Summary
Programmed necrosis, like apoptosis, eliminates pathogen infected cells as a component of host defense. Receptor interacting protein kinase (RIP) 3 (also called RIPK3) mediates programmed necrosis via RIP homotypic interaction motif (RHIM)-dependent interactions, which is induced by murine cytomegalovirus (MCMV) infection or death receptor activation and is suppressed by the MCMV-encoded viral inhibitor of RIP activation (vIRA). We find that interferon-independent expression of DNA-dependent activator of interferon regulatory factors (DAI; also known as ZBP1 or DLM-1), sensitizes cells to virus-induced necrosis and DAI knockdown or knockout cells are resistant to this death pathway. Importantly, as with RIP3−/− mice, vIRA mutant MCMV pathogenesis is restored in DAI−/− mice, consistent with a DAI-RIP3 complex being the natural target of vIRA. Thus, DAI interacts with RIP3 to mediate virus-induced necrosis analogous to the RIP1-RIP3 complex controlling death receptor-induced necroptosis. These studies unveil a role for DAI as the RIP3 partner mediating virus-induced necrosis.
Introduction
Programmed cell death may be triggered to cut short viral infection and can be targeted by pathogen-encoded cell death suppressors (Best, 2008; Lamkanfi and Dixit, 2010). Apoptosis is a well-recognized caspase-dependent host defense pathway triggered through cell-intrinsic as well as cell-extrinsic signals that also helps shape embryonic development (Hengartner, 2000; Strasser et al., 2000). Recent insights have revealed programmed necrotic cell death to be an important alternate host defense pathway (Cho et al., 2009; Upton et al., 2010). The cellular protein kinase RIP3 plays a central role in transducing signals that drive programmed necrosis whether induced by death receptor (DR) or pattern recognition receptor (PRR) activation (He et al., 2011; He et al., 2009; Zhang et al., 2009), during development (Kaiser et al., 2011; Oberst et al., 2011) or in the course of viral infection (Cho et al., 2009; Upton et al., 2010). DR-induced programmed necrosis, also called necroptosis, is revealed whenever caspase 8 activity in compromised, and requires formation of a RHIM-dependent RIP1-RIP3 complex (necrosome) and the kinase activity of both (Cho et al., 2009; He et al., 2009; Mocarski et al., 2011; Upton et al., 2010; Zhang et al., 2009).
MCMV-encoded viral inhibitor of RIP activation (vIRA), the product of the M45 gene, is a potent RHIM-containing cell death suppressor (Brune et al., 2001; Mack et al., 2008; Upton et al., 2008, 2010). vIRA has the capacity to inhibit DR- or TIR-domain-containing adaptor-inducing IFNβ (TRIF)-dependent apoptosis, necroptosis, and virus-induced programmed necrosis (Mack et al., 2008; Upton et al., 2008, 2010) as well as NF-κB activation and RIP3 auto-phosphorylation induced by overexpression of the PRR DAI (Rebsamen et al., 2009). The natural role of vIRA is to prevent rapid RIP3-dependent necrosis over the initial 8 to 12 h of infection (Upton et al., 2008, 2010). Importantly, viruses that lack vIRA, or that encode a tetra-alanine RHIM substitution (M45mutRHIM) are severely attenuated in mice (Lembo et al., 2004; Upton et al., 2010). The significance of programmed necrosis as a bona fide host defense pathway was highlighted by complete normalization of vIRA RHIM mutant virus pathogenesis in RIP3-deficient mice (Upton et al., 2010). MCMV vIRA thereby illuminated a RHIM-dependent death pathway functioning alongside apoptosis in mammalian host defense (Mocarski et al., 2011).
Whereas DR-induced necroptosis relies on a RHIM-dependent RIP1-RIP3 signaling complex, virus-induced necrosis follows a RIP3 RHIM-dependent pathway that is independent of RIP1 or TRIF (Upton et al., 2008, 2010). Because RIP3 and DAI can form a RHIM-dependent complex (Kaiser et al., 2008), this interaction may contribute to virus-induced necrosis. DAI is expressed constitutively in many tissues and is also IFN-inducible (Fu et al., 1999). As a candidate PRR triggering IFNβ expression in response to cytosolic double stranded DNA (dsDNA) (Takaoka et al., 2007), DAI has DNA binding motifs that are required for activation of downstream signaling and IRF3-mediated gene expression (Takaoka et al., 2007; Wang et al., 2008). In addition, DAI contains three RHIM-like repeats, one or two of which can bind RIP3 and/or RIP1 and activate NF-κB (Kaiser et al., 2008; Rebsamen et al., 2009). Constitutive DAI in cultured cells induces IFNβ following herpes simplex virus (HSV) (Furr et al., 2011; Takaoka et al., 2007) or human CMV (DeFilippis et al., 2010a; DeFilippis et al., 2010b) infection. However, based on the phenotype of DAI−/− mice, this protein has been considered “dispensable for both innate and adaptive immune responses to B-DNA and DNA vaccine” (Ishii et al., 2008). Given the impact of RIP3-dependent necrosis during MCMV infection, together with the potential for a RHIM-dependent complex between RIP3 and DAI (Kaiser et al., 2008), we sought to define the role of DAI as a RIP3 partner in virus-induced necrosis.
Results
RIP3 levels differentially confer susceptibility to necroptosis and virus-induced necrosis
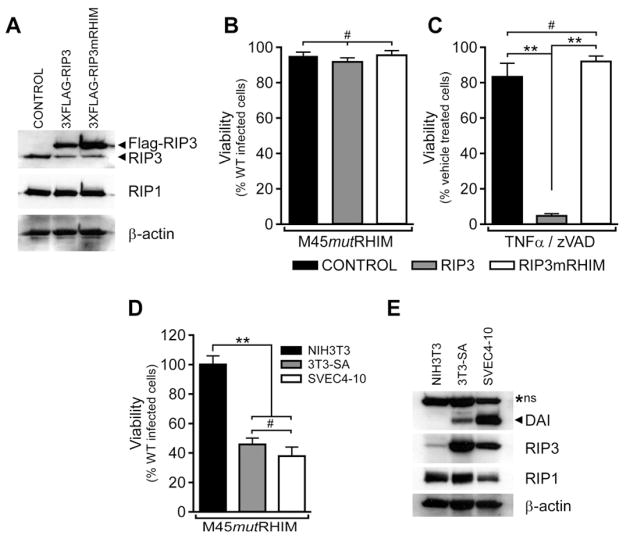
Because RIP3 is known to mediate virus-induced programmed necrosis and is targeted by vIRA during infection (Upton et al., 2010), we sought to employ sensitive (3T3-SA, SVEC4-10) and resistant (NIH3T3) cell lines to ask whether RIP3 was the sole determinant of this novel death pathway (Upton et al., 2010; Zhang et al., 2009). Introduction of WT RIP3, but not RHIM-mutant (RIP3mRHIM) into RIP3−/− MEFs sensitized cells to virus-induced necrosis (Upton et al., 2010). When naturally resistant NIH3T3 cells expressing FLAG-tagged WT RIP3, FLAG-tagged RIP3mRHIM, or vector control (Figure 1A) were assessed for sensitivity to programmed necrosis by infection with M45mutRHIM MCMV or by treatment with TNF and the caspase inhibitor, zVAD-fmk, neither WT nor mutant RIP3 was sufficient to sensitize to virus-induced necrosis (Figure 1B). In contrast, WT RIP3, but not RIP3mRHIM, sensitized NIH3T3 cells to TNF-induced necroptosis (Figure 1C). Thus, RIP3 levels were not the sole determinant of virus-induced necrosis given that over-expression does not sensitize NIH3T3 cells to this death pathway under conditions where they are sensitized to RIP1-RIP3 necroptosis. Virus-induced necrosis requires RHIM-dependent function of RIP3 independent of RIP1, while necroptosis requires RHIM-dependent association of RIP1 and RIP3 (Cho et al., 2009; He et al., 2009; Upton et al., 2010; Zhang et al., 2009). In addition, TLR3-induced necroptosis involves RIP1 and RIP3 together with RHIM-dependent interactions mediated by TRIF (Feoktistova et al., 2011; He et al., 2011). Given the fact neither RIP1 nor TRIF contribute to the viral death pathway (Upton et al., 2010), we turned attention to DAI, a constitutively expressed RHIM-containing adaptor and candidate PRR that activates NF-κB and IRF3 (Kaiser et al., 2008; Rebsamen et al., 2009; Takaoka et al., 2007). As expected (Upton et al., 2010), RIP3 levels were higher in necroptosis-susceptible cells and lower in NIH3T3 cells (Figure 1A), and RIP1 was present in all cell lines. DAI was constitutively expressed in necrosis-sensitive 3T3-SA and SVEC4-10 cells, but was not detected in necrosis-resistant NIH3T3 cells (Figure 1D–E). Importantly, both DAI and RIP3 were present in virus-induced necrosis-sensitive cells.
Figure 1. RIP3 levels differentially confer susceptibility to necroptosis and virus-induced programmed necrosis.
(A) Immunoblot (IB) analysis to detect RIP3, RIP1 and β-actin from NIH3T3 cells stably expressing 3XFLAG-tagged RIP3, RIP3mRHIM, or control. (B) Viability of RIP3-expressing NIH3T3 stable cell lines infected with M45mutRHIM MCMV at a multiplicity of infection (MOI) of 10. (n=4) (C) Viability of RIP3-expressing NIH3T3 stable cell lines after treatment with TNF (25 ng/ml) and zVAD-fmk (50 μM) to induce necroptosis (n=3–4). (D) Viability of NIH3T3, 3T3-SA and SVEC4-10 cell lines infected with M45mutRHIM MCMV (MOI=10). (n=3–7) (E) IB analysis of uninfected NIH3T3, 3T3-SA, and SVEC4-10 cells to detect DAI, RIP3, RIP1, and β-actin. A non-specific (*ns) band is characteristic for this antibody (Karayel et al., 2009). **p<0.001, #not significant (p>0.05). See also related Figure S1.
IFNβ and NF-κB signaling in necroptosis and virus-induced necrosis
DAI is constitutively expressed as well as IFN-inducible (Fu et al., 1999), is necessary for herpesvirus-induced IFNβ production (DeFilippis et al., 2010a; Furr et al., 2011; Takaoka et al., 2007), and is able to activate NF-κB (Kaiser et al., 2008). To assess any potential contribution of DAI-dependent IFNβ or NF-κB signaling in virus-induced programmed necrosis, experiments were undertaken to correlate activation events with cell death. First, DAI was not detected in NIH3T3 cells during MCMV infection although IFNβ treatment, as expected (Fu et al., 1999), induced this protein within 9 h (Figure S1A). Thus, NIH3T3 cells do not express detectable DAI during infection even though they respond to IFNβ treatment. Levels of DAI in necrosis-sensitive 3T3-SA (Figure S1A) or SVEC4-10 (data not shown) cells increased from 9 to 24 h after either MCMV infection or IFNβ treatment, consistent with a DAI-dependent IFN activation shown for other herpesviruses (DeFilippis et al., 2010a; DeFilippis et al., 2010b; Takaoka et al., 2007). These data suggest DAI must be constitutively expressed in the host cell to initiate a cell death response to MCMV infection. The same level of IFNβ mRNA is induced in SVEC4-10 cells by WT MCMV and vIRA-deficient MCMVΔ45 (Brune et al., 2001) (Figure S1B), indicating induction of IFNβ is independent of vIRA under conditions where cell death is induced by vIRA-deficient virus (Brune et al., 2001; Upton et al., 2008, 2010). Furthermore, DAI is induced to comparable levels in WT and M45mutRHIM MCMV-infected MEFs (Figure S1C). Despite the upregulation of IFNβ and DAI expression during infection with parental or vIRA-mutant virus, treatment of SVEC4-10 cells with increasing concentrations of IFNβ-neutralizing antibody did not influence levels of virus-induced programmed necrosis (Figure S1D), indicating type I IFNs induced by infection, whether via DAI or other PRR-dependent pathways do not influence virus-induced necrosis.
DAI has also been implicated as an inducer of NF-κB (Kaiser et al., 2008; Takaoka et al., 2007). Canonical NF-κB signaling is dispensable for MCMV infection (Benedict et al., 2004). and does not influence necroptotic cell death (Vanden Berghe et al., 2006). To evaluate the role of NF-κB signaling in virus-induced necrosis, BMS-345541, a specific inhibitor of IκB kinase (IKK) activity (Burke et al., 2003) was used to sensitize SVEC4-10 cells to TNF-induced apoptosis (Figure S1E), indicating the drug dose used here was sufficient to inhibit NF-κB (Katdare et al., 2007). However, parallel treatment failed to normalize the viability of SVEC4-10 cells infected with M45mutRHIM MCMV (Figure S1F) as would be expected if NF-κB signaling played a dominant role in this RIP3-dependent antiviral pathway. Together, these results demonstrate neither IFNβ nor NF-κB signaling is a major factor in virus-induced necrotic cell death.
Introduction of DAI sensitizes resistant cells to virus-induced programmed necrosis
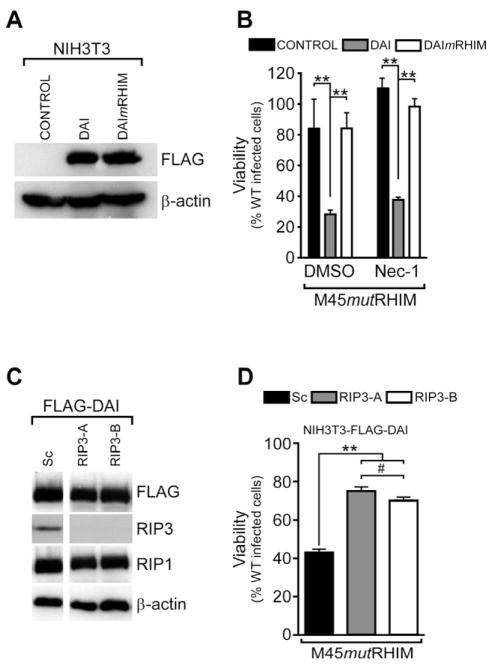
To determine a role for DAI in virus-induced programmed necrosis, NIH3T3 cells stably expressing FLAG- tagged WT DAI, FLAG- tagged RHIM mutant DAI (DAImRHIM, previously called DAImRLR-A)(Kaiser et al., 2008) and empty vector control (Figure 2A) were derived. WT DAI, but not DAImRHIM rendered these cells susceptible to virus-induced death (Figure 2B), results consistent with a role for RHIM-dependent interaction between DAI and RIP3 (Kaiser et al., 2008; Rebsamen et al., 2009). Nec-1, a potent RIP1 kinase inhibitor (Degterev et al., 2008) that blocks TNF-induced necroptosis, fails to prevent virus-induced programmed necrosis (Upton et al., 2010). Additionally, virus-induced necrosis was caspase-independent and did not require the action of TNF (Upton et al., 2010). Virus-induced death in DAI-transduced NIH3T3 cells was RIP1 kinase-independent based on resistance to Nec-1 (Figure 2B), as well as caspase- and DR-ligation-independent (Figure S2). These criteria are consistent with characteristics of virus-induced necrosis as established previously (Upton et al., 2010).
Figure 2. Increased DAI expression sensitizes resistant cells to virus-induced necrosis.
(A) IB analysis to detect FLAG and β-actin of NIH3T3 stable cells expressing FLAG-tagged WT DAI, FLAG-tagged DAImRHIM, or control. (B) Viability of DAI-expressing NIH3T3 stable cell lines infected with M45mutRHIM MCMV (MOI=10) and treated with DMSO or Nec-1 (30 μM). (n=4) (C) IB analysis to detect FLAG, RIP3, RIP1 and β-actin in stable NIH3T3 cell lines expressing FLAG-tagged WT DAI together with either a scramble control (Sc) or one of two RIP3-specific shRNAs (RIP3-A or RIP3-B). (D) Viability of DAI-expressing, RIP3 knockdown NIH3T3 stable cell lines following infection with M45mutRHIM MCMV (MOI=10)(n=3). **p<0.001, #not significant (p>0.05). See also related Figure S2.
We sought to further address the role of RIP3 by employing specific lentivirus shRNAs to knockdown expression (Figure 2C) in DAI transduced NIH3T3 cells. RIP3 now sensitized to virus-induced death (Figure 2D), in contrast to nontransduced cells. Thus, DAI and RIP3 were both required to trigger virus-induced necrotic death. The failure of DAImRHIM to sensitize NIH3T3 cells (Figure 2B) complements previous observations on RHIM mutant RIP3 (Upton et al., 2010), suggesting a RHIM-dependent RIP3-DAI complex (Kaiser et al., 2008) is involved in the death pathway. The previous demonstration that a DAI-RIP3 complex mediates NF-κB activation (Kaiser et al., 2008) that can be inhibited by vIRA (Rebsamen et al., 2009) reinforces the possibility this complex may be directly regulated by vIRA.
DAI-RIP3 complex in virus-induced programmed necrosis
We next sought to characterize whether a RIP3 and DAI interaction formed during viral infection. RIP3 co-immunoprecipitated DAI in extracts of 3T3-SA cells infected for 12 h with vIRA-mutant MCMV, consistent with a physical interaction (Figure 3A). Expression of RIP3 was constant, whereas levels of DAI increased with time. Levels of the nonfunctional tetra-alanine mutant protein expressed by M45mutRHIM MCMV increased between 1 and 12 hpi. A DAI-RIP3 interaction could not be detected during WT MCMV infection (likely a consequence of WT vIRA activity) (Figure S3A). Given the importance of RIP3 RHIM interactions in virus-induced necrosis (Upton et al., 2010), as well as the known RHIM-dependent interaction between RIP3 and DAI (Kaiser et al., 2008; Rebsamen et al., 2009), these results implicate a RIP3-DAI complex mediating programmed necrosis.
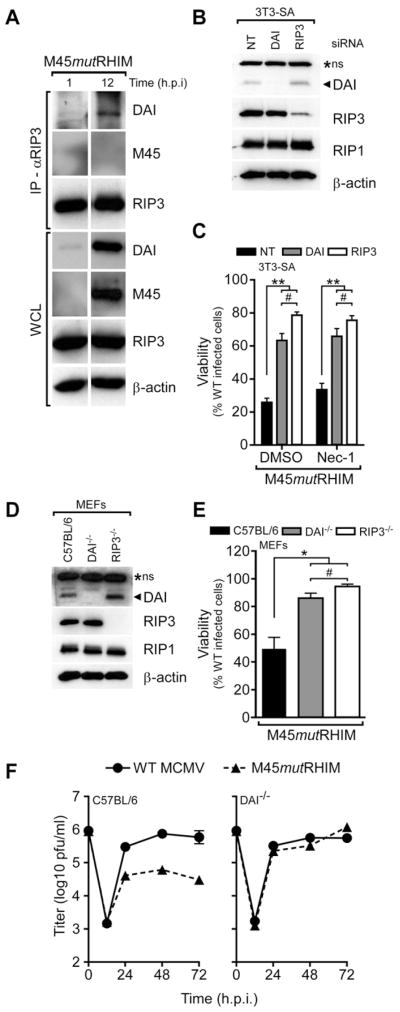
Figure 3. DAI and RIP3 cooperate in virus-induced necrosis.
(A) RIP3 and DAI interaction in the absence of vIRA. IB of immunoprecipitations (IP) and whole cell lysates (WCL; 5% total) showing DAI, M45, RIP3 and β-actin in 3T3-SA cells infected with M45mutRHIM MCMV (MOI=5) harvested at the indicated times (h.p.i.). (B) IB analysis to detect DAI, RIP3, RIP1 and β-actin in 3T3-SA cells transfected with non-targeting (NT) control, DAI, or RIP3 siRNAs (C) Viability of 3T3-SA cells transfected with the indicated siRNA and infected with M45mutRHIM (MOI=10) and treated with DMSO control or Nec-1 inhibitor (30μM). (n=4) (D) IB analysis to detect DAI, RIP3, RIP1, and β-actin in C57BL/6 control, DAI−/−, and RIP3−/− murine embryonic fibroblasts (MEFs) (E) Viability of C57BL/6 control, DAI−/−, and RIP3−/− MEFs infected with M45mutRHIM MCMV (MOI=10)(n=3). (F) Single-step replication of WT and M45mutRHIM MCMV (MOI of 5) on control C57BL/6 (left) and DAI−/− (right) MEFs. Viral titers determined by plaque assay with the first (0 h) time point representing amount of virus in the inoculum. Error bars, SD. *p<0.01, **p<0.001, #not significant (p>0.05). See also related Figure S3.
RNAi was employed in necrosis sensitive 3T3-SA cells to knockdown DAI or RIP3 protein levels compared to non-targeting control, while leaving RIP1 levels unchanged (Figure 3B). When infected with mutant virus, DAI knockdown 3T3-SA cells were protected from virus-induced necrosis at levels comparable to RIP3 knockdown cells (Figure 3C). As expected, treatment with RIP1 kinase inhibitor Nec-1 did not influence this pattern of cell death. Thus, both RIP3 and DAI were necessary to execute virus-induced programmed necrosis. Similar to siRNA knockdown, DAI−/− and RIP3−/− MEFs were resistant to virus-induced necrosis (Figure 3D–E), while control (C57BL/6) MEFs remained highly sensitive (Upton et al., 2010). Furthermore, DAI knockdown in 3T3-SA cells and DAI−/− MEFs remained sensitive to TNF-induced necroptosis (Figure S3B–C). Likewise, the RIP3-dependent necroptosis resulting in lethality of Casp8−/− mice (Kaiser et al., 2011; Oberst et al., 2011) was not rescued loss of DAI (Figure S3D), and Casp8−/−DAI−/− embryos were phenotypically indistinguishable from Casp8−/− littermate embryos (data not shown). Together, these data indicate loss of DAI ameliorates RIP3-dependent virus-induced programmed necrosis without altering the response to necroptosis requiring the RIP1-RIP3 necrosome.
To investigate the influence of DAI on virus growth, control or DAI−/− MEFs were infected and assessed for virus replication. Parental MCMV replicated to comparable high titers in all MEF lines tested, whereas mutant virus was attenuated in control cells (Figure 3F, left). However, mutant virus replication was normalized in DAI−/− MEFs (Figure 3F, right), reminiscent of normalized mutant virus growth in RIP3−/− MEFs (Upton et al., 2010). Together, these results provide evidence RIP3 and DAI coordinate to execute virus-induced programmed necrosis, and the RHIM-dependent association of RIP3 and DAI is the natural target of MCMV vIRA.
vIRA mutant virus inflammation and replication in DAI-deficient mice
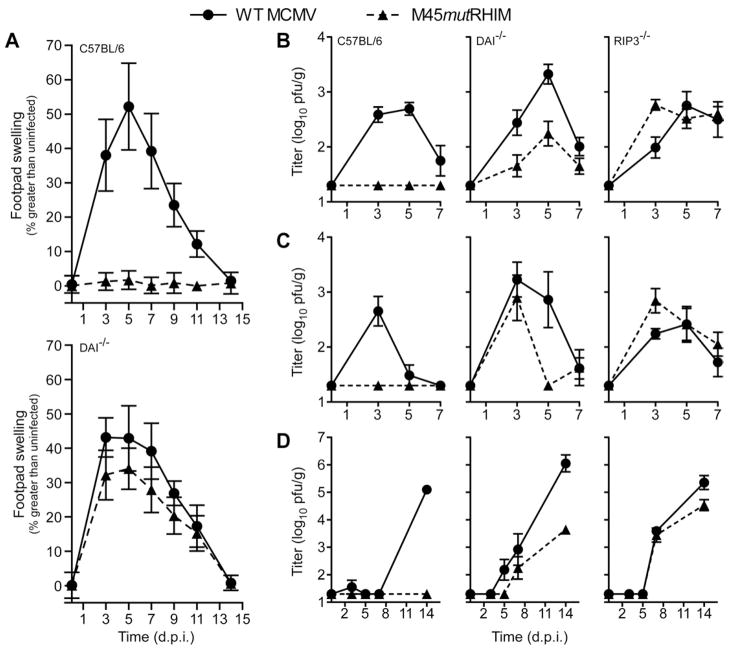
To directly address the contribution of DAI to virus-induced death, we infected DAI−/− and control (C57BL/6) mice by footpad (FP) inoculation. As in RIP3−/− mice (Upton et al., 2010), virus infection induced swelling in DAI−/− mice at levels similar to parental virus, peaking with a similar pattern to controls (Figure 4A). In contrast, mutant virus infection failed to elicit detectible swelling in C57BL/6 mice, a result expected due to attenuated replication (Upton et al., 2010). Thus, in the absence of DAI, mutant virus infection patterns exhibited inflammatory characteristics of WT MCMV infection, strongly supporting a role for DAI in host cell functions modulated by vIRA during natural infection. Interestingly, dissemination to the salivary glands (SGs) of DAI−/− mice 14 days postinfection with parental MCMV was consistently higher than control mice (Figure S4A) regardless of inoculation route, while mutant virus was comparably inefficient in reaching this organ.
Figure 4. M45mutRHIM virus attenuation in vivo is reversed in DAI−/− mice.
(A) Footpad swelling induced by parental WT or M45mutRHIM MCMV infection. Footpad thickness of C57BL/6 control (top) and DAI−/− (bottom) mice inoculated with 106 pfu was measured with a digital caliper, and mean values plotted at the indicated times over a 14 day time course (n=7–9 animals/group). (B–D) Viral titers from spleen (B), liver (C), and SGs (D) of control (C57BL/6), RIP3−/−, and DAI−/− mice infected via intraperitoneal inoculation with 106 pfu of indicated virus and harvested at the indicated time (n= 5–10 animals/group). Error bars, SEM. See also related Figure S4.
To more directly assess the role of DAI in M45mutRHIM virus replication patterns, control, DAI−/−, and RIP3−/− mice were inoculated via intraperitoneal injection. Mutant and parental virus titers were comparable in spleen (Figure 4B) and liver (Figure 4C) of DAI−/− mice throughout acute infection, while mutant virus was undetected in any organ at any time in control animals. Consistent with previous findings (Upton et al., 2010), mutant virus growth and dissemination was normalized in spleen, liver, and SG during infection of RIP3−/− animals (Figure 4B–D, right panels). Mutant virus was detected in SGs of DAI−/− mice (Figure 4D), albeit at levels lower than parental virus in control or RIP3−/− mice. Thus, DAI-deficiency permits mutant virus growth and dissemination in vivo. Parental WT virus titers in spleen and liver were elevated in DAI−/− mice compared to C57BL/6 mice, similar to observation following FP inoculation (Figure 4B–C). Mutant virus failed to replicate in highly susceptible IFNα/β receptor (IFNAR−/−) knockout mice (Figure S4B), further indicating the host defense pathway controlling type I IFN is irrelevant to virus-induced necrosis. Together, these results provide compelling evidence that virus-induced necrosis in vivo depends upon DAI and RIP3, and is the target of vIRA RHIM-mediated suppression independent of the type I IFN pathway, thereby establishing a natural function for DAI in mammalian host defense.
Discussion
In this work, we implicate the candidate cytosolic dsDNA sensor DAI in the host response to MCMV infection, providing evidence, both in vitro and in vivo, that DAI partners with RIP3 to induce RHIM-dependent programmed necrosis naturally suppressed by vIRA function. We have previously shown that RIP3-dependent, virus-induced programmed necrosis underlies the severe attenuation of M45mutRHIM MCMV (Upton et al., 2010), which is avirulent in normal or immunocompromised animals. Here, M45mutRHIM replicates in DAI deficient mice, clearly implicating DAI as a second player in this pathway. Other lines of evidence have stressed DAI is a redundant IFN-inducing DNA sensor (Lippmann et al., 2008; Wang et al., 2008), supported by findings DAI is dispensable for innate or adaptive responses to dsDNA or DNA vaccination (Ishii et al., 2008). Additionally, Cytosolic dsDNA activates multiple PRRs to induce IFNs and activate the inflammasome (Barber, 2011). Thus, although innate or inflammatory pathways may be controlled by DAI, its regulation of cell death is a critical contribution to host defense in a way that has recently emerged in evaluation of caspase 8 as well as RIP3 (Mocarski et al., 2011). This work, with our previous observations (Upton et al., 2010), highlights the importance of context in dissecting the contribution of crucial pathways in host defense. Neither RIP3−/− (Newton et al., 2004) nor DAI−/− mice (Ishii et al., 2008) exhibited outward signs of deficit until infected with an appropriate virus.
vIRA is one of several cell death suppressors encoded by MCMV that balance the host response to infection. The rapid programmed necrosis characterizing vIRA mutant virus-infected cells is likely unveiled because this mutant retains activity of at least two additional classes of cell death suppressors: viral inhibitor of caspase 8 activation (vICA) (McCormick et al., 2003; Menard et al., 2003; Skaletskaya et al., 2001) and mitochondrial inhibitors of cell death, referred to as viral mitochondrial inhibitor of apoptosis (vMIA) and viral inhibitor of Bak oligomerization (vIBO) (Cam et al., 2010; McCormick et al., 2005; McCormick et al., 2003). Here, we predict that vICA sensitizes virus-infected cells to programmed necrosis by inhibiting caspase 8 activity, which naturally holds programmed necrosis in check (Kaiser et al., 2011; Oberst et al., 2011). Similarly, Vaccinia virus B13R encodes a caspase inhibitor that predisposes infected cells to TNF-induced cell death (Li and Beg, 2000). In many situations, necrotic pathways have been unveiled when caspase 8 activity is absent or suppressed, and highlight the intricate redundancies and cross-talk between cell death pathways. Therefore, understanding the function of additional MCMV cell death suppressors, such as vICA, vMIA, and vIBO, in setting the stage for vIRA-inhibited programmed necrosis will provide crucial insight into the mechanisms initiating and executing host cell death defenses.
The most important RHIM of DAI (also known as RLR-A or RHIM-A) mediates direct interactions between DAI and RIP3 (Kaiser et al., 2008; Rebsamen et al., 2009), and facilitates transduction of necrotic death signals (Figure 2). However, the presence of two additional RHIM-like repeats (Kaiser et al., 2008; Rebsamen et al., 2009) raises the possibility additional protein-protein interactions regulate DAI activities. One current model suggests ligand recognition and binding induces DAI oligomerization to drive signal transduction (Wang et al., 2008), reminiscent of RIP1 and RIP3 oligomerization during necroptosis. Although our results demonstrate one RHIM of DAI is critical for virus-induced programmed necrosis, additional RHIM-dependent and -independent interactions may contribute to this pathway. Recent work showing the role of the “Ripoptosome” signaling platform in necroptosis (Feoktistova et al., 2011; Tenev et al., 2011) suggests that a DAI-RIP3 complex may engage or coordinate a similar signaling platform to execute virus-induced programmed necrosis. Additional studies will be necessary to delineate the contribution of the other potential functional DAI protein-protein interactions and motifs, including RHIMs and DNA binding domains, during MCMV infection.
Multiple cell-type specific pathways likely contribute to RIP3 activation (Zhang et al., 2011), and probably play a role in RIP3-dependent necrosis in vivo. Despite restoring replication, DAI−/− mice failed to completely normalize efficient dissemination of M45mutRHIM (Figure 4D, 4SA), which was different than the result in RIP3−/− animals. This behavior likely reflects the contribution of necroptosis in controlling the virus and explains differences between M45mutRHIM phenotypes in DAI- and RIP3-deficient mice, as DAI-deficient mice retain both RIP3 and RIP1 and the ability to initiate necroptoosis. In addition to a role in cell death, DAI influences the host IFN response against herpesviruses (DeFilippis et al., 2010a; Takaoka et al., 2007). It is worth noting that parental MCMV reached titers 10-fold higher in DAI−/− animals compared to controls, a result that was different from RIP3−/− mice where parental virus reached titers similar to controls. Elimination of DAI, while normalizing M45mutRHIM virus replication by preventing virus-induced necrosis, may also disrupt innate or adaptive host defense pathways necessary for efficient control of WT MCMV. Further characterization of DAI function during MCMV infection in vivo, as well as virus induced signaling events leading to RIP3-dependent necrosis, will be important future endeavors.
In summary, we have identified DAI as a critical mediator of RIP3-dependent anti-viral necrosis, providing evidence that DAI participates in an intrinsic host cell death pathway in vitro and in vivo. DAI expression is detected in settings where virus-induced necrosis occurs, and expression of DAI sensitizes resistant cells to this RIP3 RHIM-dependent pathway. A RIP3-DAI complex is detected during mutant virus infection, providing compelling evidence that modulation of this complex is critical for viral pathogenesis. Indeed, like RIP3 (Upton et al., 2010), loss of DAI permits vIRA-deficient viral replication in vivo, providing formal proof DAI participates in RIP3-dependent anti-viral necrosis. Inclusion of this PRR in the pathway of RIP3-dependent, MCMV-induced programmed necrosis adds to our current understanding of pathogen sensing and the intrinsic host responses elicited by these pathways.
Methods
Reagents and Viruses
BAC-derived parental WT K181and M45mutRHIM viruses were previously described (Upton et al., 2010). Dimethyl sulfoxide (DMSO, Sigma-Aldrich) and necrostatin (Nec-1, Calbiochem) were used as described (Upton et al., 2010). Growth curves were performed as previously described (Upton et al., 2010).
Plasmids, Transfections and Transductions
FLAG-tagged WT and mutRLR-A DAI (Kaiser et al., 2008) were cloned into the pQCXIH retroviral vector (Clontech). FLAG-tagged WT and mRHIM RIP3 retroviral and RIP3-A (TRCN0000022535), RIP3-B (TRCN0000022538), and control lentiviral shRNA constructs were previous described (Upton et al., 2010). Retro- and lentiviral production, infection and selection were previously described (Kaiser et al., 2008). siRNA transfections were performed with 200 pg of non-targeting, DAI, or RIP3 ON-TARGET plus SMARTpool siRNAs (Dharmacon) and Lipofectamine RNAiMAXX (Invitrogen) according to the manufacturer’s recommendations, and assays performed 48 h post transfection.
Mice, Infections, and Organ Harvests
C57BL/6 mice were from Jackson Laboratory. RIP3−/− mice (Ripk3tm1Vmd) were from Genentech (Newton et al., 2004), and DAI−/− mice (Zbp1tm1Aki)(Ishii et al., 2008) were from Shizuo Akira (Osaka University). Infections and organ titers were performed as previously described (Upton et al., 2010). Mice were maintained by Emory University Division of Animal Resources, and all procedures approved by the Emory University Institutional Animal Care and Use Committee.
Cell culture and Embryonic Fibroblast Isolation
NIH3T3 fibroblasts (ATCC CRL-1658), 3T3-SA (ATCC CCL-92), and SVEC4-10 (ATCC CRL-2181) were maintained as previously described (Upton et al., 2010). MEFs were isolated and maintained as previously described (Upton et al., 2010).
Cell Viability Assays
Viability assays were performed as previously described (Upton et al., 2010) using Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) according to the manufacturer’s instructions. Luminescence was measured on a Synergy HT Multi-Detection microplate reader (Bio-Tek). Values are plotted as % of vehicle treated or WT infected cells assessed in parallel with experimental samples.
IP and IB
IP and IB analyses were performed using established methods, as previously described (Upton et al., 2010). Antibodies used: mouse anti-β-actin (clone AC-74; Sigma), mouse anti-RIP1 (clone 38; BD Biosciences), anti-DAI (Karayel et al., 2009)(a gift from Tillman Burckstummer and Giulio Superti-Furga, CeMM), anti-MCMV M45 (Lembo et al., 2004)(a gift from David Lembo, University of Turin), rabbit anti-RIP3 (Imgenex), mouse anti-Flag (M2 clone) horseradish peroxidase (HRP) conjugate (Sigma-Aldrich), anti-mouse and anti-rabbit IgG-HRP (Vector Laboratories). IP were performed with goat anti-RIP3 (clone C-16; Santa Cruz Biotechnology) and protein A/G agarose (Santa Cruz Biotechnology).
Statistical Methods
Comparisons were performed by one way analysis of variation (ANOVA) with Bonferroni’s Multiple Comparison Tests. Unless otherwise noted, error bars designate standard error of the mean.
Supplementary Material
Highlights.
DAI sensitizes cells to RIP3-dependent, MCMV-induced programmed necrosis
DAI RHIM-dependent interactions mediate RIP3-dependent programmed necrosis
RIP3-DAI complex is targeted by MCMV vIRA
MCMV vIRA-mutant virus replication is restored in DAI-deficient mice
Acknowledgments
We thank Shizuo Akira (Osaka University) for DAI−/− mice, Vishva Dixit and Kim Newton (Genentech) for RIP3−/− mice, David Lembo (University of Turin) for anti-M45 antibody, and Tillman Bürckstümmer and Giulio Superti-Furga (CeMM) for anti-DAI antibody. This work was supported by the NIH (PHS grants R01 AI20211 and AI30363 to E.S.M. and F32 AI080175-01A1 to J.W.U.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barber GN. Cytoplasmic DNA innate immune pathways. Immunological reviews. 2011;243:99–108. doi: 10.1111/j.1600-065X.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- Benedict CA, Angulo A, Patterson G, Ha S, Huang H, Messerle M, Ware CF, Ghazal P. Neutrality of the canonical NF-kappaB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro. J Virol. 2004;78:741–750. doi: 10.1128/JVI.78.2.741-750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best SM. Viral subversion of apoptotic enzymes: escape from death row. Annual review of microbiology. 2008;62:171–192. doi: 10.1146/annurev.micro.62.081307.163009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune W, Menard C, Heesemann J, Koszinowski UH. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science. 2001;291:303–305. doi: 10.1126/science.291.5502.303. [DOI] [PubMed] [Google Scholar]
- Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, Yang X, Iotzova VS, Clarke W, Strnad J, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- Cam M, Handke W, Picard-Maureau M, Brune W. Cytomegaloviruses inhibit Bak- and Bax-mediated apoptosis with two separate viral proteins. Cell Death Differ. 2010;17:655–665. doi: 10.1038/cdd.2009.147. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol. 2010a;84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis VR, Sali T, Alvarado D, White L, Bresnahan W, Fruh KJ. Activation of the interferon response by human cytomegalovirus occurs via cytoplasmic double-stranded DNA but not glycoprotein B. J Virol. 2010b;84:8913–8925. doi: 10.1128/JVI.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature chemical biology. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, Macfarlane M, Hacker G, Leverkus M. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Comella N, Tognazzi K, Brown LF, Dvorak HF, Kocher O. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene. 1999;240:157–163. doi: 10.1016/s0378-1119(99)00419-9. [DOI] [PubMed] [Google Scholar]
- Furr SR, Chauhan VS, Moerdyk-Schauwecker MJ, Marriott I. A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells. Journal of neuroinflammation. 2011;8:99. doi: 10.1186/1742-2094-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011 doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Mocarski ES. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol. 2008;181:6427–6434. doi: 10.4049/jimmunol.181.9.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayel E, Burckstummer T, Bilban M, Durnberger G, Weitzer S, Martinez J, Superti-Furga G. The TLR-independent DNA recognition pathway in murine macrophages: Ligand features and molecular signature. Eur J Immunol. 2009;39:1929–1936. doi: 10.1002/eji.200939344. [DOI] [PubMed] [Google Scholar]
- Katdare M, Efimova EV, Labay E, Khodarev NN, Darga TE, Garofalo M, Nakamura S, Kufe DW, Posner MC, Weichselbaum RR. Diverse TNFalpha-induced death pathways are enhanced by inhibition of NF-kappaB. International journal of oncology. 2007;31:1519–1528. [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Lembo D, Donalisio M, Hofer A, Cornaglia M, Brune W, Koszinowski U, Thelander L, Landolfo S. The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J Virol. 2004;78:4278–4288. doi: 10.1128/JVI.78.8.4278-4288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann J, Rothenburg S, Deigendesch N, Eitel J, Meixenberger K, van Laak V, Slevogt H, N’Guessan PD, Hippenstiel S, Chakraborty T, et al. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- Mack C, Sickmann A, Lembo D, Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc Natl Acad Sci U S A. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AL, Meiering CD, Smith GB, Mocarski ES. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J Virol. 2005;79:12205–12217. doi: 10.1128/JVI.79.19.12205-12217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick AL, Skaletskaya A, Barry PA, Mocarski ES, Goldmacher VS. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology. 2003;316:221–233. doi: 10.1016/j.virol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Menard C, Wagner M, Ruzsics Z, Holak K, Brune W, Campbell AE, Koszinowski UH. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J Virol. 2003;77:5557–5570. doi: 10.1128/JVI.77.10.5557-5570.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2011;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011 doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletskaya A, Bartle LM, Chittenden T, McCormick AL, Mocarski ES, Goldmacher VS. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc Natl Acad Sci U S A. 2001;98:7829–7834. doi: 10.1073/pnas.141108798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annual review of biochemistry. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, Macfarlane M, Cain K, et al. The Ripoptosome, a Signaling Platform that Assembles in Response to Genotoxic Stress and Loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe T, Kalai M, Denecker G, Meeus A, Saelens X, Vandenabeele P. Necrosis is associated with IL-6 production but apoptosis is not. Cellular signalling. 2006;18:328–335. doi: 10.1016/j.cellsig.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Choi MK, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K, Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Zheng M, Zhao J, Li YY, Huang Z, Li Z, Han J. Multiple death pathways in TNF-treated fibroblasts: RIP3- and RIP1-dependent and independent routes. Cell Res. 2011;21:368–371. doi: 10.1038/cr.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.