Summary
Few effective measures exist to combat the worldwide obesity epidemic1, and the identification of potential therapeutic targets requires a deeper understanding of the mechanisms that control energy balance. Leptin, an adipocyte hormone that signals the status of cellular energy stores, acts via multiple types of leptin receptor (LepR-b)-expressing neurons in the brain to control feeding, energy expenditure and endocrine function2–4. The modest contributions to energy balance attributable to leptin action via many previously-studied LepR-b populations5–9 suggest that other, heretofore unidentified, hypothalamic LepR-b neurons play important roles. Here, we examine the role of LepR-b in neuronal nitric oxide synthase (NOS1)-expressing (LepR-bNOS1) neurons that comprise approximately 20% of hypothalamic LepR-b neurons. Nos1cre-mediated ablation of LepR-b (LeprNOS1KO mice) produces hyperphagic obesity, decreased energy expenditure and hyperglycemia approaching that of LepR-b-null mice. In contrast, endocrine functions in LeprNOS1KO mice are relatively spared. Thus, hypothalamic LepR-bNOS1 neurons are essential for the control of energy balance by leptin.
Commensurate with the diverse processes controlled by leptin, specialized types of LepR-b neurons lie in multiple brain regions involved in energy balance, including the brainstem, midbrain, and hypothalamus10–13. LepR-b knockdown or deletion in the hindbrain interferes with satiation, although these manipulations only slightly alter body adiposity8,9. Within the midbrain ventral tegmental area (VTA) and substantia nigra (SN), a subset of dopamine (DA) neurons contain LepR-b; leptin action via these neurons contributes minimally to body weight control, but plays a role in DA-mediated behaviors, including those linked to anxiety14–17. Midbrain serotonin neurons, while initially reported to play an important role in leptin action, neither express LepR-b nor contribute to leptin action18,19. In contrast, ablation of hypothalamic LepR-b produces a profound metabolic phenotype, demonstrating the importance of hypothalamic LepR-b signaling for leptin action20.
Within the hypothalamus, the specific set(s) of LepR-b neurons responsible for the control of energy balance by leptin remain incompletely defined. Direct leptin action via proopiomelanocortin (Pomc) neurons of the hypothalamic arcuate nucleus (ARC), ARC Agouti-related peptide (Agrp) neurons, and steroidogenic factor-1 (Sf-1) neurons in the ventromedial hypothalamic nucleus (VMH) contributes only modestly to overall energy balance5–7. LepR-b neurons in the lateral hypothalamic area (LHA), including those that contain neurotensin, mediate leptin action on orexin neurons and the mesolimbic DA system, but deletion of LepR-b from these neurons only modestly increases adiposity21–23. Thus, the identity of the hypothalamic LepR-b neurons responsible for the majority of leptin action on energy balance has remained unclear.
Nos1-expressing LepR-b (LepR-bNOS1) neurons represent a relatively small population of LepR-b neurons mainly restricted to the hypothalamus, where they are distributed in areas poised to impact output from the paraventricular hypothalamic nucleus (signaling by which mediates much of the hypothalamic control of energy balance)24–26. To study LepR-bNOS1 neurons, we inserted an internal ribosome entry site (IRES) plus the coding sequences for cre recombinase into the 3’-UTR of Nos1 in mice to promote Nos1-restricted cre expression (Nos1cre)(Figure 1a). We bred Nos1cre mice to the cre-dependent ROSA26-EGFP reporter line, generating Nos1EGFP mice. EGFP-IR overlapped with Nos1-IR soma in all regions examined (data not shown).
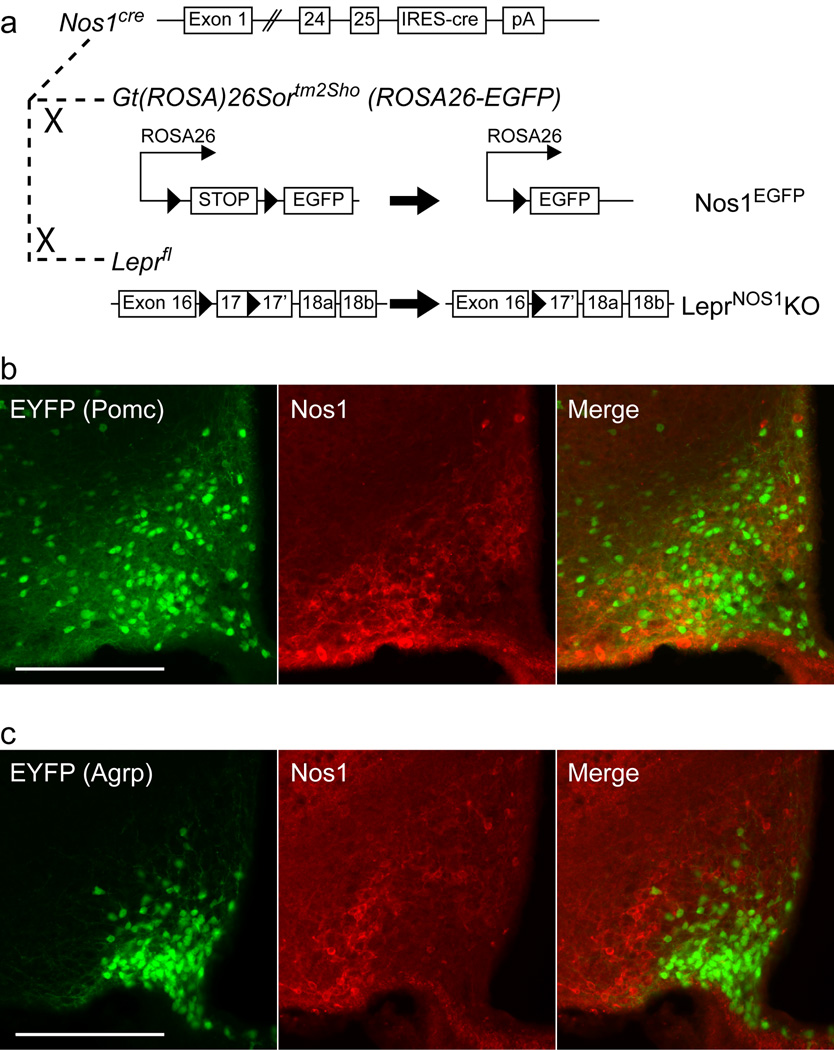
Figure 1. Generation of Nos1cre and lack of Nos1 in ARC Pomc and Agrp neurons.
(a) Schematic diagram depicting Nos1cre and its use to generate Nos1EGFP and LeprNOS1KO mice. (b) Representative images from the ARC of PomcEYFP mice demonstrating EYFP (green, left panel), Nos1-IR (red, middle panel), and merged images (right panel). (c) Representative images from the ARC of AgrpEYFP mice demonstrating EYFP (green, left panel), Nos1-IR (red, middle panel), and merged (right panel). Scale bars = 200 µm.
We examined the leptin-stimulated induction of phosphorylated signal transducer and activator-3 (pStat3; which reveals neurons containing functional LepR-b27) and its colocalization with EGFP-immunoreactivity (−IR) in Nos1EGFP mice, as well as evaluating the colocalization of Nos1-IR with EGFP-IR in LeprEGFP mice12 that express EGFP in LepR-b neurons (Supplemental Figures 1–2). As suggested by NADPH-diaphorase colocalization with leptin-stimulated pStat3-IR24, our analysis revealed that although Nos1 and LepR-b are each expressed in many brain areas, they are co-expressed predominantly in the hypothalamus; approximately 20% of hypothalamic LepR-b neurons contain Nos1. Most LepR-bNOS1 neurons lie within the ventral premammillary nucleus (PMv); the DMH and ARC also contain a substantial number; other areas contain few LepR-bNOS1 neurons. ARC Nos1-expressing cells are distinct from Pomc and Agrp neurons (Figure 1b, c; Supplemental Figure 3).
To determine the role of LepR-bNOS1 neurons in leptin action, we crossed Nos1cre mice with Leprfl animals28. Since Nos1 is expressed in gametes, germline Leprfl excision occurred in the offspring of Nos1cre;Leprfl/+ parents, generating LeprΔ. We therefore interbred Nos1cre;LeprΔ/+ to Leprfl/fl animals to generate Nos1cre;LeprΔ/fl (LeprNOS1KO) animals in which LepR-b was ablated specifically from LepR-bNOS1 neurons (Figure 1a), along with Nos1cre;LeprΔ/+ and LeprΔ/fl (control) littermates. The persistence of cre in the early embryo of some animals also produced Nos1cre;LeprΔ/Δ littermates with LepR-b inactivated in all tissues (LeprKO).
Analysis of the hypothalamus of control, LeprNOS1KO, and LeprKO animals (Supplemental Figure 4a, b) demonstrated the absence of pStat3 in LeprKO animals. We also confirmed the ablation of leptin-stimulated pStat3-IR from the PMv in LeprNOS1KO animals, along with modestly lower pStat3-IR from other areas (ARC and DMH) containing LepR-bNOS1 neurons. Thus, functional LepR-b was ablated from LepR-bNOS1 neurons in LeprNOS1KO mice. Consistent with the lack of overlap between Pomc and Nos1 neurons (Figure 1b; Supplemental Figure 3), leptin treatment stimulated pStat3-IR in similar proportions of ARC Pomc neurons in control and LeprNOS1KO mice (Supplemental Figure 4c).
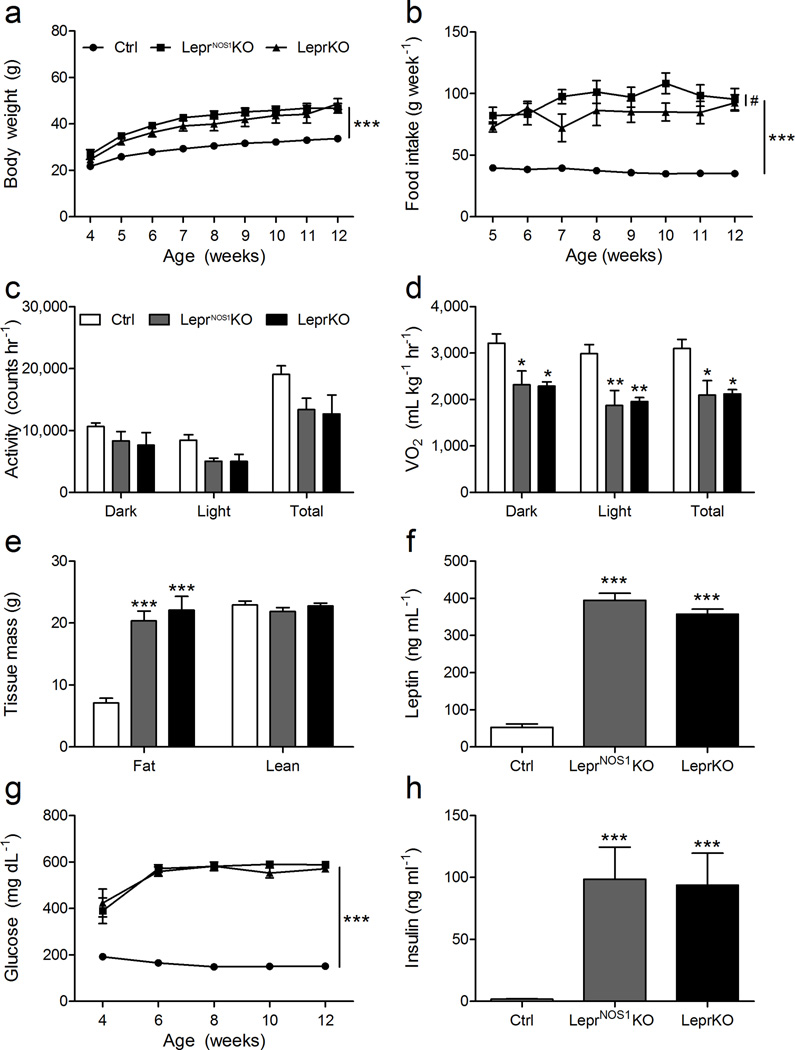
LeprNOS1KO and LeprKO males exhibited similarly high body weight and food intake relative to controls (Figure 2a, b). VO2 for LeprNOS1KO mice was lower than controls and not different from LeprKO animals; similar trends were observed for ambulatory activity (Figure 2c, d; Supplemental Figure 5). The excess weight in LeprNOS1KO and LeprKO mice was largely due to increased adipose mass (Figure 2e), which was reflected by high leptin concentrations relative to controls (Figure 2f). We observed similar trends in LeprNOS1KO females (Supplemental Figure 6), although the obesity and metabolic dysfunction in LeprNOS1KO females was less severe relative to LeprKO females. Thus, direct leptin action on LepR-bNOS1 neurons is crucial for the regulation of feeding, activity, and energy expenditure, and therefore for the control of body weight and adiposity.
Figure 2. LepR-bNOS1 neurons regulate energy balance and glucose homeostasis.
(a) Body weight and (b) food intake from 4–12 weeks of age for male control (Ctrl), LeprNOS1KO and LeprKO mice. CLAMS analysis of 12–14 week old male mice determined (c) ambulatory locomotor activity and (d) VO2. Data are shown for dark cycle (Dark), light cycle (Light) and averaged over 24 h (Total). (e) Body composition analysis of 12–14 week old animals. (f) Serum leptin concentrations for 12 week old animals. (g) Biweekly blood glucose concentrations from 4–12 weeks of age for male Ctrl, LeprNOS1KO and LeprKO mice. (h) Serum insulin concentrations for 8 week old animals. Graphed data represent average values ± SEM; n ≥ 8 for all measurements. ANOVA: *, p < 0.05 vs Ctrl; **, p < 0.01 vs Ctrl; ***, p < 0.001 vs Ctrl; #, p < 0.05; all other comparisons, p = NS.
To determine the potential role for leptin action via LepR-bNOS1 neurons in glucose homeostasis, we examined circulating blood glucose and insulin concentrations in control, LeprNOS1KO and LeprKO mice (Figure 2g, h). As with body weight and adiposity, male LeprNOS1KO and LeprKO mice exhibited similarly high blood glucose concentrations relative to controls from an early age, despite high insulin concentrations relative to controls. Thus, leptin action via LepR-bNOS1 neurons is indispensible for the regulation of glucose homeostasis, in addition to energy balance, and LepR-bNOS1 neurons represent crucial regulators of metabolic leptin action.
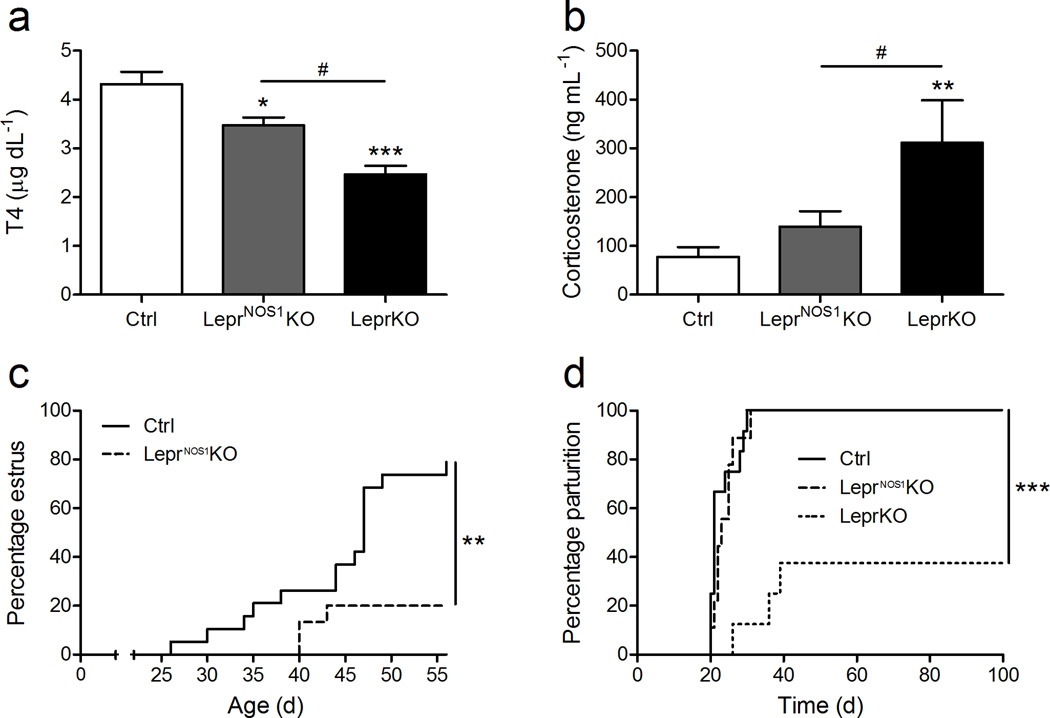
To interrogate the contribution of LepR-bNOS1 neurons to the control of endocrine systems by leptin, we examined circulating hormone concentrations along with parameters of reproductive function in LeprNOS1KO mice (Figure 3). While circulating T4 concentrations in LeprNOS1KO males were lower than for controls, they remained higher than in LeprKO mice (Figure 3a). Furthermore, while circulating corticosterone was higher in LeprKO males than in LeprNOS1KO and control animals, corticosterone concentrations were not different between LeprNOS1KO and controls (Figure 3b). The onset of vaginal estrus was delayed relative to controls in singly-housed LeprNOS1KO females (Figure 3c); upon mating with C57Bl/6 males, all LeprNos1KO females delivered litters with similar timing as controls (Control = 23 ± 1 d; LeprNos1KO = 24 ± 1 d), and with similar litter sizes (Control = 6.9 ± 1.1; LeprNos1KO = 7.1 ± 0.7 pups litter−1), however (Figure 3d). In contrast, the majority of LeprKO females failed to deliver pups within 6 weeks of mating.
Figure 3. LepR-bNOS1 neurons contribute modestly to endocrine functions.
Serum T4 (a) and corticosterone (b) concentrations from 7–8 week old male control (Ctrl), LeprNOS1KO and LeprKO mice. All measurements, n ≥ 5. Graphed data represent average values ± SEM. (c) Analysis of time to first estrus for female Ctrl and LeprNOS1KO mice; percent exhibiting vaginal estrus is plotted by age. All measurements, n ≥ 9. (d) Analysis of reproductive competence in female Ctrl, LeprNOS1KO and LeprKO mice. Experimental animals were mated with C57Bl/6 males and the delivery of pups was monitored daily. Percent delivering pups by days post-mating is plotted. All measurements, n ≥ 8. ANOVA: *, p < 0.05 vs Ctrl; **, p < 0.01 vs Ctrl; ***, p < 0.001 vs Ctrl; #, p < 0.05; all other comparisons, p = NS.
Thus, while deletion of LepR-b from LepR-bNOS1 neurons promoted obesity and metabolic dysfunction to an extent similar to that observed in animals with Leprfl deleted throughout the body, the control of the thyroid and adrenal axes remained relatively intact, and female reproductive function was partially impacted. Hence, our present findings reveal that leptin action via LepR-bNOS1 neurons contributes modestly to the control of endocrine function, but is indispensible for the control of energy balance and glucose homeostasis.
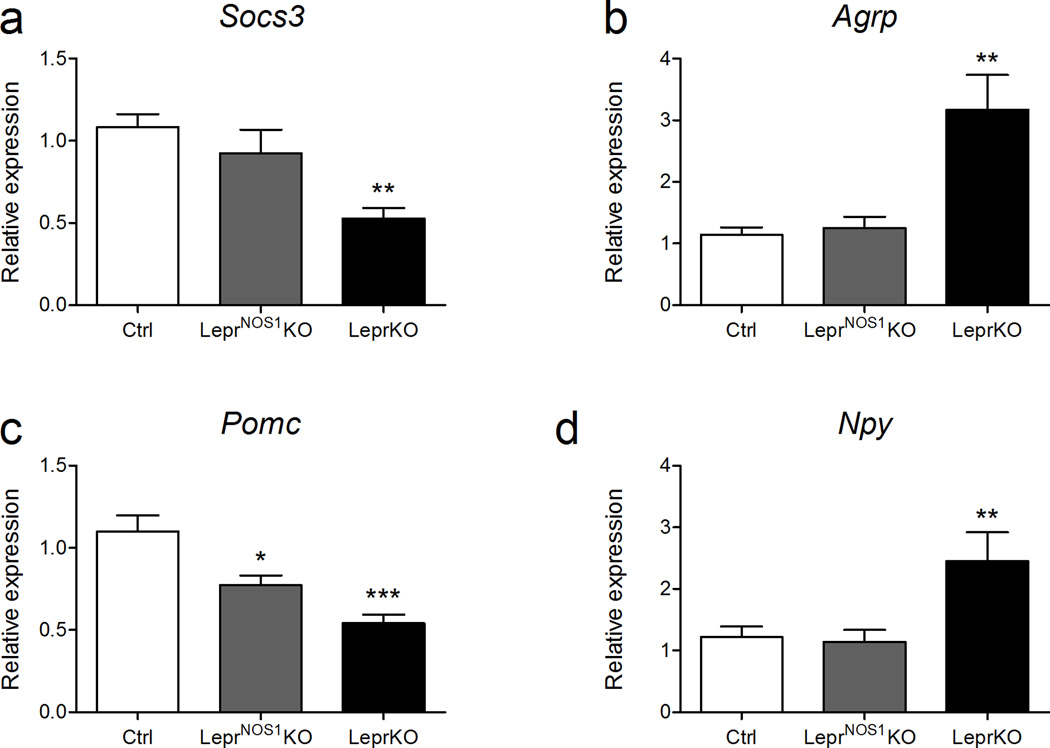
Relatively few ARC LepR-b neurons express Nos1, and the deletion of LepR-b from LepR-bNOS1 neurons was insufficient to detectably alter ARC Socs3 expression (a surrogate for LepR-b signaling in the ARC29)(Figure 4a). Altered ARC physiology may contribute to the phenotype of LeprNOS1KO mice, however; while the expression of Npy and Agrp were unaltered in LeprNOS1KO mice relative to controls, Pomc expression was lower in LeprNOS1KO mice than in controls (Figure 4b–d), even though LepR-bNOS1 neurons are distinct from Pomc neurons (Figures 1b; Supplemental Figures 3, 4c). Thus, LepR-bNOS1 cells may indirectly mediate aspects of melanocortin action, consistent with recent findings revealing that leptin action via non-Pomc cells controls important aspects of Pomc neuron function and the regulation of energy balance5,30. Given the severity of the metabolic defects exhibited by LeprNOS1KO mice, LepR-bNOS1 neurons may act by other mechanisms, as well.
Figure 4. ARC gene expression in LeprNOS1KO animals.
(a) Socs3, (b) Agrp, (c) Pomc, and (d) Npy mRNA expression by qPCR of microdissected ARC samples from ad libitum-fed male and female 7–8 week old control (Ctrl), LeprNOS1KO and LeprKO mice. All measurements n ≥ 14. Graphed data represent average values ± SEM. ANOVA: *, p < 0.05 vs Ctrl; **, p < 0.01 vs Ctrl; ***, p < 0.001 vs Ctrl; all other comparisons, p = NS.
Thus, leptin action via LepR-bNOS1 neurons is crucial for the control of energy balance and metabolism and for the regulation of ARC Pomc neurons. While the frequency of Nos1cre-mediated excision in gametes dictated that we study mice on the heterozygous LeprΔ/fl background, LeprΔ/fl mice express the wild-type receptor on all LepR-b neurons and have no detectable phenotype; it is therefore unlikely that this genetic background contributed substantially to the dramatic phenotype of LeprNOS1KO mice. While Nos1 expression identifies LepR-bNOS1 neurons important for metabolic control, Nos1 signaling is unlikley to mediate downstream leptin action; although leptin regulates the phosphorylation and presumably the activity of Nos1 in hypothalamic regions containing LepR-bNOS1 neurons24, NO is a retrograde transmitter31 and animals null for Nos1 exhibit no obvious primary metabolic phenotype32. It is therefore likely that other transmitters in the LepR-bNOS1 neurons play crucial roles.
Recent data suggest that GABAergic (vGat-expressing) LepR-b neurons (which represent many ARC, DMH, and LHA LepR-b neurons- approximately 60–75% of total hypothalamic LepR-b neurons) are important for the control of energy balance. Approximately 20–30% of ARC and DMH LepR-bNOS1 neurons contain Gad1 (which produces GABA), and a similar fraction of ARC/DMH LepR-bNOS1 neurons are activated by leptin (Supplemental Figure 7). By itself, direct leptin action on glutamatergic (vGlut2-expressing) LepR-b neurons (essentially all PMv and VMH LepR-b neurons) contributes only modestly to the control of energy balance by leptin30. Leptin activates the vast majority of PMv LepR-b neurons (most of which are LepR-bNOS1 neurons) 25,33, and a variety of data support a role for PMv LepR-bNOS1 neurons (~70% of total LepR-bNOS1 neurons) in fertility, consistent with the delayed estrus observed in LeprNos1KO females34. Since the PMv represents a sexually-dimorphic nucleus rich in androgen receptor 35, sex-specific differences in LepR-b neurons in this nucleus could contribute to the sexual dimorphism observed in the LeprNos1KO phenotype. The apparently minor role played by glutamatergic LepR-b neurons in energy balance suggests that PMv LepR-bNOS1 neurons alone do not mediate the majority of the metabolic phenotype displayed by LeprNos1KO mice, suggesting either that the modest number of LepR-bNOS1 neurons elsewhere (e.g., in the DMH and ARC) mediate this dramatic phenotype, or that the PMv LepR-b neurons may reinforce the action of the DMH and ARC LepR-bNOS1 neurons via supporting action on similar neural networks. It will be important to understand the detailed mechanisms of action for LepR-bNOS1 neurons in the future, since this small group of cells controls energy balance and thus represents a potential therapeutic target for obesity and related diseases.
Methods
Materials
Leptin was the generous gift of Amylin Pharmaceuticals, Inc. (San Diego, CA).
Animals
We bred all mice in our colony in the Unit for Laboratory Animal Medicine at the University of Michigan. All animals and procedures used were in accordance with the guidelines and with the approval of the University Committee on the Use and Care of Animals. We provided all animals ad libitum access to food and water. We purchased male C57Bl/6J animals for breeding studies and Gt(ROSA)26Sortm1(EYFP)Cos (ROSAEYFP) mice from Jackson Labs. We produced PomcEYFP and AgrpEYFP animals by crossing PomcCre and AgrpCre animals5,36 onto the ROSAEYFP background.
To generate Nos1cre, PCR amplification produced a 6 kb fragment containing the mouse genomic Nos1 sequence centered on the STOP codon in the final (3’) exon for insertion into pCR2.1. PCR mutagenesis created an AscI site 60 bp 3’ to the STOP codon, for the introduction of the IRES-Cre-Frt-Neo-Frt sequences to generate pCRNos1-IRES-Cre-Frt-Neo-Frt. NotI/NheI digestion excised the entire insert for subcloning into NotI/XbaI-cut pPNT backbone to generate pPNT-Nos1-IRES-Cre for targeting. NotI digestion linearized the vector for electroporation into R1 ES cells. We used Taqman-based qPCR screening to initially identify correctly targeted clones37, followed by Southern blotting for final confirmation. We injected correctly targeted ES cells into blastocysts to generate chimeras, which we bred to C57Bl/6 animals to establish germline transmission.
We bred Nos1cre mice with ROSAEGFP mice to generate Nos1cre;ROSAEGFP (Nos1EGFP) animals for the analysis of Nos1cre expression. We also bred Nos1cre animals with Leprfl/fl mice. Due to the periodic expression of Nos1cre during gametogenesis, we bred Nos1cre;leprΔ/+ to Leprfl/fl mice in order to obtain littermate Nos1cre;LeprΔ/fl (LeprNOS1KO), Nos1cre; LeprΔ/+ and LeprΔ/fl (control) and Nos1cre;LeprΔ/Δ (LeprKO) animals for study. We genotyped the offspring by PCR.
Perfusion and immunolabeling
We anesthetized the mice with an overdose of intraperitoneal (IP) pentobarbital and transcardially perfused them with 10% neutral buffered formalin. We sectioned the brains coronally (30 µm) using a sliding microtome followed by immunofluoresent analysis. We visualized the antigens via immunofluorescence using species-specific AlexaFluor-488 or -568 secondary antibodies (Invitrogen, Cat# A11039 and A10042; 1:200), and processed and imaged the sections as previously described38. Antibodies used were GFP (Abcam, chicken, Cat# ab13970; 1:1000) and Nos1 (ImmunoStar, rabbit, Cat# 24287; 1:5000). We purchased normal donkey serum and biotinylated donkey anti-rabbit (Cat# 711-065-152) from Jackson ImmunoResearch. We counted cells using Adobe Photoshop software.
Phenotypic studies
We individually housed mice for study from the time of weaning at 21 d. Beginning at 28 d, we monitored body weight and chow (Purina Lab Diet #5001) intake weekly. We collected blood for serum and measured blood glucose with a glucometer biweekly. We monitored female mice for vaginal opening and then for vaginal estrus by cellular histology until eight weeks of age. We collected all data between 13:00 and 16:00. We analyzed mice for body fat and lean mass between 12–14 weeks of age using an NMR-based analyzer (Minispec LF90II, Bruker Optics). We also analyzed a subset of mice (13–16 weeks old) for oxygen consumption (VO2) and locomotor activity using the Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments). We analyzed serum for insulin and leptin using assays from Crystal Chem; we purchased assays for T4 and corticosterone from Siemens and Arbor Assays, respectively.
RNA extraction and qPCR
We prepared RNA from microdissected ARC using Trizol (Invitrogen) and converted 1 µg samples to cDNA using the Superscript First Strand Synthesis System for RT-PCR (Invitrogen). We analyzed these cDNAs in triplicate via quantitative RT-PCR for Gapdh and Socs3 (Applied Biosystems) or Pomc, Npy, and Agrp39 using an Applied Biosystems 7500. We calculated relative mRNA expression values by the 2−ΔΔCt method, with normalization of each sample ΔCt value to the average ΔCt from Ctrl mice.
Statistics
We used one-way ANOVA followed by Bonferroni post hoc test for multiple comparisons using Graphpad Prism software for PC. Differences were considered significant for p < 0.05.
Supplementary Material
Acknowledgements
We thank Amylin Pharmaceuticals (San Diego, CA) for the generous gift of leptin; Drs. S. Chua (Albert Einstein College of Medicine), B. Lowell (Beth Israel-Deaconess Medical Center), and G. Barsh (Stanford University) for the gift of Leprfl/fl, Agrpcre, and Pomccre mice, respectively; and members of the Myers lab for helpful discussions and technical support. Core support (Animal Phenotyping, CMB, Clinical, MIAC) was provided by the Michigan Diabetes Research and Training Center and Nutrition and Obesity Research Center. Supported by the Marilyn H. Vincent Foundation and grants from the American Diabetes Association (M.G.M.), American Heart Association (M.G.M., R.L.L.) and the US National Institutes of Health (DK057768 to M.G.M.). M.G.Y. is supported by NIH T32GM008322; C.M.P. was supported by T32HL007853.
Footnotes
Contributions:
R.L.L., M.G.Y., C.M.P., and I.E.G. carried out the experiments (with Core and other technical assistance). R.L.L., M.G.Y. and C.M.P. analyzed and prepared data for publication. M.G.M. guided the overall approach in collaboration with R.L.L. and M.G.Y.; M.G.M., R.L.L. and M.G.Y. co-wrote the manuscript.
REFERENCES
- 1.Bray GA, Ryan DH. Drug treatment of obesity. Psychiatr Clin North Am. 2011;34:871–880. doi: 10.1016/j.psc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi IS, O'Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980S–984S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 5.Balthasar N, et al. Leptin Receptor Signaling in POMC Neurons Is Required for Normal Body Weight Homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 7.van de WE, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes MR, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest. 2011;121:2413–2421. doi: 10.1172/JCI43703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott MM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson CM, Leshan RL, Jones JC, Myers MG., Jr Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011 doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity.(Silver.Spring) 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 14.Fulton S, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Hommel JD, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Perez SM, Zhang W, Lodge DJ, Lu XY. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leshan RL, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam DD, et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav VK, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest. 2010;120:2931–2941. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leinninger GM, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leinninger GM, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donato J, Jr, Frazao R, Fukuda M, Vianna CR, Elias CF. Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology. 2010;151:5415–5427. doi: 10.1210/en.2010-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leshan RL, et al. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 27.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 28.McMinn JE, et al. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome. 2004;15:677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 29.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Molecular Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 30.Vong L, et al. Leptin Action on GABAergic Neurons Prevents Obesity and Reduces Inhibitory Tone to POMC Neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feil R, Kleppisch T. NO/cGMP-dependent modulation of synaptic transmission. Handb Exp Pharmacol. 2008:529–560. doi: 10.1007/978-3-540-74805-2_16. [DOI] [PubMed] [Google Scholar]
- 32.Huang PL. Mouse models of nitric oxide synthase deficiency. J. Am. Soc.Nephrol. 2000;11(Suppl 16):S120–S123. [PubMed] [Google Scholar]
- 33.Williams KW, et al. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J Neurosci. 2011;31:13147–13156. doi: 10.1523/JNEUROSCI.2602-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donato J, Jr, et al. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokosuka M, Prins GS, Hayashi S. Co-localization of androgen receptor and nitric oxide synthase in the ventral premammillary nucleus of the newborn rat: an immunohistochemical study. Brain Res Dev Brain Res. 1997;99:226–233. doi: 10.1016/s0165-3806(96)00217-9. [DOI] [PubMed] [Google Scholar]
- 36.van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soliman GA, et al. A simple qPCR-based method to detect correct insertion of homologous targeting vectors in murine ES cells. Transgenic Res. 2007;16:665–670. doi: 10.1007/s11248-007-9110-2. [DOI] [PubMed] [Google Scholar]
- 38.Munzberg H, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bates SH, et al. STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.