Abstract
It is increasingly recognized that the non-neoplastic stromal compartment in most solid cancers plays an active role in tumor proliferation, invasion and metastasis. Cancer associated fibroblasts (CAFs) are one of the most abundant cell types in the tumor stroma, and these cells are pro-tumorigenic. Evidence that CAFs are epigenetically and possibly also genetically distinct from normal fibroblasts is beginning to define these cells as potential targets of anti-cancer therapy. Here, we review the cell of origin and molecular biology of CAFs, arguing that such knowledge provides a rational basis for designing therapeutic strategies to coordinately and synergistically target both the stromal and malignant epithelial component of human cancers.
Introduction
The original concept of cancer as a non-healing wound has been dramatically extended by recent research on the cancer stroma. Although the mesenchymal proliferation that surrounds carcinoma cells shares some features with the proliferative fibroblastic reactions seen in benign ulcers and wounds, accumulating evidence suggests that cancer-associated fibroblasts (CAFs) are a special cell type that actively contributes to tumor growth and malignant behavior. A fast moving and sometimes controversial area of investigation concerns the origins of CAFs and the genetic and epigenetic changes that may account for the tumor-promoting phenotype of these cells. Here we argue that research on the molecular biology of CAFs is beginning to suggest approaches for targeting these pro-tumorigenic stromal cells in anti-cancer therapy.
The phenotype of CAFs
In addition to their plump spindle-shaped mesenchymal appearance, several specific molecular markers have been used to define CAFs in tissue sections. The terms ‘peritumoral fibroblast’, ‘cancer associated fibroblast’ and ‘cancer associated myofibroblast’ are often used interchangeably (1). The presence of mesenchymal markers (alpha-smooth muscle actin - ASMA, vimentin, paladin 4Ig, podoplanin) and absence of epithelial (cytokeratin), endothelial (CD31) and fully differentiated smooth muscle (smoothelin) markers have been used to define myofibroblasts (both tumor-associated and non-tumor associated), but there may be a population of morphologically similar cells in the peri-tumor microenvironment that does not express all these markers yet shares certain functional and lineage traits with CAFs (2, 3). In addition none of these markers by themselves are unique to CAFs. In this review we use the term CAFs and include under this term cells that are found in the tumor stroma, are morphologically fibroblast-like, express ASMA, and are negative for epithelial markers such as cytokeratin and E-cadherin.
Origin and function of myofibroblasts in tissue repair and cancer
In wound healing, the differentiation of local precursor cells into myofibroblasts results in a new phenotype with enhanced contractile and secretory abilities, that allows mobility of these cells for wound contraction (hence the importance of ASMA expression) and synthesis and deposition of extracellular matrix (ECM) (3). Among the important signals driving this transition are local cytokines and inflammatory mediators (4). While it is believed that myofibroblastic transdifferentiation of resident fibroblasts is numerically most important at many tissue sites of injury, there is also evidence that a smaller subpopulation of myofibroblasts is recruited from the bone marrow. Although engraftment of bone-marrow derived cells (BMDCs) was seen in tissue stroma even in the absence of injury (5), it has been shown that the percentage of BMDCs among ASMA positive cells increases after injury. It appears that there may be tissue specific differences in the degree to which BMDCs contribute to the stroma in wound healing. In one early study, which examined this question in the mouse small intestine and colon by a protocol of male bone marrow transplantation in females followed by Y-chromosome in the recipients, 40–60% of pericryptal ASMA positive cells after radiation injury were found to be bone-marrow derived (6). The numbers have been somewhat lower, though still significant, in injury paradigms with transplantation of tagged bone marrow cells followed by examination of the lung, kidney and pancreas (5). Although in certain tissues (stomach) BMDCs have been shown to contribute significantly to both epithelial (7) and stromal cells after injury, in other tissue (pancreas), the majority of BMDCs were seen only in the reactive stroma (8).
In terms of cell numbers, data from mouse models of cancer suggest that in some situations, such as gastric cancer, bone marrow derived mesenchymal cells may contribute as much as 15–25% to the CAF population (9, 10). Bone marrow derived stem cells are divided into hematopoetic stem cells and mesenchymal stem cells (MSCs). The latter group makes a contribution, both in health and disease, to connective tissue like bone, cartilage, muscle and adipose tissue (11). After early development it appears to be chronic inflammation and tissue remodeling that drives these cells to target tissues. Thus, the recruitment of BMDCs to cancer or precancerous tissue is another and perhaps newest evidence of the similarity between benign and malignant wound healing (12, 13). Similar to the ability of cancer cells to transform resident fibroblasts or myofibroblasts to tumor-promoting CAFs, it has been shown that cancer-derived soluble factors (conditioned media or malignant ascites) and direct co-culture with carcinoma cells can transform mesenchymal stem cells to a CAF phenotype (14, 15).
From experiments in transgenic mouse models it also appears that some CAFs can arise locally from an endothelial-mesenchymal transformation at the invasive edge of the cancer (16). A related possibility is that at least a sub-population of these cells is derived from epithelial cells via epithelial-mesenchymal transition (EMT). There is evidence that in vitro epithelial cells can transdifferentiate into myofibroblasts (17) and that in fibrosing diseases of the lung and kidney several cells with myofibroblast markers also share epithelial markers. In a TGF-β1 induced model of lung fibrosis using β-gal tagged epithelial cells it was concluded that the majority of myofibroblasts were epithelial derived (18). Nonetheless, this field of research remains incomplete. So far, it has been more exciting to probe the contributions of non-resident cells, but it will be interesting to see future experiments directly evaluating the more mundane contribution of tissue-resident fibroblasts to populations of CAFs in various tumor models.
PDGF (platelet derived growth factor) and TNF-α are examples of cytokines that induce proliferation of resident fibroblasts, but they are not sufficient to induce expression of ASMA. TGF-β1 has been shown to induce differentiation to myofibroblasts (19, 20) but it appears that this process is dependent on the expression ECM proteins, including ED-A fibronectin (21). In addition, expression of other proteins involved in cell adhesion (vinculin, paxillin, tensin) is increased (2). TGF-β1 controls both fibronectin production and signalling, as it also induces the focal adhesion-associated kinase FAK, which is important for integrin signalling. Inhibition of FAK signalling inhibited the ability of TGF-β1 to mediate ASMA production and myofibroblastic differentiation (22). Secretion of ECM proteins and matrix metalloproteases (MMPs) also increases, leading to restructuring of the ECM. The source of TGF-β1 in tumors may not only be the epithelial cells but also macrophages and other cells in the tumor microenvironment. Another important regulator of transdifferentiation is endothelin, which is produced by the tumor neo-vasculature (23).
The term CAF in the broadest sense refers simply to myofibroblasts that are found physically associated with carcinoma cells, but this term is also appropriately used with the more specific functional connotation of “tumor promoting cancer-derived myofibroblasts” (24, 25). In addition to the circumstantial evidence that these cells constitute a substantial volume of the tumor mass, (in certain epithelial tumors such as pancreatic, gastric and breast cancers as much as 50–70% (1)) there are now multiple lines of functional evidence supporting an active role for CAFs in tumor formation. In particular, experiments in multiple laboratories have shown that: (i) CAFs are synergistic with epithelial cell and accelerate carcinogenesis in xenograft models; and (ii) CAFs can influence the invasiveness and metastatic pattern of tumors. The evidence for synergies between CAFs and malignant epithelial cells in tumor initiation, proliferation and invasion has been reported in cell mixing studies with tumor xenografting, and in other in vivo studies. One of the earliest observations was that polyoma virus induced transformation of epithelial cells into malignant neoplastic cells (that is, carcinoma cells) occurred only when these cells were co-cultured with induced mesenchymal cells, but not when they were cultured alone (26). In the prostate, CAFs from human tumors were able to induce transformation of non-tumorigenic prostatic epithelial cell lines (SV40T-expressing, immortalized but non tumorigenic BPH-1 cell lines) while normal prostate fibroblasts were not. While non-tumorigenic fibroblasts may play a role in enhancing proliferation of malignant and pre-malignant epithelial cells, they are not able to initiate the malignant transformation of epithelial cells de novo (27). Furthermore, co-culturing CAFs with induced epithelial cells resulted in a tumorigenic epithelial cell phenotype that persisted even in the absence of CAFs (24). In addition to irradiation of stromal fibroblasts, which has been shown to enhance the tumor promoting ability of CAFs in some cancers (28), senescence and an inflammatory microenvironment may also contribute to this functional CAF phenotype (29, 30).
Transformation or expansion of local mesenchymal cells occurs under the influence of cytokines and as noted above several other precursors of CAFs have been demonstrated or postulated, including smooth muscle cells, endothelial cells, fibroblasts, stellate cells (in the liver) or adipocytes via transdifferentiation, and even epithelial cells via EMT. In the gastrointestinal tract myofibroblasts are already present in low but easily detectable numbers in the mucosa, and histopathology suggests that phenotypically similar (ASMA-positive) cells of the thin muscularis mucosae layer may also proliferate to generate CAFs in gastrointestinal cancers as the malignant epithelial cells invade and breach this layer. In-vitro studies have demonstrated that cancer epithelial cells can induce proliferation and altered gene expression in naive fibroblasts (31). The acquisition of myofibroblastic properties by naive fibroblasts (such as following treatment with TGF-β) increased the ability of these cells to synergize with cancer cells and promote invasion. However, in side-by-side comparisons CAFs treated with TGF-β, compared to non-tumor-associated myofibroblasts treated with this same cytokine, were much more efficient in promoting invasive behavior of malignant epithelial cells (32). Importantly, the tumor promoting phenotype can persist even when CAFs are passaged in culture without continuous exposure to cancer cells (33).
Taken together these observations suggest that an as yet poorly defined process of “activation” of fibroblasts is essential for a pro-carcinogenic role. An important question is whether myofibroblast precursor cells require innate (genetic or epigenetic) changes to fully transform into cancer-promoting myofibroblasts (i.e. functional CAFs), or alternatively whether paracrine signals of the tumor microenvironment (factors secreted by tumor epithelial cells or other TME components) are chiefly responsible for inducing this phenotypic transformation. There is now a growing body of data suggesting that while the latter is quite important, there are indeed cell-autonomous and metastable differences between CAFs and normal or reactive (non-cancer-associated) myofibroblasts. One possibility is that these cells are of different origin and lineage than locally activated myofibroblasts, while the other is that they are of the same lineage but acquire specific genetic and/or epigenetic changes. As should be evident from the preceding discussion on the multiple origins of CAFs, these 2 possibilities are not mutually exclusive.
While a major premise of this review is that restructuring of the microenvironment often promotes tumor progression, for completeness it is important to recognize that in some special circumstances excess stroma might inhibit tumor invasion. Notably, clinical observations have shown that certain fibrotic conditions may provide a less appropriate “soil” for cancer cells, e.g. in cirrhosis the chance of seeding of the liver by metastatic colon cancer is decreased (34). On the other hand, an activated stroma, such as a non-malignant wound, supports a far more invasive phenotype then implantation into normal tissue (35). The protective ability of fibroblasts appears to decrease even as non neoplastic wound healing begins. While not itself carcinogenic, the transition from fibroblast to reactive myofibroblast in non-neoplastic conditions does seem to create a microenvironment supportive of tumor formation. While some of the mediators of this transition, as described above, are similar in benign and malignant wounds (PDGF, TGF-β), in cancers they are often tumor cell derived. In a sense this may explain why reactive myofibroblasts in a normal wound are transient, while CAFs persist in cancers. Interestingly, Bhowmick et al have shown that the same TGF-β that supports CAF proliferation serves as a mediator of fibroblast derived tumor-suppressive function. In their experiment the TGF-β2 receptor was selectively inactivated in fibroblasts, and they found that the animals developed stromal proliferation and epithelial neoplasia in the prostate and the foregut (36). This would suggest that TGF-β signaling in stroma plays a role in the suppression of epithelial transformation, and that myofibroblasts may need to acquire additional defects (such as TβRII mutation or comparable epigenetic lesions) to permit and accelerate epithelial carcinogenesis.
Lastly regarding the origins of mesenchymal stromal cells in cancer, it is important to note that while this review focuses largely on epithelial cancers (carcinomas) the roles of mesenchymal stromal cells are also being intensively investigated in hematopoietic cancers, such as leukemias and multiple myeloma (MM), which arise in the bone marrow. In this special location mesenchymal cells can support hematopoietic stem cells, and treatment of mice with the proteasome inhibitor Bortezomib, which is an effective drug against MM, led to differentiation of mesenchymal stromal cells to normal bone forming osteoblasts, suggesting that this drug may have a side benefit of restoring bone formation in patients with this aggressive osteolytic cancer (37).
Genetic alterations in CAFs
Is the acquisition of tumor promoting activities by CAFs due to genomic alterations? Accumulating evidence from in-vitro and in-vivo studies suggests that CAFs may have a metastable pro-tumorigenic phenotype at least in part because of genetic and/or epigenetic alterations of their DNA. An alternative though not mutually exclusive explanation would require continued autocrine or paracrine stimulation to sustain their phenotype in the absence of genomic alterations. Clearly stating that a given cell type has an epigenetic or genetic abnormality requires a comparison with the presumed normal counterpart or precursor of that cell, so this area of research has to proceed hand in hand with ongoing studies on the cell-of-origin of CAFs – an issue that is not yet fully resolved for any organ or tumor type. In reviewing the evidence for both genetic and epigenetic changes in CAFs we particularly emphasize studies that have demonstrated these differences when comparing CAFs in the tumor stroma to myofibroblasts in reactive non-cancer-associated stroma of the same organ. Nonetheless, knowledge of the genomic and epigenomic status of CAFs may translate into new therapeutic strategies even before the origin of these cells is precisely determined.
A technical hurdle in studies of the tumor microenvironment is the need to dissociate the relevant tissues into defined cell populations. Laser capture microdissection (LCM) allows remarkable precision in isolating tissue components, but it introduces several potential confounders. Some studies have used basic cell morphology to distinguish tumor cells from CAFs, but various cell populations other then CAFs - notably invasive epithelial cells that have undergone an EMT and pericytes/endothelial cells - can take on a plump spindle shape, and this may influence findings. The geographic relationship of the CAFs to the epithelial tumor cells may also be important as some epigenetic changes may vary between “peri-tumoral” stroma immediately abutting the nests and glands of malignant epithelial cells and “intratumoral” stroma farther away from the epithelial component (38). In addition, artifact may be introduced after LCM and DNA amplification from formalin-fixed paraffin-embedded (FFPE) tissue sections; as the PCR can require extra cycles the danger of PCR contamination is increased and this can potentially lead to false positive results in molecular studies (39). No single method is perfect, as cell sorting methods that rely on a defined cell population may be too exclusive (40) and primary cultures of myofibroblasts from tissue explants may be affected by clonal selection (41).
These limitations are especially important as some findings regarding the presence or absence of genetic alterations (mutations, loss of heterozygosity - LOH) in CAFs have differed between studies. Analyses in breast (42), ovarian (43), prostate (44), bladder (45). head and neck (46), colon (47) pancreatic (48) and other cancers have identified the possibility that LOH and genetic mutations may exist in the tumor stroma and that some mutations in a given tumor are in fact unique to the stromal component. All of these studies have utilized FFPE sections and amplified DNA. Two studies from the same group have also suggested that certain stroma specific LOH in breast and head and neck cancer may be predictive of nodal metastasis (42, 46). However, contrary findings and counterarguments have arisen – for example, transgenic animal models show that mutations in epithelial cells are sufficient for both carcinogenesis and desmoplasia. More directly, studies that have used cell sorting (40) and primary culture of stroma did not confirm a significant number of genetic mutations or LOH in the stromal component of tumors (41, 49). In several recent studies aimed to confirm the presence of p53 mutations in cancer-associated stromal cells no mutations were found (50, 51). At this point it is certainly possible, in fact likely, that a population of cells in the tumor associated stroma acquires novel mutations, but it is not yet evident whether these events are sufficient or necessary for clonal expansion and acquisition of the CAF phenotype.
Epigenetic alterations in CAFs
Epigenetic changes, that is, somatically heritable changes that are not due to DNA sequence alterations, make a major contribution to cancer initiation and progression and in some instances, such as several types of childhood cancers, clearly precede genetic changes in the earliest phase of tumor initiation (52). The main current paradigm in cancer epigenetics is that the malignant cells undergo a global reduction in genomic methylation and also acquire site-specific gains of methylation in the promoter regions of some loci, including tumor suppressor genes. These aspects, and the availability of well tolerated drugs that induce DNA demethylation, make epigenetic alterations attractive markers and targets for detecting and treating cancer. Relevant to this review, recent findings are beginning to show that epigenetic changes can provide a potential mechanism for CAFs to acquire their novel phenotype.
Gene specific methylation patterns are potentially very important in distinguishing CAFs from non-cancer-associated myofibroblasts. In breast cancer, using immunomagnetic purification and methylation-specific digital karyotyping, methylation differences were identified between normal stroma and tumor stroma and were found to be distinct between myofibroblasts, myoepitheial cells and epithelial cells (49). These findings have been broadly consistent with observations by tissue microdissection followed by methylation-sensitive Pyrosequencing in prostate cancer, where tissue compartment-specific DNA methylation patterns were observed in tumor epithelium, tumor-associated stroma and non-tumor stroma (25, 38). Another study also indicated compartment-specific DNA methylation in prostate cancer (53). In that study the extent of promoter hypermethylation of the GSTP1 gene differed in tumor stroma that was sampled in the center of the tumor compared to at the periphery. An interesting twist in this area is the recent demonstration that CAFs from breast cancers can interact with breast epithelial cells to induce hypermethylation of a putative tumor suppressor gene, cystatin M (CST6) in the epithelial cells (54).
Research linking gains or losses of DNA methylation in stromal cells to specific functional aspects of these cells is still in its very early days and much more work needs to be done. However, from cell line studies it already appears that some potentially relevant genes, such as the stromelysin MMP-3, can be activated by hypomethylation (55). Arguably a more comprehensive list of epigenetically regulated genes is needed before functional correlations can be done. With the exception of the papers on prostate cancer and breast cancer stroma cited above, little information is available so far on gene specific methylation in stromal cells – in this regard new optimized methods for bisulfite sequencing from FFPE tissues may be helpful (56).
In addition to the challenges in characterizing gene-specific DNA methylation in stromal cells, global epigenetic alterations are also of considerable interest. Recently, our lab found that CAFs in human gastric carcinomas (GCAs) and in a mouse model of GCA have globally reduced DNA methylation, compared to normal gastric myofibroblasts (57). The evidence for this phenomenon, which broadly parallels the well documented global loss of methylation in cancerous epithelial cells, is as follows:
Microarray analysis (MSNP) of CpG methylation in DNA from short-term cultures of gastric myofibroblasts isolated from within and distant from (gastrectomy margins) human primary gastric cancers.
Measurement of global CpG methylation in these same DNA samples by a cytosine incorporation (HpaII fill-in) assay.
Immunohistochemistry (IHC) and immunofluorescence (IF) microscopy, on tissue sections of human primary GCAs double-stained for methyl-cytosine (5meC) and alpha smooth muscle actin (ASMA).
IHC and double IF for 5meC/ASMA on tissue sections from an IL1-β transgenic mouse model of progressive gastric epithelial dysplasia and GCA.
Capitalizing on the ability of IHC for 5-meC to yield data at single cell resolution in native tissue sections, we were able to appreciate that while hypomethylation is a feature of both the tumor epithelium and the surrounding CAFs, it largely spares the tumor infiltrating leukocytes. In addition, the findings in the IL1-β and hypergastrinemic (INS GAS) transgenic (Figure 1.) mice suggested that genomic hypomethylation in CAFs, at least in this model, may occur at an even earlier stage than epithelial demethylation. As a control, we showed in the supplementary data that the decrease in 5-meC staining is not seen in the fibroblastic stromal proliferation of healing benign gastric ulcers from human surgical specimens (57). The cause of global demethylation is not known, but we can speculate that a combination of local methyl donor deficiency and insufficient methyltransferase enzyme activity in the context of continuous cellular proliferation may play a role. DNMT1 is the key methyltransferase enzyme responsible for maintaining CpG methylation. Although we did not find a significant difference in DNMT1 mRNA expression between cancer and normal tissue derived myofibroblasts, in our preliminary data gastric, colon and pancreatic cancer show less immunoreactive DNMT1 in tumor stroma compared to tumor epithelium (Figure 2). DNMT1 expression detected by IHC is low in the non tumor associated stroma as well, suggesting that baseline expression of DNMT1 may be generally lower in this cell lineage.
Fig. 1. Anti–5-methyl-C immunohistochemistry showing loss of CpG methylation in myofibroblasts in a mouse model of gastric carcinoma.
These transgenic hypergastrinemic mice (INS GAS) on an FVB background develop gastric carcinoma in situ after infection with Helicobacter felis. Top (A,C) and bottom (B,D), wild type (FVB) and INS GAS + H. felis infected stomach sections stained for anti-αSMA and anti-5-methylcytosine (anti-5meC) in serial sections. The αSMA-positive spindle-shaped myofibroblasts of the normal muscularis mucosae, as well as the few αSMA-positive intraepithelial myofibroblasts, showed uniformly strong staining in multiple sections examined. In contrast, in a lesion with carcinoma-in-situ, the anti-5-methylcytosine staining of the more abundant αSMA-positive cells is significantly reduced (arrows).
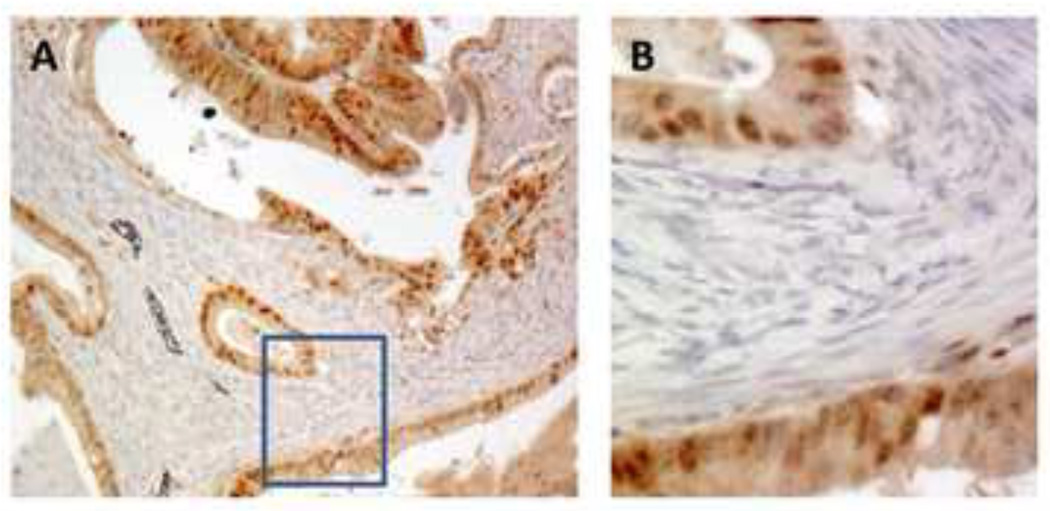
Fig. 2. Anti-DNMT1 immunohistochemistry in human intestinal type gastric cancer.
A. 10×; B. 40×. Almost all DNMT1 positive cells are seen in the tumor epithelium and not in the stroma. Thus while DNMT1 mRNA expression can be detected in the stromal cells by sensitive RT-PCR, the protein levels appear much reduced relative to the levels in the malignant epithelial cells. On serial sections the stromal cells were strongly αSMA positive (not shown).
The mechanism by which global hypomethylation may contribute to the CAF phenotype, if indeed it does, is also not yet known. Studies have found that cancers with a low global methylation index may have higher genomic instability (58, 59), however in our analysis by MSNP we did not observe LOH or copy number aberrations in the CAFs, which appeared to be fully diploid, with no evidence of chromosomal instability. Alternatively, global hypomethylation may lead to transcriptional dysregulation. Recent studies have demonstrated that several key stromal genes are epigenetically regulated and many of those expressed in stroma are hypomethylated in cancer (MMP3, 9 and 10) (41, 55, 60). Of course a third hypothetical possibility is that rather than being a functional pathway, global hypomethylation may simply be a passive result of local conditions of methyl donor deficiency, surrounding inflammatory signals, or tumor hypoxia (61, 62). As we discuss in the next section, regardless of the precise biological role of global hypomethylation, it may be possible to take advantage of this phenomenon in therapeutic strategies to inhibit the function of CAFs.
CAFs as potential targets of anti-cancer therapy
Several aspects of the biology of CAFs suggest that targeting these cells (in conjunction with the malignant epithelial cells) may be helpful in making anti-cancer therapy more effective:
CAFs can support tumor epithelial proliferation, angiogenesis and invasion;
Even in the absence of genetic alterations, epigenetic and gene expression differences between epithelium and normal and cancer-associated stroma can point to novel therapeutic targets;
De novo acquisition of genetic mutations may be less common in stromal cells than in malignant epithelial cells, so CAFs may be less prone to escape or resistance to therapy via genomic instability;
Current cancer therapy (both chemo- and radiation therapy) often results in residual fibrosis and it has been pointed out recently that adjuvant therapy may need to target this fibrosis as well as residual tumor embedded within it (63);
CAF derived factors may interfere with anti-cancer therapies (64);
CAF derived factors can contribute to the recruitment of BMDCs to tumors (33);
Tumor stroma may prevent effective immune surveillance or anti-tumor immune response (65);
A negative correlation may exist between the magnitude of the desmoplastic stromal reaction and survival in certain cancers (66).
In this discussion we approach stroma-targeted therapy from two angles: therapies that are directed at CAFs or the epithelial-CAF interaction (Table 1), and therapies that likely affect both epithelium and stroma but may exert their effect at least in part on the stroma. The synergistic relationship between stroma and epithelium suggested a priori that stroma-targeted intervention may have a synergistic role in primary cancer therapy. However, the wound healing or fibrosis that follows surgery, chemotherapy and radiation therapy may also be a source of factors that can support recurrence and metastasis and thus stromal therapies may also emerge as important in the adjuvant setting.
Table 1.
Stroma targeted anti-cancer strategies.
| Proposed Mechanism | Target | Drugs, drug classes |
|---|---|---|
| CAF Differentiation | DNMT1 | 5-aza-2’-deoxycitidine |
| FAPa | Sibrotozumab | |
| CAF-Epithelial Interaction | HGF/Met | NK4, anti-HGF mAbs |
| MMPs | Non-peptidic MMP inhibitors (*) TIMPs |
|
| SDF1/CXCL2 | AMD-3100 | |
| Smo (Hedgehog pathway) | IPI-926 | |
| CAF-ECM Interaction | MMPs | (as above) |
| Tenascin-C | Radioactive labeled antibody, siRNA | |
| PAI-1/uPAR | Radioactive labeled PAI, Å6 | |
| FAPα | Sibrotozumab, small molecule inhibitors | |
| CAF-Endothelial Interaction | PDGF-C | Antibodies used in synergy with anti-VEGF-A |
| CAF targeted anti-Inflammatory signaling | CD11b+ myeloid stromal cells (MDSCs) COX-2 |
CTL |
| CAF DNA methylation | DNMT1 | 5-aza-2’-deoxycitidine |
Examples of MMP inhibitors include Marimastat, Rebimastat (broad spectrum); Tanomastat (MMP2,3, 9); Prinomastat (MMP 2,3,9,13,14).
As the differentiation into an activated CAF is increasingly viewed as a key carcinogenic event, inhibition of differentiation has become another attractive therapeutic target. While not yet done in a cancer system, myofibroblast differentiation from hepatic stellate cells was halted by inhibition of DNMT1 by 5-aza-2’-deoxycitidine (5aza-dC) (67). Especially in the context of the importance of epigenetic regulations in myofibroblast function, this finding may be particularly relevant. In addition, certain epithelial cell derived MMPs (i.e. MMP7) may have a role in myofibroblast differentiation and can be targeted in therapy (68, 69).
Perhaps the richest harvest of stroma-targeted therapies has emerged from the inhibition of signaling between epithelial cells, endothelial cells and myofibroblastss. Several of these targets are expressed by multiple stromal components, such as tumor endothelial cells, tumor associated macrophages, epithelial tumor cells or CAFs. Here we focus on CAF targeted approaches only. Stroma-directed therapies can target growth factors secreted by CAFs that act on epithelial transformation and enhance invasiveness. The HGF/Met pathway has been the target of early pre-clinical studies using NK4, a competitive antagonist of Met and anti-HGF monoclonal antibodies (70, 71). These studies have shown a remarkable decreased ability of metastastatic potential and tumor cell growth and human studies are planned. Importantly, as previously noted, this pathway is under the regulation of TGF-β. This is particularly important as it emphasize the complexity of stromal targeted interventions. At this stage TGF-β inhibition may not only be a “foe”, but rather a “friend” and it maybe one of the important reasons to be concerned that anti-TGF-β therapies may accelerate pre-malignant lesions (72). Most importantly, at later stages, TGF-β is an important immunosuppressant and also enhances myofibroblast differentiation and maybe a promising target against tumor progression and invasion (73). MMP inhibitors are promising as they may play a role in both epithelial-CAF signaling and remodeling of the ECM. Increased expression of several MMPs (MMP 9, 2, 3) or decreased expression of their inhibitors (i.e. TIMP2) has been associated with tumor progression or an invasive phenotype (74, 75). However, their efficacy has been limited due to both adverse effects (possibly due to their role in normal tissue homeostasis, wound healing and angiogenesis) and unpredictable behavior in different tumors or tumor stages (68). Other (at least partially) CAF derived factors such as uPA, its inhibitor PAI-1 and Tenascin-C have been targets of therapy. Inhibitors of uPA and radioactive labeled PAI-1 has shown efficacy in inhibiting tumor growth and invasion in preclinical studies (76, 77). Tenascin-C inhibitors have been effective in early clinical trials in astrocytomas and NHL (78). Similar to PAI-1, the tumor stroma specific expression of Tenascin-C may also be used to target delivery of radiotherapies. Inhibition of FAP (by monoclonal Ab, Sibrotozumab), which may also play a role in the differentiation of fibroblasts to myofibroblasts, has also shown efficacy in early clinical trials (79). An important axis to target is the SDF1/CXCL2-CXCR4 signaling pathway. The CXCR4 antagonist, AMD3100 has shown tumor growth inhibitory effects in pre-clinical studies and may also reduce the recruitment of BMDCs (80, 81). Most recently, a CAF directed therapeutic potential has been demonstrated in overcoming loss effect by VEGF inhibitors. CAF derived PDGF-C has been shown to play a major role in stimulating VEGF production and antibodies targeted against PDGF-C were synergistic with anti-VEGF-A antibodies (64).
Another innovative recent advance among stroma targeted therapies has been the demonstration that in pancreatic cancer, the inhibition of stromal cell proliferation may allow improved delivery of cytotoxic agents to the tumor cell. Olive et al have argued that in the vasculature-poor but stroma-rich K-ras/p53 murine pancreatic cancer model, using a stroma specific inhibitor of a hedgehog receptor (IPI-926) they were able to inhibit stromal proliferation, increase tumor vasculature, increase delivery of gemcitabine to tumor cells and most importantly demonstrate an increased survival (82). This study adds to accumulating evidence that inhibition of the Hedgehog pathway can reduce tumor associated stromal proliferation. A study by Yauch et al has shown that the effectiveness of HhAntag was due to its ability to inhibit stromal proliferation and this in turn led to inhibition of tumor growth (83). Interestingly, using another inhibitor of Smo, Olive et al did not find a reduction in tumor growth and has observed an increase in tumor vasculature. This raises the possibility of a paradigm shift where stroma is not necessarily the primary target but possibly more importantly an intermediate obstacle that needs to be eliminated to enhance anti-tumor drug delivery.
Modification of the inflammatory response is another approach that is stroma-directed. Recent evidence has emerged that stroma might play a barrier role in evading immune surveillance. When adoptively transferred effector T cells were targeted against only cancer cells these cells eventually escaped immune recognition by loss of their tumor antigen. However, when stroma was also targeted this led to significant tumor growth retardation and when combined with cancer targeted effector T cells it is also resulted in tumor elimination (84, 85). This important recognition has opened the possibility that targeting T cell anti-tumor responses to stromal cells, in addition to cancer cells, may be a new focus of anti-cancer immune therapy. Despite the promise that acute inflammatory response may halt tumor growth, the chronic inflammatory responses in tumors most often propagate cancer growth. It is suggested that chronic inflammation results in a tumorigenic transformation of both the stroma and epithelium and plays an important role in recruitment of BMDCs to these components. A key mediator of inflammatory stimuli, COX-2, has been shown to be both stromal and epithelial derived (86) and its expression is markedly increased when fibroblasts or fibroblast derived factors are co-cultured with cancer cells (87). It has recently been suggested that COX-2 was able to inhibit the invasion promoting effect of fibroblasts in a co-culture system (88). Proposed mechanisms for this effect include a fibroblast induced epithelial induction of COX-2 (88) or a direct up-regulation of COX-2 expression in fibroblasts (87). In either scenario COX-2 inhibition as a strategy to prevent cancer progression or invasion can now begin to be viewed as a type of stromal intervention.
Lastly, targeting epigenetic alterations (DNA methylation) may be an opportunity to preferentially inhibit the function and/or proliferation of CAFs. As described above, certain gene specific methylation changes have been found to be specific for cancer associated stroma as compared to both normal stroma and neoplastic epithelium (89, 90). In addition, our studies in gastric cancer have suggested that global genomic hypomethylation occurs in CAFs and in some situations it may even occur earlier in these stromal cells than in the epithelium (Figure 3). We have also shown that despite a lack of difference in DNMT1 expression between CAFs and non-neoplastic adjacent myofibroblastss, DNMT1 expression is much less in the stroma compared to the epithelium. Based on these observations we can suggest that these stromal cells may be more susceptible to hypomethylating drugs like 5aza-dC, which might be expected to produce a “hypomethylation crisis” by causing further demethylation in the already hypomethylated CAF genomes.
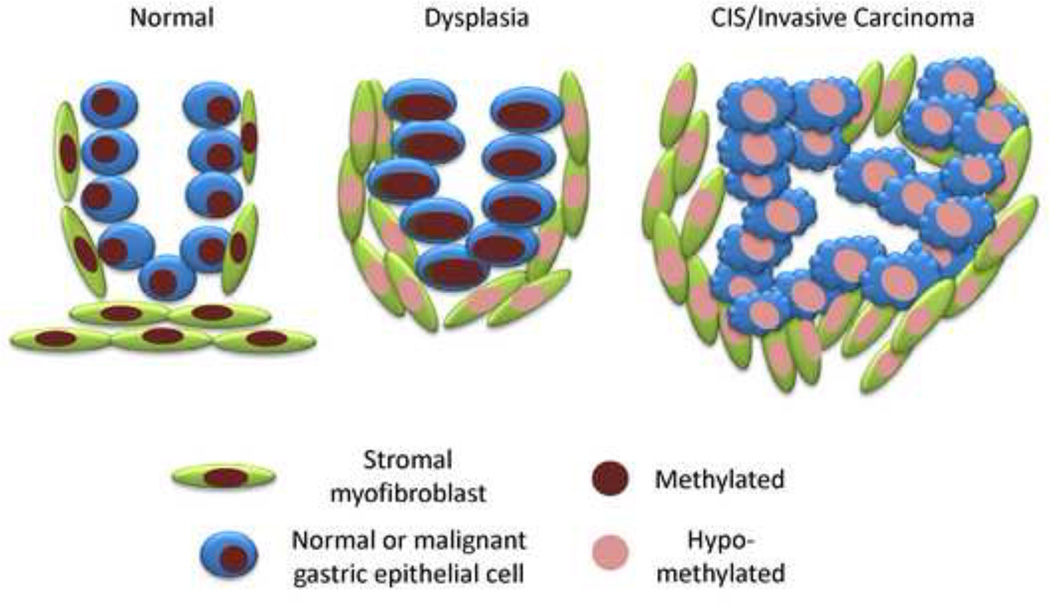
Fig. 3. Changes in global methylation in malignant epithelium and CAFs.
Two populations of cells (epithelial, blue; stromal, green) are shown at three stage of neoplastic progression. The nuclear color corresponds to the global methylation index. Based on our findings in gastric cancers, hypomethylation, as indicated by decreased nuclear staining, is seen early in CAFs and occurs somewhat later in the malignant epithelial cells. Hypomethylation is seen in both compartments at the carcinoma in situ/invasive cancer stage. How this scenario may generalize to other types of epithelial cancers is under investigation.
Currently available epigenetic interventions that target DNA methylation fall into 2 categories: (i) methyl donor modifiers and (ii) methyltransferase inhibitors. The former are folate, betaine and choline, whereas the main methyltransferase inhibitors are nucleoside (5–azacytidine, 5-aza-2′-deoxycytidine and zebularine) and non-nucleoside inhibitors (procaine, procainamide, EGCG and RG108) (91). The role of methyl donors in carcinogenesis is an area of some controversy and growing clinical interest (92, 93). Both human and animal studies have shown a correlation between methyl donor deficiency and cancer (94), whereas others suggested an acceleration of carcinogenesis following supplementation (95). An emerging concept in methyl donor supplementation may be the timing of these interventions with relation to neoplastic stage. It is conceivable that early methyl donor supplementation may reverse global demethylation, whereas when given at later stages it may fuel hypermethylation (96, 97).
Inhibition of DNA methyltransferases (DNMT1, DNMT3a, and DNMT3b) may offer an opportunity to inhibit CAFs. There are two possibilities for a beneficial effect of anti-DNMT therapy: as noted above, further demethylation in cells that already have hypomethylated genomes tissue may induce sensescence and growth arrest (a hypomethylation crisis). It is possible that given the apparently low expression of DNMT1 in CAFs compared to cancerous epithelium, anti-DNMT therapy may be particularly effective against this stromal component. But on the other hand there are some genes that may be important to the carcinogenic properties of CAFs that have shown over-expression after demethylation. Experiments with demethylating drugs in mouse models of stroma-rich human cancers should be able to answer whether this class of drug can inhibit the net proliferation of CAFs in vivo. Because of the extensive cross-talk between CAFs and the malignant epithelium, specificity for CAFs will not be demonstrated by such experiments alone. However, it may be possible to prove direct activity against the stromal compartment by using reconstitution systems, in which CAFs could be treated with the demethylating drug prior to mixing these cells with untreated malignant epithelial cells. Using demethylating drugs together with directly cytotoxic drugs that have a non-overlapping mechanism of action and target both the cancerous epithelium and the stroma may be necessary for curative therapy.
Summary and Conclusions
The recognition of the active role that CAFs play in carcinogenesis adds a new level of complexity to cancer biology but also brings an opportunity for new therapeutic strategies. Understanding the genetics and epigenetics of this stromal compartment will be especially important in order to differentiate CAFs as therapeutic targets. Altered DNA methylation in CAFs has been a common finding across several recent animal and human studies and as this epigenetic mark can be targeted by available drugs epigenetic therapy against CAFs will be an interesting avenue for future research. At the same time, pharmacological and biological agents that interfere with signaling between the malignant epithelial cells and the supporting CAFs will likely continue to be tested. If the progress that has been made in the field of anti-angiogenesis therapies is any indication, there is hope that effective anti-stroma therapies will soon begin to emerge.
Acknowledgements
The authors thank Martha Salas for technical assistance. This work was supported by a grant from the NIH (U54CA126513), to T.C.W. and B.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48(5–6):509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 2.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250(2):273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 3.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12(1):22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 5.Direkze NC, Forbes SJ, Brittan M, et al. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21(5):514–520. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 6.Brittan M, Hunt T, Jeffery R, et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50(6):752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8(9):1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 8.Marrache F, Pendyala S, Bhagat G, Betz KS, Song Z, Wang TC. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut. 2008;57(8):1113–1120. doi: 10.1136/gut.2007.143271. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. J Biol Chem. 2008;283(28):19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 10.Direkze NC, Hodivala-Dilke K, Jeffery R, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 13.Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137(6):491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeon ES, Moon HJ, Lee MJ, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26(3):789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 15.Mishra PJ, Humeniuk R, Medina DJ, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68(11):4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67(21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 17.Oldfield MD, Bach LA, Forbes JM, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108(12):1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257(1):180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 20.Simmons JG, Pucilowska JB, Keku TO, Lund PK. IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;283(3):G809–G818. doi: 10.1152/ajpgi.00057.2002. [DOI] [PubMed] [Google Scholar]
- 21.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thannickal VJ, Lee DY, White ES, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278(14):12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 23.Shi-Wen X, Chen Y, Denton CP, et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15(6):2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70(9–10):473–485. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 26.Dawe CJ, Morgan WD, Slatick MS. Influence of epithelio-mesenchymal interactions on tumor induction by polyoma virus. International journal of cancer. 1966;1(5):419–450. doi: 10.1002/ijc.2910010504. [DOI] [PubMed] [Google Scholar]
- 27.Hayward SW, Wang Y, Cao M, et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61(22):8135–8142. [PubMed] [Google Scholar]
- 28.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60(5):1254–1260. [PubMed] [Google Scholar]
- 29.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2008 doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98(21):12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fromigue O, Louis K, Dayem M, et al. Gene expression profiling of normal human pulmonary fibroblasts following coculture with non-small-cell lung cancer cells reveals alterations related to matrix degradation, angiogenesis, cell growth and survival. Oncogene. 2003;22(52):8487–8497. doi: 10.1038/sj.onc.1206918. [DOI] [PubMed] [Google Scholar]
- 32.Casey TM, Eneman J, Crocker A, et al. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat. 2008;110(1):39–49. doi: 10.1007/s10549-007-9684-7. [DOI] [PubMed] [Google Scholar]
- 33.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Gervaz P, Pak-art R, Nivatvongs S, Wolff BG, Larson D, Ringel S. Colorectal adenocarcinoma in cirrhotic patients. J Am Coll Surg. 2003;196(6):874–879. doi: 10.1016/S1072-7515(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 35.Dingemans KP, Zeeman-Boeschoten IM, Keep RF, Das PK. Transplantation of colon carcinoma into granulation tissue induces an invasive morphotype. Int J Cancer. 1993;54(6):1010–1016. doi: 10.1002/ijc.2910540625. [DOI] [PubMed] [Google Scholar]
- 36.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee S, Raje N, Schoonmaker JA, et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118(2):491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Canales J, Hanson JC, Tangrea MA, et al. Identification of a unique epigenetic sub-microenvironment in prostate cancer. J Pathol. 2007;211(4):410–419. doi: 10.1002/path.2133. [DOI] [PubMed] [Google Scholar]
- 39.Kern SE, Winter JM. Elegance, silence and nonsense in the mutations literature for solid tumors. Cancer Biol Ther. 2006;5(4):349–359. doi: 10.4161/cbt.5.4.2551. [DOI] [PubMed] [Google Scholar]
- 40.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Gonda TA, Gamble MV, et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68(23):9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patocs A, Zhang L, Xu Y, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357(25):2543–2451. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 43.Tuhkanen H, Anttila M, Kosma VM, et al. Frequent gene dosage alterations in stromal cells of epithelial ovarian carcinomas. Int J Cancer. 2006;119(6):1345–1353. doi: 10.1002/ijc.21785. [DOI] [PubMed] [Google Scholar]
- 44.Condon MS. The role of the stromal microenvironment in prostate cancer. Semin Cancer Biol. 2005;15(2):132–137. doi: 10.1016/j.semcancer.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Paterson RF, Ulbright TM, MacLennan GT, et al. Molecular genetic alterations in the laser-capture-microdissected stroma adjacent to bladder carcinoma. Cancer. 2003;98(9):1830–1836. doi: 10.1002/cncr.11747. [DOI] [PubMed] [Google Scholar]
- 46.Weber F, Xu Y, Zhang L, et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297(2):187–195. doi: 10.1001/jama.297.2.187. [DOI] [PubMed] [Google Scholar]
- 47.Bian Y, Knobloch TJ, Sadim M, et al. Somatic acquisition of TGFBR1*6A by epithelial and stromal cells during head and neck and colon cancer development. Hum Mol Genet. 2007;16(24):3128–3135. doi: 10.1093/hmg/ddm274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6(4):1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 49.Qiu W, Hu M, Sridhar A, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40(5):650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell IG, Qiu W, Polyak K, Haviv I. Breast-cancer stromal cells with TP53 mutations. N Engl J Med. 2008;358(15):1634–1635. doi: 10.1056/NEJMc086024. author reply 6. [DOI] [PubMed] [Google Scholar]
- 51.Walter K, Omura N, Hong SM, Griffith M, Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7(6):882–888. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 53.Hanson JA, Gillespie JW, Grover A, et al. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98(4):255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 54.Lin HJ, Zuo T, Lin CH, et al. Breast cancer-associated fibroblasts confer AKT1-mediated epigenetic silencing of Cystatin M in epithelial cells. Cancer Res. 2008;68(24):10257–10266. doi: 10.1158/0008-5472.CAN-08-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Couillard J, Demers M, Lavoie G, St-Pierre Y. The role of DNA hypomethylation in the control of stromelysin gene expression. Biochem Biophys Res Commun. 2006;342(4):1233–1239. doi: 10.1016/j.bbrc.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich D, Lesche R, Tetzner R, et al. Analysis of DNA methylation of multiple genes in microdissected cells from formalin-fixed and paraffin-embedded tissues. J Histochem Cytochem. 2009;57(5):477–489. doi: 10.1369/jhc.2009.953026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang L, Gonda T, Gamble M, et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-08-1319. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richards KL, Zhang B, Baggerly KA, et al. Genome-wide hypomethylation in head and neck cancer is more pronounced in HPV-negative tumors and is associated with genomic instability. PLoS ONE. 2009;4(3):e4941. doi: 10.1371/journal.pone.0004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuzaki K, Deng G, Tanaka H, Kakar S, Miura S, Kim YS. The relationship between global methylation level, loss of heterozygosity, and microsatellite instability in sporadic colorectal cancer. Clin Cancer Res. 2005;11(24 Pt 1):8564–8569. doi: 10.1158/1078-0432.CCR-05-0859. [DOI] [PubMed] [Google Scholar]
- 60.Chicoine E, Esteve PO, Robledo O, Van Themsche C, Potworowski EF, St-Pierre Y. Evidence for the role of promoter methylation in the regulation of MMP-9 gene expression. Biochem Biophys Res Commun. 2002;297(4):765–772. doi: 10.1016/s0006-291x(02)02283-0. [DOI] [PubMed] [Google Scholar]
- 61.Shahrzad S, Bertrand K, Minhas K, Coomber BL. Induction of DNA hypomethylation by tumor hypoxia. Epigenetics. 2007;2(2):119–125. doi: 10.4161/epi.2.2.4613. [DOI] [PubMed] [Google Scholar]
- 62.Lim SO, Gu JM, Kim MS, et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology. 2008;135(6):2128–2140. 40, e1–e8. doi: 10.1053/j.gastro.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 63.Harless WW. Revisiting perioperative chemotherapy: the critical importance of targeting residual cancer prior to wound healing. BMC Cancer. 2009;9(1):118. doi: 10.1186/1471-2407-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Zhang B. Targeting the stroma by T cells to limit tumor growth. Cancer Res. 2008;68(23):9570–9573. doi: 10.1158/0008-5472.CAN-08-2414. [DOI] [PubMed] [Google Scholar]
- 66.Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H, Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95(12):2546–2554. doi: 10.1002/cncr.11006. [DOI] [PubMed] [Google Scholar]
- 67.Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14(2):275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 68.McCaig C, Duval C, Hemers E, et al. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130(6):1754–1763. doi: 10.1053/j.gastro.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Hemers E, Duval C, McCaig C, Handley M, Dockray GJ, Varro A. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: implications for epithelial-mesenchymal signaling. Cancer Res. 2005;65(16):7363–7369. doi: 10.1158/0008-5472.CAN-05-0157. [DOI] [PubMed] [Google Scholar]
- 70.Kim KJ, Wang L, Su YC, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12(4):1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 71.Wen J, Matsumoto K, Taniura N, Tomioka D, Nakamura T. Hepatic gene expression of NK4, an HGF-antagonist/angiogenesis inhibitor, suppresses liver metastasis and invasive growth of colon cancer in mice. Cancer Gene Ther. 2004;11(6):419–430. doi: 10.1038/sj.cgt.7700705. [DOI] [PubMed] [Google Scholar]
- 72.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 74.van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 75.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qu CF, Song EY, Li Y, et al. Pre-clinical study of 213Bi labeled PAI2 for the control of micrometastatic pancreatic cancer. Clin Exp Metastasis. 2005;22(7):575–586. doi: 10.1007/s10585-005-5788-9. [DOI] [PubMed] [Google Scholar]
- 77.Pulukuri SM, Gondi CS, Lakka SS, et al. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J Biol Chem. 2005;280(43):36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Hofmeister V, Schrama D, Becker JC. Anti-cancer therapies targeting the tumor stroma. Cancer Immunol Immunother. 2008;57(1):1–17. doi: 10.1007/s00262-007-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scott AM, Wiseman G, Welt S, et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9(5):1639–1647. [PubMed] [Google Scholar]
- 80.Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22(6):1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 81.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100(23):13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science. 2009 doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 84.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang B, Zhang Y, Bowerman NA, et al. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 2008;68(5):1563–1571. doi: 10.1158/0008-5472.CAN-07-5324. [DOI] [PubMed] [Google Scholar]
- 86.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105(11):1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sato N, Maehara N, Goggins M. Gene expression profiling of tumor-stromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64(19):6950–6956. doi: 10.1158/0008-5472.CAN-04-0677. [DOI] [PubMed] [Google Scholar]
- 88.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106(9):3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bloushtain-Qimron N, Yao J, Snyder EL, et al. Cell type-specific DNA methylation patterns in the human breast. Proc Natl Acad Sci U S A. 2008;105(37):14076–14081. doi: 10.1073/pnas.0805206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res. 2006;66(5):2794–2800. doi: 10.1158/0008-5472.CAN-05-2821. [DOI] [PubMed] [Google Scholar]
- 92.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004;13(4):511–519. [PubMed] [Google Scholar]
- 93.Ziegler RG, Lim U. One-carbon metabolism, colorectal carcinogenesis, chemoprevention--with caution. J Natl Cancer Inst. 2007;99(16):1214–1215. doi: 10.1093/jnci/djm105. [DOI] [PubMed] [Google Scholar]
- 94.Pufulete M, Al-Ghnaniem R, Leather AJ, et al. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology. 2003;124(5):1240–1248. doi: 10.1016/s0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 95.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 96.Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60(19):5434–5440. [PubMed] [Google Scholar]
- 97.Song J, Sohn KJ, Medline A, Ash C, Gallinger S, Kim YI. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer Res. 2000;60(12):3191–3199. [PubMed] [Google Scholar]