al. Autocrine hepatocyte growth factor provides a local mechanism for promoting axonal growth. J Neurosci. 1998;18:8369–8381. doi: 10.1523/JNEUROSCI.18-20-08369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forgie A, Wyatt S, Correll PH, Davies AM. Macrophage stimulating protein is a target-derived neurotrophic factor for developing sensory and sympathetic neurons. Development. 2003;130:995–1002. doi: 10.1242/dev.00329. [DOI] [PubMed] [Google Scholar]
- 22.Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 23.Belliveau DJ, et al. NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J Cell Biol. 1997;136:375–388. doi: 10.1083/jcb.136.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suttles J, et al. CD40 signaling of monocyte inflammatory cytokine synthesis through an ERK1/2-dependent pathway. A target of interleukin (il)-4 and il-10 anti-inflammatory action. Journal of Biological Chemistry. 1999;274:5835–5842. doi: 10.1074/jbc.274.9.5835. [DOI] [PubMed] [Google Scholar]
- 25.Zheng B, et al. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood. 2003;102:1019–1027. doi: 10.1182/blood-2002-11-3507. [DOI] [PubMed] [Google Scholar]
- 26.Davies AM. Regulation of neuronal survival and death by extracellular signals during development. Embo J. 2003;22:2537–2545. doi: 10.1093/emboj/cdg254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies AM. The survival and growth of embryonic proprioceptive neurons is promoted by a factor present in skeletal muscle. Dev. Biol. 1986;115:56–67. doi: 10.1016/0012-1606(86)90227-7. [DOI] [PubMed] [Google Scholar]
- 28.Davies AM, Lee KF, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- 29.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 30.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–352. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- 31.Barker V, Middleton G, Davey F, Davies AM. TNFalpha contributes to the death of NGF-dependent neurons during development. Nat Neurosci. 2001;4:1194–1198. doi: 10.1038/nn755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.