INTRODUCTION
Genetic studies and tumor biopsies have shown the importance of stromal components for cancer progression, but much remains to be learned about the dynamic interactions among the distinct tumor components within live animals. One challenge of studying cell behavior in progressively developing tumors has been the difficulty of maintaining live mice on the microscope stage. To prepare mice for long-term intravital imaging, auxiliary equipment is necessary to enable and to control anesthesia (such as the anesthesia gas mixer itself, a gas humidifier, indwelling lines for saline, and heat blanket). The other important component is to gain optical access to the mammary gland. This protocol describes a surgical technique that creates a skin flap with the mammary gland. The method is relatively easily taught, does not compromise the peritoneal cavity or any major blood vessels, and is generally well tolerated by the mice. There is minimal inflammatory response to the surgery itself if the solutions and tools are sterile, the surgical work area is clean, and aseptic techniques are used. This protocol works well for a single long-term image session, but does not enable repeated imaging sessions. For such approaches, methods for implanting imaging windows over the inguinal mammary gland should be used instead.
RELATED INFORMATION
A description of the experimental setup for long-term intravital imaging with spinning-disk confocal microscopy is found in Dynamic, Long-Term In Vivo Imaging of Tumor-Stroma Interactions in Mouse Models of Breast Cancer Using Spinning-Disk Confocal Microscopy (Ewald et al. 2011a). A monitoring protocol for long-term imaging (up to 40 h) is described in Monitoring of Vital Signs for Long-Term Survival of Mice Under Anesthesia (Ewald et al. 2011b). For repeated imaging sessions with implanted mammary imaging windows, see Shan et al. (2003) and Kedrin et al. (2008).
MATERIALS
CAUTIONS AND RECIPES: Please see the end of this protocol for appropriate handling of materials marked with <!>, and recipes for reagents marked with <R>.
Reagents
<!>Cyanoacrylate adhesive (Instant Krazy Glue, advanced formula gel; Elmer's Products)
-
<!>Isoflurane
The microscope room must be properly ventilated for use of isoflurane; see Dynamic, Long-Term In Vivo Imaging of Tumor-Stroma Interactions in Mouse Models of Breast Cancer Using Spinning-Disk Confocal Microscopy (Ewald et al. 2011a) for a discussion of the drawbacks of the various anesthetic options.
The following combination can be used as an alternative to isoflurane (see Steps 5.iii-5.iv):
<!>2,2,2-Tribromoethanol (Avertin) to induce a nonresponsive state
<!>Urethane (10% w/v in saline) to allow long-term (6–12 h) anesthesia during imaging
Mouse (breast cancer model, e.g., mouse mammary tumor virus long terminal repeat [LTR]-driven polyomavirus middle T antigen [MMTV-PyMT] model)
<!>Nitrogen (for use with oxygen as carrier gas with isoflurane)
<!>Oxygen
Saline solution (to be delivered at 50 μL/h)
Equipment
<!>Alcohol swabs (isopropyl alcohol, sterile)
Anesthesia apparatus (including gas mixer, isoflurane vaporizer, induction chamber, nose cones, tubing, and waste gas scavenging system; Molecular Imaging Products Co.)
Coverslips (no. 1, 24 × 40 mm)
Forceps (surgical, sterile)
Gauze sponges (Curity, 4 × 4 in, USP type VII gauze, sterilized)
Heated blanket (with recirculating water system; Gaymar Industries) or heating lamp, heated stage, or environmental chamber (see Step 14)
Laboratory tape
Line for saline
- Microscope (spinning-disk confocal, with x-y-z stage and epifluorescence capability; seeDynamic, Long-Term In Vivo Imaging of Tumor-Stroma Interactions in Mouse Models of Breast Cancer Using Spinning-Disk Confocal Microscopy [Ewald et al. 2011a])
Microscope slides (precleaned, 25 × 75 × 1 mm)
- Microscope stage insert (custom; Applied Scientific Instrumentation [ASI])The stage insert must be strong enough to support the weight of the mouse so that there are no movements in the z plane when the mouse is breathing. We use a custom stage insert with holes cut corresponding to the location of the inguinal mammary gland on the mouse.
Mustache trimmer (battery operated) or chemical hair remover (e.g., Nair) (see Step 7)
Nebulizer (Salter Labs 8901), for use as a humidifier
Oximeter probe (MouseOx; STARR Life Sciences), for imaging sessions of >3 h
Oxygen analyzer (for anesthesia gas; Hudson RCI 5801)
Oxygen sensor (for anesthesia gas; Hudson RCI 5803)
Packing foam, compressed gauze, or other semiflexible material (optional; see Step 4)
Scissors (surgical, sterile, 2 pairs)
Syringe with needle (for administration of Avertin and urethane)
Syringe (3-mL) (for saline infusion in experiments >12 h)
Tuberculin syringe (slip tip, 1 mL; BD) (for saline infusion in experiments <12 h)
Winged infusion set (23 g × 3/4 in., Surflo; Terumo)
METHOD
Preparation of the Microscope Stage (5 min)
-
1.
Tape coverslips over the imaging ports of the stage insert with laboratory tape as shown in Figure 1.
-
2.
Disinfect the stage and the coverslips with sterile isopropyl alcohol swabs.
-
3.
Place the stage insert in the x-y-z stage.
-
4.
Secure the isoflurane anesthesia line and the nose cone to the stage with laboratory tape.
Packing foam, compressed gauze, or other semiflexible material can be positioned under the anesthesia line to help secure the line and the nose cone with the proper angle relative to the mouse.
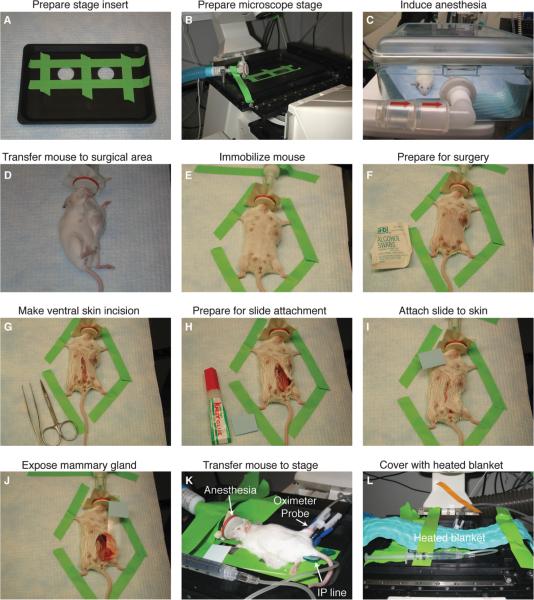
FIGURE 1.
Surgical preparation of mice for long-term intravital imaging of tumor-stroma interactions in the mammary gland. (A) Coverslips are taped over the imaging ports of a custom stage insert (ASI). (B) The stage insert is placed in the x-y-z stage, and the isoflurane anesthesia line is secured to the stage. (C) The mouse is initially anesthetized with 4% isoflurane (21% oxygen, balance nitrogen) until breathing is deep and slow (2–4 min), and thereafter (D) transferred to the surgical area, and isoflurane is reduced to 2.5%. (E) The mouse is immobilized with laboratory tape, (F) the ventral surface of the mouse is prepared for surgery with an isopropyl alcohol swab, and (G) a ventral midline incision is made with sterilized scissors. (H,I) To position the mammary gland on the microscope stage, a glass slide is attached to the skin and fur (outside surface) of the mouse using Krazy Glue. (J) The glass slide is rotated to expose the inner surface of the mammary gland, and (K) the mouse is transferred to the stage and isoflurane is reduced to 0.9%–1.1%. An indwelling IP line is inserted with a winged infusion set (for administration of saline 50 μL/h), and an oximeter probe is attached to the thigh of the mouse. (L) A recirculating heated water blanket is placed over the mouse during imaging.
Surgical Procedure (10–20 min)
Time for this section depends on experience.
-
5.
Anesthetize the mouse:
If the microscope room is properly ventilated for the use of isoflurane-
i.Use 4% isoflurane (using 21% oxygen and balance nitrogen as carrier gas) to anesthetize the mouse in an induction chamber.Use an oxygen sensor and analyzer to adjust the concentration of oxygen in the carrier gas.
-
ii.Transfer the mouse to the surgical area once the mouse is breathing deeply and slowly (after 2–4 min), and reduce the isoflurane concentration to 2.5%.Always minimize the amount of time the mouse is anesthetized with high concentrations of isoflurane. If the mouse is kept deeply anesthetized for too long, the survival time during the following imaging session is decreased. Throughout the anesthesia protocol, humidify the carrier gas with a nebulizer and sterile water.
-
i.
- If the microscope room is insufficiently ventilated for use of isoflurane
-
iii.Use Avertin to anesthetize the mouse during the surgical procedure.
-
iv.Use urethane once the mouse is transferred to the microscope stage (see Step 11.vi).
-
iii.
-
6.Immobilize the mouse for surgery
-
i.Verify that the level of anesthesia is deep enough for surgery by pinching a hind paw hard with forceps.
-
ii.If the mouse does not react (i.e., when the mouse is sufficiently anesthetized), immobilize it with laboratory tape as shown in Figure 1E.
-
i.
-
7.
Prepare the ventral surface of the mouse for surgery with isopropyl alcohol swabs
If loose hairs in the surgical area become a problem, the ventral surface can be shaved (using, e.g., a mustache trimmer). Alternatively, hairs can be removed using Nair or a similar hair removal product. Chemical hair removal is best done the day before surgery, because the product is greasy. -
8.Make a single ventral midline incision with sterile scissors from ~5 mm above the vaginal opening until ~4 mm above the tip of the sternum (see Fig. 1G).
-
i.For the first cut, use sterile forceps to gently pull the skin up and away from the abdominal wall.
-
ii.After the initial cut, gently separate the skin and the abdominal wall with blunt use of the scissors before cutting further through the skin.The major blood vessels that supply the mammary glands and the skin run ~0.5 cm to each side of the midline and should not be damaged by this technique. Be careful not to cut through the abdominal wall. Use gauze sponges to stop any bleeding resulting from cuts.
-
i.
-
9
Switch to a new set of sterile scissors, and gently free the skin over the inguinal mammary gland from the underlying abdominal wall (see Fig. 1H).
The primary method should be a gentle pull in the skin with sterile forceps and blunt use of the scissors. It should only be necessary to do a minimal amount of cutting through the connective tissue. The skin should not be cut beyond the midline cut. -
10.
Attach a glass microscope slide to the outside surface of the skin (see Fig.1I).
This slide is to facilitate the positioning of the mammary gland on the microscope stage, because it enables a stable handle on the skin flap with the mammary gland. The imaging is not done through the slide.-
i.
Position the glass slide without glue to determine where to apply glue for best placement.
Be careful not to position the slide so the mouse's hind limb interferes with the placement on the microscope stage. -
ii.
Use superglue to attach the slide.
Several brands of superglue have been tested; best results were obtained with Krazy Glue (advanced formula gel).
-
i.
Positioning of the Mouse on the Stage (5 min)
-
11.Transfer the mouse to the microscope stage (see Fig. 1J,K) and proceed as follows:
-
i.Rotate and expose the inner surface of the mammary gland so that it is located directly on top of the taped coverslips.
-
ii.Loosely secure the glass slide by taping one side of it to the stage, so that later manipulations of the mouse do not result in major displacement of the imaging fields.Take care not to squeeze the tissue and impair the blood supply.
-
iii.Use the microscope's epifluorescence capabilities and eyepieces to determine the optimal location of the mouse.
-
iv.Adjust the position of the anesthesia line to fit the individual mouse, and then tape the line down.
-
v.Reduce the isoflurane concentration. Start with 1% isoflurane, and adjust to between 0.8% and 1.2%, depending on the individual mouse (see Monitoring of Vital Signs for Long-Term Survival of Mice Under Anesthesia [Ewald et al. 2011b]).Mice from different genetic backgrounds have very different sensitivity to isoflurane. The recommended values are for the FVB/n background, although mice on the C3H background may require lower concentrations to achieve survival under long-term anesthesia.
-
vi.If the microscope room is insufficiently ventilated for isoflurane, use urethane (1.0–1.2 g/kg administered intraperitoneally as a 10% solution in saline) once the mouse is being transferred to the microscope stage.On average, 300 μL of the urethane solution will keep an adult female mouse anesthetized for ~6 h. Supplementary doses of urethane, if needed, can be administered to maintain the mouse under anesthesia, starting at one-tenth of the initial dosage of urethane. Expected survival time under continuous urethane anesthesia is 6–8 h. The maximal survival, which we have achieved with urethane anesthesia, is just over 12 h.
-
i.
-
12.
Position an indwelling intraperitoneal (IP) line with a winged infusion set attached to a 1-mL (for experiments of <12 h) or 3-mL (for experiments of >12 h) syringe for administration of 50 μL/h of saline. Secure the needle to the stage with laboratory tape.
The administration of saline during the long-term anesthesia is critical for survival. -
13.
For imaging sessions of >3 h, attach an oximeter probe to the thigh of the mouse (on the opposite side of the mammary gland that is subject to imaging).
This will allow accurate measurements of the status of the mouse and greatly facilitates controlling the level of anesthesia. Alternatively, carefully monitor the breath rate and corneal (blinking) reflex as described in Monitoring of Vital Signs for Long-Term Survival of Mice Under Anesthesia (Ewald et al. 2011b). -
14.
Place a heated blanket (e.g., one using a recirculating water system) over the mouse to avoid hypothermia during imaging. Tape the blanket down so that the mouse is well covered (see Fig. 1L).
Avoiding hypothermia is critical for long-term survival under anesthesia. Alternative methods of keeping the mouse from becoming hypothermic are the use of a heating lamp, a heated stage, or an environmental chamber.
TROUBLESHOOTING
Problem
An acute inflammatory response develops during the procedure.
Solution
Minimize the risk of inducing an acute inflammatory response by using sterilized surgical tools, a clean surgical work area, sterile solutions, and aseptic techniques.
DISCUSSION
Using the method described here, access to the inguinal mammary gland for imaging is easily achieved with minimal risk of bleeding or disruption of blood supply. We have successfully used the method on more than 80 mice, and it has been performed by six different operators. Thus, it is easily mastered and well tolerated by the mice. Compared with techniques that involve the insertion of glass windows, the surgical procedure is simpler and faster to perform, and there is no window to constrain the growth of the tissue (e.g., the tumor). However, our method will not work for repeated imaging sessions. The method is specifically for the imaging of the mammary gland but may be adapted for imaging of other organs (e.g., the skin).
CAUTIONS
[NOTE: For reagents marked with the <!> symbol not listed below, please consult the manufacturer's Material Safety Data Sheet for further information.]
2,2,2-Tribromoethanol
2,2,2-Tribromoethanol may be harmful by inhalation, ingestion, or skin absorption. The vapor is also irritating to the eyes, mucous membranes, and upper respiratory tract. Wear appropriate gloves and safety glasses.
Cyanoacrylate adhesives
Cyanoacrylate adhesives are harmful by inhalation, ingestion, and skin absorption. Immediate bonding of tissues can occur. Do not pull apart. Inhalation may cause lightheadedness. Wear appropriate gloves and safety glasses. Follow manufacturer's safety guidelines.
Isoflurane
Isoflurane is an irritant and may be harmful by inhalation, ingestion, or skin absorption. Chronic exposure may be harmful. Wear appropriate gloves and safety glasses.
Isopropyl alcohol (Isopropanol, 2-Propanol)
Isopropyl alcohol (isopropanol, 2-propanol) is flammable and irritating. It may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves and safety glasses. Do not breathe the vapor. Keep away from heat, sparks, and open flame.
Nitrogen (gaseous or liquid)
Nitrogen (gaseous or liquid) may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves and safety glasses. Consult your local safety office for proper precautions.
Oxygen (O2)
Oxygen (O2) presents a fire and explosion hazard. The gas is heavier than air and is a strong oxidant. Vapors may cause dizziness or asphyxiation without warning. Keep away from heat, sparks, and open flame.
Urethane
Urethane is a mutagen and suspected carcinogen. It is also highly toxic and is readily absorbed through the skin. It may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves and safety glasses. Do not breathe the dust and use only in a chemical fume hood.
ACKNOWLEDGMENTS
We thank Dr. Bryan Welm for his ideas and work on preliminary studies related to this project. This work was supported by the National Institutes of Health grants CA057621, CA105379, and ES012801 to Z.W.
REFERENCES
- Ewald AJ, Werb Z, Egeblad M. Dynamic, long-term in vivo imaging of tumor-stroma interactions in mouse models of breast cancer using spinning-disk confocal microscopy. Cold Spring Harb Protoc. 2011a doi: 10.1101/pdb.top97. doi: 10.1101/pdb.top97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Werb Z, Egeblad M. Monitoring of vital signs for long-term survival of mice under anesthesia. Cold Spring Harb Protoc. 2011b doi: 10.1101/pdb.prot5563. doi: 10.1101/pdb.prot5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S, Sorg B, Dewhirst MW. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc Res. 2003;65:109–117. doi: 10.1016/s0026-2862(02)00017-1. [DOI] [PubMed] [Google Scholar]