Abstract
Object
Thalamopeduncular tumors arise at the junction of the inferior thalamus and cerebral peduncle and present with a common clinical syndrome of progressive spastic hemiparesis. Pathologically, these lesions are usually juvenile pilocytic astrocytomas and are best treated with resection with the intent to cure. The goals of this study are to define a common clinical syndrome produced by thalamopeduncular tumors and to discuss imaging characteristics as well as surgical adjuncts, intraoperative nuances, and postoperative complications relating to the resection of these neoplasms.
Methods
The authors present a retrospective review of their experience with 10 children presenting between 3 and 15 years of age with a thalamopeduncular syndrome. Formal preoperative MR imaging was obtained in all patients, and diffusion tensor (DT) imaging was performed in 9 patients. Postoperative MR imaging was obtained to evaluate the extent of tumor resection. A prospective analysis of clinical outcomes was then conducted by the senior author.
Results
Pilocytic astrocytoma was the pathological diagnosis in 9 cases, and the other was fibrillary astrocytoma. Seven of 9 pilocytic astrocytomas were completely resected. Radical surgery was avoided in 1 child after DT imaging revealed that the corticospinal tract (CST) coursed through the center of the tumor, consistent with the infiltrative nature of fibrillary astrocytoma as identified by stereotactic biopsy. In 8 patients, tractography served as an important adjunct for designing a surgical approach that spared the CST. In 6 cases the CSTs were pushed anterolaterally, making a transsylvian approach a poor choice, as was evidenced by the first patient in the series, who underwent operation prior to the advent of tractography, and who awoke with a dense contralateral hemiparesis. Thus, subsequent patients with this deviation pattern underwent a transcortical approach via the middle temporal gyrus. One patient exhibited medial deviation of the tracts and another had lateral deviation, facilitating a transtemporal and a transfrontal approach, respectively.
Conclusions
The thalamopeduncular syndrome of progressive spastic hemiparesis presenting in children with or without symptoms of headache should alert the examiner to the possibility of a tumoral involvement of CSTs. Preoperative tractography is a useful adjunct to surgical planning in tumors that displace motor pathways. Gross-total resection of pilocytic astrocytomas usually results in cure, and therefore should be entertained when developing a treatment strategy for thalamopeduncular tumors of childhood.
Keywords: thalamopeduncular astrocytoma, juvenile pilocytic astrocytoma, diffusion tensor imaging, tractography, pediatric neurosurgery, congenital
The thalamopeduncular syndrome is a common clinical presentation exhibited by children harboring a lesion at the interface between the thalamus and cerebral peduncle. This subclassification was introduced by Puget et al.21 in an effort to better describe thalamic tumors of childhood. However, these lesions have also been designated as “basal ganglia,” “brainstem,” or “thalamic” tumors by other authors, specifically focal midbrain gliomas arising from the tegmentum.3,4,8,9,11,14–17,21,24,27–29 In addition to anatomical location, classification schema have been proposed for brainstem tumors based on growth patterns, to include diffuse, cervicomedullary, and focal tumors, which help guide therapeutic decisions based on natural history.7,8,11,26 Differences in nomenclature make the incidence of these lesions difficult to quantify, although they do represent a small subset of supratentorial pediatric astrocytomas in general.
With advances in functional imaging, coupled with frameless stereotaxy, the treatment of deep-seated intracerebral tumors has shifted. These lesions were once considered unresectable because of high morbidity and mortality rates; however, contemporary microsurgical techniques have fostered attempts at resecting these neoplasms, although the approach has been somewhat variable. 11,14–17,20,21,27,29 Given the location of these lesions, obtaining a curative resection without injury to either the CST, the oculomotor nerve, or the optic tract has proven challenging. Chemotherapy and radiotherapy have been used to treat lesions in the region of the diencephalon, with variable success.10,12,24 The application of tractography via DT imaging has proven to be a valuable asset for surgical planning,5,16,19 and we used it to evaluate the course of the CST preoperatively. We describe our experience with 10 children presenting with this syndrome of progressive spastic hemiparesis without obstructive hydrocephalus whose treatment algorithm was tailored by preoperative functional neuroimaging.
Methods
Between 2003 and 2010, 10 children were referred to the pediatric brain tumor program of Le Bonheur Children’s Medical Center and St. Jude Children’s Research Hospital in Memphis, Tennessee, with tumors of the thalamopeduncular region. Five boys and 5 girls between 3 and 15 years of age presented with a history of gradual, progressive, spastic hemiparesis (Table 1). In 2 cases, patients reported intermittent headaches, but symptoms of raised intracranial pressure were otherwise not a part of their clinical presentation.
TABLE 1.
Clinical data of the 10 children in the series*
| Case No. | Age (yrs), Sex | Presentation | FU (mos) | Prior Treatment | Complications |
|---|---|---|---|---|---|
| 1 | 7, M | hemiparesis | 90 | none | worse paresis |
| 2 | 12, F | hemiparesis | 49 | STR & chemo | HH, ION |
| 3 | 3, M | hemiparesis | 46 | biopsy & chemo | |
| 4 | 8, F | hemiparesis | 33 | none | |
| 5 | 10, F | hemiparesis | 32 | biopsy & chemo | |
| 6 | 13, M | hemiparesis | 31 | biopsy | 3rd CN |
| 7 | 3, F | hemiparesis | 30 | STR & chemo | |
| 8 | 6, F | hemiparesis | 21 | biopsy | 3rd CN |
| 9† | 10, M | hemiparesis | 21 | biopsy & chemo | tremor, STR |
| 10‡ | 15, M | hemiparesis | 8 | none | HH, STR |
Chemo = chemotherapy; CN = cranial nerve; FU = follow-up; HH = homonymous hemianopia; ION = ischemic optic neuropathy; STR = subtotal resection.
Patient harbored a multifocal tumor that was debulked but could not be cured surgically.
Patient had a small residual component noted on postoperative imaging.
All patients were studied using detailed Gd-enhanced MR imaging. Diffusion-weighted images were acquired and analyzed as has been previously reported.19 Briefly, images were acquired with a double-spin echo planar imaging sequence (Reese et al.23) with an initial b = 0 value and 12 gradient directions with b = 1000 seconds/mm.2 These images were acquired in 4 repetitions and were averaged after realignment to improve the signal-to-noise ratio in the final product images. Diffusion tensor echo planar imaging pulse-sequence parameters were as follows: TR 7 seconds; TE 101 msec; field of view 192 mm; matrix 128 × 128. Diffusion tensor images were processed using the DTI Toolkit (http://sourceforge.net/projects/spmtools) with a statistical parametric mapping program (version SPM2; http://www.fil.ion.ucl.ac.uk/spm/). The images that were analyzed for this study were acquired for the clinical management of these patients’ disease, and a retrospective review of the clinical and imaging data was approved by the St. Jude Institutional Research Board.
Results
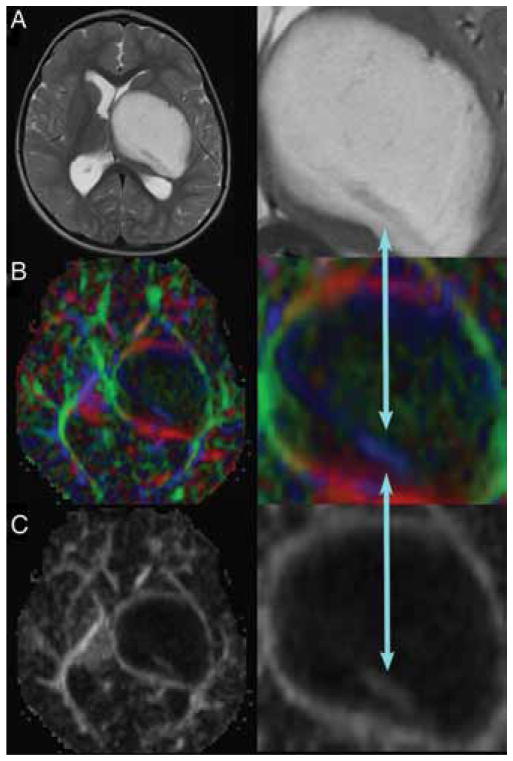
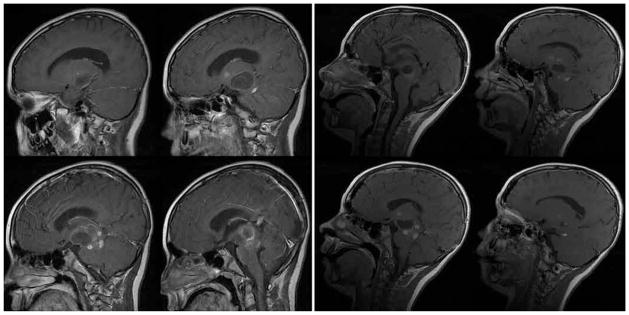
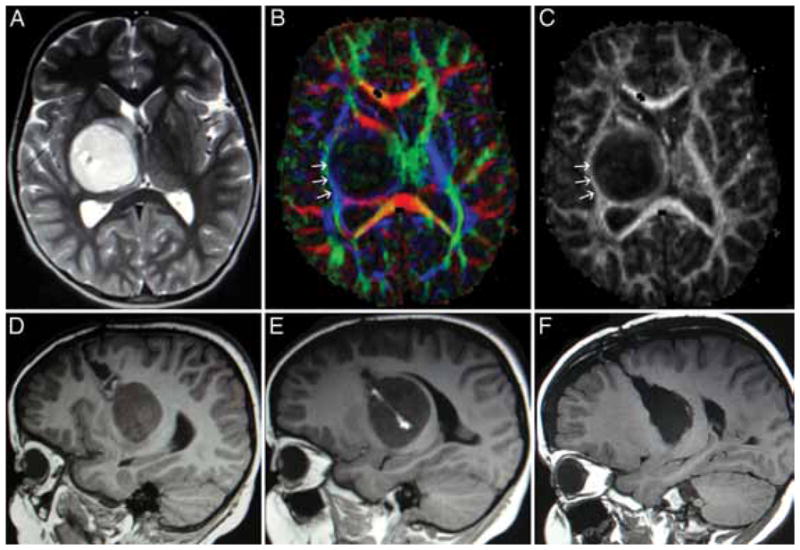
Seven of the 10 children in this series were referred for evaluation after previous biopsy procedures or attempted resection, and 5 experienced radiographic progression of their tumor while being treated with chemotherapy. All of the patients underwent detailed MR imaging studies, which revealed a variety of imaging characteristics, as exemplified in Fig. 1. The earliest patient in this series was surgically treated before DT imaging and tractography were available. In this patient’s case, a transsylvian approach was used to enter the tumor where it invaded the temporal horn of the lateral ventricle, and a gross-total resection was accomplished; however, his hemiparesis was worsened by the resection, without a clear-cut explanation from his postoperative MR images. In the 9 subsequent cases, DT imaging and tractography were used to study the displacement of the CSTs. In 1 child, tractography demonstrated the CSTs coursing through the midportion of the tumor (Fig. 2). A stereotactic biopsy was performed, and pathological findings were consistent with fibrillary astrocytoma. This patient went on to receive chemotherapy as opposed to resection.
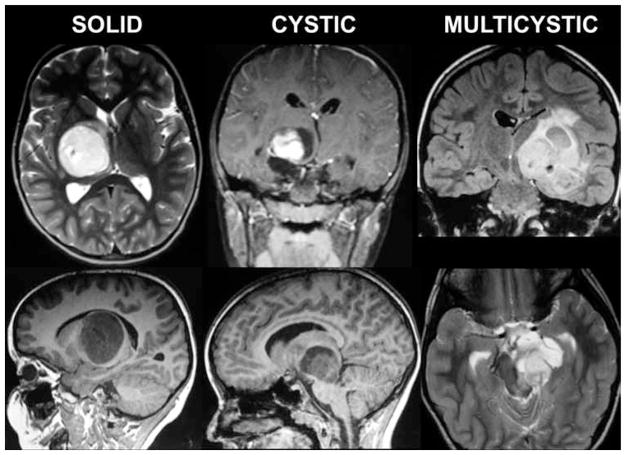
Fig. 1.
Illustrative neuroimaging studies. Eight of 9 tumors in this series demonstrated pilocytic histological features. Some tumors were solid and some included cystic components. All lesions displayed variable contrast enhancement. One tumor was thought to be a glioblastoma based on imaging findings because of the heterogeneous enhancement pattern; however, results of stereotactic biopsy sampling proved it to be pilocytic.
Fig. 2.
Large left thalamopeduncular tumor. Axial T2-weighted MR image (A) revealing a hypodense structure passing through the posterior aspect of the lesion. The DT imaging color map (B) and fractional anisotropy map (C) demonstrated that the hypodense structure on T2- weighted imaging was the CST (blue arrows) passing directly through the tumor. The right-hand panels are enlarged views of the areas of interest.
For the other 8 patients, the information from tractography was used to define a frameless stereotactic trajectory to the tumor, allowing resection of the neoplasm while sparing the CSTs. Anterolateral deviation of the CST was the most common pattern noted in this series, which prompted a transcortical, middle temporal gyral approach in 6 cases (Fig. 3). This approach was also used to approach a lesion that displaced the tracts medially (Fig. 4). Finally, 1 patient underwent a frontal transcortical approach through a previous biopsy tract after tractography revealed lateral displacement of the tracts (Fig. 5).
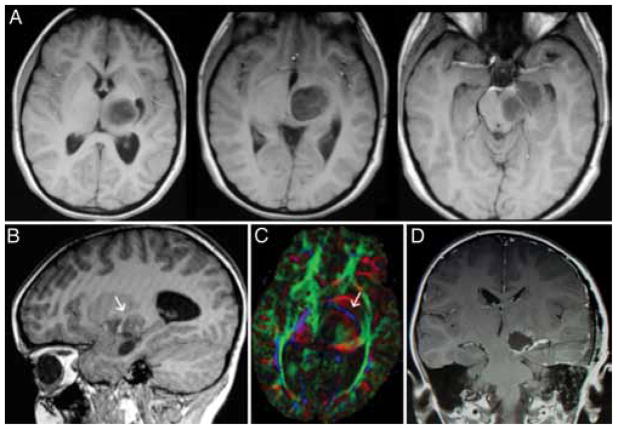
Fig. 3.
Left thalamopeduncular pilocytic astrocytoma. A: Composite of 3 axial T1-weighted Gd-enhanced MR images demonstrates that the tumor arises from the lateral aspect of the peduncle underneath the thalamus, pushing the normal thalamus superiorly. The thalamic displacement made a transcallosal approach to the tumor a poor choice, because the surgeon would have violated the normal thalamus to reach the tumor. B: The optic tract, a structure that must be carefully avoided in removing the tumor, is deviated superior and lateral to the tumor. The arrow designates the optic tract. C: An axial DT image of the same tumor shows the CSTs deviated anteriorly and laterally (arrow; CSTs are in blue); this was the most common pattern of CST displacement in this series, noted in 7 of 10 patients. This pattern of tract displacement made a transsylvian approach unattractive; one would have to transect the tracts to reach the tumor. D: The authors chose an approach through the middle temporal gyrus by using frameless stereotactic navigation to approach the tumor just posterior to the CSTs.
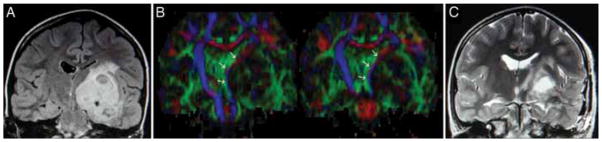
Fig. 4.
Left thalamopeduncular cystic and solid pilocytic astrocytoma. A: A coronal FLAIR MR image demonstrating that the tumor grows from the thalamopeduncular junction, across the ambient cistern, and through the choroidal fissure of the temporal horn of the lateral ventricle to invade the middle fossa. B: Coronal DT images showing that the CSTs are displaced medial to the tumor. Medial tract displacement made an image-guided approach through the middle temporal gyrus optimal to reach both the superior and inferior extent of the neoplasm. C: A coronal T2-weighted MR image obtained the day after surgery, demonstrating the approach through the temporal lobe and a gross-total resection of the tumor, sparing the CSTs.
Fig. 5.
Pilocytic tumor in a 3-year-old child presenting with spastic hemiparesis. A: Axial T2-weighted MR image showing displacement of the thalamus anterior and posterior to the tumor. B: A DT imaging color map. The arrows (tracts in blue) designate the CST. C: A fractional anisotropy map demonstrating that in this case the CSTs (arrows) were draped around the lateral aspect of the tumor, which made a lateral approach unattractive. D and E: Sagittal T1-weighted MR images showing previous biopsy track; note enhancement of the track. F: The authors chose to use the previous biopsy track, a transfrontal corticotomy, to remove the tumor; the patient’s hemiparesis improved postoperatively.
Based on results of follow-up studies done to date, a gross-total resection was accomplished in all but 2 of the pilocytic astrocytomas (Table 1). Initially thought to be a confluent mass, 1 of the 9 pilocytic tumors was noted to be multifocal in nature on postoperative imaging. Although stable for 18 months postoperatively, the patient’s residual tumor showed evidence of radiographic progression on his 21-month follow-up, and will be treated with focal irradiation (Fig. 6). A second patient was thought to have had a complete resection, but a 3-month follow-up scan demonstrated a small focus of residual tumor that is being followed expectantly at present. With the exception of the patient with multifocal disease, none of the other 8 patients whose tumors had pilocytic histological features have required further adjuvant therapy. However, the mean follow-up period of 36 months is too brief at this time to comment on the rate of recurrence.
Fig. 6.
Left: Thalamopeduncular tumor with cystic component and heterogeneous enhancement. Right: Postoperative sagittal Gd-enhanced MR images reveal the resection cavity and illustrate the multifocal nature of the tumor.
Five patients displayed postoperative neurological deficits that are attributed directly to surgical intervention. As previously stated, the first patient in the series exhibited a significant hemiparesis secondary to probable violation of the descending motor tracts. In another patient, the oculomotor nerve was indistinguishable in the tumor capsule and was inadvertently sacrificed. A third patient developed a transient partial oculomotor nerve palsy postoperatively from manipulation of the nerve as it was stretched around the tumor in the ambient cistern. Finally, 2 patients’ tumors involved the ipsilateral optic tract, and resection resulted in a contralateral homonymous hemianopia. One of these patients also experienced an ipsilateral ischemic optic neuropathy involving the intraorbital optic nerve that resulted in severe visual loss in that eye as a consequence. Patients uniformly demonstrated worsening of their contralateral hemiparesis immediately after surgery, but as of this writing, all of the patients in the series who underwent preoperative DT imaging with tractography followed by resection have returned to their preoperative motor status or better. However, all patients continue to exhibit a mild thalamopeduncular syndrome, evidenced by a lack of fine-motor coordination of the affected side, a subtle spasticity, and a circumduction gait.
Discussion
Approximately 20% of pediatric intracranial tumors arise from the thalamus or brainstem, with an incidence rate of 5% and 15%, respectively.1,4,22 Lesions arising at the interface of the diencephalon and mesencephalon have lacked classification specificity. In fact, these neoplasms have been termed “thalamic,” “brainstem,” or “basal ganglia” tumors in the past. Puget et al.21 divided tumors of the thalamic region into unilateral, bilateral, and thalamopeduncular; a term that best describes the anatomical features of these tumors without designating a specific structure of origin, which is largely unknown. These tumors appear to arise in the cerebral peduncle at its junction with the inferior thalamus, and the majority of these tumors are confluent lesions with histological features consistent with pilocytic tumors. They are slow growing, displacing the CSTs, which accounts for the clinical presentation of slowly progressive spastic hemiparesis. Based on this series, the CSTs may be displaced anterolaterally (7 of 10 cases); medially (1 of 10 cases); laterally (1 of 10 cases); or encased within the substance of the tumor, as exhibited by the fibrillary astrocytoma.
Interestingly, the CSTs were never displaced posteriorly, which may offer a clue as to the point of origin of these neoplasms. As the glioma enlarges, it appears to follow the path of least resistance, growing across the ambient cistern and pushing the thalamus superiorly. The optic tract is typically stretched laterally and superiorly around the tumor capsule, and may be difficult to discern intraoperatively. For those tumors extending along the length of the peduncle into the posterior fossa, the third cranial nerve is stretched inferiorly. In 1 case, the third cranial nerve was indistinguishable from the tumor capsule beneath the tentorial edge, resulting in its inadvertent sacrifice while removing the capsule.
Early on, these tumors seem to cause little in the way of symptoms while growing unconstrained across the ambient cistern. Once they enlarge enough to reach the tentorial edge, the lesions expand the fimbria of the hippocampus until it tears, at which point the tumor grows through the choroidal fissure and into the temporal horn of the lateral ventricle. It is in this location that they are most surgically accessible. Two patients had complaints of headaches as part of their clinical syndrome, but none had signs or symptoms of raised intracranial pressure. Early ventricular enlargement was found on 1 patient’s preoperative MR imaging studies and this patient would probably have become symptomatic from hydrocephalus had the tumor not been addressed surgically. Thus, an additional benefit of radical tumor resection is the avoidance of CSF shunting.
Tomita and Cortes27 reported a series of 7 tumors arising from the midbrain tegmentum and extending in an exophytic fashion to arise from the cerebral peduncle. These lesions were resected via a subtemporal approach with acceptable morbidity, and pathological findings proved juvenile pilocytic astrocytoma in all cases. The subtemporal approach, while avoiding penetration of neocortex, does require significant retraction of the temporal lobe. Furthermore, this trajectory limits access to the superior aspect of the tumor; hence, these authors were only able to perform a gross-total resection in 3 of 7 tumors. Although it is commonly accepted that juvenile pilocytic astrocytoma is a surgically treatable disease, there are still centers that forgo operative intervention because of the morbidity associated with resection of deep-seated lesions, as evidenced by the fact that half of the patients in our series were referred to us after their disease had progressed on various chemotherapeutic regimens.
A transsylvian approach was used for the first patient in the series to access the tumor, because it had grown into the temporal horn of the lateral ventricle. A complete resection was accomplished, but postoperatively the patient’s hemiparesis worsened. Based on our subsequent understanding from tractography, we suspect that the patient’s CSTs were displaced anterolaterally by the tumor, and that our approach violated the tracts. Whether the increase in paresis could have been improved upon with the addition of subcortical motor tract stimulation at the time of tumor resection is a matter of conjecture.2 In our experience, these tumors are gray and fleshy, making them easily distinguishable from the subjacent white matter tracts. The mesial aspect of the tumor within the brainstem easily separates from the delicate neural structures with ultrasonic aspiration.
The second patient in the series had previously undergone a left frontal transcortical approach to the tumor at another institution for subtotal resection that resulted in transient aphasia and spastic hemiparesis. Because the patient’s DT imaging studies obtained prior to our surgery demonstrated that the CSTs had been previously disrupted and yet her speech had returned to normal, a transsylvian approach was again used in this case. This allowed a gross-total resection of the neoplasm, without speech deficit or worsening hemiparesis. However, the patient awoke with severe visual deficits consisting of only light perception in her left eye and a right temporal field hemianopia. The right hemianopia can be explained by damage to the left optic tract as it traverses the surgical field superolaterally. However, the complete loss of useful vision on the left is not explained anatomically, and is best explained by an ischemic optic neuropathy, which was supported by a postoperative MR imaging study that demonstrated subtle enhancement of the intraorbital portion of the optic nerve. Ischemic optic neuropathy is a recognized complication of neurosurgical procedures, most commonly described after prolonged spinal surgery in the prone position with significant blood loss and periods of hypotension.6,18,25 The incidence of optic nerve dysfunction following craniotomy is exceedingly rare, but it does exist as a complication reported most frequently with surgery of intracranial aneurysms. But, barring direct neural injury, intraorbital ischemia results from a disturbance in arterial or venous blood flow. This is most likely caused by ocular compression by the retracted skin flap.13 Therefore, meticulous attention to the course and manipulation of the optic tract and oculomotor nerve, as well as standard practices to prevent ocular compression, are as essential to a functional outcome as sparing of the CSTs.
The utility of tractography in neurosurgical planning has been previously reported.2,5,16,19 Berman et al.2 demonstrated the utility and accuracy of DT imaging for the preoperative evaluation of deep white matter motor tracts, substantiated by intraoperative subcortical stimulation, in patients undergoing craniotomy for glioma. Phillips et al.19 reported 2 cases in which DT imaging proved to be a useful tool in establishing differences between tumor and normal brainstem white matter architecture, aiding the resection of low-grade brainstem gliomas. Mapping the CSTs preoperatively allowed us to minimize significant postoperative motor deficits in this series. Although it has been suggested that these tumors arise from the cerebral peduncle proper, this study demonstrates that the CSTs are displaced in variable directions, making the crus cerebri an unlikely site of origin.27 The basal ganglia are displaced forward and medially by these tumors, and although all of these children had a spastic hemiparesis, none had chorea or athetosis preoperatively. However, despite the lack of evidence of infarction or radiologically confirmed change in residual tumor volume, the patient with a multifocal neoplasm developed athetosis 2 weeks postoperatively. In retrospect, this was probably related to involvement of the basal ganglia by his multifocal astrocytoma. His symptoms are currently controlled well with medical therapy, allowing the child to ride his bicycle and paddle his kayak.
To date, there have been no recurrences after grosstotal resection. Seven patients have exhibited improvement in their hemiparesis, although all have varying degrees of residual motor deficit, exhibited by upper-extremity fine motor incoordination and/or a circumduction gait. Observation has been chosen for one of the patients who proved to have a subtotal resection, but repeat resection will be recommended should his tumor progress. The patient with multifocal progression is undergoing focal irradiation.
Conclusions
Tumors arising at the junction of the thalamus and cerebral peduncle typically present between early childhood and puberty with a thalamopeduncular syndrome characterized by progressive spastic hemiparesis. If confluent, resection of these lesions should be entertained, which may provide both a cure and avoidance of CSF shunting. Diffusion tensor imaging is a valuable tool for preoperative planning, because these tumors tend to displace the CSTs in various directions. Given their growth patterns, thalamopeduncular tumors tend to encroach on the ipsilateral optic tract, basal vein of Rosenthal, and ipsilateral third cranial nerve. An approach should be tailored to avoid these delicate neural structures, with a goal of gross-total resection. In this series, the tumors that had been treated with lowgrade chemotherapy had all progressed, suggesting that perhaps conventional chemotherapy is of marginal benefit in the management of these neoplasms.
Abbreviations used in this paper
- CST
corticospinal tract
- DT
diffusion tensor
Footnotes
Portions of this work were presented in 2009 at the American Society of Pediatric Neurosurgeons’ annual meeting, Fairmont Orchid Hotel, Kohala Coast, Hawaii.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Boop. Acquisition of data: Boop, Ogg, Scoggins, Sanford, Patay. Drafting the article: Boop, Broadway, Ogg. Study supervision: Sanford.
References
- 1.Barkovich AJ. Pediatric Neuroimaging. 3. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 2.Berman JI, Berger MS, Chung SW, Nagarajan SS, Henry RG. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107:488–494. doi: 10.3171/JNS-07/09/0488. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein M, Hoffman HJ, Halliday WC, Hendrick EB, Humphreys RP. Thalamic tumors in children. Long-term followup and treatment guidelines. J Neurosurg. 1984;61:649–656. doi: 10.3171/jns.1984.61.4.0649. [DOI] [PubMed] [Google Scholar]
- 4.Cuccia V, Monges J. Thalamic tumors in children. Childs Nerv Syst. 1997;13:514–521. doi: 10.1007/s003810050128. [DOI] [PubMed] [Google Scholar]
- 5.DaSilva AF, Tuch DS, Wiegell MR, Hadjikhani N. A primer on diffusion tensor imaging of anatomical substructures. Neurosurg Focus. 2003;15(1):E4. doi: 10.3171/foc.2003.15.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative risk factors for posterior ischemic optic neuropathy. J Am Coll Surg. 2002;194:705–710. doi: 10.1016/s1072-7515(02)01210-3. [DOI] [PubMed] [Google Scholar]
- 7.Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg. 1986;64:11–15. doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- 8.Epstein FJ, Farmer JP. Brain-stem glioma growth patterns. J Neurosurg. 1993;78:408–412. doi: 10.3171/jns.1993.78.3.0408. [DOI] [PubMed] [Google Scholar]
- 9.Grigsby PW, Thomas PR, Schwartz HG, Fineberg B. Irradiation of primary thalamic and brainstem tumors in a pediatric population. A 33-year experience. Cancer. 1987;60:2901–2906. doi: 10.1002/1097-0142(19871215)60:12<2901::aid-cncr2820601210>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Hadjipanayis CG, Kondziolka D, Flickinger JC, Lunsford LD. The role of stereotactic radiosurgery for low-grade astrocytomas. Neurosurg Focus. 2003;14(5):e15. doi: 10.3171/foc.2003.14.5.16. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman HJ, Soloniuk DS, Humphreys RP, Drake JM, Becker LE, De Lima BO, et al. Management and outcome of lowgrade astrocytomas of the midline in children: a retrospective review. Neurosurgery. 1993;33:964–971. doi: 10.1227/00006123-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Jenkin RD, Boesel C, Ertel I, Evans A, Hittle R, Ortega J, et al. Brain-stem tumors in childhood: a prospective randomized trial of irradiation with and without adjuvant CCNU, VCR, and prednisone. A report of the Childrens Cancer Study Group. J Neurosurg. 1987;66:227–233. doi: 10.3171/jns.1987.66.2.0227. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien) 1997;139:221–226. doi: 10.1007/BF01844755. [DOI] [PubMed] [Google Scholar]
- 14.Kelly PJ. Stereotactic biopsy and resection of thalamic astrocytomas. Neurosurgery. 1989;25:185–195. [PubMed] [Google Scholar]
- 15.McGirr SJ, Kelly PJ, Scheithauer BW. Stereotactic resection of juvenile pilocytic astrocytomas of the thalamus and basal ganglia. Neurosurgery. 1987;20:447–452. doi: 10.1227/00006123-198703000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Moshel YA, Elliott RE, Monoky DJ, Wisoff JH. Role of diffusion tensor imaging in resection of thalamic juvenile pilocytic astrocytoma. Clinical article. J Neurosurg Pediatr. 2009;4:495–505. doi: 10.3171/2009.7.PEDS09128. [DOI] [PubMed] [Google Scholar]
- 17.Moshel YA, Link MJ, Kelly PJ. Stereotactic volumetric resection of thalamic pilocytic astrocytomas. Neurosurgery. 2007;61:66–75. doi: 10.1227/01.neu.0000279725.13521.a3. [DOI] [PubMed] [Google Scholar]
- 18.Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145:604–610. doi: 10.1016/j.ajo.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips NS, Sanford RA, Helton KJ, Boop FA, Zou P, Tekautz T, et al. Diffusion tensor imaging of intraaxial tumors at the cervicomedullary and pontomedullary junctions. Report of two cases. J Neurosurg. 2005;103 (6 Suppl):557–562. doi: 10.3171/ped.2005.103.6.0557. [DOI] [PubMed] [Google Scholar]
- 20.Pierre-Kahn A, Hirsch J-F, Vinchon M, Payan C, Sainte-Rose C, Renier D, et al. Surgical management of brain-stem tumors in children: results and statistical analysis of 75 cases. J Neurosurg. 1993;79:845–852. doi: 10.3171/jns.1993.79.6.0845. [DOI] [PubMed] [Google Scholar]
- 21.Puget S, Crimmins DW, Garnett MR, Grill J, Oliveira R, Boddaert N, et al. Thalamic tumors in children: a reappraisal. J Neurosurg. 2007;106 (5 Suppl):354–362. doi: 10.3171/ped.2007.106.5.354. [DOI] [PubMed] [Google Scholar]
- 22.Reardon DA, Gajjar A, Sanford RA, Heideman RL, Walter AW, Thompson SJ, et al. Bithalamic involvement predicts poor outcome among children with thalamic glial tumors. Pediatr Neurosurg. 1998;29:29–35. doi: 10.1159/000028681. [DOI] [PubMed] [Google Scholar]
- 23.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twicerefocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 24.Souweidane MM, Hoffman HJ. Current treatment of thalamic gliomas in children. J Neurooncol. 1996;28:157–166. doi: 10.1007/BF00250196. [DOI] [PubMed] [Google Scholar]
- 25.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997;22:1319–1324. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Stroink AR, Hoffman HJ, Hendrick EB, Humphreys RP. Diagnosis and management of pediatric brain-stem gliomas. J Neurosurg. 1986;65:745–750. doi: 10.3171/jns.1986.65.6.0745. [DOI] [PubMed] [Google Scholar]
- 27.Tomita T, Cortes RF. Astrocytomas of the cerebral peduncle in children: surgical experience in seven patients. Childs Nerv Syst. 2002;18:225–230. doi: 10.1007/s00381-002-0576-1. [DOI] [PubMed] [Google Scholar]
- 28.Tovi D, Schisano G, Liljeqvist B. Primary tumors of the region of the thalamus. J Neurosurg. 1961;18:730–740. doi: 10.3171/jns.1961.18.6.0730. [DOI] [PubMed] [Google Scholar]
- 29.Vandertop WP, Hoffman HJ, Drake JM, Humphreys RP, Rutka JT, Amstrong DC, et al. Focal midbrain tumors in children. Neurosurgery. 1992;31:186–194. doi: 10.1227/00006123-199208000-00003. [DOI] [PubMed] [Google Scholar]