Abstract
Objective
The receptor for advanced glycation endproducts (RAGE) recognizes a variety of ligands that play an important role in the posttraumatic inflammatory response. However, whether soluble RAGE (sRAGE) is released early after trauma-hemorrhage in humans and whether such a release is associated with the development of an inflammatory response and coagulopathy is not known and therefore constitutes the aim of the present study.
Methods
One hundred sixty eight patients were studied as part of a prospective cohort study of severe trauma patients admitted to a single Level 1 Trauma center. Blood was drawn within 10 minutes of arrival to the Emergency Department (ED) before the administration of any fluid resuscitation. sRAGE, TNF-a, IL-6, von Willebrand Factor (vWF), Angiopoietin-2 (Ang-2), Prothrombin time, (PT), prothrombin fragments 1+2 (PF1+2), soluble thrombomodulin (sTM), protein C (PC), plasminogen activator inhibitor-1 (PAI-1), and D-Dimers (fibrin degradation products) were measured using standard techniques. Base deficit was used as a measure of tissue hypoperfusion. Measurements were compared to outcome measures obtained from the electronic medical record and trauma registry.
Results
Plasma levels of sRAGE were increased within 30 minutes after severe trauma in humans and correlated with the severity of injury, early posttraumatic coagulopathy and hyperfibrinolysis as well as with endothelial cell activation (angiopoietin-1 and complement). Furthermore, we found that there was a significant relationship between plasma levels of sRAGE and the development of acute renal failure. This relationship was not quite significant for patients who developed acute lung injury (p=.11), although patients with less than 26 ventilator-free days had significantly higher plasma levels of sRAGE than those with more than 26 ventilator-free days. Finally, there was no relationship between plasma levels of sRAGE and mortality rate in trauma patients.
Conclusions
The results of this study demonstrate that the release of sRAGE in the bloodstream of trauma patients requires severe injury and is associated with coagulation abnormalities and endothelial cell and complement activation.
Keywords: sRAGE, Trauma, Coagulopathy, Protein C, Complement
Introduction
Trauma remains the leading cause for mortality for patients between 1 and 40 years of age and eclipses cancer, heart disease and HIV/AIDS (1). While there remain a large proportion of trauma victims who die early from overwhelming injury, trauma patients who survive their initial injury do not die from their injury per se, but from an overwhelming inflammatory dysregulation leading to organ dysfunction, nosocomial infection, and ultimately multiorgan failure (2, 3). Recent studies have provided new information on the molecular mechanisms involved in this early inflammatory response. For example, the receptor for advanced glycation end-products (RAGE) is activated by several ligands, including alarmins, such as high mobility group box 1 (HMGB1) that has recently been shown to be released early after trauma (4, 5). Activation of RAGE by HMGB1 is known to initiate a rapid and sustained inflammatory response culminating in the activation of mitogen-activated protein kinases (MAPkinases), nuclear factor kappaB (NF-kB) and RhoGTPases (6).
Two carboxy-terminal truncated isoforms of RAGE, such as soluble RAGE (sRAGE) and endogenous secreted RAGE (esRAGE) have been identified in human and mice (7). However, whether sRAGE is released in the plasma early after trauma in humans is unknown and constitutes the first aim of this study. Furthermore, because we have previously described an activation of both the protein C and complement systems nearly immediately after trauma (8, 9), we also sought to define the relationships between plasma levels of sRAGE, activation of coagulation and of the protein C system and the release of other markers of inflammation, endothelial cell and complement activation early after trauma.
Materials and Methods
The Institutional Review Board of the University of California at San Francisco approved the research protocol for this prospective cohort study and granted a waiver of consent for the blood sampling as a minimal risk intervention.
Patients
Consecutive major trauma patients admitted to the San Francisco General Hospital (level 1 trauma center) were studied. All adult trauma patients who met criteria for full trauma team activation (highest triage criteria) were eligible for enrollment. Patients less than 18 years old or transferred from other hospitals were excluded. In addition, patients later found to be taking anti coagulant medications or with known pre existing coagulation abnormalities were also excluded from the study.
Sample collection and measurements
The methodology has been described previously in detail (8). Briefly, a 10 ml sample of blood was drawn in citrated tubes within 10 minutes of arrival in the emergency department. The samples were immediately transferred to the central laboratory, centrifuged and the plasma extracted and stored at −80°C. Samples were analyzed at the conclusion of the study by researchers who were blinded to all patients' data. In this study, we measured soluble RAGE in plasma (Quantikine soluble RAGE, R&D Systems Inc., Minneapolis, MN). These results were compared to plasma levels of IL-1b, IL-6, IL-10 and TNF-a (R&D Systems Inc., Minneapolis, MN), Angiopoietin-2 (Ang-2) (Quantikine Ang-2 EIA, R&D Systems Inc., Minneapolis, MN), soluble C5b-9 to assess the late phase of terminal complement activation (sC5b-9 EIA, Quidel Corp., San Diego, CA), prothrombin fragments (PF 1+2) (Enzygnost F1+2 EIA, Dade Behring, Germany), soluble thrombomodulin (sTM) (Asserachrom Thrombomodulin EIA, Diagnostica Stago Inc., Parsippany, NJ), plasminogen activator inhibitor 1 (PAI-1) (Oxford Biochemicals, Oxford MI). Protein C activity (PC) and D-Dimers (fibrin degradation products) were measured with a Stago Compact Functional Coagulation Analyzer (Diagnostica Stago Inc., Parsippany, NJ), All measurements were done in accordance with the manufacturer's instructions.
Data collection, outcome measures
Data were collected prospectively on patient demographics, injury time, mechanism (blunt or penetrating), pre-hospital fluid administration, time of arrival in the trauma room and admission vital signs. The Injury Severity Score (ISS) was used as a measure of the degree of tissue injury (10). An arterial blood gas was drawn at the same time as the research sample as part of the standard management of major trauma patients. The base deficit was used as a measure of the degree of tissue hypoperfusion. Base deficit is a clinically useful early marker of tissue hypoperfusion in trauma patients and an admission base deficit greater than 6 mmol/l has previously been identified as predictive of worse outcome in trauma patients (11, 12).
Outcome measures
Patients were followed until hospital discharge or death. For mortality analysis, patients surviving to hospital discharge were assumed to still be alive. Secondary outcome measures were also recorded for 28-day ventilator-free days, acute lung injury (American-European consensus conference definition) (13) and acute renal injury (Acute Dialysis Quality Initiative consensus conference definition) (14) and blood transfusions required in the first 24 hours.
Statistical analysis
Data analysis was performed by the investigators. Normal-quantile plots were used to test for normal distribution. Relationships between quartile of sRAGE and continuous variables were tested with the Kruskall-Wallis test followed by a non-parametric test for trend. Two-group analysis was performed using the Wilcoxon rank-sum method. Correlation was assessed by Spearman correlation coefficients. Logistic regression was used to examine the relationship between mortality and sRAGE levels. A p-value of ≤ 0.05 was chosen to represent statistical significance.
Results
Patient population
Table 1 shows the characteristics of our severely injured trauma patients enrolled in the study. We enrolled 168 consecutive traumatized patients over a 15-month period into the study. Due to short transport times from the scene of injury to our trauma center in San Francisco, the mean time from injury to blood sampling was 32 ± 6 minutes. Patients received an average of 150 ± 100 ml of intravenous crystalloid prior to blood sample collection, but did not receive any vasopressor, colloid or emergency blood prior to blood sample collection.
Table 1.
Clinical characteristics of trauma patients.
| Demographic data | |
| Age, yrs | 41(27-63) |
| Sex, Female/Male | n = 50 (30%)/n = 118 (70%) |
| Characteristics on injury | |
| Injury Severity Score | 17 (9-26) |
| Penetrating injury | n = 43 (25%) |
| Severe head injury (AIS head > 3) | n = 47 (27%) |
| Physiology | |
| Heart rate > 100/min | n = 76 (45%) |
| Systolic blood pressure <100 mmHg | n = 38 (22%) |
| Base deficit > 6 mmol/l | n = 56 (27%) |
| Blood samples | |
| Time from injury to emergency department arrival (min) | 28 (23-29) |
| Time from emergency department arrival to sample (min) | 4 (1-9) |
| Intravenous fluids prior to initial blood sample (ml) | 100 (0-200) |
Total number of patients included is n = 168. Data are presented as mean and numbers (%). Time of injury is defined as the time of pre-hospital emergency medical service activation.
Plasma levels of sRAGE correlate with ISS score in trauma patients
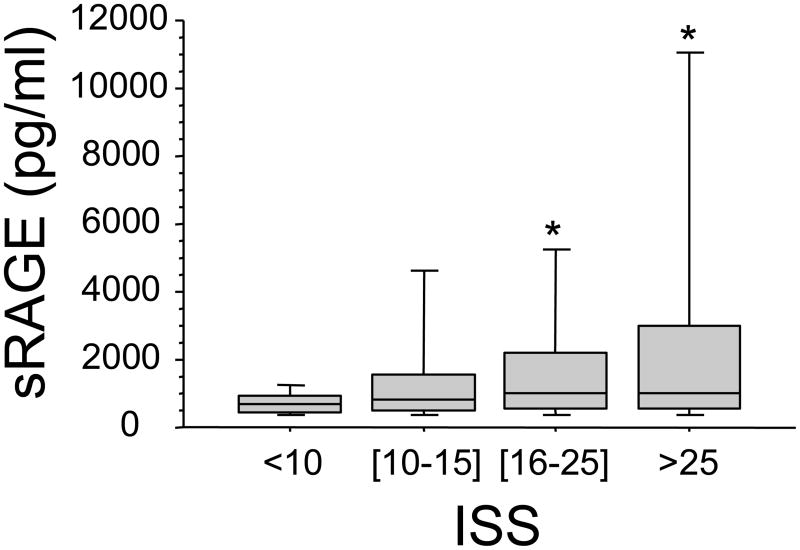
Our results indicate that plasma levels of sRAGE increased with increasing ISS (p<.001 by rank and p<.001 by trend), but not with BD (p=.11 by rank). Furthermore, there was a positive correlation between sRAGE and ISS (r=0.25 p=.0017) (Figure 1).
Figure 1. Effect of severity of injury on plasma levels of sRAGE early after trauma.
Blood samples were obtained from 168 consecutive major trauma patients immediately upon admission to the hospital. Plasma levels of sRAGE correlated with the Injury Severity Score (ISS). Data are presented in quartiles, *p ≤ 0.05 based on test for rank and trend.
Plasma levels of sRAGE and endothelial cell and complement activation in trauma patients
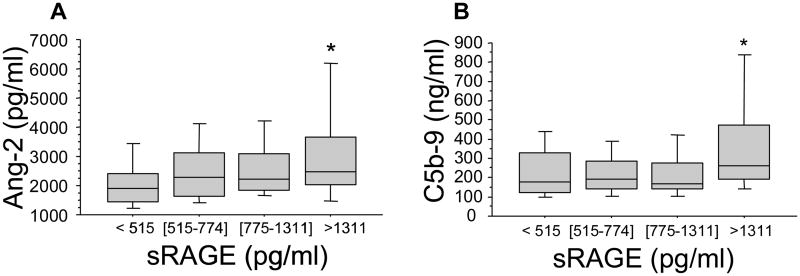
We have previously reported that there is an increase in plasma levels of makers of endothelial cell activation and inflammation (15) and an activation of complement (9) within 45 min after severe trauma in humans. Thus, we next determined the relationship between plasma levels of sRAGE and of markers of endothelial cell and complement activation and inflammation early after trauma. We found plasma levels of sRAGE increased with increasing plasma levels of Ang-2 (p=.003 by rank, but p<.0001 by trend, Spearman correlation r=.32, p<.0001) (Figure 2A). Recent experimental studies have indicated that complement appears to be the early mediators of the sterile inflammatory response associated with hemorrhagic shock (16). We thus examined whether there was a correlation between plasma levels of sRAGE and activation of complement early after trauma. The results indicate that trauma patients who had the higher plasma levels of sRAGE had significantly higher plasma levels of C5b-9 (membrane attack complex) generated as the final common pathway of complement activation (p=.003 by rank and p=.004 by trend, Spearman correlation r=.21 p=.008) (Figure 2B). In contrast, there was no significant correlation between plasma levels of sRAGE and those of pro-inflammatory mediators, such as, IL-1b, IL-6, IL-10 or TNF-a (data not shown).
Figure 2. High plasma levels of sRAGE are associated with the release of markers of endothelial cell and complement activation in trauma patients.
Blood samples were obtained from 168 consecutive major trauma patients immediately upon admission to the hospital. Panels A&B. Plasma levels of sRAGE are associated with increased plasma levels of Ang-2 and with increased complement activity, as indicated by elevated soluble C5b-9 plasma levels that are generated during the late phase of complement activation. Data are presented in quartiles, *p ≤ 0.05 based on test for rank and trend.
Plasma levels of sRAGE and early coagulation derangements in trauma patients
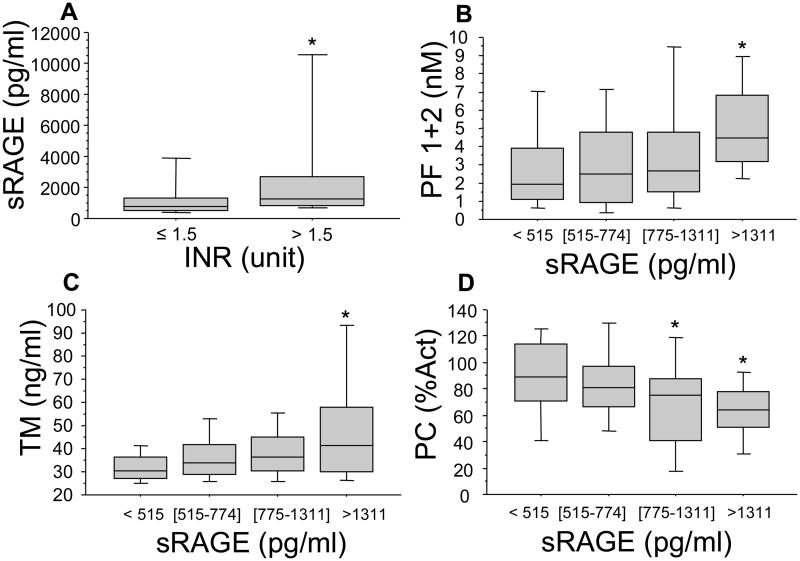
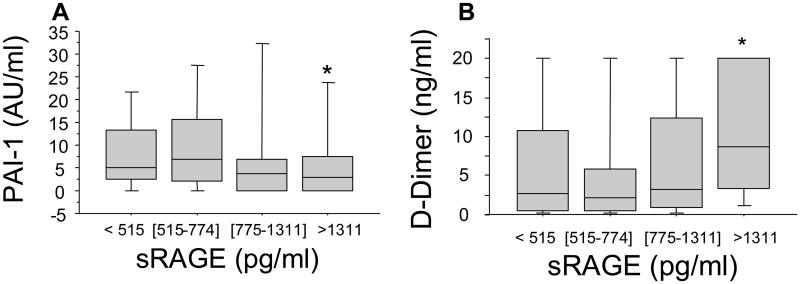
Coagulation abnormalities are common following major trauma and are directly related to worse clinical outcome (17). We have recently shown that only patients who are severely injured and in shock are coagulopathic at the admission to the Emergency Department within 45 min after injury and that the development of this coagulopathy correlates with the activation of the protein C pathway rather than with the consumption of coagulation factors (8). We next sought to identify whether the release of sRAGE in the plasma in our patients was related to coagulation abnormalities. Patients with clinically significant coagulation abnormalities (INR = international normalized ratio > 1.5) had significantly higher plasma levels of sRAGE (p<.05) (Figure 3A). Furthermore, increasing plasma levels of sRAGE were associated with increase in the plasma levels of soluble PF 1+2, a marker of thrombin generation (p=.0006 by rank and p<.0001 by trend, Spearman correlation r=.34 p<.0001), soluble thrombomodulin (p=.0007 by rank and p<.0001 by trend, Spearman correlation r=.35 p<.0001) and a fall in protein C levels (p=.002 by rank and p<.0001 by trend, Spearman correlation -.31 p=.0001) (Figure 3B,C&D). Finally, plasma levels of sRAGE were negatively correlated with those of PAI-1 (p<.05 by rank and p=.03 by trend, Spearman correlation r=-.18 p=.02), and positively correlated with D-Dimer (fibrin degradation products) levels (p=.005 by rank and p=.002 by trend, Spearman correlation r=.29 p=.0002 (Figure 4A&B), suggesting an increased fibrinolytic activity in patients with elevated plasma levels of sRAGE.
Figure 3. High plasma levels of sRAGE are associated with coagulation abnormalities in trauma patients.
Blood samples were obtained from 168 consecutive major trauma patients immediately upon admission to the hospital. Panel A. Trauma patients with coagulation abnormalities (INR = international normalized ratio > 1.5) had significantly higher levels of sRAGE. *p ≤ 0.05 from patients with INR = international normalized ratio < 1.5. Panels B&D. High plasma levels of HMGB1 were associated with coagulation derangements early after trauma that are not due to coagulation factor deficiency as shown by the rise in the levels of soluble PF 1+2, a marker of thrombin generation and soluble thrombomodulin as well as a fall in protein C levels. Data are presented in quartiles, *p ≤ 0.05 based on test for rank and trend.
Figure 4. High plasma levels of sRAGE are associated with increased fibrinolytic activity in trauma patients.
Blood samples were obtained from 168 consecutive major trauma patients immediately upon admission to the hospital. Panels A&B. High plasma levels of sRAGE are associated with increased fibrinolytic activity early after trauma, as shown by the plasma levels of PAI-1 and D-Dimers (fibrin degradation products). Data are presented in quartiles, *p ≤ 0.05 based on test for rank and trend.
Plasma levels of sRAGE and clinical outcome in trauma patients
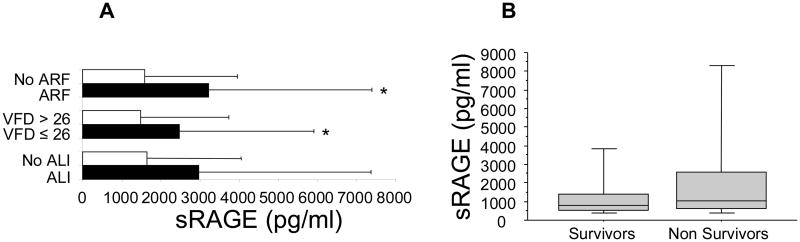
We found that there was a significant relationship between plasma levels of sRAGE and the development of acute renal failure (p<.05). This relationship was not quite significant for patients who developed acute lung injury (p=.11), although patients with less than 26 ventilator-free days had significant higher plasma levels of sRAGE than those with more than 26 ventilator-free days (p<.05). Finally, there was no relationship between plasma levels of sRAGE and mortality rate in trauma patients (p=.20).
Discussion
The results of this study demonstrate for the first time that (a) sRAGE, is released in the plasma within 30-45 min after severe trauma in humans; (b) the release of sRAGE in the plasma requires severe tissue injury and (c) is associated with posttraumatic coagulation abnormalities and endothelial cell and complement activation.
The first important finding of our study is that plasma levels of sRAGE are increased in severely traumatized patients within 30-45 minutes after injury. There was no significant fluid resuscitation or other potentially confounding treatment prior to blood sampling and, therefore, our findings represent the direct effects of the injury and shock on the release of sRAGE into the bloodstream. The soluble form of RAGE is devoid of the transmembrane and cytosolic domains and arises from two sources: proteolysis of the full-length receptor and alternative splicing. Recent studies have reported that RAGE is subjected to protein ectodomain shedding by metalloproteases (18, 19). The second form of soluble RAGE, named endogenous secretory RAGE (esRAGE), is formed by alternative splicing of RAGE mRNA (20). The plasma levels of esRAGE are fourfold lower that sRAGE released by proteolysis, but might be expected to reflect levels of receptor expression in a more direct way than sRAGE (7). As RAGE is activated by multiple ligands, including HMGB1, it is likely that the major function of RAGE is to propagate cellular inflammation and dysfunction due to sustained ligand-receptor interaction at sites of ligand deposition. However, soluble RAGE takes on a dominant-negative phenotype and blocks signaling of RAGE and other receptors activated by the multiple ligands of RAGE, as it has been reported in an experimental model of E. coli-mediated acute lung injury (21).
In our trauma patient population, sRAGE was elevated early after trauma and before any fluid resuscitation. Furthermore, sRAGE correlated with the severity of injury and the plasma levels of an important mediator of endothelial activation (angiopoietin-2) and complement that are both known to increase vascular permeability (22, 23). These results are not surprising, as a previous study has demonstrated the critical role of RAGE signaling in an experimental model of intestinal barrier dysfunction after hemorrhagic shock (24). As we and other investigators have reported that plasma levels of Ang-2, complement and HMGB1 are elevated early after severe trauma (4, 9, 15), the increase in plasma levels of sRAGE early after trauma may be interpreted as both a marker of cellular damage as well as a compensatory mechanism to modulate the severity of the inflammatory response observed after severe trauma in humans.
The second important finding of our study is that plasma levels of sRAGE correlate with coagulation abnormalities observed after severe trauma and hypovolemic shock. We found that increasing plasma levels of sRAGE correlated with increasing plasma levels of PF 1+2, a marker of thrombin activation, increasing plasma levels soluble thrombomodulin and decreasing plasma levels of protein C. Furthermore, there was a correlation between plasma levels of sRAGE and activation of fibrinolysis early after trauma. There are several potential explanations for these correlations. First, the activation of RAGE signaling by advanced glycation end-products has been shown in monocytes to increase the expression of tissue factor, a major mediator of the initiation of coagulation after tissue injury (25), thus suggesting that the activation of RAGE signaling may contribute to the activation of coagulation in the microcirculation after trauma. Second, it has recently been reported that HMGB1, an important ligand of RAGE, is released in the plasma early after trauma (4). It is known that the activation of RAGE signaling by its ligands induces a positive feedback on the expression of the receptor at the cell membrane (26). Furthermore, HMGB1 has been shown to cause fibrin deposition and activation of the coagulation in healthy rat (27). Taken together, these results indicate that RAGE and its ligands may contribute to the activation of the coagulation in the microcirculation early after trauma.
Do plasma levels RAGE measured early after trauma correlate with the later development of organ injury and outcome? Our results clearly show that there is no correlation between mortality and the plasma levels of sRAGE early after severe trauma. A previous study has reported that plasma levels of sRAGE are significantly more elevated in septic patients who ultimately died than in survivors (28). The significance of plasma levels of sRAGE measured in trauma or septic patients is still uncertain. sRAGE levels may represent a marker of cellular damage as well as be part of a counterregulatory mechanism that modulates the severity of the posttraumatic or septic inflammatory response. Indeed it is possible that the role of sRAGE may change from a pro-inflammatory one (reflecting ligand signaling and subsequent solubilization) to an anti-inflammatory decoy effect over time after a traumatic or septic insult. Another possible reason that might explain the difference between both studies is likely related to the fact that we sampled the blood of our trauma patients within 45 min after injury while the plasma levels of sRAGE were measured later in septic patients within 24 hours after diagnosis of sepsis (4). Indeed it is possible that our blood sampling was performed too early to detect the maximum plasma level of sRAGE after severe trauma.
Despite a lack of correlation between sRAGE levels and mortality, we found a significant correlation between plasma levels of sRAGE and the later development of acute renal failure in severely traumatized patients. Several previously published studies have reported the association between an increase in plasma levels of sRAGE and compromised renal function (29). One hypothesis is that renal insufficiency can suppress handling and excretion of sRAGE, although the major site(s) where sRAGE is processed is currently unknown. However, the time-course of change in the renal function early after trauma using specific markers of the renal function has not be studied. Thus, it is possible that there is an early decline of the renal function after severe trauma that would explain the correlation between increase in plasma levels of sRAGE and the later diagnosis of renal dysfunction.
In summary, the results of this study demonstrate for the first time that sRAGE is released into the bloodstream early after severe trauma in humans. The release of sRAGE requires severe injury and is associated with posttraumatic coagulation abnormalities and endothelial cell and complement activation. Furthermore, we found that there was a significant relationship between plasma levels of sRAGE and the development of acute renal failure. In contrast, this relationship was not quite significant for patients who developed acute lung injury, although patients with less than 26 ventilator-free days had significantly higher plasma levels of sRAGE than those with more than 26 ventilator-free days. Finally, there was no relationship between plasma levels of sRAGE and mortality rate in trauma patients. Thus, future studies will be needed to determine the role of plasma levels of sRAGE in modulating the inflammatory response after severe trauma.
Figure 5. High plasma levels of sRAGE are associated with less ventilator-free days and acute renal failure in trauma patients.
Panel A. Trauma patients with less ventilator free days (VFD) and acute renal failure (ARF) had significantly higher plasma levels of sRAGE. Data are mean ± SD; *p ≤ 0.05 compared to patients without organ injury or with more than 26 VFD. Panel B. There was no significant relationship between plasma levels of sRAGE and mortality rate after severe trauma. Data are mean ± SD.
Acknowledgments
Supported in Part by NIH K08 GM-085689 (MJC), NIH RO1 GM-62188 (JFP), NIH K23 HL090833 (CC) and AAST Hemostasis and Resuscitation Scholarship (MJC).
Footnotes
Institution at which work was performed: The Departments of Surgery and Anesthesia at San Francisco General Hospital, University of California San Francisco, CA.
Contributor Information
Mitchell J. Cohen, The Department of Surgery, University of California San Francisco, CA.
Michel Carles, The Department of Anesthesia, University of California San Francisco, CA.
Karim Brohi, The Royal London Hospital, London, UK.
Carolyn S. Calfee, The Departments of Medicine and Anesthesia, University of California San Francisco, CA.
Pamela Rahn, The Department of Surgery, University of California San Francisco, CA.
Mariah S Call, The Department of Anesthesia, University of California San Francisco, CA.
Brian B. Chesebro, The Department of Anesthesia, University of California San Francisco, CA.
Michael A. West, The Department of Surgery, University of California San Francisco, CA.
Jean-François Pittet, The Departments of Surgery and Anesthesia, University of California San Francisco, CA.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. discussion 510-502. [DOI] [PubMed] [Google Scholar]
- 3.Stahel PF, Smith WR, Moore EE. Role of biological modifiers regulating the immune response after trauma. Injury. 2007;38:1409–1422. doi: 10.1016/j.injury.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Peltz ED, Moore EE, Eckels PC, et al. Hmgb1 Is Markedly Elevated within Six Hours of Mechanical Trauma in Humans. Shock. 2008 doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bopp C, Bierhaus A, Hofer S, et al. Bench-to-bedside review: The inflammation-perpetuating pattern-recognition receptor RAGE as a therapeutic target in sepsis. Crit Care. 2008;12:201. doi: 10.1186/cc6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 7.Kalea AZ, Schmidt AM, Hudson BI. RAGE: a novel biological and genetic marker for vascular disease. Clin Sci (Lond) 2009;116:621–637. doi: 10.1042/CS20080494. [DOI] [PubMed] [Google Scholar]
- 8.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganter MT, Brohi K, Cohen MJ, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 10.Baker SP, O'Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 11.Davis JW, Parks SN, Kaups KL, et al. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. 1996;41:769–774. doi: 10.1097/00005373-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rutherford EJ, Morris JA, Jr, Reed GW, et al. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 16.Fink MP. Bench-to-bedside review: High-mobility group box 1 and critical illness. Crit Care. 2007;11:229. doi: 10.1186/cc6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 18.Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Bukulin M, Kojro E, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008;283:35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- 20.Malherbe P, Richards JG, Gaillard H, et al. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res. 1999;71:159–170. doi: 10.1016/s0169-328x(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Tasaka S, Shiraishi Y, et al. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med. 2008;178:356–362. doi: 10.1164/rccm.200707-1069OC. [DOI] [PubMed] [Google Scholar]
- 22.Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 23.Lister KJ, James WG, Hickey MJ. Immune complexes mediate rapid alterations in microvascular permeability: roles for neutrophils, complement, and platelets. Microcirculation. 2007;14:709–722. doi: 10.1080/10739680701404879. [DOI] [PubMed] [Google Scholar]
- 24.Raman KG, Sappington PL, Yang R, et al. The role of RAGE in the pathogenesis of intestinal barrier dysfunction after hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2006;291:G556–565. doi: 10.1152/ajpgi.00055.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa K, Yoshinari M, Iwase M, et al. Advanced glycosylation end products induced tissue factor expression in human monocyte-like U937 cells and increased tissue factor expression in monocytes from diabetic patients. Atherosclerosis. 1998;136:281–287. doi: 10.1016/s0021-9150(97)00221-9. [DOI] [PubMed] [Google Scholar]
- 26.Herold K, Moser B, Chen Y, et al. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J Leukoc Biol. 2007;82:204–212. doi: 10.1189/jlb.1206751. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Kawahara K, Nakamura T, et al. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J Thromb Haemost. 2007;5:109–116. doi: 10.1111/j.1538-7836.2006.02255.x. [DOI] [PubMed] [Google Scholar]
- 28.Bopp C, Hofer S, Weitz J, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147:79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Kalousova M, Hodkova M, Kazderova M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–411. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]