Abstract
Background
Considerable disparities exist in colorectal cancer (CRC) incidence and mortality rates between blacks and whites in the US. We estimated how much of these disparities could be explained by differences in CRC screening and stage-specific relative CRC survival.
Methods
We used the MISCAN-Colon microsimulation model to estimate CRC incidence and mortality rates in blacks aged 50 years and older from 1975 to 2007 assuming they had: 1) the same trends in screening rates as whites instead of observed screening rates (incidence and mortality); and 2) the same trends in stage-specific relative CRC survival rates as whites instead of observed (mortality only); and 3) a combination of both. The racial disparities in CRC incidence and mortality rates attributable to differences in screening and/or stage-specific relative CRC survival were then calculated by comparing rates from these scenarios to the observed black rates.
Results
Differences in screening account for 42% of disparity in CRC incidence and 19% of disparity in CRC mortality between blacks and whites. 36% of the disparity in CRC mortality could be attributed to differences in stage-specific relative CRC survival. Together screening and survival explained a little over 50% of the disparity in CRC mortality between blacks and whites.
Conclusion
Differences in screening and relative CRC survival are responsible for a considerable proportion of the observed disparities in CRC incidence and mortality rates between blacks and whites.
Impact
Enabling blacks to achieve equal access to care as whites could substantially reduce the racial disparities in CRC burden.
Keywords: Colorectal neoplasms, healthcare disparities, early detection of cancer, survival rate, computer simulation
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States. In 2010, 142,570 people were estimated to be newly diagnosed with CRC and 51,370 were estimated to die of the disease (1). While age-adjusted CRC incidence and mortality rates have been falling for white men and women since the late 1970s to early 1980s, the decreases began later and were slower in black men and women (2). Prior to 1980, CRC incidence and mortality rates were lower in blacks than in whites (2). It is therefore unlikely that the disparity in CRC rates is the result of biological differences. Differences in social economic status and the resulting differential access to screening and treatment are more likely candidates (3).
Screening has been estimated to be the most important driver of the observed decline in CRC incidence and mortality (4). Although dissemination of CRC screening continues to be suboptimal in both whites and blacks despite recommendations of the US Preventive Services Task Force (5) and the US Multi-Society Task Force on Colorectal Cancer (6), dissemination is considerably lower for blacks than for whites (7). According to the 2008 National Health Interview Survey, 51.4% of blacks aged 50 years and older reported having either an FOBT within the past year, a sigmoidoscopy in the past 5 years, and/or colonoscopy in the past 10 years, compared to 57.0% among whites (7). In earlier years, the disparity in screening uptake was even bigger.
Treatment has been estimated to be another important driver of the observed decrease in CRC mortality (4). Several studies have shown that blacks are treated less frequently with chemotherapy and radiation therapy than whites (8–10), although randomized controlled trials showed that equal treatment leads to equal outcomes (11, 12). This treatment disparity has contributed to the poorer CRC survival in blacks compared to whites, even after correcting for potential confounding factors (13–15).
These differences in screening uptake and survival are both assumed to have contributed to the observed disparity in CRC incidence and mortality between blacks and whites (3). Differences in lifestyle factors known to influence CRC risk, such as obesity, smoking and physical activity (16), have also likely contributed to the observed disparity in CRC incidence and mortality rates. However, while differences in screening and stage-specific relative CRC survival are mostly the result of differential access to care and may be mitigated by enabling equal access, lifestyle factors such as dietary pattern and leisure time physical activity, might be more difficult to modify. In this analysis, we therefore focused on access to care: we determined to what extent equal access to care could reduce observed disparities in CRC incidence and mortality rates between blacks and whites by estimating how much of these disparities can be explained by differences in CRC screening and stage-specific relative CRC survival.
METHODS
We estimated the contribution of differences in CRC screening and stage-specific relative CRC survival between blacks and whites to disparities in CRC rates using the MISCAN-Colon microsimulation model of the Cancer Intervention and Surveillance Modeling Network (CISNET) (17, 18).
The MISCAN-Colon Model
Appendix 1 describes the MISCAN model. Briefly, the model simulates the life histories of a large population of individuals from birth to death and has a natural history component that tracks the progression of underlying colorectal disease in the absence of screening (Figure 1). As each simulated individual ages, there is a chance that one or more adenomas may develop depending on age, sex, race and individual risk. Adenomas can progress from small (1–5 mm) to medium (6–9 mm) to large (≥10 mm) size, and some may eventually become malignant. A preclinical cancer (i.e., not detected) has a chance of progressing through stages I to IV and may be detected by symptoms at any stage. With screening, adenomas and preclinical cancers may be detected depending on the sensitivity of the test for that lesion and, for endoscopic tests, whether the lesion is within reach of the endoscope.
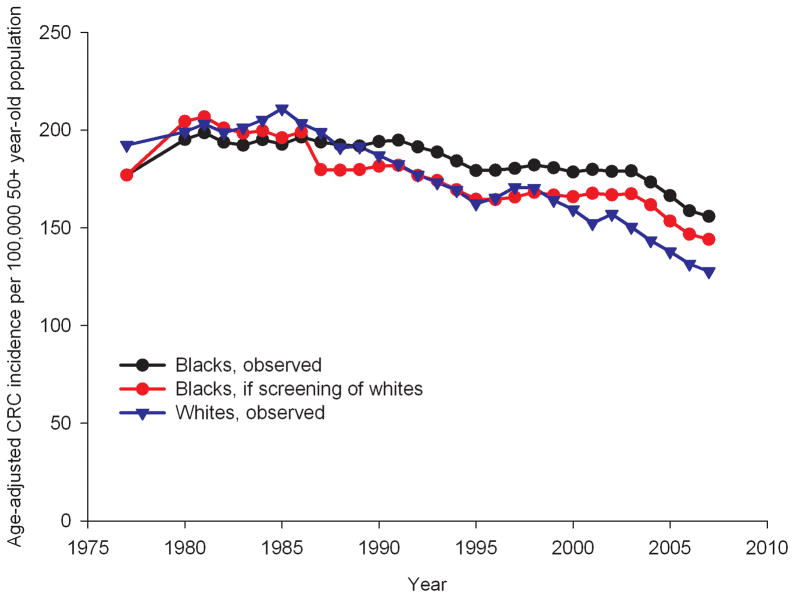
Figure 1.
Age-standardized CRC incidence rates in the 50+ year-old population from 1975 to 2007, as observed for whites and blacks, and as expected for blacks if they would have had the same screening pattern as whites*
*The rates for blacks have been smoothed by taking 3-yearly averages for each datapoint.
For each age and sex, the natural history model outcomes were calibrated to pre-screening data from autopsy studies (19–29) and clinical incidence data from the Surveillance, Epidemiology, and End-Results (SEER) Program before the introduction of screening (1975–1979) (30). The model uses race- and sex-specific all-cause mortality estimates from the US life tables. Trends in race- and sex-specific relative survival following CRC diagnosis from 1975 to 2003 were obtained from SEER (30) (Appendix 2). Estimates for screening uptake over time by age, sex and race were obtained from 7 waves of the National Health Interview Survey (31) (Table 1). We assumed no screening prior to 1978. For years for which no data were available, we linearly extrapolated trends from years for which data were available. The assumptions for the sensitivity and specificity of screening tests were based on a literature review (32). We assumed that colonoscopy reached the cecum in 98% of subjects. For sigmoidoscopy, we assumed that 80% of examinations reached the junction of the sigmoid and descending colon and 40% reached the beginning of the splenic flexure (33, 34). We assumed that 1 in 10,000 colonoscopies led to a fatal complication.
Table 1.
Age-standardized estimates* from 7 waves of the National Health Interview Survey (31) for the percent of the population aged 50 years and older ever having had a CRC test, having had home-based FOBT in the past 2 years or endoscopy in the past 10 years used by survey year, sex and race.
| Population subgroup | % Ever had CRC test | % FOBT within past two years† | % Endoscopy in past 10 years† | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1987 | 1992 | 1998 | 2000 | 2003 | 2005 | 2008 | 1992 | 1998 | 2000 | 2003 | 2005 | 2008 | 2000 | 2003 | 2005 | 2008 | |
| White Men | 35.9 | 45.7 | 52.9 | 54.4 | 58.1 | 60.4 | 66.0 | 15.6 | 19.6 | 22.5 | 22.0 | 18.3 | 16.0 | 37.1 | 42.7 | 47.4 | 55.3 |
| White Women | 35.7 | 43.9 | 47.1 | 54.8 | 55.3 | 60.9 | 64.1 | 16.9 | 19.2 | 24.9 | 21.0 | 18.0 | 15.3 | 32.0 | 36.9 | 43.9 | 49.7 |
| Black Men | 18.4 | 37.2 | 39.8 | 49.1 | 51.9 | 48.7 | 56.3 | 7.8 | 13.9 | 17.7 | 22.4 | 15.5 | 13.8 | 37.3 | 38.0 | 36.2 | 47.1 |
| Black Women | 19.5 | 36.3 | 34.1 | 43.3 | 48.4 | 51.3 | 56.6 | 11.3 | 12.0 | 20.5 | 20.4 | 16.4 | 14.4 | 22.8 | 30.4 | 37.9 | 47.0 |
FOBT = Fecal Occult Blood Test;
Although we present age-standardized estimates in this table, age-specific estimates served as inputs for the model
No questions were asked about FOBT use in the past 2 years in the first NHIS wave of 1987. No questions were asked about endoscopy use in the past 10 years in the NHIS waves of 1987, 1992 and 1998.
The validity of the model has previously been tested using the data from several large randomized screening and surveillance studies, such as the CoCap sigmoidoscopy study (35), the Minnesota Colon Cancer Control Study (35), and the National Polyp Study (36). Additionally, the model was able to reproduce the observed CRC incidence and mortality trends in the US while accounting for secular trends in risk factor prevalence, screening practice, and chemotherapy treatment (37).
Study Population
We used the model to simulate the US population from 1975 to 2007 for black men and women. We restricted our analysis to the simulated population aged 50 years and older, because this is the group for whom screening is recommended (5, 6) and incidence and mortality in this group accounts for almost 90% of total CRC incidence and mortality (38).
Colorectal Cancer Screening and Survival Runs
We used the simulation model to estimate annual age-standardized CRC incidence and mortality rates in black men and women ages 50 years and older for each year from 1975–2007 assuming trends in:
CRC screening and stage-specific relative CRC survival rates as observed for blacks (Run 1);
CRC screening rates as observed for whites and in stage-specific relative CRC survival rates as observed for blacks (Run 2);
CRC screening rates as observed for blacks and in stage-specific relative survival rates as observed for whites (Run 3);
CRC screening and stage-specific relative CRC survival rates as observed for whites (Run 4).
Analysis
Expected Colorectal Cancer Incidence and Mortality Rates
Using the results of the above runs, we then calculated three sets of expected incidence and mortality rates: 1. expected annual CRC incidence and mortality rates if blacks would have had the same screening pattern as whites; 2. expected annual CRC mortality rates if blacks would have had the same stage-specific relative CRC survival pattern as whites; and 3. expected annual CRC mortality rates if blacks would have had the same screening and stage-specific relative CRC survival pattern as whites.
The expected CRC incidence and mortality rates for blacks, assuming that they had patterns of screening similar to those in whites from 1975 to 2007, were estimated by applying the percent difference in age-adjusted incidence (mortality) rate in each year between Run 1 and Run 2 to the observed CRC incidence (mortality) rate for blacks in that year. The expected CRC mortality rates for blacks, assuming they had the same stage-specific relative CRC survival as whites, were estimated by applying the percent difference in mortality rate each year between Run 1 and Run 3 to the observed CRC mortality rate for blacks. Finally, the expected CRC mortality rates for blacks, assuming they had the same patterns of screening and stage-specific relative CRC survival as whites, were estimated by applying the percent difference in mortality rate each year between Run 1 and Run 4 to the observed CRC mortality rate for blacks.
Calculation of Disparities and Proportion of Disparities Explained by Screening and Survival
We calculated observed and expected disparities in CRC incidence and mortality as the absolute difference in age-standardized rates (the recommended measure for pairwise comparisons (39)) in 2007, the most recent year for which both incidence and mortality data are available from SEER. The observed disparity was calculated as the absolute difference in CRC incidence (mortality) rates between blacks and whites (Formula 1, Appendix 3). The expected disparity (in 2007) if blacks would have had the same screening as whites was calculated as the absolute difference between the expected CRC incidence (mortality) rate in blacks if they had had white trends in screening rates and the observed CRC incidence (mortality) in whites (Formula 2, Appendix 3). Similarly, the expected disparity in CRC mortality if blacks would have had the same stage-specific relative CRC survival as whites was calculated as the absolute difference between the expected CRC mortality rate in blacks if they had white trends in relative survival rates and the observed CRC incidence (mortality) in whites (Formula 3, Appendix 3). Finally, the expected disparity in CRC mortality if blacks would have had the same CRC screening and stage-specific relative CRC survival as whites was calculated as the absolute difference between the expected CRC mortality rate in blacks if they had white trends in screening and relative survival rates and the observed CRC incidence (mortality) in whites (Formula 4, Appendix 3).
The proportion of disparity in CRC incidence (mortality) in 2007 that was explained by screening was then calculated as the difference between the observed disparity in CRC incidence (mortality) rates and the expected disparity if blacks had white patterns of screening, expressed as the percentage of the observed disparity in CRC incidence (mortality) rates (Formula 5, Appendix 3). The proportion of disparity in CRC mortality explained by CRC survival was calculated as the difference between the observed disparity in CRC mortality rates and the disparity if blacks had white patterns of stage-specific relative CRC survival (Formula 6, Appendix 3). The total proportion of disparity that could be explained by screening and survival was calculated as the difference between the observed disparity in CRC mortality rates and the expected disparity if blacks had white patterns of CRC screening and stage-specific relative CRC survival expressed as the percentage of the observed disparity in CRC mortality rates (Formula 7, Appendix 3).
Sensitivity analyses
We performed three sensitivity analyses. First we performed an analysis in which blacks not only received less screening but also lower quality screening, assuming 25% lower adenoma detection rates with endoscopy. We then re-estimated the proportion of disparity explained by differences in screening assuming white screening adherence and quality. Second, we explored the robustness of our results to the assumption that equal access to care resulted in the same stage-specific relative CRC survival for blacks and whites by assuming that 25% of the difference in relative survival between blacks and whites could not be taken away with equal access to care. Finally, we evaluated the impact on mortality disparity if equal access to care not only resulted in the same stage-specific relative CRC survival for blacks as for whites, but also in the same stage distribution.
RESULTS
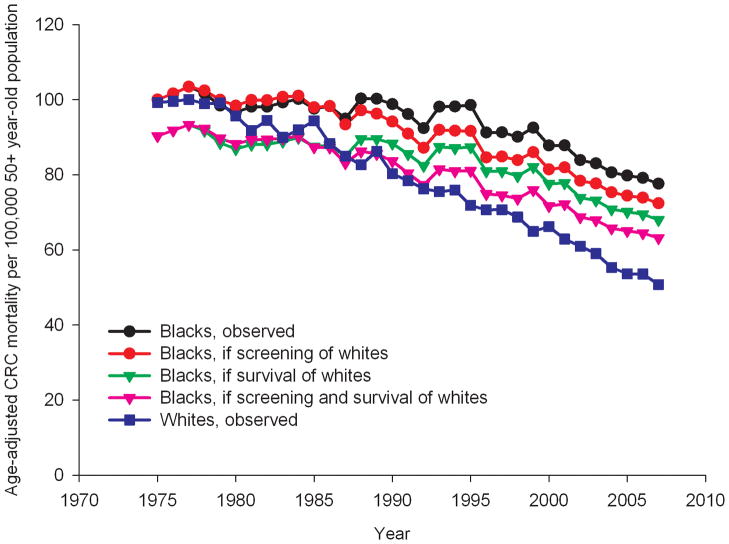
If blacks aged 50 years and older would have had the same screening pattern as observed in whites, their expected CRC incidence rate per 100,000 in 2007 would have been 144.1, 7.6% lower than the observed rate for blacks in 2007 (Figure 1). The expected CRC mortality rate would have been 72.4 per 100,000 (6.7% lower than observed, Figure 2). If blacks would have had the same trend in stage-specific relative CRC survival as whites, CRC mortality would drop to 68.0 per 100,000 in 2007, 12.4% lower than the observed rate in 2007. With the same trend in screening and stage-specific relative CRC survival as whites, CRC mortality in blacks would have been 19.6% lower than observed for a rate of 63.2 per 100,000 in 2007.
Figure 2.
Age-standardized CRC mortality rates in the 50+year old population from 1975 to 2007, as observed for whites and blacks, and as expected for blacks if they would have had the same screening pattern as whites, or the same survival pattern or a combination*
*The rates for blacks have been smoothed by taking 3-yearly averages for each datapoint.
The observed disparities in age-standardized CRC incidence and mortality rates (per 100,000) in 2007 between blacks and whites aged 50 years and older were 28.2 incident cases and 26.8 deaths, respectively (Table 2). With the same screening pattern for blacks as for whites the disparity in these rates would decrease to 16.4 incident cases and 21.6 deaths per 100,000. With the same stage-specific relative CRC survival for blacks as for whites the disparity in CRC mortality rates would decrease to 17.2 deaths per 100,000. If blacks would both have the same screening and stage-specific relative CRC survival as whites, the disparity in CRC mortality would further decrease to 12.4 deaths per 100,000. As such, differences in CRC screening explained 42% of the observed disparity in CRC incidence between blacks and whites and 19% of disparity in CRC mortality. Stage-specific relative CRC survival differences explained 36% of the disparity in CRC mortality. Together differences in screening and survival explained 54% of disparity in CRC mortality.
Table 2.
Base case analysis: Disparities in CRC incidence and mortality between blacks and whites aged 50 years and older, as observed in 2007 and as expected if blacks had the same CRC screening and stage-specific relative CRC survival patterns as whites.
| Sex | CRC incidence disparity* | CRC mortality disparity* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Expected with same screening | % explained by screening | Observed | Expected with same screening | % explained by screening | Expected with same survival | % explained by survival | Expected with same screening and survival† | % explained by screening and survival† | |
| Male | 30.0 | 14.8 | 51% | 35.3 | 28.7 | 19% | 23.4 | 34% | 17.4 | 51% |
| Female | 29.8 | 20.3 | 32% | 23.2 | 18.9 | 19% | 15.0 | 35% | 11.0 | 53% |
| Total | 28.2 | 16.4 | 42% | 26.8 | 21.6 | 19% | 17.2 | 36% | 12.4 | 54% |
Per 100,000 50+-year old population. Disparity is defined as the absolute difference in rates in 2007. For example, the observed disparity in CRC incidence (28.2 per 100,000 50+-year old population) is calculated as the observed CRC incidence rate in blacks in 2007 (155.9 final datapoint of black line in Figure 1) minus the observed CRC incidence rate in whites in 2007 (127.7; final datapoint of red line in Figure 1).
Because of synergy in effects separate effects of screening and survival do not add up.
Results for men and women separately were similar (Table 2), with the exception of the percent of disparity in CRC incidence explained by screening. For men, 51% of the observed disparity in CRC incidence between blacks and whites could be explained by screening, where for women this was 32%.
Sensitivity analysis
If blacks not only receive less screening but also lower-quality screening, the percent of disparity in CRC incidence and mortality explained by screening increased to 68% and 34%, respectively (Table 3). If 25% of difference in stage-specific relative CRC survival between blacks and whites cannot be removed through equal access to care, only 28% of the disparity in CRC mortality could be attributed to differences in treatment. Finally, if equal access to care not only results in the same stage-specific relative CRC survival in blacks as in whites but also in the same stage distribution, 60% of the disparity in CRC mortality was attributable to differences in access to care.
Table 3.
Sensitivity analyses: Percent of disparities in CRC incidence and mortality between blacks and whites aged 50 years and older explained by screening and survival.
| Sensitivity analysis | CRC incidence disparity % explained by screening | CRC mortality disparity | ||
|---|---|---|---|---|
| % explained by screening | % explained by survival | % explained by screening and survival* | ||
| Base case analysis | 42% | 19% | 36% | 54% |
| Lower-quality endoscopy in blacks | 68% | 34% | 36% | 66% |
| 25% remaining survival difference | 42% | 19% | 28% | 46% |
| Same stage distribution as whites | 42% | 19% | 57% | 60% |
Because of synergy in effects separate effects of screening and survival do not add up.
DISCUSSION
This study shows that more than 40% of disparity in CRC incidence and approximately 20% of disparity in CRC mortality between blacks and whites can be explained by differences in screening uptake. Approximately 35% of the disparity in CRC mortality can be explained by differences in stage-specific relative CRC survival. Together differences in screening and relative survival explain a little over 50% of disparity in CRC mortality between blacks and whites.
Although differences in screening uptake and survival explain approximately half of the racial disparities in CRC incidence and mortality, the other half cannot be explained by these factors. A closer look at the data reveals that this finding would have been anticipated. Where racial disparities in CRC incidence and mortality have been increasing over the years (3), the disparity in screening has been stable, and even decreasing slightly (Table 1). The most recent estimates for colorectal screening uptake report a less than 5% difference in uptake between whites (59.8%) and blacks (55.0%) (40). If screening would have explained the vast majority of the difference in CRC incidence and mortality, we would have expected the same diverging trend for screening rates as for CRC rates. Moreover, part of the observed disparity, especially in males, is caused by an increase in CRC incidence and mortality rates in blacks over the period 1975–1980 (38), which was before the introduction or wide dissemination of screening modalities and/or adjuvant chemotherapies. Since this increase in CRC incidence and mortality cannot be a result of screening or survival, part of the disparity could never be explained by these factors.
Other factors that may have contributed to the racial disparity in CRC incidence and mortality rates are differences in susceptibility, quality of care, and lifestyle. The prevalence of polymorphisms associated with CRC risk has been shown to differ between whites and blacks (41). On the other hand, several studies have shown that most of black/white differences in CRC outcomes such as stage of disease at diagnosis or survival are no longer present when correcting for socioeconomic status, including health insurance status (8). Furthermore, a follow-up study of Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial participants after a positive sigmoidoscopy revealed a lower uptake of diagnostic colonoscopies among blacks when compared with whites but little differences in the yield of colorectal neoplasia (42). Finally, CRC incidence and mortality in blacks were lower or comparable to whites in the 1970s (30), making it unlikely that susceptibility is an important driver of current disparities.
In our primary analysis, we only considered uptake of screening, assuming equal quality of screening among blacks and whites. Quality of endoscopy has been shown to be dependent on the skill of the endoscopist performing the procedure, with adenoma detection rates of high adenoma detectors being double that of low adenoma detectors (43–47). Adenoma detection rate is an independent predictor of the risk of interval CRC after screening colonoscopy (47). Physicians treating black patients are known to be less well trained and have less access to clinical resources than physicians treating white patients (48). Different quality of screening between blacks and whites is therefore not improbable. The sensitivity analysis indeed showed that if blacks received lower-quality screening, a larger proportion of the CRC disparity would be explained by screening. We have eliminated disparities in quality of stage-specific treatments, by evaluating the impact of assuming the stage-specific relative CRC survival of whites for blacks. However, we have not eliminated potential disparities in timeliness of treatment. Blacks are known to present with more advanced stage of disease than whites (41). Although part of this difference may be explained by differences in screening uptake, timeliness of care-seeking may also play an important role. In a sensitivity analysis assuming the same stage distribution for blacks as for whites (in the absence of screening) the proportion of disparity in CRC mortality explained by access to care indeed increased.
Known or unknown lifestyle factors are the most likely candidates to explain the remaining 34–46% (Table 3) of the disparity in CRC incidence and mortality that cannot explained by screening and stage-specific relative CRC survival differences. Several lifestyle factors are known to be associated with CRC risk. Alcohol, smoking, obesity, and meat consumption increase the risk of CRC, whereas physical activity and post menopausal hormone replacement therapy (in women) decrease risk (49). Smoking prevalence had been higher in black men than white men until the late nineties (50). Furthermore, obesity has been consistently higher in blacks than in whites since 1970, while rates of physical activity have consistently been lower (50). We have not explicitly evaluated the effect of these established lifestyle factors. Instead, we decided to focus on the more actionable items of screening and survival, because lifestyle factors might be more difficult to modify.
Five limitations are noteworthy. First, we assumed black-white differences in adenoma onset, CRC location and stage distribution between blacks and whites, but not in types of cancers or tumor aggressiveness. This assumption is supported by the fact that CRC incidence and mortality used to be lower in blacks than in whites (30). Second, we did not incorporate risk factors into the model. As a result of that limitation and because of other potential exogenous factors, the simulated CRC incidence and mortality levels will not correspond with the observed. Instead, we assumed that the simulated relative benefit of white screening and stage-specific relative CRC survival patterns over black would be applicable to the observed CRC incidence and mortality. This approach mirrors a relative-risk approach, where it is assumed that the relative risk of e.g. screening is constant irrespective of the background incidence and mortality level. This assumption seems reasonable: all three randomized controlled trials on biennial guaiac FOBT screening found similar percent mortality reductions ranging from 15–21% despite being performed in populations with a different background incidence level (51–53).
Third, screening uptake in the model was based upon estimated test rates from multiple waves of the National Health Interview Survey. These tests may have been performed for other reasons than screening. Furthermore, the estimates from these surveys may be biased because they are based on self-report; the data are not longitudinal so we had to make assumptions for screening patterns within individuals in the model; the questions on CRC screening have changed from survey to survey; and the respondents may not be representative for the US population as a whole. This last bias in particular may have influenced our results if the bias differs for whites versus blacks. If we have overestimated screening rates in blacks, the contribution of screening differences to the observed disparities in CRC rates may be higher than the estimated 40–50%.
Fourth, CRC incidence and mortality data for blacks are sparse. For this analysis, we therefore used 3-year pooled estimates for CRC incidence and mortality rates over time. However, even with this pooling, an interesting discrepancy exists between the racial disparities in CRC incidence and mortality for women. The absolute racial disparity in CRC incidence is larger than that for mortality, where the opposite would have been expected (as can be seen for men). As a result, the proportion of CRC incidence disparity that can be explained by screening is much smaller for women than for men, whereas the results for CRC mortality are similar. Fifth, we restricted our analysis to the simulated population aged 50 years and older, because this is the group for whom screening is recommended (5, 6) and incidence and mortality in this group accounts for almost 90% of total CRC incidence and mortality (38). CRC incidence and mortality is disproportionally higher in blacks among people younger than 50. Racial disparities in survival could have played a role in this difference.
Finally, we have not explicitly considered racial differences in treatment but used racial differences in stage-specific relative CRC survival as a proxy. Data on use and quality of CRC treatment by race are sparse, especially for the population below 65 years of age. There is some information on chemotherapy use (9, 10, 41), but data on other types of treatment such as surgery and radiotherapy are hard to come by. If part of the racial differences in survival cannot be explained by differences in (quality of) treatment, we have overestimated the potential for reducing disparities in CRC mortality. A systematic review of cancer-specific survival differences between blacks and whites showed that only modest cancer-specific survival differences are evident for blacks and whites treated comparably for similar-stage cancer. For CRC, no difference was found (54). Therefore, differences in cancer biology between racial groups are unlikely to be responsible for a substantial portion of the observed discrepancy in stage-specific relative CRC survival (54). We explored the impact of our assumption in a sensitivity analysis and found that the effect was limited.
Although differences in screening and stage-specific relative CRC survival do not explain all of the observed racial disparities in CRC incidence and mortality, they do explain roughly half. Measures should therefore be taken to eliminate the gaps in screening use and survival between blacks and whites. The National Institutes of Health’s State-of-the-Science conference has concluded that elimination of financial barriers should be the first priority area to enhance the use of CRC screening (55). The Affordable Care Act may be an important step towards this elimination. The Act aims to improve access to quality health care for all Americans (56). Furthermore, all new health plans must cover certain preventive services including CRC screening without charging a deductible, co-pay or coinsurance. Several studies have shown that in situations with equal access to care, such as military medical centers, Department of Defense facilities, Medicare, Medicaid or clinical trials, no differences in screening uptake or CRC treatments between blacks and whites exist (57–62).
In conclusion, this study shows that approximately half of the disparities in CRC incidence and mortality rates between blacks and whites can be explained by differences in screening and survival. Enabling blacks to achieve equal access to care as whites could therefore substantially reduce the racial disparities in CRC burden.
Supplementary Material
Acknowledgments
Funding
This research was financially supported by the National Cancer Institute at the National Institutes of Health (U01-CA-088204, U01-CA-097426, U01-CA-115953 and U01-CA-152959). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
This research was conceived and developed while the first author (Dr. Iris Lansdorp-Vogelaar) was a visiting scientist at American Cancer Society.
Abbreviations
- CRC
Colorectal Cancer
- SEER
Surveillance, Epidemiology, and End-Results
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Colorectal Cancer Facts & Figures 2008–2010. Atlanta, GA: American Cancer Society; 2008. [Google Scholar]
- 3.DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2908–12. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Screening for colorectal cancer: U S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 6.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 7.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry J, Bumpers K, Ogunlade V, Glover R, Davis S, Counts-Spriggs M, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27(1):59–83. doi: 10.1080/07347330802614840. [DOI] [PubMed] [Google Scholar]
- 9.Hao Y, Landrine H, Jemal A, Ward KC, Bayakly AR, Young JL, Jr, et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health. 2010 doi: 10.1136/jech.2009.096008. [DOI] [PubMed] [Google Scholar]
- 10.Obeidat NA, Pradel FG, Zuckerman IH, Trovato JA, Palumbo FB, DeLisle S, et al. Racial/ethnic and age disparities in chemotherapy selection for colorectal cancer. Am J Manag Care. 2010;16(7):515–22. [PubMed] [Google Scholar]
- 11.Dignam JJ, Ye Y, Colangelo L, Smith R, Mamounas EP, Wieand HS, et al. Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy randomized trials. J Clin Oncol. 2003;21(3):413–20. doi: 10.1200/JCO.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander DD, Waterbor J, Hughes T, Funkhouser E, Grizzle W, Manne U. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomark. 2007;3(6):301–13. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960–2005. Am J Public Health. 2010;100(10):1912–6. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116(19):4622–31. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–42. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32(1):13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 18.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis [see comments] J Natl Cancer Inst. 2000;92(7):557–63. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 19.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7:249–61. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 20.Blatt L. Polyps of the Colon and Rectum: Incidence and Distribution. Dis Colon Rectum. 1961;4:277–282. [Google Scholar]
- 21.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61(7):1472–6. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–6. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark JC, Collan Y, Eide TJ, Esteve J, Ewen S, Gibbs NM, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36(2):179–86. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 24.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 25.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33(11):1508–14. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24(7):799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 27.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43(5):1847–57. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 28.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49(4):819–25. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23(10):835–42. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surveillance Epidemiology and End Results (SEER) Program. Based on the November 2003 submission. Bethesda, MD: National Cancer Institute; Apr, 2004. SEER*Stat Database: Incidence - SEER 9 Regs Public Use. Nov 2003 Sub (1973–2001), DCCPS, Surveillance Research Program, Cancer Statistics Branch. www.seer.cancer.gov. [Google Scholar]
- 31.National Health Interview Survey. Centers for Disease Control and Prevention, National Center for Health Statistics; www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- 32.Zauber A, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Cost-effectiveness of DNA stool testing to screen for colorectal cancer; Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models; 2007. [PubMed] [Google Scholar]
- 33.Adam Ali. Shorthouse. How accurate is the endoscopist’s assessment of visualization of the left colon seen at flexible sigmoidoscopy? Colorectal Disease. 2000;2(1):41–44. doi: 10.1046/j.1463-1318.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 34.Doria-Rose VP, Levin TR, Selby JV, Newcomb PA, Richert-Boe KE, Weiss NS. The incidence of colorectal cancer following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology. 2004;127(3):714–22. doi: 10.1053/j.gastro.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 35.Loeve F, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JDF. Final report MISCAN-COLON microsimulation model for colorectal cancer: report to the National Cancer Institute Project No. NO1-CN55186. Rotterdam: Department of Public Health, Erasmus University; 1998. [Google Scholar]
- 36.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, et al. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111(4):633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 37.Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, et al. How much can current interventions reduce colorectal cancer mortality in the U.S? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107(7):1624–33. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 38.American Cancer Society. Colorectal Cancer Facts & Figures 2011–2013. Atlanta: 2011. [Google Scholar]
- 39.Harper S, Lynch J. Methods for Measuring Cancer Disparities: Using Data Relevant to Healthy People 2010 Cancer-Related Objectives. Bethesda, MD: National Cancer Institute; 2005. NIH Publication No. 05-5777. [Google Scholar]
- 40.Klabunde C, Brown M, Ballard-Barbash R, White MC, Thompson T, Plescia M, Coleman King S. Cancer Screening — United States, 2010. Morbidity and Mortality Weekly Report. 2012;61(03):41–45. [PubMed] [Google Scholar]
- 41.Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol. 2009;15(30):3734–43. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laiyemo AO, Doubeni C, Pinsky PF, Doria-Rose VP, Bresalier R, Lamerato LE, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010;102(8):538–46. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkin W, Rogers P, Cardwell C, Cook C, Cuzick J, Wardle J, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126(5):1247–56. doi: 10.1053/j.gastro.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Bretagne JF, Hamonic S, Piette C, Manfredi S, Leray E, Durand G, et al. Variations between endoscopists in rates of detection of colorectal neoplasia and their impact on a regional screening program based on colonoscopy after fecal occult blood testing. Gastrointest Endosc. 2010;71(2):335–41. doi: 10.1016/j.gie.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 45.Bretthauer M, Skovlund E, Grotmol T, Thiis-Evensen E, Gondal G, Huppertz-Hauss G, et al. Inter-endoscopist variation in polyp and neoplasia pick-up rates in flexible sigmoidoscopy screening for colorectal cancer. Scand J Gastroenterol. 2003;38(12):1268–74. doi: 10.1080/00365520310006513. [DOI] [PubMed] [Google Scholar]
- 46.Imperiale TF, Glowinski EA, Juliar BE, Azzouz F, Ransohoff DF. Variation in polyp detection rates at screening colonoscopy. Gastrointest Endosc. 2009;69(7):1288–95. doi: 10.1016/j.gie.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 47.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 48.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351(6):575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 49.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125(1):171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 50.Cancer Trends Progress Report. Bethesda, MD: 2010. [Google Scholar]
- 51.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 52.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 53.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 54.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 55.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):663–7. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 56.U.S. Department of Health & Human Services. The Health Care Law & You. 2012 http://www.healthcare.gov/law/introduction/index.html.
- 57.Hassan MO, Arthurs Z, Sohn VY, Steele SR. Race does not impact colorectal cancer treatment or outcomes with equal access. Am J Surg. 2009;197(4):485–90. doi: 10.1016/j.amjsurg.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Hofmann LJ, Lee S, Waddell B, Davis KG. Effect of race on colon cancer treatment and outcomes in the Department of Defense healthcare system. Dis Colon Rectum. 2010;53(1):9–15. doi: 10.1007/DCR.0b013e3181bdcdb2. [DOI] [PubMed] [Google Scholar]
- 59.Berry J, Caplan L, Davis S, Minor P, Counts-Spriggs M, Glover R, et al. A black-white comparison of the quality of stage-specific colon cancer treatment. Cancer. 2010;116(3):713–22. doi: 10.1002/cncr.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanoff HK, Sargent DJ, Green EM, McLeod HL, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27(25):4109–15. doi: 10.1200/JCO.2009.21.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DuBard CA, Yow A, Bostrom S, Attiah E, Griffith B, Lawrence W. Racial/ethnic differences in quality of care for North Carolina Medicaid recipients. N C Med J. 2009;70(2):96–101. [PubMed] [Google Scholar]
- 62.Shih YC, Zhao L, Elting LS. Does Medicare coverage of colonoscopy reduce racial/ethnic disparities in cancer screening among the elderly? Health Aff (Millwood) 2006;25(4):1153–62. doi: 10.1377/hlthaff.25.4.1153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.