INTRODUCTION
Anesthesia protocols for mice have been optimized, as described here, to achieve long-term imaging (up to 40 h) and facilitate survival through careful monitoring of the mice during anesthesia. Isoflurane anesthesia is the preferred method, because it can be adjusted quickly as needed during the experiment. Critical for the long survival times under anesthesia is the use of the lowest possible dose of anesthesia, which is identified by corneal reflex and monitoring of breath and heart rate, blood-oxygenation levels, and vascular distension using an oximeter probe. It is critical that the carrier gas for isoflurane is humidified. In addition, it is essential to keep mice warm and to compensate for loss of fluid by supplementing with saline. Alternative approaches rely on injectable anesthetics, which do not require dedicated equipment or high-ventilation rates in the imaging room. However, injectable anesthetics are harder to dose for image sessions of >6–10 h.
RELATED INFORMATION
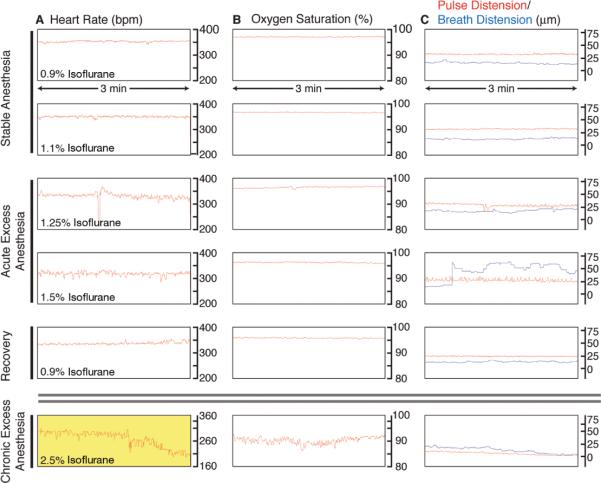
A description of the experimental setup for long-term intravital imaging with spinning-disk confocal microscopy is found in Dynamic, Long-Term In Vivo Imaging of Tumor-Stroma Interactions in Mouse Models of Breast Cancer Using Spinning-Disk Confocal Microscopy (Ewald et al. 2011a). A simple, fast method for accessing the inguinal mammary gland for imaging is described in Preparation of Mice for Long-Term Intravital Imaging of the Mammary Gland (Ewald et al. 2011b). Sample readouts of heart rate, oxygen saturation, and the distension of blood vessels caused by the pulse and breathing during anesthesia are shown in Figure 1.
FIGURE 1.
Monitoring of vital signs during long-term anesthesia and imaging. An oximeter probe attached to the thigh of the mouse is used to measure (A) the heart rate (bpm), (B) the arterial oxygen saturation of the blood (%), and (C) the distension of blood vessels caused by the pulse and the breathing (μm). Traces are 3 min long, originating from a single 9-h image session. (A) Under stable anesthesia (0.9%–1.1% isoflurane), the heart rate is steady and typically between 300 bpm and 450 bpm. With acute excess anesthesia, 1.25%–1.5% isoflurane, the heart rate lowers and becomes erratic. Recovery is achieved by reducing isoflurane concentration to 0.9%, and the heart rate stabilizes. At chronic excess anesthesia levels (2.5% isoflurane), the heart rate declines to critically low levels (yellow, <210 bpm). (B) Oxygen saturation stays at ~97% at anesthesia levels of 0.9%–1.25% isoflurane. It falls toward 90%–95% at 1.5% isoflurane and is slower to recover than heart rate when isoflurane is restored to 0.9%. At chronically high anesthesia levels, oxygen saturation dips to <90%. (C) Under stable anesthesia, the vascular distension caused by the pulse is relatively constant and is of higher amplitude than the vascular distension caused by breathing. At 1.25% isoflurane, the breath distension increases in amplitude, and, at 1.5% isoflurane, the mouse begins gasping, and the distension caused by breathing becomes irregular and exceeds pulse distension. At 0.9% isoflurane, the mouse recovers. Chronic exposure to excess isoflurane (2.5% isoflurane) results in a decline in the distension caused by pulse and breathing. (For color figure, see doi: 10.1101/pdb.prot5563 online at www.cshprotocols.org.)
MATERIALS
CAUTIONS AND RECIPES: Please see the end of this protocol for appropriate handling of materials marked with <!>, and recipes for reagents marked with <R>.
Reagents
-
<!>IsofluraneThe microscope room must be properly ventilated for use of isoflurane; see Dynamic, Long-Term In Vivo Imaging of Tumor-Stroma Interactions in Mouse Models of Breast Cancer Using Spinning-Disk Confocal Microscopy (Ewald et al. 2011a).
Mouse (breast cancer model, e.g., mouse mammary tumor virus long terminal repeat [LTR]-driven polyomavirus middle T antigen [MMTV-PyMT] model, prepared for imaging as in Preparation of Mice for Long-Term Intravital Imaging of the Mammary Gland [Ewald et al. 2011b])
<!>Nitrogen (for use with oxygen as carrier gas with isoflurane)
<!>Oxygen
Equipment
Anesthesia apparatus (including gas mixer, isoflurane vaporizer, induction chamber, nose cones, tubing, and waste gas scavenging system; Molecular Imaging Products Co.)
Forceps (sterile)
Nebulizer (Salter Labs 8901), for use as a humidifier
Oximeter probe (MouseOx; STARR Life Sciences)
Oxygen analyzer (for anesthesia gas; Hudson RCI 5801)
Oxygen sensor (for anesthesia gas; Hudson RCI 5803)
METHOD
Using an Oximeter Probe to Monitor Anesthesia
-
1.
Attach the oximeter probe to the thigh of the mouse during preparation for imaging (see Step 13 of Preparation of Mice for Long-Term Intravital Imaging of the Mammary Gland [Ewald et al. 2011b]).
-
2.Use the probe to measure the heart rate (beats per minute [bpm]), the arterial oxygen saturation of the blood (%), the distension of blood vessels caused by the pulse and the breathing (μm), and the breath rate (see Fig. 1). Typical values are as follows:
- Under optimal anesthesia: The heart rate is steady and between 300 bpm and 450 bpm, oxygen saturation stays at 97%–98%, and the vascular distension caused by the pulse is relatively constant and of higher amplitude than the vascular distension caused by breathing. The breath rate is ~55–65 breaths per minute.
- Under too deep anesthesia: The heart rate falls <300 bpm and may become erratic, oxygen saturation drops <95%, the breath distension increases in amplitude, and the distension caused by breathing exceeds the pulse distension. The breath rate slows to <50 breaths per minute.
- Under too light anesthesia: The heart rate may increase to >450 bpm, and breath rates will be >70 breaths per minute.
-
3.For imaging experiments >12 h, take the following steps to relieve the pressure of the oximeter probe, which somewhat impairs the blood supply to the leg:
- Remove the probe after ~3 h (once anesthesia levels have been stable for at least 1 h).
- Allow the leg to recover for ~3 h. Rely on visual inspection and corneal reflex as described in Step 4.
- Reattach the probe for another ~3-h imaging period aided by the oximeter measurements.
Observing Mouse Anesthesia Through Visual Inspection
The methods described here serve as a backup to the use of the oximeter probe. Although the detailed information on oxygen saturation, pulse rate, and pulse versus breath distention allows finer adjustment of the isoflurane and oxygen concentrations for the individual mouse (and therefore results in longer survival time), one can choose to rely solely on visual monitoring. Without the oximeter probe, we have achieved survival times under anesthesia of 18 h versus >40 h with the aid of the probe.
-
4.Visually inspect the mouse for breath rate and depth every 15 min, and regularly test the corneal (blinking) reflex by lightly touching the eyeball (e.g., with the tip of sterile forceps).
- Under optimal anesthesia, the breath rate should be ~55–65 breaths per minute.
- Under too deep anesthesia: The breath rate falls to <50 breaths per minute and the mouse breathes heavily (it gasps).
- Under too light anesthesia: Breath rates are >70 breaths per minute and the mouse breathes superficially. The mouse blinks or the eye muscles twitch in response to testing the corneal reflex.
Adjusting the Anesthesia
-
5.Adjust the isoflurane as necessary.
- For mice on the FVB/n background, the optimal level of isoflurane is almost always 0.9%–1%.
- The required levels often become lower as time under anesthesia progresses.
- It usually takes 10–15 min until the mouse has fully responded to a change in isoflurane concentration.
-
6.To maintain the oxygen saturation in the mouse's blood, monitor and adjust the concentration of oxygen in the carrier gas using an oxygen sensor and analyzer.
- The oxygen is balanced with nitrogen and used as carrier gas for the isoflurane.
- Mice will often need increased concentrations of oxygen during the time course of imaging to maintain blood saturation >98%. We typically use ~40% oxygen after the first 6–8 h and 80%–100% after 24 h of continuous anesthesia.
-
7.Throughout the anesthesia protocol, humidify the carrier gas by forcing it to flow through a nebulizer with sterile water.Humidification of the carrier gas is critical to long-term survival with isoflurane anesthesia. Clean and dry the nebulizer carefully after each image session to avoid bacterial growth (e.g., Legionella).
DISCUSSION
This monitoring protocol reproducibly allows for keeping mice alive under anesthesia for >12 h. Critical for the long survival times are the use of the lowest possible dose of anesthesia and a humidified carrier gas for the isoflurane. In addition, it is essential to keep mice warm and to compensate for loss of fluid by supplementing with saline. Alternative approaches rely on injectable anesthetics, which do not require dedicated equipment or high-ventilation rates in the imaging room, but these are generally harder to adapt to the individual mice and difficult to dose for image sessions >6–10 h.
CAUTIONS.
[NOTE: For reagents marked with the <!> symbol not listed below, please consult the manufacturer's Material Safety Data Sheet for further information.]
Isoflurane
Isoflurane is an irritant and may be harmful by inhalation, ingestion, or skin absorption. Chronic exposure may be harmful. Wear appropriate gloves and safety glasses.
Nitrogen (gaseous or liquid)
Nitrogen (gaseous or liquid) may be harmful by inhalation, ingestion, or skin absorption. Wear appropriate gloves and safety glasses. Consult your local safety office for proper precautions.
Oxygen (O2)
Oxygen (O2) presents a fire and explosion hazard. The gas is heavier than air and is a strong oxidant. Vapors may cause dizziness or asphyxiation without warning. Keep away from heat, sparks, and open flame.
ACKNOWLEDGMENTS
We thank Dr. Bryan Welm for his ideas and work on preliminary studies related to this project. This work was supported by the National Institutes of Health grants CA057621, CA105379, and ES012801 to Z.W.
REFERENCES
- Ewald AJ, Werb Z, Egeblad M. Dynamic, long-term in vivo imaging of tumor-stroma interactions in mouse models of breast cancer using spinning-disk confocal microscopy. Cold Spring Harb Protoc. 2011a doi: 10.1101/pdb.top97. doi: 10.1101/pdb.top97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Werb Z, Egeblad M. Preparation of mice for long-term intravital imaging of the mammary gland. Cold Spring Harb Prot. 2011b doi: 10.1101/pdb.prot5562. doi: 10.1101/pdb.prot5562. [DOI] [PMC free article] [PubMed] [Google Scholar]