Abstract
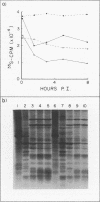
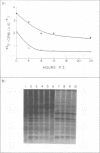
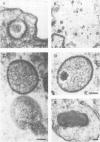
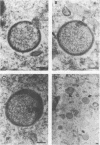
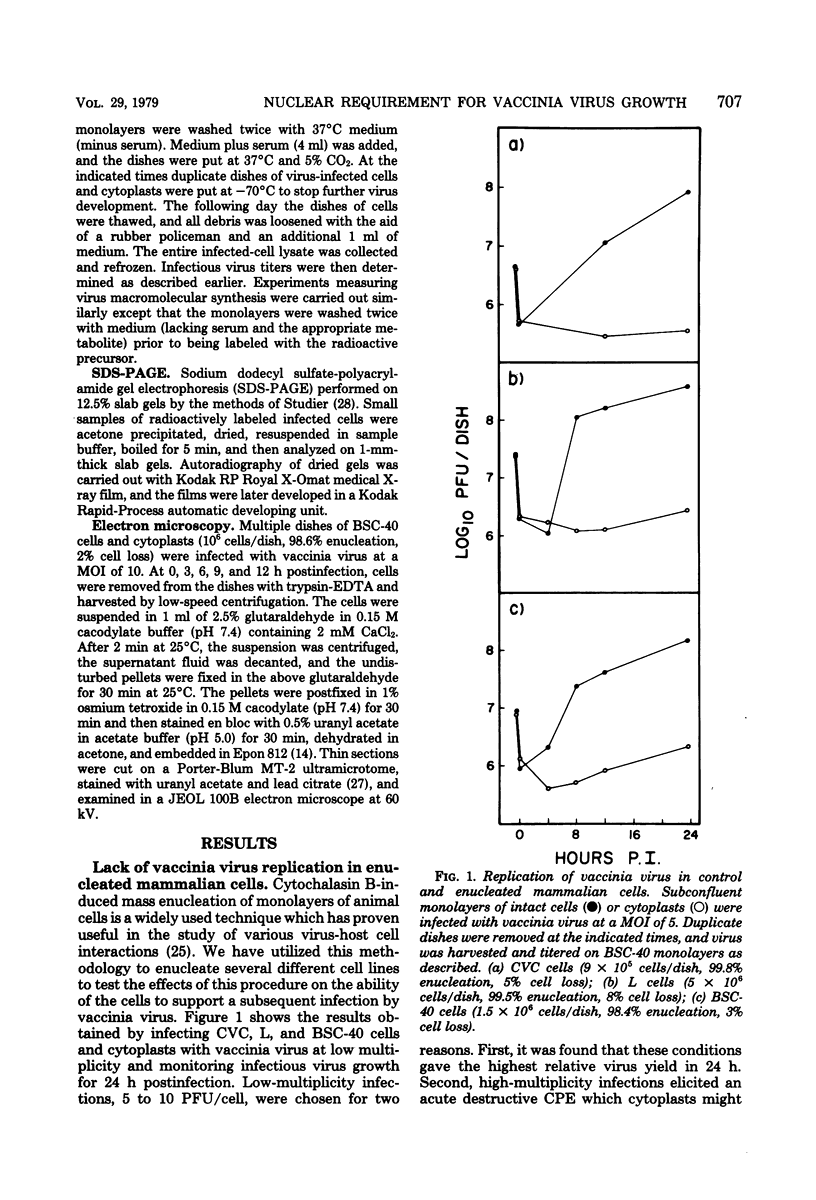
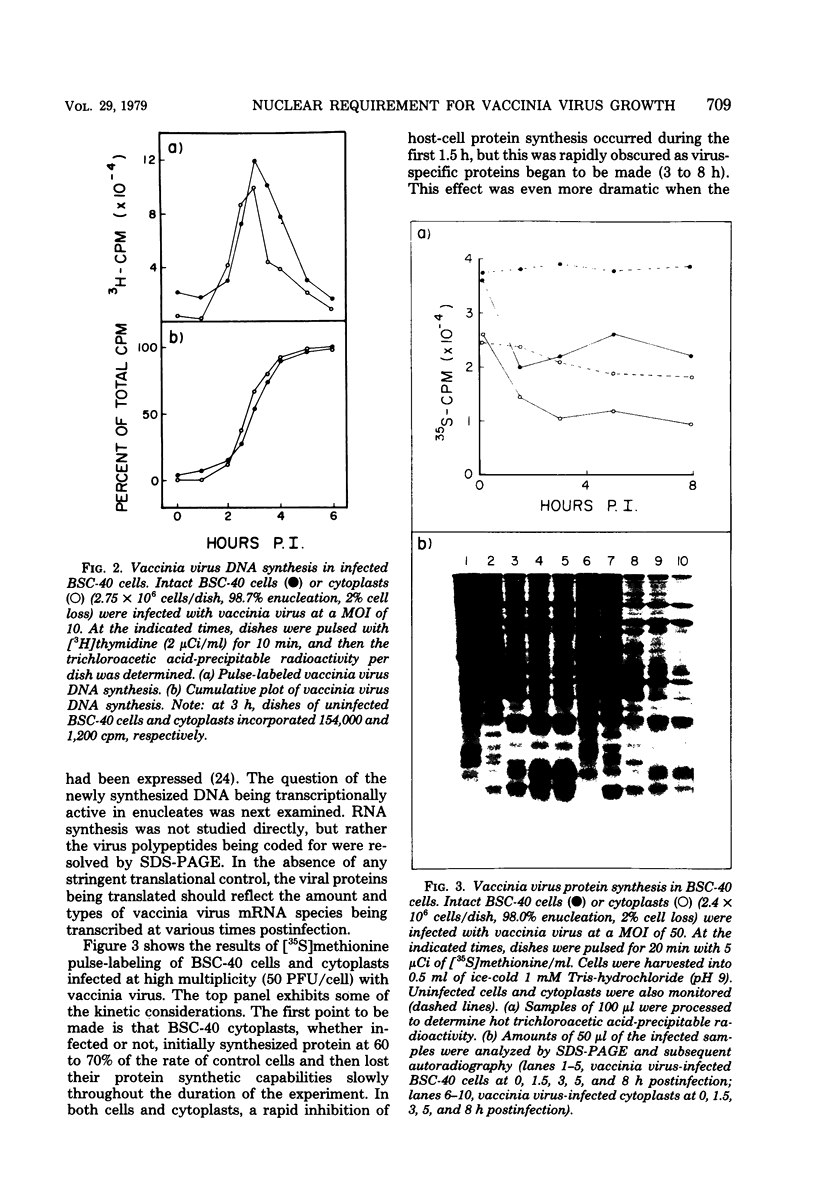
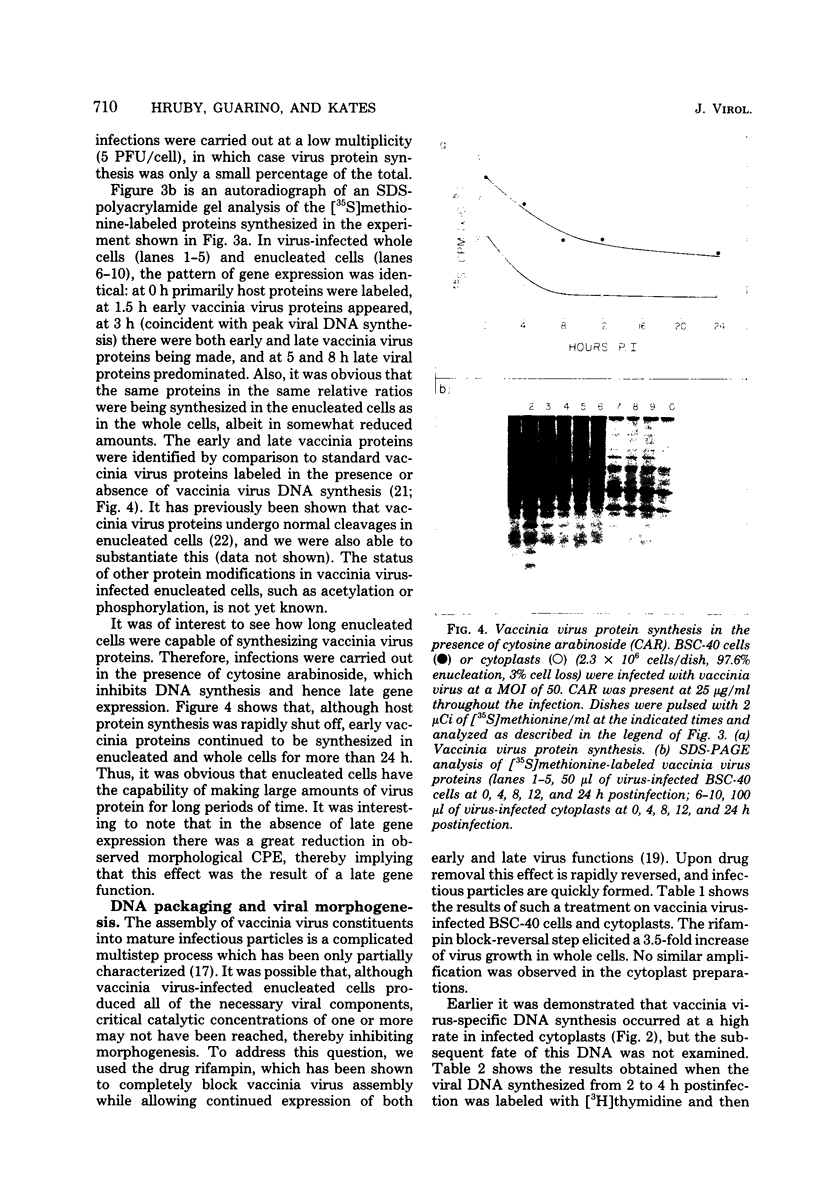
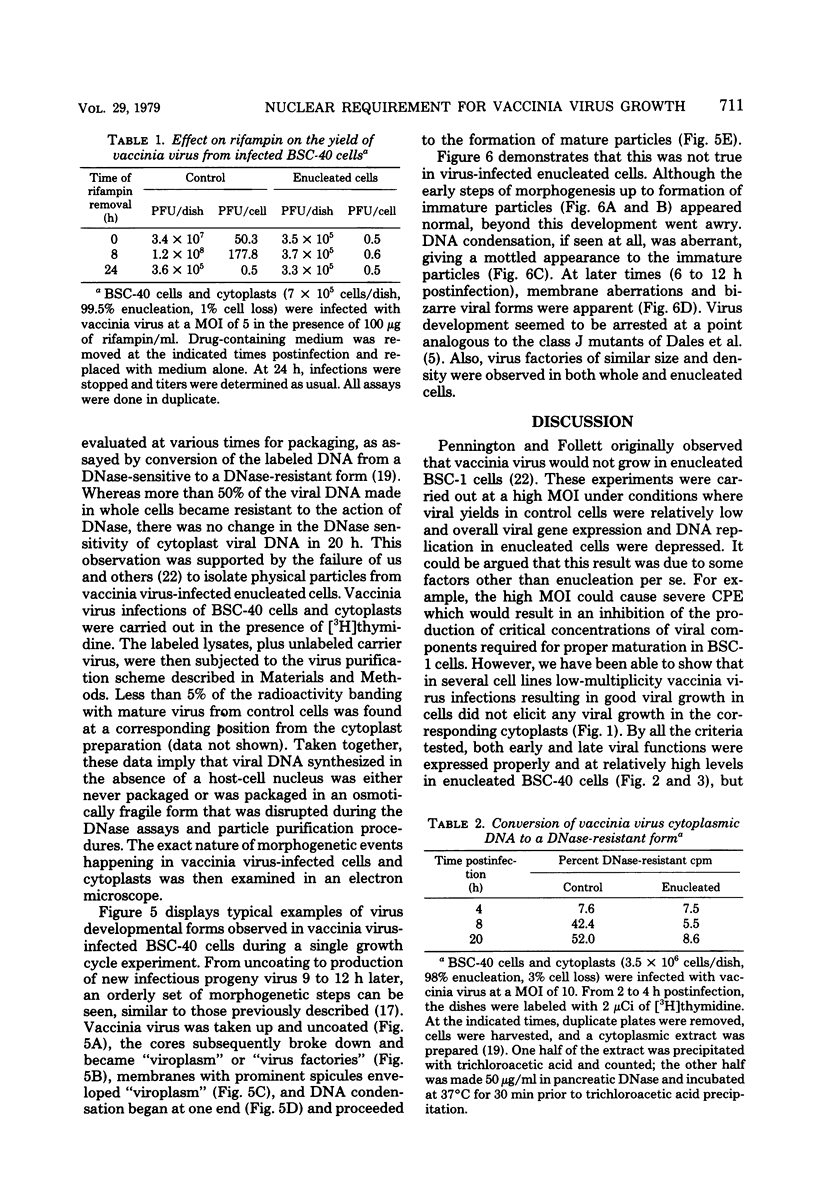
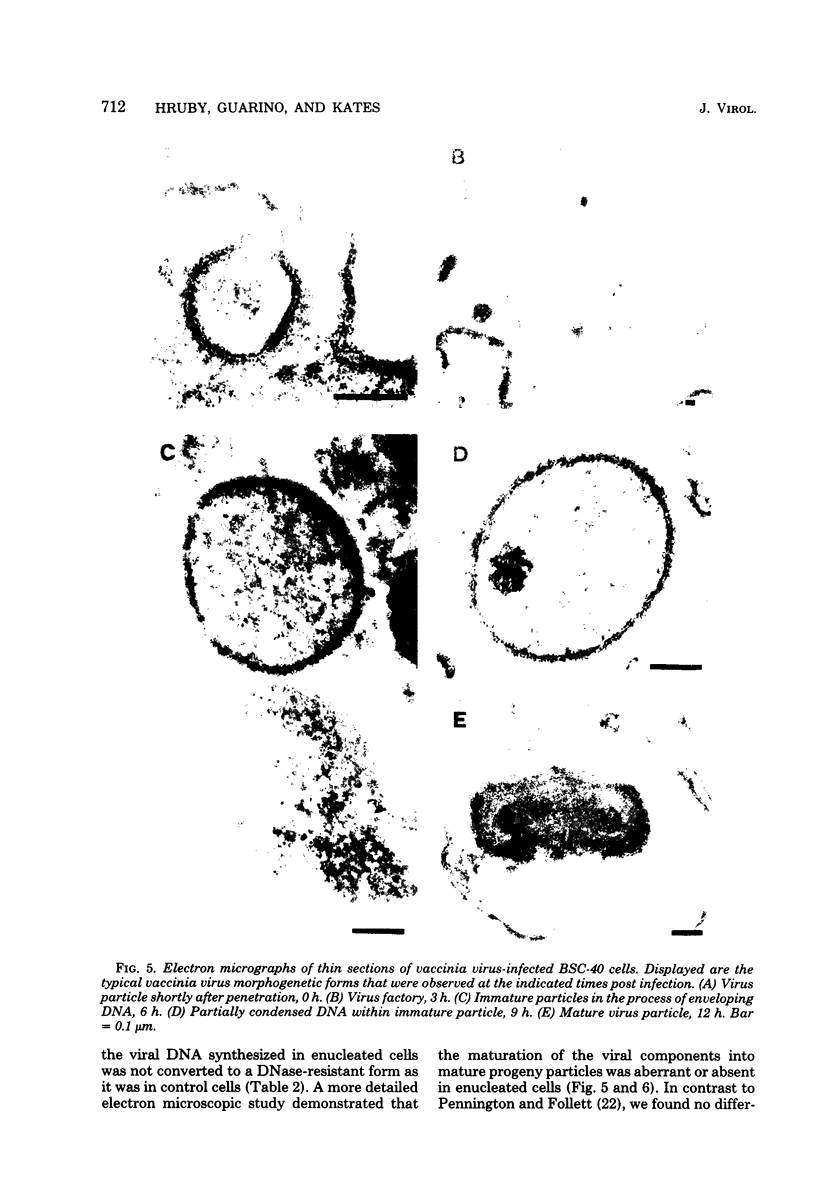
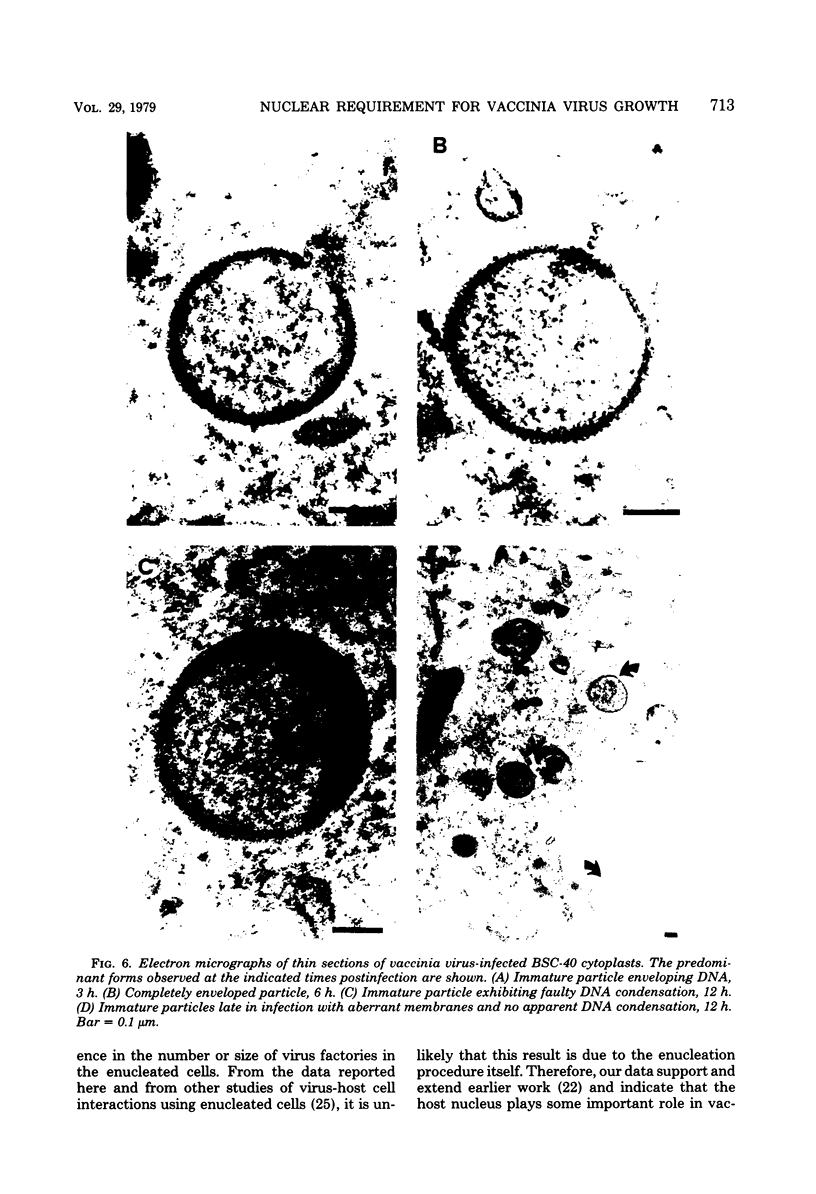
Using cytochalasin B-induced enucleation techniques, we examined the ability of vaccinia virus to replicate in the absence of the host-cell nucleus in several mammalian cell lines. It was found that virus-infected enucleated cells (cytoplasts) prepared from BSC-40, CVC, and L cells were incapable of producing infectious progeny virus. The nature of this apparent nuclear involvement was studied in detail in BSC-40 cells. Modulations designed to maximize cytoplast integrity and longevity, such as reduction of the growth temperature and initial multiplicity of infection, did not improve virus growth in cytoplasts. Sodium dodecyl sulfate-polyacrylamide gel analysis of the [35S]methionine pulse-labeled proteins synthesized in vaccinia virus-infected cytoplasts demonstrated that both early and late viral gene products were being expressed at high levels and with the proper temporal sequence. Vaccinia virus cytoplasmic DNA synthesis, as measured by [3H]thymidine incorporation, peaked at 3 h postinfection and was 70 to 90% of control levels in cytoplasts. However, in the cytoplasts this DNA was not converted to a DNase-resistant form late in infection, which was consistent with the failure to isolate physical particles from infected cytoplasts. Treatment of vaccinia virus-infected cells with 100 μg of rifampin/ml from 0 to 8 h to increase the pools of viral precursors, followed by subsequent removal of the drug, resulted in a threefold increase virus yield. This treatment had no effect on virus-infected cytoplasts. Finally, vaccinia virus morphogenesis was studied under an electron microscope in thin sections of virus-infected cells and cytoplasts which had been prepared at various times during a single-step growth cycle. It was apparent that, although early virus morphogenetic forms appeared, there was no subsequent DNA condensation or particle maturation in the cytoplasts. These results suggest that vaccinia virus requires some factor or function from the host-cell nucleus in order to mature properly and produce infectious progeny virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D. Radiation enhanced reactivation of nuclear replicating mammalian viruses. Photochem Photobiol. 1977 May;25(5):477–482. doi: 10.1111/j.1751-1097.1977.tb09173.x. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Deitch A. D., Sawicki S. G., Godman G. C., Tanenbaum S. W. Enhancement of viral infectivity by cytochalasin. Virology. 1973 Dec;56(2):417–428. doi: 10.1016/0042-6822(73)90046-9. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J Gen Virol. 1975 Feb;26(2):183–196. doi: 10.1099/0022-1317-26-2-183. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford L. G., Randall C. C. Virus-specific RNA and DNA in nuclei of cells infected with fowlpox virus. Virology. 1976 Jan;69(1):1–14. doi: 10.1016/0042-6822(76)90189-6. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Katz E., Margalith E., Winer B. Inhibition of vaccinia virus growth by the nucleoside analogue 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ribavirin). J Gen Virol. 1976 Aug;32(2):327–330. doi: 10.1099/0022-1317-32-2-327. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaColla P., Weissbach A. Vaccinia virus infection of HeLa cells. I. Synthesis of vaccinia DNA in host cell nuclei. J Virol. 1975 Feb;15(2):305–315. doi: 10.1128/jvi.15.2.305-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Initiation of vaccinia virus infection in actinomycin D-pretreated cells. J Virol. 1968 Jul;2(7):678–685. doi: 10.1128/jvi.2.7.678-685.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Enders J. F. Vaccinia virus replication and cytopathic effect in cultures in phytohemagglutinin-treated human peripheral blood leukocytes. J Virol. 1968 Aug;2(8):787–792. doi: 10.1128/jvi.2.8.787-792.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. Vaccinia virus reexamined: development and release. Virology. 1976 Aug;73(1):43–58. doi: 10.1016/0042-6822(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Vińuela E. Requirement of cell nucleus for African swine fever virus replication in Vero cells. J Virol. 1977 Mar;21(3):902–905. doi: 10.1128/jvi.21.3.902-905.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Sykes J. M., Hunt T. Characteristics of a coupled cell-free transcription and translation system directed by vaccinia cores. Eur J Biochem. 1978 Jan 2;82(1):199–209. doi: 10.1111/j.1432-1033.1978.tb12012.x. [DOI] [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Vaccinia virus replication in enucleate BSC-1 cells: particle production and synthesis of viral DNA and proteins. J Virol. 1974 Feb;13(2):488–493. doi: 10.1128/jvi.13.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Enucleation as a technique in the study of virus-host interactions. Curr Top Microbiol Immunol. 1977;76:49–82. doi: 10.1007/978-3-642-66653-7_2. [DOI] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M. Effect of mitomycin C on the growth of some animal viruses. Proc Natl Acad Sci U S A. 1961 Aug;47:1212–1217. doi: 10.1073/pnas.47.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Walen K. H. Nuclear involvement poin xvirus infection. Proc Natl Acad Sci U S A. 1971 Jan;68(1):165–168. doi: 10.1073/pnas.68.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]