Abstract
SnoN/SkiL (TGFβ regulator) is dysregulated in ovarian cancer, a disease associated with acquired drug-resistance. Arsenic trioxide (As2O3used in treating APL) induces SnoN to oppose the apoptotic response in ovarian cancer cells. We now report that As2O3 increases phosphorylation of EGFR/p66ShcA and EGFR degradation. As2O3 activates Src(Y416) whose activity (inhibited by PP2) modulates EGFR activation, its interaction with Shc/Grb2, and p-AKT. Inhibition of PI3K reduces SnoN and cell survival. Although EGFR or MAPK1 siRNA did not alter SnoN expression, As2O3–induced cleaved PARP was reduced together with increased XIAP. Collectively, As2O3 mediates an initial rise in pY-Src(416) to regulate the PI3K/AKT pathway which increases SnoN and cell survival; these early events may counter the cell death response associated with increased pY-EGFR/MAPK activation.
Keywords: ovarian cancer, SnoN/SkiL, epidermal growth factor receptor (EGFR), Src tyrosine kinase, PI3K/AKT, As2O3
1. Introduction
Ovarian cancer is one of the leading causes of cancer deaths among women in the United States. Regrettably, the current chemotherapeutics (platinum/taxane-based drugs) have not markedly increased recurrence-free survival of this deadly disease. Thus, there is an urgent need for the development of novel treatment strategies. Specific genes located in regions of genomic aberrations associated with ovarian cancers include two co-transcriptional repressors, SnoN/SkiL [1] and ecotropic viral integration site-1 (EVI1) [2], amplified at the 3q26.2 locus and involved in the TGF-β signaling cascade. Other genetic alterations include amplification of the catalytic subunit of phosphatidylinositol 3-kinase (PI3K, PIK3CA) located at the 3q26.3 locus [3]. Inhibition of this pathway sensitizes multiple cancer cell types to chemotherapeutic agents [4].
As2O3, a clinically approved drug in the treatment of acute promyelocytic leukemia (APL), elicits antitumor properties in cells derived from solid tumors such as ovarian cancers [5]. As2O3 treatment leads to cytotoxicity via induction of apoptosis [5]. We have shown that As2O3 treatment in epithelial ovarian cancer cells alters expression of certain TGFβ mediators [6]. This cytotoxic agent markedly induces SnoN/SkiL expression concurrent with pro-survival autophagy in a reactive oxygen species (ROS) dependent manner. This protective pathway antagonizes the As2O3-induced apoptotic response [6]. Indeed, small interfering RNA-mediated SnoN knockdown increases the sensitivity of ovarian cancer cells to As2O3 [6]. However, the mechanisms through which As2O3 induce SnoN expression and the consequent cell death response are not clearly understood.
Herein, we assess the contribution of EGFR and downstream pathways including activation of the Src/PI3K/AKT and ShcA/Grb2/MAPK signaling pathways to As2O3-induced SnoN expression and the cell death response. We identified that As2O3 activates EGFR and promotes phosphorylation of p66 ShcA and its interaction with the Grb2 adaptor protein with slower kinetics compared to EGF-mediated EGFR activation. Furthermore, EGFR is degraded upon As2O3 treatment in combination with cycloheximide. Inhibitors of Src (PP2 but not SU6656), PI3K (LY294002 or GDC0941 (to a lesser degree)), and knockdown of PIK3CA altered As2O3-induced changes in SnoN expression. In contrast to EGF, PP2 modulated As2O3-induced EGFR activation and interaction with Shc/Grb2. We also noted reduced Grb2-EGFR interaction with p66 ShcA knockdown in the presence of As2O3 implicating p66 ShcA isoform in mediating this interaction. With MAPK1 and EGFR (to a lesser extent) siRNA, we noted a significant increase in cell survival. Together, our results implicate activation of the pro-survival PI3K pathway in As2O3–induced changes in SnoN expression and cell survival. These events occur prior to full activation of the EGFR/MAPK pathway which may contribute to the As2O3-induced cell death response.
2. Materials and Methods
2.1 Cell Culture
HEY ovarian carcinoma cells were kindly provided by Dr. Gordon Mills (MD Anderson Cancer Center, Texas) and cultured in RPMI 1640 supplemented with 8% FBS and penicillin/streptomycin. Cells were maintained in a 37°C humidified incubator containing 95% air and 5% CO2.
2.2 Cell Treatments with EGF, As2O3, and Signaling Pathway Inhibitors
EGF, SU6656, and PP2 were obtained from Calbiochem (Rockland, MA). As2O3 was obtained from Sigma-Aldrich (St. Louis, MO). U0126 and LY294002 were obtained from Cell Signaling Technology (Danvers, MA). PD153035 was obtained from A.G. Scientific (San Diego, CA). GDC0941 was obtained from Selleckchem (Houston, TX). Actinomycin D was obtained from MP Biomedicals (Solon, OH). PP2, U0126, PD153035, LY294002, SU6656, and GDC0941 were dissolved in dimethylsulfoxide (DMSO). Cells were pretreated with PP2, U0126, and PD153035 for 2 h prior to treatment with either EGF or As2O3. All other inhibitors were added concurrently with EGF or As2O3.
2.3 siRNA Treatment of Ovarian Carcinoma Cell Lines
siRNA targeting EGFR (L-003114-00), pp60 c-Src (L-003175-00), MAPK1 (L-003555-00), PIK3CA (L-003018-00), non-targeting ON-TargetPLUS control siRNA (D-001810-10), and Dharmafect I transfection reagent were obtained from Dharmacon (Lafayette, CO). ShcA p66 siRNA was custom designed (obtained from Dharmacon) based on a published sequence towards its CH2 domain [7]. The sense sequence is 5’-GAAUGAGUCUCUGUCAUCGUU-3’ and antisense sequence is 5’-CGAUGACAGAGACUCAUUCUU-3’. The siRNA transfection method was followed according to our previously published studies [6]. Mock transfection was performed in the absence of siRNA, as control.
2.4 Protein Isolation and Immunoprecipitation (IP)
Cells were lysed in lysis buffer (1% Triton X-100, 50 mM HEPES, 150 mM NaCl, 1 mM MgCl2, 1 mM EGTA, 10% glycerol, and protease inhibitor cocktail (Roche, Madison, WI)) for 1 h at 4°C. Cells lysates were harvested and centrifuged at 14,000 rpm for 10 min at 4°C. An aliquot of the supernatant was collected for analysis (inputs). Quantification of total protein was performed using the Bicinchoninic Acid assay (Fisher Scientific, Pittsburgh, PA) and then the samples were normalized to a minimum concentration of 1 mg/ml. For IP, 5 µl of anti-EGFR (200 µg/ml of EGFR rabbit polyclonal (sc-03)) or anti-Shc (250 µg/ml) antibody was added to the cell lysates and incubated on a shaking platform for 3 h at 4°C. This was followed by the addition of 40 µl of Protein G Sepharose 4 Fast Flow Beads (Amersham Biosciences, Piscataway, NJ) followed by a further 1 h incubation at 4°C. The immunoprecipitates were sedimented and washed extensively with lysis buffer as well as PBS. Proteins were eluted from the beads by heating to 95°C with 5% SDS sample buffer.
2.5 SDS-PAGE and Western Blot Analyses
Protein samples were analyzed on appropriate percentage (8%, 10%, or 12%) SDS-PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes and used for western blotting according to previously published methods [6]. Bound antibody was detected using enhanced chemiluminescence reagent (Bio-Rad, Hercules, CA) followed by exposure to film.
Primary antibodies were used at the following dilutions. PY99 (HRP conjugated, SC-7020, 1:1000), SnoN rabbit polyclonal (SC-914, 1:1000), and EGFR rabbit polyclonal (SC-03, 1:1000) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). PARP rabbit polyclonal (#9542, 1:1000), p-AKT (Ser473) rabbit polyclonal (#4060, 1:1000), AKT rabbit polyclonal (#4685, 1:1000), PIK3CA rabbit polyclonal (#4249, 1:1000), p-Src (416) rabbit monoclonal (#2113, 1:1000), c-Src mouse monoclonal (#2110, 1:1000), XIAP rabbit monoclonal (#2045, 1:1000), Bcl-XL rabbit monoclonal (#2764, 1:1000), and GAPDH rabbit polyclonal (#2118, 1:4000 dilution) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Shc rabbit polyclonal (#06-203, 1:1000) antibody was obtained from Millipore (Billerica, MA). Grb2 mouse monoclonal (#610111, 1:2000) antibody was obtained from BD Biosciences (San Jose, CA). pSer-p66 Shc mouse monoclonal antibody (#566807, 1:1000) was obtained from Calbiochem (Rockland, MA).
2.6 Quantitative PCR
Total RNA was isolated from HEY cells using the RNeasy Mini kit (Qiagen, Valencia, CA). Quantitative PCR was performed using One-Step PCR Taqman master mix, primers/probes for SnoN (Assays-by-Design, Hs00180524_m1), and One-Step-Plus Detection System (Applied Biosystems, Bedford, MA) using β-actin as endogenous control. PCR conditions and analyses were followed according to previously published methods [6].
2.7 Apoptosis Assays
Using the kit obtained from Calbiochem (Rockland, MA), assays were performed as previously published [6]. Samples were analyzed by flow cytometry (College of Medicine, University of South Florida). Signals were detected at 518 nm for FL1 and at 620 nm for FL2, respectively.
2.8 Caspase Assays
Caspase Glo-3/7 activity assays (Promega, Madison, WI) were performed according to the manufacturer’s instructions. Briefly, 50,000 cells were seeded per well in an opaque 96-well plate and appropriate drug treatments were then performed, followed by the addition of 100 µl of caspase activity substrate. Luminescence readings were measured using a BioTek plate reader.
2.9 Phosphatase Assays
Phosphatase assay was performed using the 1-Step PNPP as substrate which was obtained from ThermoScientific. To cell lysates, 100 µl of 1-Step PNPP was added and mixed. The reaction was incubated at room temperature for 30 minutes followed by addition of 50 µl of 2N NaOH to terminate the reactions. Absorbance was measured at 405 nm.
2.10 Statistical Analyses
The number of replicates conducted for each experiment is indicated in the figure legends. Error bars displayed on the bar graphs represent standard deviations. Data represented in tabular format (Fig. 3K and 4B) are displayed as averages ± standard deviations. Western blots were analyzed by densitometric analyses using Image J program (Image Processing and Analysis in Java, NIH Image Software, http://rsb/info/nioh/gov.ij/) as previously described [6]. P-values were calculated based on the student’s t-test (p-value<0.05, *; p-value<0.001, **).
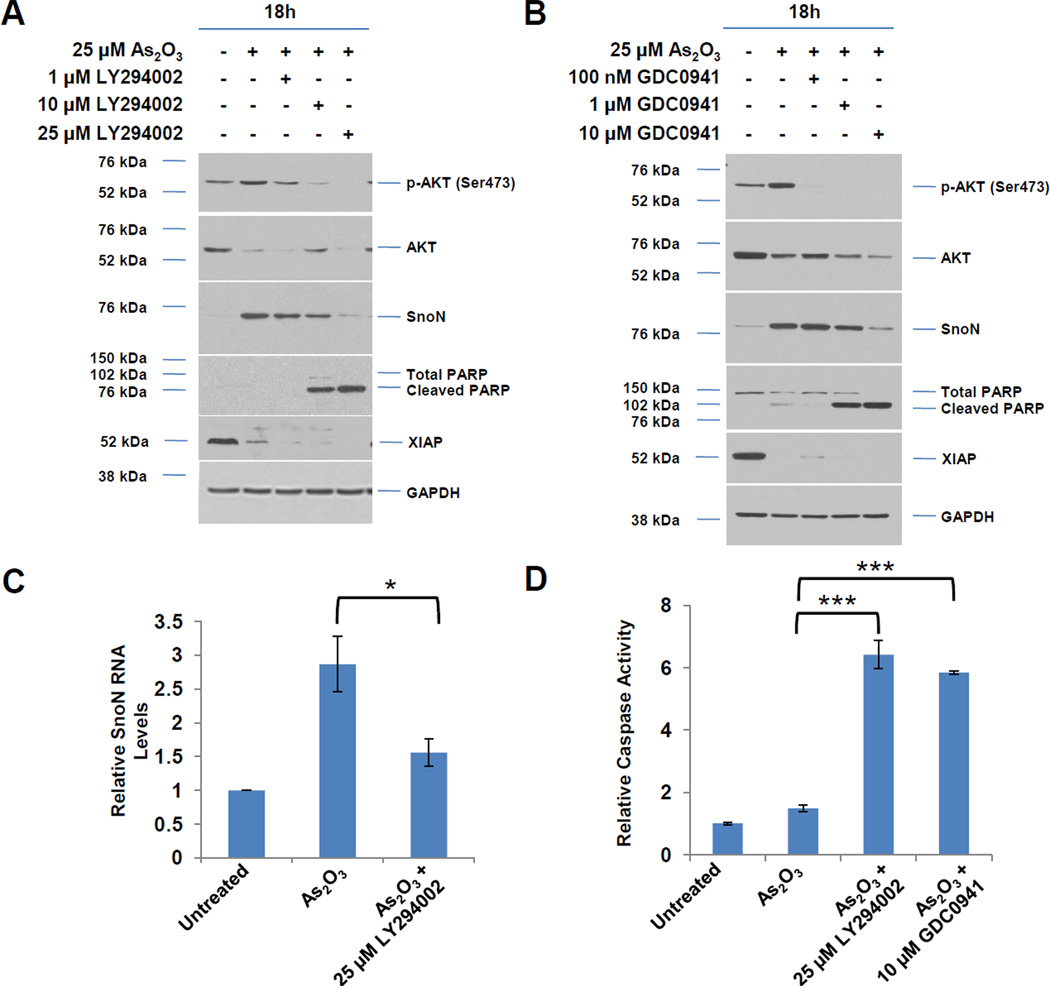
Fig. 3. PI3K inhibitors (LY294002 and GDC0941) and PIK3CA siRNA alter SnoN expression and the apoptotic response to As2O3.
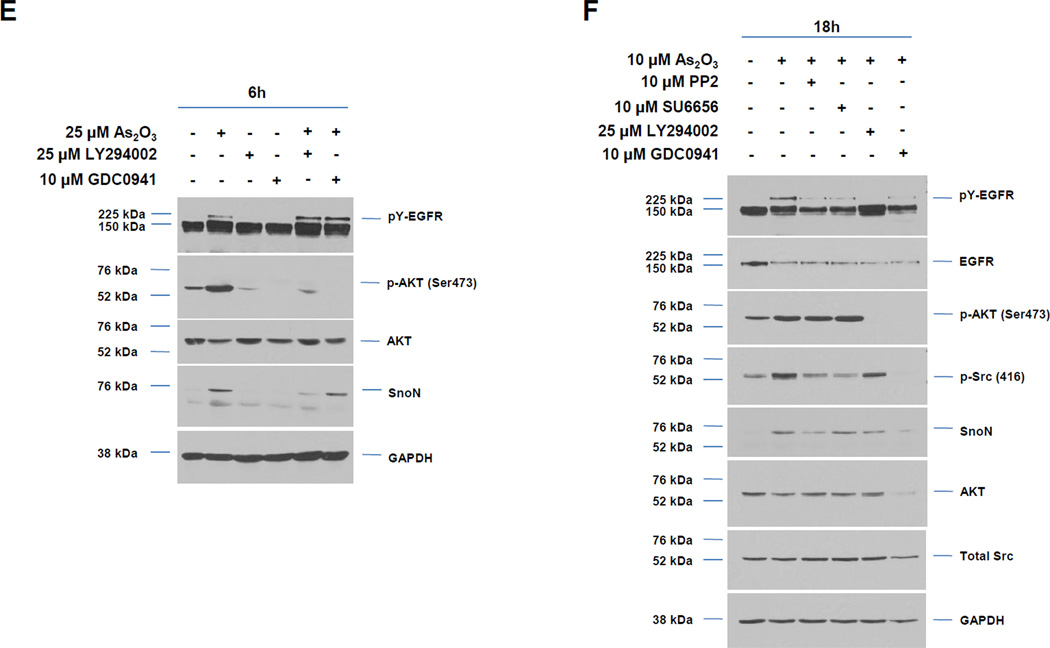
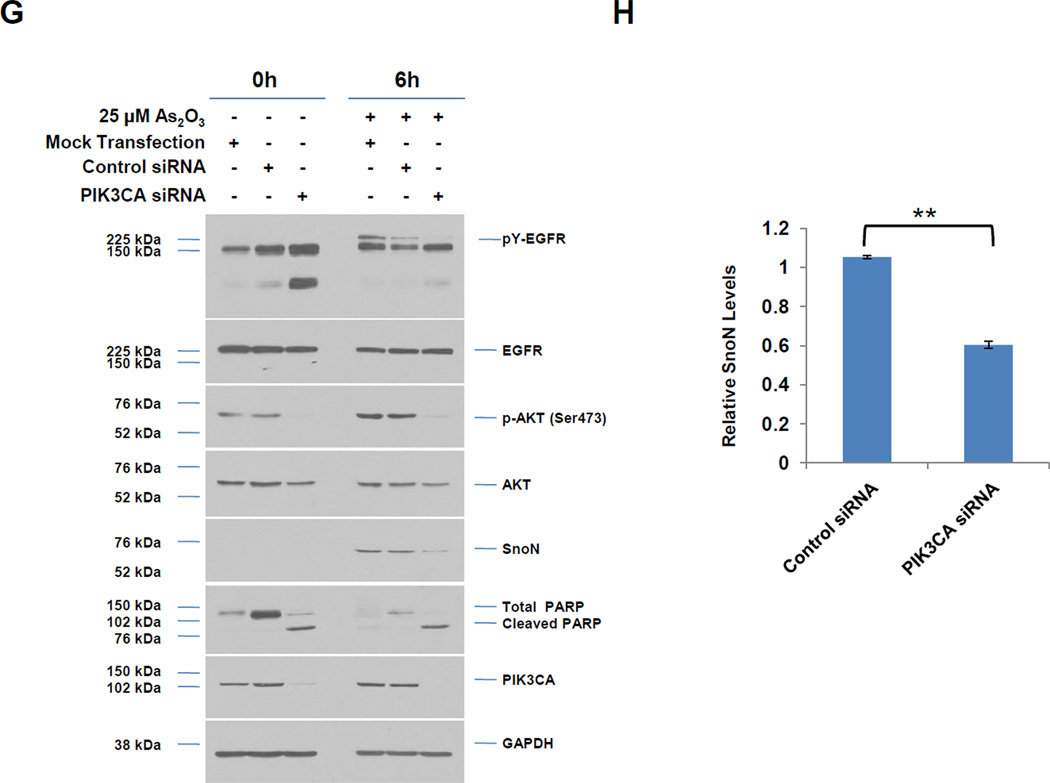
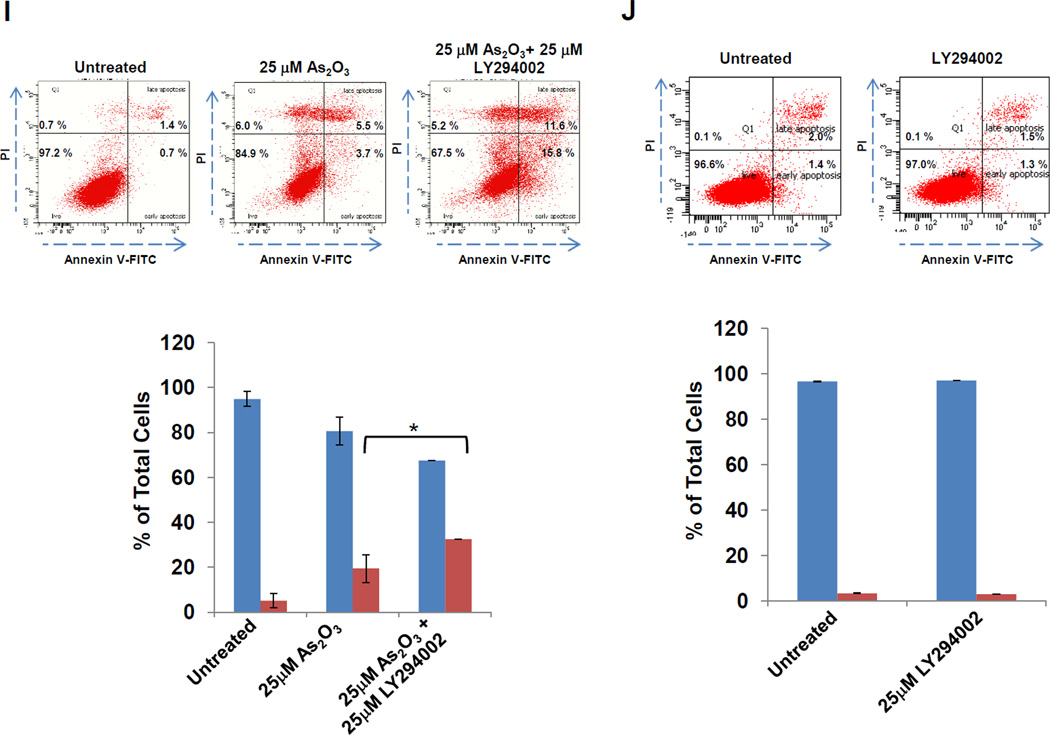
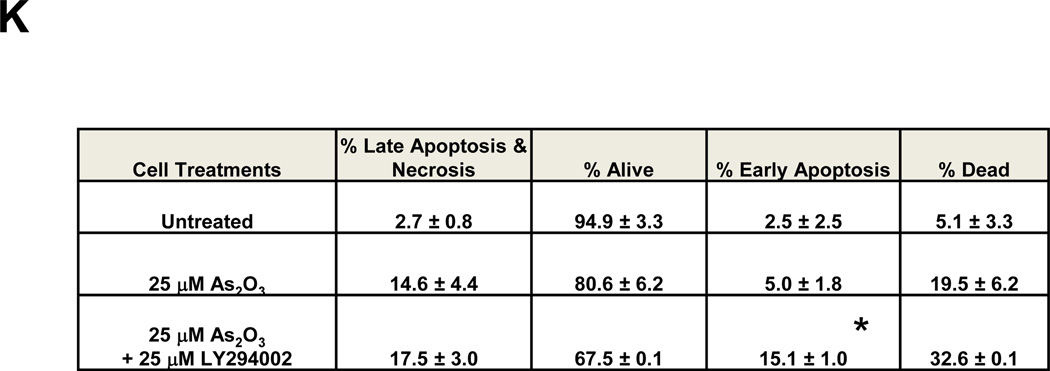
A, Following seeding and adherence of HEY, cells were co-treated with LY294002 (1–25 µM) and As2O3 (25 µM) for 18 h. Cells lysates were harvested and western analyses performed. [n=3] B, Following seeding and adherence of HEY cells, cells were co-treated with GDC0941 (100 nM-10 µM) and As2O3 (25 µM) for 18 h. Cells lysates were harvested and western analyses performed. [n=2] C, Following seeding and adherence of HEY, cells were co-treated with LY294002 and As2O3 for 18 h. RNA was harvested and qPCR performed. [n=3] D, Caspase activity assay was performed on HEY cells treated with As2O3, As2O3 with LY294002, or As2O3 with GDC0941 for 18 h. [n=3]. E, Following seeding and adherence of HEY, cells were co-treated with LY294002 (25 µM) or GDC0941 (10 µM) with As2O3 (25 µM) for 6 h. Cells lysates were harvested and western analyses performed. [n=2]. F, Following seeding and adherence of HEY, cells were treated with PP2 10 µM), SU6656 (10 µM), LY294002 (25 µM), or GDC0941 (10 µM) with As2O3 (10 µM) for 18 h. Cells lysates were harvested and western analyses performed. [n=3]. G, HEY cells were treated with non-targeting (control) siRNA or PIK3CA siRNA. At 72 h after transfection, cells were treated with As2O3 for 6 h. Cell lysates were then collected and western analyses performed. H, Densitometric analyses of the SnoN from data presented in E is shown. I, HEY cells were treated with As2O3 alone or in combination with LY294002 for 18 h at which time both the floating and adherent cells were collected. Cells were stained with annexin V-FITC and propidium iodide (PI) followed by flow cytometric analysis. Raw data plots are shown (top panels). The percentage of viable and dead cells are shown in lower panel. J, HEY cells were treated with LY294002 alone for 18 h at which time both the floating and adherent cells were collected. Cells were stained with annexin V-FITC and propidium iodide (PI) followed by flow cytometric analysis. Raw data plots are shown (top panels). The percentage of viable and dead cells are shown in lower panel. K, The data presented in I is also represented in tabular format showing % of late apoptotic/necrotic, alive, early apoptotic and dead cells. [n=2]
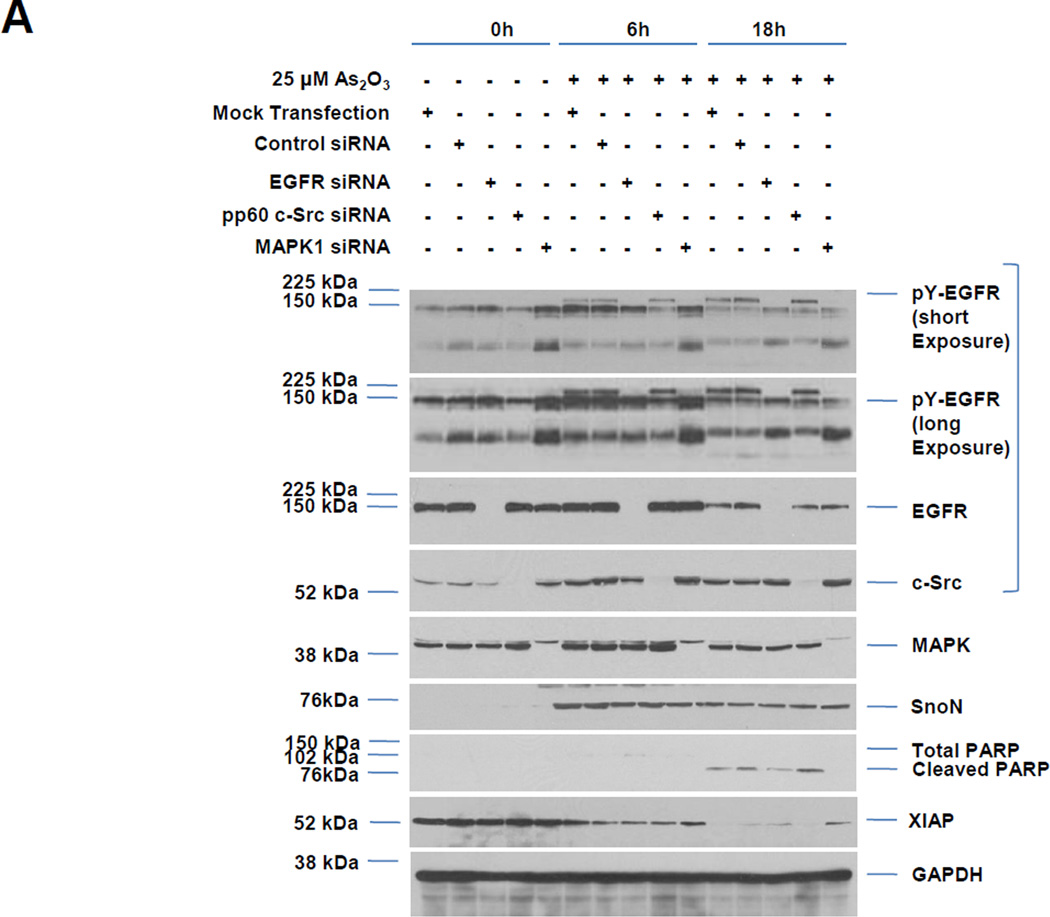
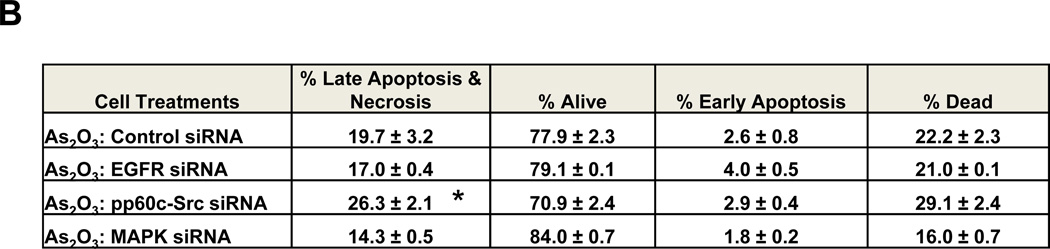
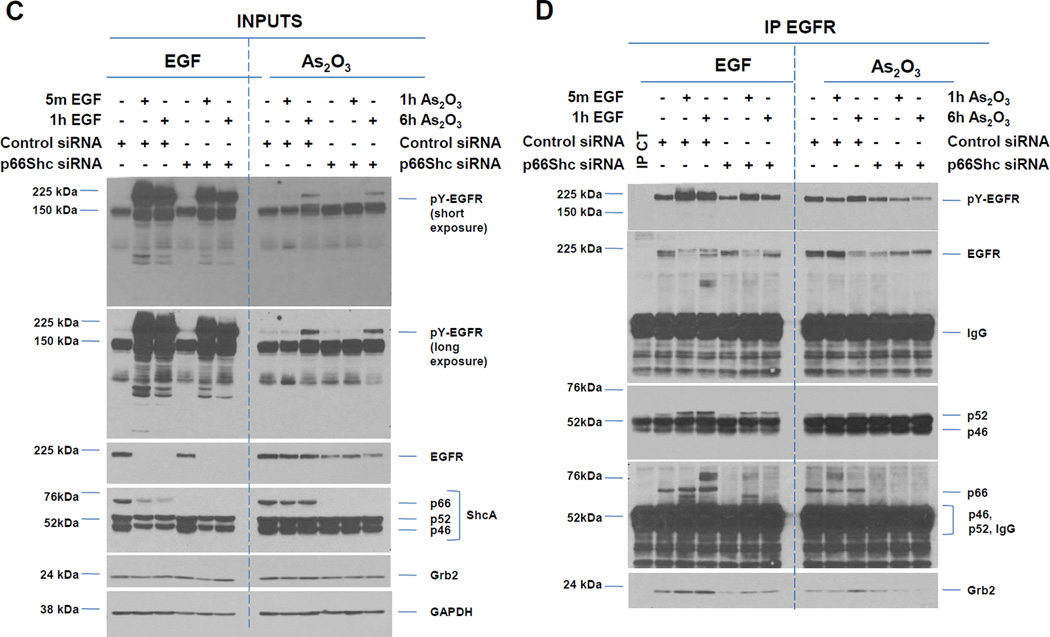
Fig. 4. Knockdown of EGFR/MAPK1/pp60 c-Src modulate cell survival in response to As2O3.
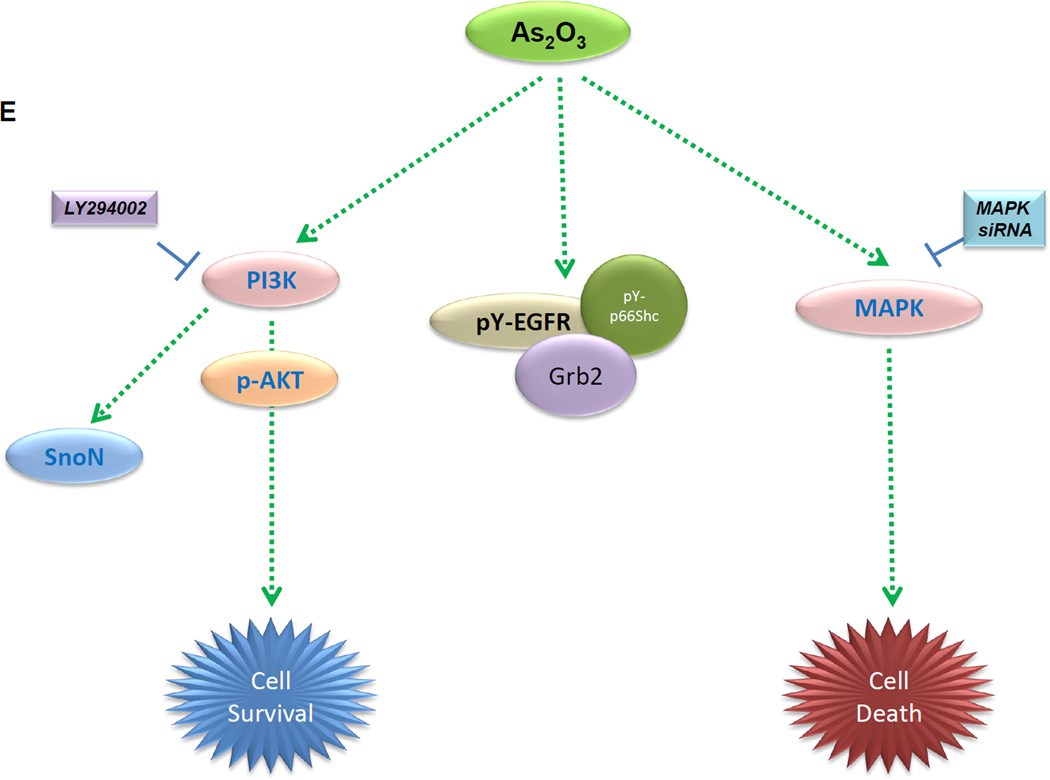
A, HEY cells were treated with siRNA presented followed by treatment with As2O3 for 6 or 18 h. Cell lysates were collected and western analyses performed. [n=2] B, The data from the apoptosis assay for cells as described in A is represented in tabular format. [n=2] C, HEY cells were treated with EGF or with As2O3. Cell lysates were collected (“inputs”) and western analyses performed. [n=2] D, Using lysates presented from C, immunoprecipitation (IP) was performed using anti-EGFR rabbit polyclonal antibody followed by western analyses. [n=2]. E, Proposed model of the action of As2O3 on regulation of SnoN levels. In response to As2O3, the Src and AKT pathways are activated. Inhibition via LY294002 (and PP2 but not SU6656) leads to a reduction in As2O3-induced SnoN mRNA and protein levels. As2O3 also leads to tyrosine phosphorylation of the EGF receptor and phosphorylation of p66ShcA together with increased binding of Grb2. Inhibition of the MAPK pathway via siRNA targeting MAPK1 leads to increased cellular survival in response to As2O3.
3. Results
3.1 As2O3 Induces EGFR and ShcA Phosphorylation and Interaction with Grb2 Adaptor Proteins in HEY Ovarian Carcinoma Cells
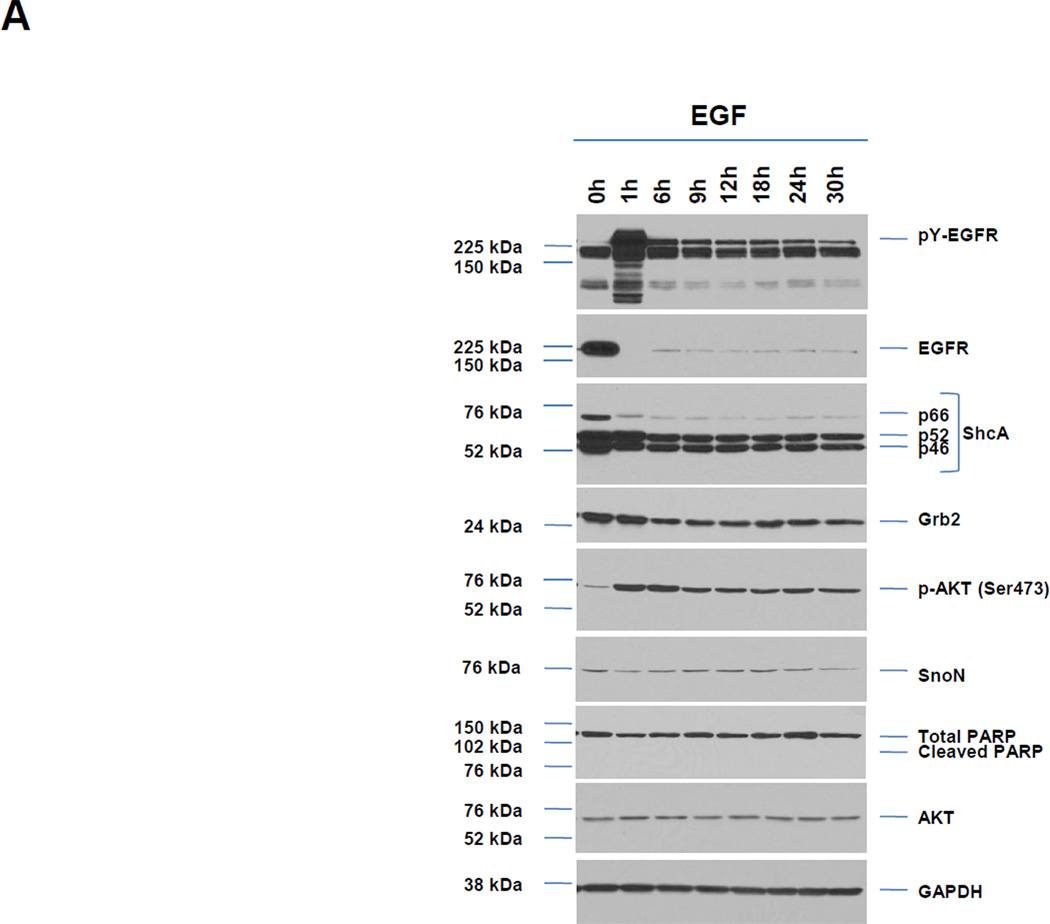
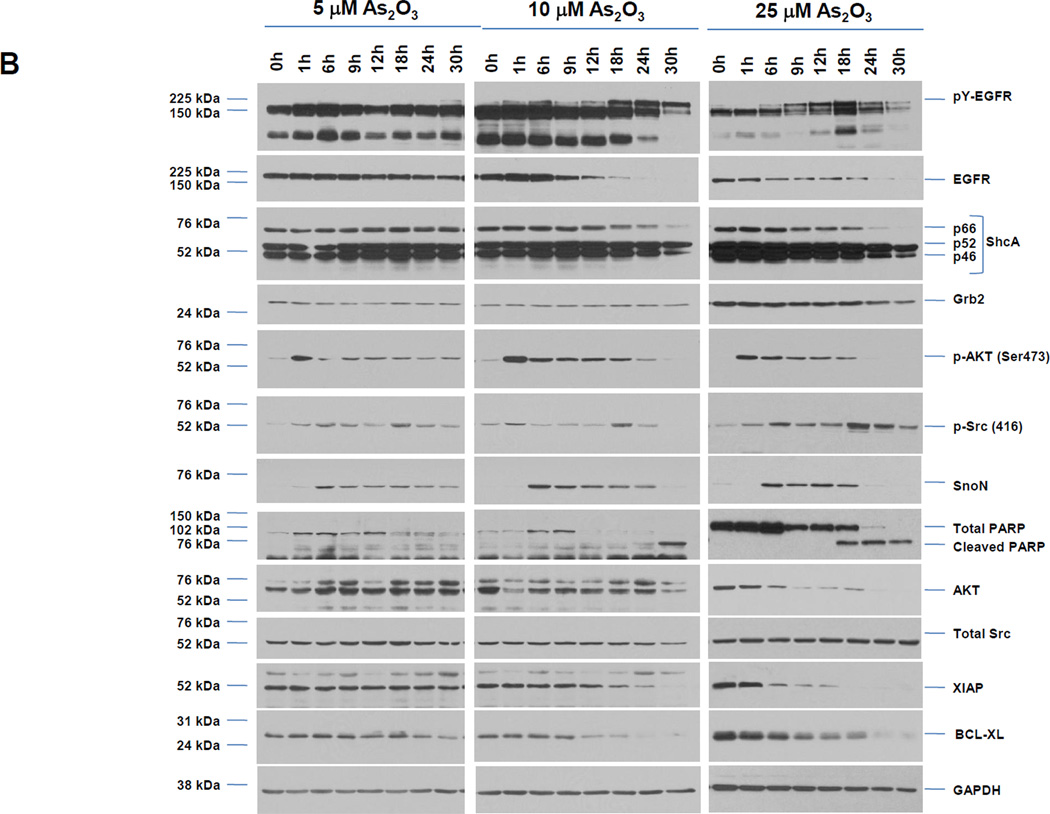
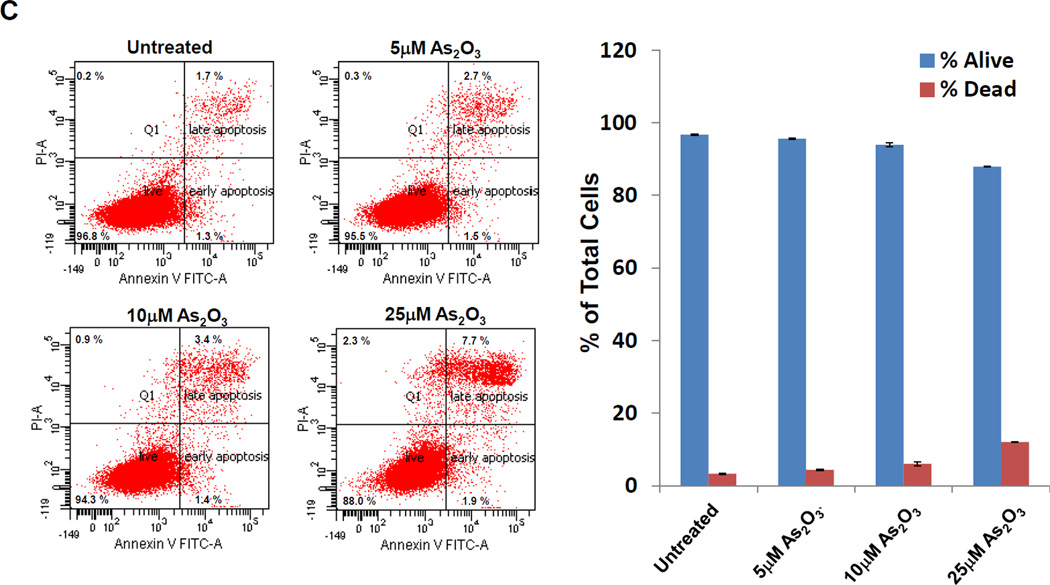
To assess whether HEY cells undergo EGFR activation in response to As2O3, we treated HEY cells with 5, 10, or 25 µM As2O3 (doses previously reported [6]) and contrasted their cellular responses to EGF-treated cells (Fig. 1A). Following 1 h EGF, we observed a marked increase in pY-EGFR, reduced mobility of ShcA isoforms, and activation of AKT (Ser473). EGFR levels appear to rapidly disappear upon 1 h treatment when it becomes heavily phosphorylated; this may be due to the antibody detecting unphosphorylated form of EGFR compared to total EGFR or to receptor internalization which is not extracted or detected under our conditions. Protein levels of ShcA and Grb2 decreased rapidly at 6 h EGF. The results with the three doses of As2O3 were similar although with increased intensity with elevated doses. In contrast, As2O3 induced pY-EGFR with slower kinetics peaking at 18 h post stimulation and expression of ShcA/Grb2 decreased gradually until 30 h (Fig. 1B). Similar to EGF, p-AKT occurred 1 h post As2O3 treatment followed by a rapid decay. Subsequent to p-AKT activation, SnoN increased at 6 h followed by a rapid decay with As2O3. In contrast, there were no reproducible changes in SnoN with EGF. We have assessed phospho-Src (Y416) which shows a biphasic profile (initial rise at 1–6 h and second increase at 18 h). At least 10 µM As2O3 is needed in HEY cells to observe cleaved PARP with a significant apoptotic response (Fig. 1B and 1C). In addition, the expression levels of anti-apoptotic markers (Bcl-XL and XIAP) were markedly reduced with As2O3 treatment. Thus, upon cellular treatment with As2O3, the kinetics of EGFR phosphorylation and downstream consequences on ShcA/Grb2 and SnoN expression suggest a different mechanism of EGFR and downstream signaling pathway activation in contrast to EGF.
Fig. 1. As2O3 induces EGFR activation and interactions with ShcA/Grb2 adaptor proteins in HEY cells.
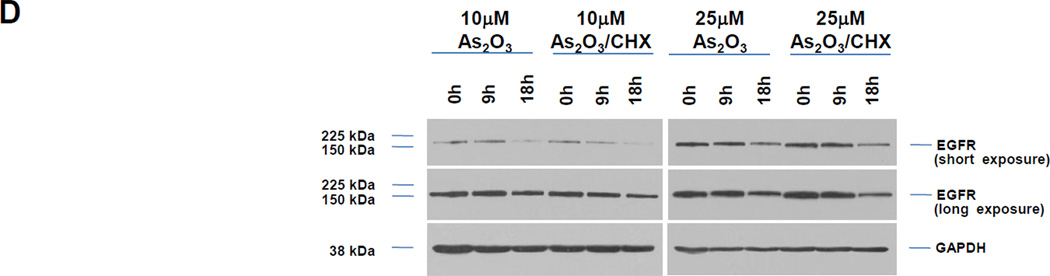
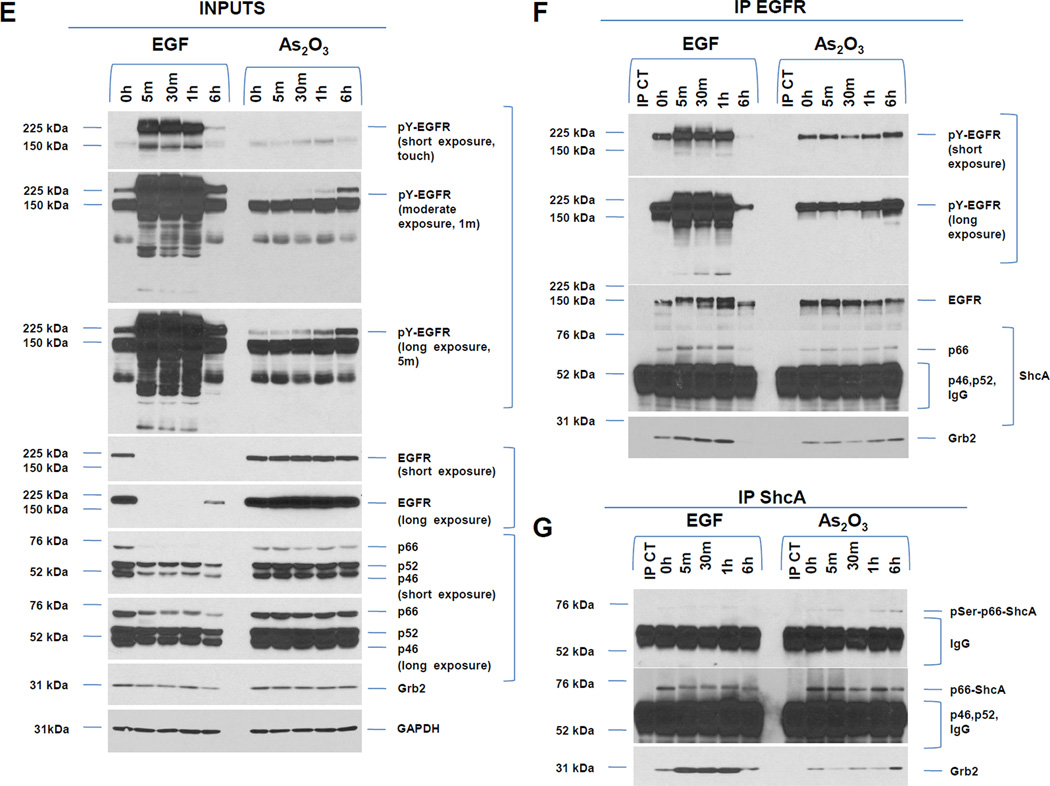
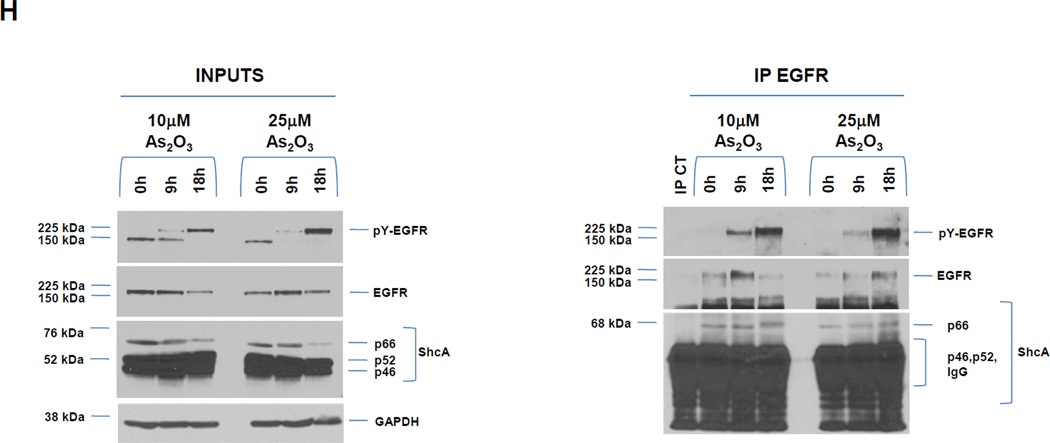
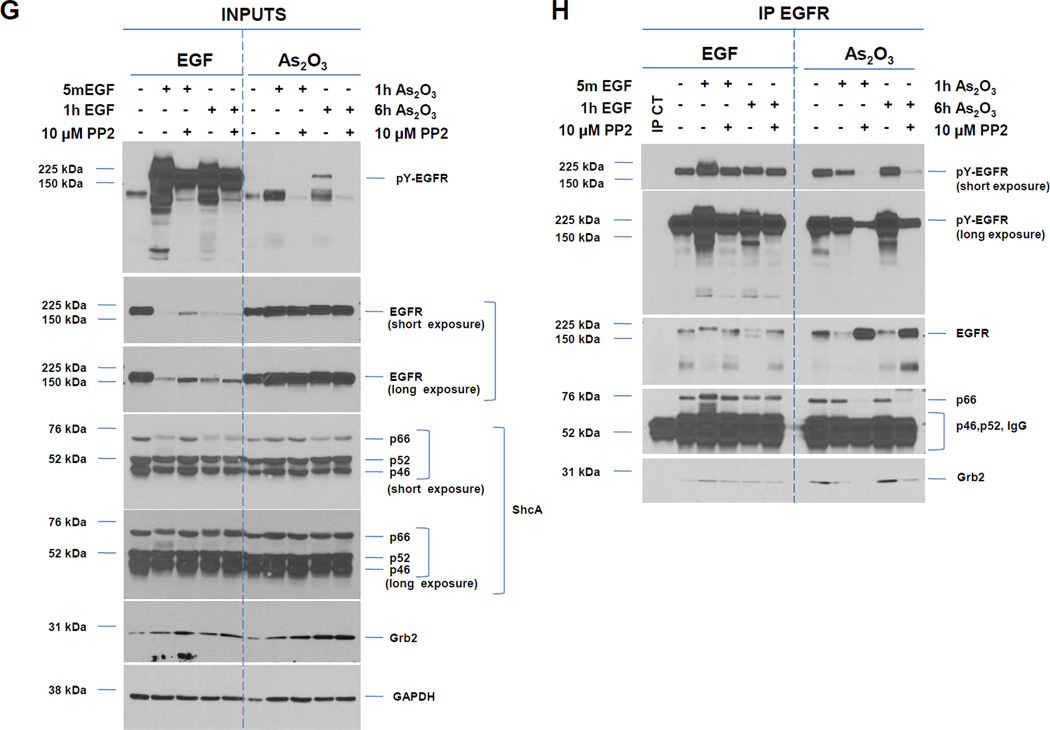
A, HEY cells were treated with 100 ng/ml EGF from 1–30 h and then cell lysates were analyzed by western analyses. [n=4]. B, HEY cells were treated with 5, 10, or 25 µM As2O3 from 1–30 h and then cell lysates were analyzed by western analyses. [n=2]. C, HEY cells were treated with 5, 10, or 25 µM As2O3 for 18 h at which time both the floating and adherent cells were collected. Cells were stained with annexin V-FITC and propidium iodide (PI) followed by flow cytometric analysis. Raw data plots are shown (left panel). The percentage of viable and dead cells for data presented (right panel). [n=2]. D, HEY cells were treated with As2O3 in the presence/absence of cycloheximide (CHX, 0.05 ng/ml) across time points indicated. Cell lysates were harvested and western analyses performed. [n=2]. E, HEY cells were stimulated with EGF or As2O3 across time points indicated. Cell lysates were harvested (“inputs”) and western analyses performed. [n=4] F, Using lysates from E, immunoprecipitation (IP) was performed using anti-EGFR rabbit polyclonal antibody followed by western analyses. IP CT refers to immunoprecipitation control using antibody and protein G beads. [n=4]. G, IP was performed using anti-ShcA rabbit polyclonal antibody followed by western analyses. H, Similar to experiments performed in E (“inputs”, left panel) and F (IP EGFR, right panel) except HEY cells were treated with As2O3 at 10µM at 9 and 18 h.
To determine whether altered phosphatase activity could lead to the increased pY-EGFR with As2O3 as previously reported to occur with arsenite treatment [8], we measured phosphatase activity using PNPP as substrate between 1 – 30 h As2O3 across all doses (5, 10, and 25 µM) (results not shown). However, no changes in phosphatase activity were observed suggesting that other mechanisms are responsible for As2O3-mediated changes in pY-EGFR. Similar to EGF treatment which is well-established to lead to EGFR degradation in lysosomes following ubiquitination, we have noted that co-treatment of cells with As2O3 (10 and 25 µM) and cycloheximide, an inhibitor of protein translation, did not alter reduction in EGFR levels (Fig. 1D) suggesting that EGFR is degraded upon As2O3 treatment in HEY cells.
We next determined whether there was increased interaction of adaptor molecules (ShcA and Grb2) upon As2O3-induced activation of EGFR. Similar to EGF, immunoprecipitated pY-EGFR associated with p66 ShcA in both the absence and presence of As2O3 treatment. Since the p46/52 ShcA isoforms co-migrated with IgG, we could not assess their phosphorylation status or kinetics of interaction with EGFR. However, we observed increased Grb2 binding to pY-EGFR (for both EGF and As2O3) (Fig. 1F and G). The mobility of p66 ShcA was reduced at 6 h As2O3 treatment suggestive of a phosphorylation event; indeed, p66 ShcA was positive for pSer36 (Fig. 1G). Longer incubation (9 and 18 h) times (Fig. 1H) leads to increased phosphorylation of p66 Shc with 10 and 25 µM As2O3. Collectively, these results indicate that As2O3 induces increased interaction of Grb2 with pY-EGFR/Shc complex whose kinetics and phosphorylation pattern differ markedly to that of EGF.
3.2 Src Tyrosine Kinase Inhibitor, PP2 but not SU6656, Alters As2O3-Induced EGFR Activation, its Interaction with ShcA/Grb2 Adaptor Proteins, and SnoN Expression
To identify the signaling pathways that are responsible for As2O3-induced changes in SnoN expression [6], we first determined the optimal doses of the following inhibitors in EGF treated HEY ovarian carcinoma cells: PP2 (general Src family tyrosine kinase (SFK) inhibitor, 10 µM), U0126 (MAPK inhibitor, 10 µM), and PD153035 (EGFR and HER2/neu kinase inhibitor, 100 nM) (results not shown). With 25 µM As2O3 co-treated with PP2 (Fig. 2A, B, and C), we observed a marked reduction of SnoN, together with a slight reduction in p-AKT compared to As2O3 alone or PP2 alone. Similar results were obtained in cells treated with 10 µM As2O3 for 18 h (Fig. 3F).
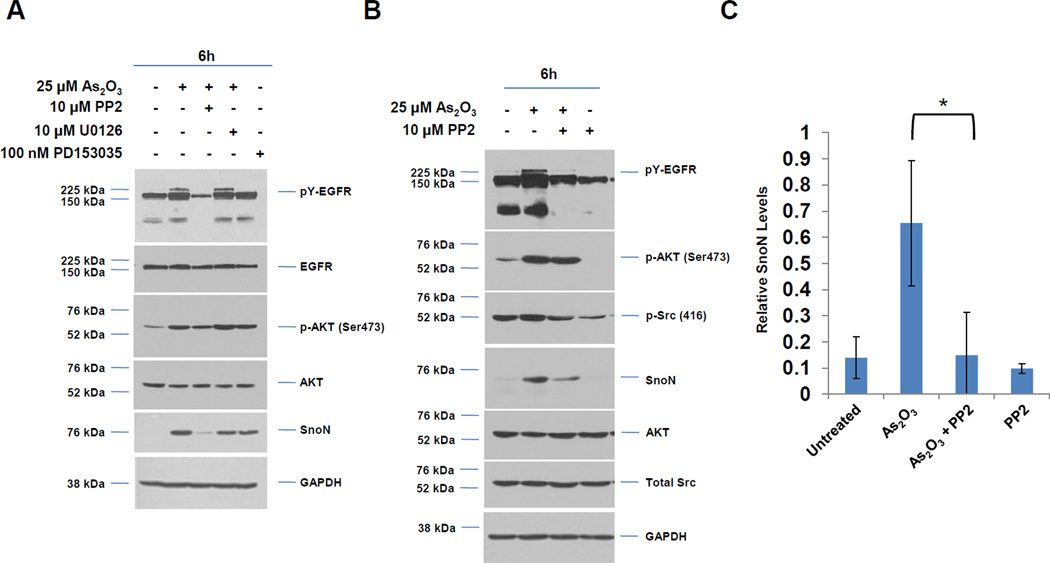
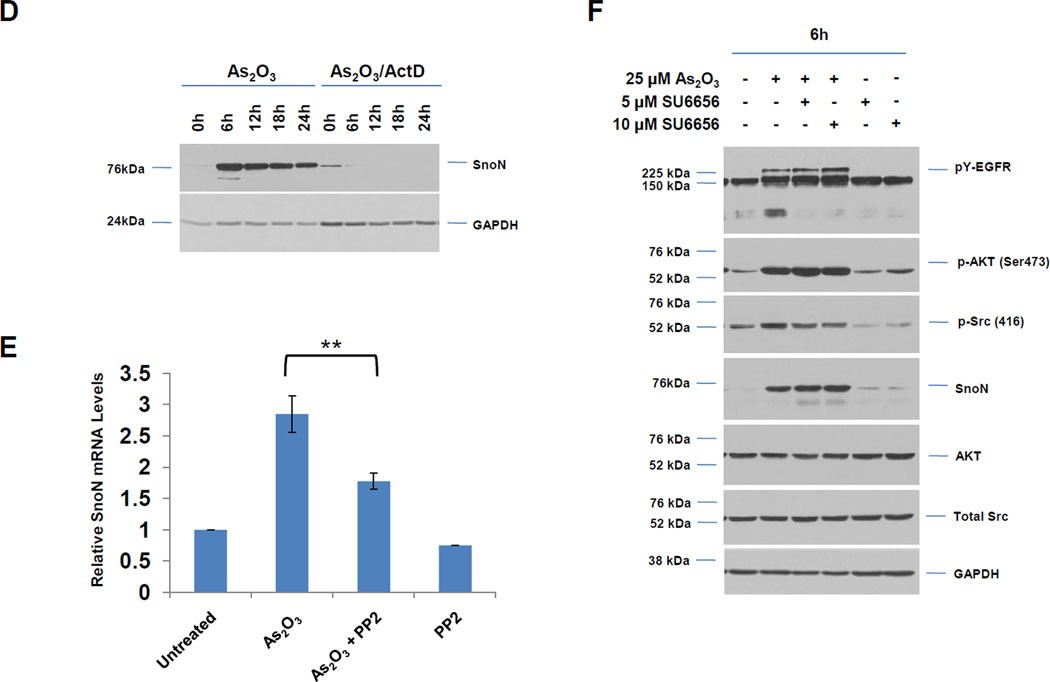
Fig. 2. Src tyrosine kinase inhibitor (PP2) alters As2O3-mediated EGFR activation, interaction of EGFR with ShcA/Grb2 adaptor proteins, and SnoN expression.
A, HEY cells were pretreated with PP2 (10 µM), U0126 (10 µM), or PD153035 (100 nM) for 2 h prior to stimulation with As2O3 (25 µM) for 6 h. Cells lysates were harvested and western analyses performed. [n=4] B, HEY cells were treated with PP2 (10 µM) only or for 2 h prior to stimulation with As2O3 (25 µM) for 6 h. Cells lysates were harvested and western analyses performed. [n=2]. C, Densitometric analyses of SnoN (6 h) is shown for data presented in B. D, HEY cells were treated with 25 µM As2O3 in the presence/absence of actinomycin D (ActD, 0.5 µg/ml) across time points indicated. Cell lysates were harvested and western analyses performed. [n=2]. E, Following seeding and adherence of HEY, cells were pretreated with PP2 (10 µM) for 2 h prior to stimulation with As2O3 (25 µM) for 6 h. RNA was isolated and used for qPCR. Relative RNA-fold changes are presented for SnoN. [n=3]. F, HEY cells were pretreated with SU6656 (5 or 10 µM) for 2 h prior to stimulation with As2O3 (25 µM) for 6 h. Cells lysates were harvested and western analyses performed. [n=2]. G, HEY cells were pretreated with PP2 (10 µM) for 2 h followed by treatment with EGF for either 5 min or 1 h or As2O3 for either 1 h or 6 h. Cell lysates were collected (“inputs”) and western analyses performed. [n=2]. H, Using lysates presented from G, immunoprecipitation (IP) was performed using anti-EGFR rabbit polyclonal antibody followed by western analyses.
To determine if As2O3-induced changes in SnoN expression are due to transcriptional upregulation, we co-treated HEY cells with actinomycin D (transcriptional inhibitor) and As2O3 (Fig. 2D). With actinomycin D, induction of SnoN protein was markedly reduced suggesting that SnoN levels induced by As2O3 are due to transcriptional upregulation. To determine whether Src tyrosine kinase mediates changes in SnoN mRNA transcripts, we performed real-time PCR. We observed a 2.8-fold increase in SnoN expression with As2O3, which was markedly reduced by PP2 (40% reduction, Fig. 2E). In contrast, U0126 did not alter SnoN mRNA while PD153035 led only to only a slight reduction in SnoN mRNA (results not shown). Inhibition of Src kinase activity was validated via western analyses for p-Src (Y416) (Fig. 2B). Although the use of PP2 suggests that Src contributes to transcriptional upregulation of SnoN with As2O3, this could not be validated with SU6656 [9], another specific Src kinase inhibitor (Fig. 2F). With this inhibitor, we did not observe marked changes in SnoN or pAKT/pY-EGFR. SU6656 exhibits higher specificity to specific Src family kinases relative to PP2; thus, the differential response may be due to effective inhibition of different Src family kinases by PP2 and SU6656 involved in regulating the downstream effects of As2O3.
We next assessed whether As2O3-induced activation of Src modulates expression of ShcA/Grb2 or the ability of EGFR to interact with these adaptor proteins. Thus, we stimulated cells with As2O3 (1 and 6 h) in the absence/presence of 10 µM PP2 and contrasted this response with EGF (5 min and 1 h). With both treatment conditions, we observed a dramatic reduction in pY-EGFR with PP2 treatment. As expected, the reduced mobility of p66 ShcA isoform (phosphorylated form) observed with EGF treatment was reduced in the presence of PP2 (Fig. 2G). Together, these results suggest that activation of Src is necessary for phosphorylation of EGFR in response to As2O3 (and EGF). To assess whether Src modulates the As2O3-induced interaction of EGFR with ShcA/Grb2, we performed immunoprecipitation of EGFR in the presence/absence of PP2 (Fig. 2H). With As2O3, PP2 reduces pY-EGFR and co-immunoprecipitating p66 ShcA/Grb2. This contrasts with 5 min EGF/PP2 treatment where an equivalent amount of p66 ShcA co-immunoprecipitated with EGFR (although increased mobility was observed with only a subtle reduction in bound Grb2). Collectively, these results indicate that upon As2O3 treatment, Src activity contributes to EGFR tyrosine phosphorylation and its interaction with downstream adaptor molecules (ShcA/Grb2) with contrasting characteristics to that of EGF-mediated EGFR/Shc/Grb2 interactions.
3.3 PI3K Inhibitors Alter As2O3-Mediated SnoN Expression and Cell Survival
Since AKT was activated 1 h post As2O3 treatment prior to changes in SnoN expression (see Fig. 1B), we next assessed whether the PI3K pathway contributes to As2O3-mediated increases in SnoN expression via the use of PI3K inhibitors (LY294002 and GDC0941). With increasing doses of these inhibitors with 18 h As2O3, we observed a dramatic reduction in p-AKT, SnoN, and XIAP, with a corresponding increase in cleaved PARP and caspase 3/7 activity (Fig. 3A, B, and D). The PI3K/AKT pathway could transcriptionally regulate SnoN as noted with a 50% reduction in mRNA transcripts upon co-treatment of As2O3 with LY294002 (Fig. 3C). With 6 h 25µM As2O3 treatment, we observed dramatic reduction in SnoN levels (as well as XIAP) particularly with LY294002 (and to a lesser extent with GDC0941) (Fig. 3E). Similar results were obtained with cells treated at 10 µM As2O3 for 18 h (Fig. 3F). We also targeted PIK3CA (catalytic subunit of PI3K) with siRNA in combination with As2O3 and observed a marked reduction in SnoN expression with 6 h As2O3 (Fig. 3G and H). Co-treatment of LY294002 (or GDC0941, results not presented) with As2O3 increased apoptosis as determined via annexin V staining (Fig. 3I, J, and K). No dramatic changes were observed with LY294002 or GDC0941 treatments alone. Collectively, these results implicate As2O3-induced activation of the PI3K/AKT pathway in altering SnoN expression and cell survival.
3.4 siRNA Targeting EGFR, MAPK1, and pp60 c-Src Alter As2O3-Mediated Cell Survival
To further examine whether pp60 c-Src (one of the SFK family members) may be involved in altering the As2O3-mediated cell death response, we assessed the effect of siRNAs targeting this specific Src family member. Additionally, we examined the effect of siRNAs against MAPK1 (targets p42 ERK1, shown to be sufficient for reducing tumor cell viability [10]) and EGFR (one of the proteins tyrosine phosphorylated upon As2O3 treatment). In the presence of As2O3 and EGFR or MAPK1 siRNA, we observed a marked reduction in pY-EGFR (Fig. 4A). Further, we observed a marked reduction in cleaved PARP with increased XIAP upon knockdown of MAPK1/EGFR; in addition, we observed increased cleaved PARP with reduction in XIAP with pp60 c-Src siRNA relative to mock or non-targeting siRNA treated cells (Fig. 4A). These results suggest that knockdown of MAPK1, EGFR, and pp60 c-Src leads to changes in cell viability via modulation of anti-apoptotic signaling mediators. Indeed, the changes in cell viability for MAPK1 and pp60 c-Src siRNA were confirmed via Annexin V/PI staining (Fig. 4B). Although reduction of EGFR and MAPK themselves does not alter SnoN expression, there was also no change with pp60 c-Src knockdown which contrasts with the PP2 results but supports the SU6656 inhibitor studies. The absence of SnoN changes with pp60 c-Src siRNA suggests that other members of the Src tyrosine kinase family (of which multiple members exist including Yes, Lyn, or Fyn) may be responsible for the changes in SnoN expression. The EGFR and MAPK pathway appear to be disconnected to the regulation of SnoN expression.
Since reduced p66 ShcA isoform is associated with increased survival [11], we next assessed whether p66 ShcA could be involved in regulating As2O3-mediated increased interaction between EGFR with Grb2. We immunoprecipitated EGFR in cells treated with non-targeting or p66 ShcA siRNA in the presence/absence of As2O3. The p66 ShcA siRNA, designed to target the CH2 homology domain, is specific to p66 (not p46/p52) (Fig. 4C). Upon As2O3 (and EGF) treatment, we noted a subtle but reproducible reduction in pY-EGFR with p66 siRNA in EGFR immunoprecipitates (Fig. 4D). Although interaction of p52 ShcA with EGFR was not disrupted, the abundance of Grb2 and EGFR was markedly reduced following p66 ShcA knockdown with As2O3. Together, these results suggest that p66 ShcA is required for the interaction of Grb2 with upon As2O3 treatment.
4. Discussion
We previously demonstrated that As2O3 induces autophagy in ovarian carcinoma cells and that increased expression of SnoN, a TGFβ co-transcriptional repressor, modulates As2O3-induced protective autophagy [6]. Since EGFR and downstream signaling pathways (PI3K/AKT) are amplified and/or dysregulated in cancers [3, 12, 13], we assessed their contribution to As2O3-induced cell death response and to regulation of SnoN expression in ovarian cancer cells. Since HEY cells are very resistant to As2O3, we assessed three independent doses of As2O3 (5, 10, and 25 µM) which elicits similar expression profiles of the assessed markers; however, we noted increased intensity of cleaved PARP and cell death with higher doses of As2O3 (cleaved PARP together with reduction in anti-apoptotic markers (i.e. Bcl-XL and XIAP) is observed weakly with 10µM As2O3). In A431 cells, a dose of 20 µM As2O3 was utilized to investigate the cellular response on the EGFR/ERK signaling cascade [14, 15]; this dose is markedly higher to doses used in the treatment of APL (~2 µM As2O3). As2O3-treated ovarian carcinoma cells show a rapid and transient AKT activation prior to pY-EGFR. We now demonstrate that the PI3K pathway transcriptionally regulates SnoN expression and the consequent cell death response (apoptosis) via the use of PI3K inhibitors (LY294002/GDC0941) and specific siRNA targeting PIK3CA (Fig. 3G and 4E). These responses are similar to TG-interacting factor (TGIF), another transcriptional TGFβ co-repressor, whose expression levels are increased post-transcriptionally as a result of activation of the PI3K/AKT pathway in HepG2 cells in response to As2O3 [16].
We observed that Src activity increased upon treatment with As2O3 (5, 10 and 25 µM) eliciting a biphasic profile (early (1–6 h) and late response (18 h)). We propose that the initial activation may be responsible for the early cytoprotective response while the later activation may be involved in eliciting the cell death response. Indeed, Src family members has been described to elicit both functionalities (pro- and anti-apoptotic responses) which may depend on the involvement of different Src family members [17]. The fate of the cell will likely depend, however, on the overall balance of survival and death inducing signals. Cell treatments performed in the presence of PP2 could markedly reduce SnoN mRNA and protein. In contrast, SU6656 and siRNA targeting pp60 c-Src did not alter SnoN levels (or pY-EGFR/p-AKT). Since this differential response may be due to effective inhibition of different Src family kinases by PP2 and SU6656, future directions would include identification of the specific Src family kinase member that os activated upon As2O3 treatment which modulates SnoN expression.
Since phosphatase activity was not altered markedly with As2O3 treatment in our cells, other mechanisms leading to EGFR activation may include Src activity which is influenced by reactive oxygen species (ROS) generated with As2O3 [18]. Interestingly, SnoN induction following As2O3 treatment could be reverted by co-treatment with an anti-oxidant, N-acetyl-L-cysteine (NAC) [6]. Thus, in response to As2O3, Src activity may be upregulated via ROS leading to increased SnoN levels. It has been reported that activation of Src leads to transactivation of the EGF receptor; in response to As2O3, EGFR can be phosphorylated at Y845 and Y1173, which are well-established c-Src phosphorylation sites [15]. In our studies, we observed that the kinetics of EGFR/p66 ShcA activation and interaction with Grb2 upon As2O3 treatment was markedly slower relative to EGF and further, was modulated differently in response to PP2 and p66 ShcA siRNA. Thus, in contrast to EGF treatment, a ligand-independent mechanism (i.e. activation of non-receptor Src tyrosine kinases) is likely to be involved under our conditions leading to receptor activation in cells treated with As2O3 [19].
Interestingly, activation of EGFR signaling cascade can also counteract the cell death response to various chemotherapeutic agents such as cisplatin [20]. These results could be explained by the level of stress induced; under moderate oxidative stress conditions, pY-EGFR elicits a pro-survival role while under severe oxidative stress, pY-EGFR leads to increased cell death [21, 22]. Although knockdown of EGFR and MAPK1 did not alter SnoN levels, they increased cell survival. Indeed, siRNA towards MAPK1 is similar to that reported to ERK1/ERK2 which suppresses cell viability in ovarian cancer cells with chemotherapeutic agents [10]. However, in contrast to cisplatin treatment where disruption of EGFR activation increases cell death [23], disruption of EGFR/MAPK signaling with As2O3 in our system leads to increased cell survival with recovery of XIAP expression. Indeed, As2O3 can induce apoptosis by preventing induction of XIAP and Bcl-XL [24] together with induction of Noxa and Bim [25]. The EGFR and MAPK pathway appears to be disconnected to the regulation of SnoN expression. The cytoprotective pathway may involve an early burst of Src activity leading to SnoN upregulation which is eventually overcome by the strength of the pro-death EGFR/Src (late burst of activity)/MAPK pathway.
Since the catalytic subunit of PI3K (PIK3CA) is located at 3q26.2, upstream of the chromosomal location of SnoN at 3q26.2, we propose that oncogenic cooperativity may occur between PI3K and SnoN in ovarian cancer chemoresponsiveness. Collectively, our results implicate the cytoprotective Src/PI3K pathway as contributing to SnoN expression; this may be a valuable pathway to target or develop new strategies for increasing the therapeutic effectiveness of As2O3 and other chemotherapeutic agents for treatment of cancer patients.
Highlights.
SnoN, a TGFβ regulator at 3q26.2, is dysregulated in ovarian cancer.
Expression of SnoN/SkiL is induced with As2O3 in ovarian cancer cells.
As2O3 activates EGFR/p66 ShcA and increases their interaction with Grb2
As2O3 activates Src and PI3K/AKT pathway to modulate SnoN expression and ovarian cancer cell survival.
Pathways of As2O3–induced SnoN induction may provide targets for cancer treatment.
Acknowledgements
This work was supported by RO1 CA123219 to MN and University of South Florida Start-up Funds to MN. This work has been supported in part by the Flow Cytometry Core Facility at the College of Medicine, University of South Florida.
Abbreviations
- As2O3
arsenic trioxide
- DMSO
dimethyl sulfoxide
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- MAPK
mitogen activated protein kinase
- p-AKT
phosphorylated protein kinase B
- PARP
poly (ADP-ribose) polymerase
- PBS
phosphate buffered saline
- PI
propidium iodide
- PI3K
phosphatidylinositol 3-kinase
- pY-EGFR
tyrosine phosphorylated EGFR
- qPCR
quantitative/real-time polymerase chain reaction
- siRNA
small inhibitory RNA
- TGFβ
transforming growth factor β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nanjundan M, Cheng KW, Zhang F, Lahad J, Kuo WL, Schmandt R, Smith-McCune K, Fishman D, Gray JW, Mills GB. Overexpression of SnoN/SkiL, amplified at the 3q26.2 locus, in ovarian cancers: a role in ovarian pathogenesis. Mol Oncol. 2008;2:164–181. doi: 10.1016/j.molonc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanjundan M, Nakayama Y, Cheng KW, Lahad J, Liu J, Lu K, Kuo WL, Smith-McCune K, Fishman D, Gray JW, Mills GB. Amplification of MDS1/EVI1 and EVI1, located in the 3q26.2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007;67:3074–3084. doi: 10.1158/0008-5472.CAN-06-2366. [DOI] [PubMed] [Google Scholar]
- 3.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 4.Westfall SD, Skinner MK. Inhibition of phosphatidylinositol 3-kinase sensitizes ovarian cancer cells to carboplatin and allows adjunct chemotherapy treatment. Mol Cancer Ther. 2005;4:1764–1771. doi: 10.1158/1535-7163.MCT-05-0192. [DOI] [PubMed] [Google Scholar]
- 5.Lallemand-Breitenbach V, Zhu J, Chen Z, de The H. Curing APL through PML/RARA degradation by As2O3. Trends in molecular medicine. 2012;18:36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell death and differentiation. 2010;17:1867–1881. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi G, Shen X, Clemmons DR. p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment. Mol Endocrinol. 2008;22:2162–2175. doi: 10.1210/me.2008-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza K, Maddock DA, Zhang Q, Chen J, Chiu C, Mehta S, Wan Y. Arsenite activation of P13K/AKT cell survival pathway is mediated by p38 in cultured human keratinocytes. Mol Med. 2001;7:767–772. [PMC free article] [PubMed] [Google Scholar]
- 9.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng P, Wagoner HA, Pescovitz OH, Steinmetz R. RNA interference (RNAi) for extracellular signal-regulated kinase 1 (ERK1) alone is sufficient to suppress cell viability in ovarian cancer cells. Cancer Biol Ther. 2005;4:961–967. doi: 10.4161/cbt.4.9.1912. [DOI] [PubMed] [Google Scholar]
- 11.Le S, Connors TJ, Maroney AC. c-Jun N-terminal kinase specifically phosphorylates p66ShcA at serine 36 in response to ultraviolet irradiation. J Biol Chem. 2001;276:48332–48336. doi: 10.1074/jbc.M106612200. [DOI] [PubMed] [Google Scholar]
- 12.Siwak DR, Carey M, Hennessy BT, Nguyen CT, McGahren Murray MJ, Nolden L, Mills GB. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. Journal of oncology. 2010;2010:568938. doi: 10.1155/2010/568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao C, Lu S, Jiang Q, Wang WJ, Song X, Kivlin R, Wallin B, Bagdasarian A, Tamakloe T, Chu WM, Marshall J, Kouttab N, Xu A, Wan Y. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830–1838. doi: 10.1016/j.cellsig.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu ZM, Huang HS. As2O3-induced c-Src/EGFR/ERK signaling is via Sp1 binding sites to stimulate p21WAF1/CIP1 expression in human epidermoid carcinoma A431 cells. Cell Signal. 2006;18:244–255. doi: 10.1016/j.cellsig.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Tseng HY, Liu ZM, Huang HS. NADPH oxidase-produced superoxide mediates EGFR transactivation by c-Src in arsenic trioxide-stimulated human keratinocytes. Archives of toxicology. 2012;86:935–945. doi: 10.1007/s00204-012-0856-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu ZM, Huang HS. Inhibitory role of TGIF in the As2O3-regulated p21 WAF1/CIP1 expression. Journal of biomedical science. 2008;15:333–342. doi: 10.1007/s11373-007-9232-9. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annual review of cell and developmental biology. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Wu S, Xing D. The reactive oxygen species-Src-Stat3 pathway provokes negative feedback inhibition of apoptosis induced by high-fluence low-power laser irradiation. The FEBS journal. 2010;277:4789–4802. doi: 10.1111/j.1742-4658.2010.07884.x. [DOI] [PubMed] [Google Scholar]
- 19.Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer. 2011;104:1241–1245. doi: 10.1038/bjc.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed SM, Salgia R. Epidermal growth factor receptor mutations and susceptibility to targeted therapy in lung cancer. Respirology. 2006;11:687–692. doi: 10.1111/j.1440-1843.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 21.Arany I. Dual role of the activated epidermal growth factor receptor in renal tubular cells during stress. Kidney Int. 2008;73:5–7. doi: 10.1038/sj.ki.5002583. [DOI] [PubMed] [Google Scholar]
- 22.Arany I, Faisal A, Nagamine Y, Safirstein RL. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. J Biol Chem. 2008;283:6110–6117. doi: 10.1074/jbc.M708799200. [DOI] [PubMed] [Google Scholar]
- 23.Benhar M, Engelberg D, Levitzki A. Cisplatin-induced activation of the EGF receptor. Oncogene. 2002;21:8723–8731. doi: 10.1038/sj.onc.1205980. [DOI] [PubMed] [Google Scholar]
- 24.Momeny M, Zakidizaji M, Ghasemi R, Dehpour AR, Rahimi-Balaei M, Abdolazimi Y, Ghavamzadeh A, Alimoghaddam K, Ghaffari SH. Arsenic trioxide induces apoptosis in NB-4, an acute promyelocytic leukemia cell line, through up-regulation of p73 via suppression of nuclear factor kappa B-mediated inhibition of p73 transcription and prevention of NF-kappaB-mediated induction of XIAP, cIAP2, BCL-XL and survivin. Med Oncol. 2010;27:833–842. doi: 10.1007/s12032-009-9294-9. [DOI] [PubMed] [Google Scholar]
- 25.Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008;111:5152–5162. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]