Abstract
Positron emission tomography (PET) findings suggesting lower D2-type dopamine receptors and dopamine concentration in brains of stimulant users have prompted speculation that increasing dopamine signaling might help in drug treatment. However, this strategy needs to consider the possibility, based on animal and postmortem human data, that dopaminergic activity at the related D3 receptor might, in contrast, be elevated and thereby contribute to drug-taking behavior. We tested the hypothesis that D3 receptor binding is above normal in methamphetamine (MA) polydrug users, using PET and the D3-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin ([11C]-(+)-PHNO). Sixteen control subjects and 16 polydrug users reporting MA as their primary drug of abuse underwent PET scanning after [11C]-(+)-PHNO. Compared with control subjects, drug users had higher [11C]-(+)-PHNO binding in the D3-rich midbrain substantia nigra (SN; +46%; p < 0.02) and in the globus pallidus (+9%; p = 0.06) and ventral pallidum (+11%; p = 0.1), whereas binding was slightly lower in the D2-rich dorsal striatum (approximately −4%, NS; −12% in heavy users, p = 0.01) and related to drug-use severity. The [11C]-(+)-PHNO binding ratio in D3-rich SN versus D2-rich dorsal striatum was 55% higher in MA users (p = 0.004), with heavy but not moderate users having ratios significantly different from controls. [11C]-(+)-PHNO binding in SN was related to self-reported “drug wanting.” We conclude that the dopamine D3 receptor, unlike the D2 receptor, might be upregulated in brains of MA polydrug users, although lower dopamine levels in MA users could have contributed to the finding. Pharmacological studies are needed to establish whether normalization of D3 receptor function could reduce vulnerability to relapse in stimulant abuse.
Introduction
Animal data have long suggested that the neurotransmitter dopamine (DA) plays a role in several aspects of addiction, including addiction to the dopaminergic stimulant drugs methamphetamine (MA) and cocaine. However, despite years of intense investigation, no pharmacotherapy, DA related or other, has been approved for treatment of stimulant dependence (Kish, 2008). Whereas evidence points to low levels of DA and DA D2 receptor number in stimulant users (Wilson et al., 1996; Volkow et al., 2001, 2009; Martinez et al., 2004, 2011; Lee et al., 2009; Wang et al., 2011), some animal and postmortem human data in cocaine users (Staley and Mash, 1996; Mash, 1997; Segal et al., 1997; Sokoloff et al., 2001) suggest that activity at the D3 receptor, a member of the D2-like receptor family, may be pathologically increased in addiction. If correct, a therapeutic strategy aimed at a generalized increase in DA signaling might successfully address a D2 deficiency but exacerbate an already exaggerated D3-related process.
Over the past 20 years, interest in the novel D3 receptor, for which the function is still unknown, has developed in large part because of the strikingly high preferential expression of the receptor in limbic brain areas associated with reward and motivation (e.g., ventral limbic striatum) (Sokoloff et al., 1990; Murray et al., 1994), whereas the D2 receptor is distributed uniformly throughout the striatum (Sokoloff et al., 1990). In animal models, D3-selective antagonists decrease drug-seeking behavior, raising the possibility that this receptor modulates motivation to self-administer drugs (Le Foll et al., 2005; Heidbreder and Newman, 2010). Animal data also suggest that sensitization to DA-elevating drugs [long hypothesized to explain stimulant addiction in humans (Robinson and Berridge, 1993)] secondary to repeated dopaminergic stimulation coincides with increased D3 receptor expression in ventral, but also dorsal striatal, regions that do not normally express high levels of D3 (Bordet et al., 1997). Together, the findings tentatively suggest that increased transmission at the D3 receptor in limbic striatal and ectopic regions could underlie some aspects of psychostimulant addiction.
With the recent development of a D3-preferring positron emission tomography (PET) radiotracer, [11C]-(+)-propyl-hexahydro-naphtho-oxazin ([11C]-(+)-PHNO) (Wilson et al., 2005), it has become possible to investigate the contribution of the D3 receptor in living human brain. [11C]-(+)-PHNO binding (∼20-fold selectivity for D3 over D2) can be interpreted in a region-dependent manner, with binding in dorsal striatum (high D2/low D3 expression) more likely reflecting D2 receptor availability and binding in hypothalamus and substantia nigra (SN) reflecting predominantly D3 availability. The ventral pallidum (VP) and globus pallidus (GP) are areas of mixed D2/D3 binding where the D3 fraction has been estimated to represent 75 and 65%, respectively (Tziortzi et al., 2011).
Based on the above data, we tested the hypothesis that MA users would have above-normal [11C]-(+)-PHNO binding in a D3-rich brain area (midbrain/SN) and decreased binding a high D2/low D3-expressing brain area (dorsal striatum) .
Materials and Methods
Subjects.
Sixteen healthy and 16 MA-using volunteers participated in a PET imaging study approved by the Centre for Addiction and Mental Health Research Ethics Board. Brain PET measures of the vesicular monoamine transporter have been reported previously for some of these cases (Boileau et al., 2008).
All participants underwent a comprehensive medical and psychiatric screening interview and completed a comprehensive drug-history questionnaire (structured and open ended, locally developed), which included questions about drug-use frequency, typical dose and route of administration, years of use, recent drug use, withdrawal symptoms, time spent in drug-related activities (e.g.: using, seeking, recovering from drug effects), number of failed attempts to quit, impact on daily activities, and readiness to change use. MA users and control subjects were healthy males or females (age, 19–45 years) and were free of significant medical conditions and current or previous Diagnostic and Statistical Manual of Mental Disorders, fourth revision (DSM-IV) Axis I disorders (First et al., 1996) (excluding stimulant abuse/dependence in the MA group and nicotine dependence in both groups). Study inclusion criteria for the MA group included the following: (1) self-reported use of MA as the primary drug of abuse; (2) meeting DSM-IV criteria for MA abuse or dependence (all subjects also met proposed DSM-V criteria for “amphetamine use disorder”); (3) testing positive for MA in scalp hair; and (4) no current (12 months) self-reported abuse of or dependence on drugs other than MA (except nicotine). On screening day, subjects completed mood scales, a general IQ test, and the Eysenck Personality Inventory (Eysenck, 1953) (Table 1).
Table 1.
Subject demographic information
| Control subjects (n = 16)a | Methamphetamine users (n = 16)a | Group difference (p value) | |
|---|---|---|---|
| Age (years) | 28.43 ± 5.01 (16) | 27.93 ± 5.66 (16) | 0.32 |
| Gender | 14 (M) | 12 (M) | 0.33 |
| Ethnicity | 13 (W) | 13 (W) | 0.67 |
| Weight (kg) | 74.95 ± 17.96 (16) | 80.53 ± 14.52 (16) | 0.21 |
| Years of education | 16.68 ± 2.62 (16) | 12.75 ± 2.56 (16) | <0.01 |
| Premorbid IQ (NART) | 117.43 ± 5.77 (12) | 115.4 ± 4.50 (16) | 0.31 |
| Beck Depression Inventory | 1.18 ± 2.16 (16) | 6 ± 6.83 | 0.02 |
| Inventory of Depressive Symptomatology | 4.13 ± 4.45 (15) | 10 ± 8.59 (16) | 0.02 |
| Nicotine smokers (>5 cigarettes d) | 1 (16) | 7 (16) | 0.02 |
| Cigarettes per day | 0.7 ± 1.65 (16) | 4.8 ± 4.8 (16) | <0.01 |
| Cannabis (≥1 time per month) | 3 (16) | 9 (16) | 0.02 |
| Alcohol use (>14 drinks per week) | 0 (16) | 1 (16) | 0.3 |
| Purdue Pegboard Task | |||
| Dominant | 15.5 ± 1.89 (16) | 13.93 ± 2.97 (16) | 0.03 |
| Nondominant | 14.62 ± 1.70 (16) | 14 ± 1.93 (16) | 0.34 |
| Both hands | 12.31 ± 1.62 (16) | 11.5 ± 1.5 (16) | 0.15 |
| Trails | |||
| A | 21.83 ± 4.96 (12) | 25.46 ± 4.50 (15) | 0.03 |
| B | 45.75 ± 9.70 (12) | 51.06 ± 11.73 (15) | 0.1 |
| Digit symbol substitution | 78.6 ± 12.2 (10) | 59.2 ± 14.8 (15) | <0.01 |
| Written | 63.0 ± 11.4 (10) | 54.8 ± 8.6 (15) | 0.02 |
| Eysenck Personality Inventory | |||
| Extroversion | 10.5 ± 5.05 (16) | 14.07 ± 4.32 (14) | <0.01 |
| Neuroticism | 4.87 ± 3.24 (16) | 9.57 ± 3.56 (14) | <0.01 |
| Impulsivity | 3.31 ± 1.66 (16) | 5.07 ± 2.05 (14) | 0.02 |
M, Male; W, White.
aData are mean ± SD (n).
Italics indicate p < 0.05.
PET imaging session and region-of-interest analyses.
MA users were asked to withhold all illicit drug use for a minimum of 14 d before the scan. On the day of the scan, all subjects were required to test negative on a urine drug screen (9-Drug Test Panel; BTNX) and complete the Profile of Mood States (POMS) questionnaire (McNair et al., 1992), visual analog scales (VAS) measuring drug craving, and the Purdue Pegboard Task of motor dexterity (Lafayette Instrument Company). A short battery of neuropsychological tests was administered after the PET scan (Table 1).
On a separate session (<7 d after the PET scan), all subjects received an oral dose of dextro-amphetamine (0.4 mg) and reported mood and drug-related feelings (results of this study will be reported separately).
[11C]-(+)-PHNO synthesis and image acquisition protocols on the CPS-HRRT neuro-PET camera system (Siemens Medical Imaging) were described in detail previously (Graff-Guerrero et al., 2008). Scans were initiated after bolus injection of [11C]-(+)-PHNO (mean dose, 303.4 MBq; specific activity, 1263.89 mCi/μmol; mean mass, 2.3 μg). Raw data were reconstructed by filtered-back projection. Spin echo proton-density weight magnetic resonance images (MRIs; slice thickness, 2 mm; repetition time, >5300 ms; echo time, 13 ms; flip angle, 90°; number of excitations, 2; acquisition matrix, 256 × 256; FOV, 22 cm) were obtained (Signa 1.5T MRI scanner; General Electric Medical Systems) for region-of-interest (ROI) delineation.
ROI delineation and time activity curve analyses were performed using in-house image analysis software for automated quantification of PET data [ROMI; details by Rusjan et al. (2006)]. Bilateral subcompartments of the striatum, including sensorimotor striatum (SMST), associative striatum (AST), and limbic striatum (LST), were automatically segmented (Rusjan et al., 2006) as described by Martinez et al. (2003). The (whole) GP was delineated with the procedure described and validated by Rusjan (2008). The ROI identified as the midbrain SN corresponded to contiguous midbrain gray matter voxels extending from planes z = −4 to z = −14 on six consecutive transverse slices in stereotaxic space (2 mm, MNI space). Identification of midbrain gray matter voxels within this region was performed by using the automated procedure described by Rusjan et al. (2006). The automatically selected VP covered approximately five coronal slices starting at the interhemispheric anterior commissural connection and was defined laterally and medially as described by Tziortzi et al. (2011). Cerebellar cortex (excluding vermis, lobules IX and X) served as the reference region. [11C]-(+)-PHNO time activity curves were obtained from dynamic data, and specific binding (BPND) was estimated in each ROI using the simplified reference tissue method (SRTM) (Lammertsma and Hume, 1996). Parameter estimation was performed with PMOD (version 2.8.5; PMOD Technologies).
Voxel-wise parameter estimation.
Voxel-wise parameter estimation of [11C]-(+)-PHNO binding was generated using the basis function implementation of SRTM (Lammertsma and Hume, 1996) with the tissue time activity curve of cerebellar cortex as the reference region. Normalized BPND maps (SPM2; Wellcome Trust Centre for Neuroimaging, London, UK) were statistically investigated to assess significant contrasts between groups at every voxel, using independent sample t test analysis (SPM8). The threshold for significant clusters was set to a family-wise error (FWE) corrected p < 0.05. This approach is aimed at detecting differences in neuroreceptor ligand binding at the voxel level, with no a priori anatomical hypothesis, and enables circumvention of some limitations of ROI placement, as well as investigation of regions not included in our ROI template (e.g., the hypothalamus).
Statistical approach.
Group comparisons of [11C]-(+)-PHNO binding across ROIs were conducted using standard repeated-measures ANOVAs or ANCOVAs (ROIs × group). When indicated, sphericity corrections were made with Greenhouse-Geisser adjustments. Least significant difference t tests, Bonferroni corrected for planned comparisons, were applied to determine the significance of regional differences in BPND between groups. The ratio of SN [11C]-(+)-PHNO BPND [100% D3 (Tziortzi et al., 2011)] to SMST [11C]-(+)-PHNO BPND [0% D3 (Tziortzi et al., 2011)] was estimated as an index of individual D3 levels, and t tests were used to assess group differences. One-tailed tests were selected to investigate potential decreased binding in D2-rich dorsal striatum. Relationships between continuous variables were analyzed with the Pearson product moment correlation coefficient and Spearman's rank test for categorical data.
Results
Demographic characteristics and drug profiles
MA polydrug users matched control subjects on age, gender, and ethnicity but had slightly lower education levels. They scored lower than control subjects on the Purdue test of motor dexterity and on tests of working memory and attention, but groups did not significantly differ with respect to estimated premorbid IQ (NART) (Table 1). MA polydrug users self-rated as being more impulsive, and although not clinically depressed, had significantly greater self-reported depressive symptoms. They also used more cannabis and tobacco but did not report drinking more alcohol (Table 1).
Hair analysis confirmed use of MA in all subjects with the exception of one MA user, who did not have scalp hair but provided an MA-positive urine sample at interview. Although MA-using subjects reported MA as the primary drug of abuse, hair analysis disclosed presence of other drugs in hair, particularly cocaine metabolites, confirming, as expected, that the MA users were polydrug users (Table 2).
Table 2.
Co-used substances
| Control subjects (n = 16) | Methamphetamine users (n = 16) | |
|---|---|---|
| Methamphetamine/amphetamine | 0% | 100% |
| 0 (11) | 15 (15) | |
| Cocaine/cocaine metabolitesa | 0% | 80% |
| 0 (11) | 12 (15) | |
| MDMA/MDA/MDEAa | 0% | 53.3% |
| 0 (11) | 8 (15) | |
| Benzopdiazepinea | 0% | 33% |
| 0 (11) | 5 (15) | |
| Morphine/codeinea | 0% | 46% |
| 0 (11) | 7 (15) | |
| THC (>1 month)b | 18.7% | 56.2% |
| 3 (16) | 9 (16) | |
| Ketamineb | 0% | 25% |
| 0 (16) | 4 (16) |
aHair.
bSelf-report.
MDMA, 3,4-Methylenedioxymethamphetamine; MDA, 3,4-Methylenedioxyamphetamine; MDEA, 3,4-Methylenedioxy-N-ethylamphetamine.
The pattern of MA use was variable across the sample (Table 3). Sixty-three percent (10 of 16) of the sample was composed of “heavy” MA users who preferred smoking or injecting MA, often consumed doses >300 mg per occasion, and used MA >3 d/week for at least 3 months. The remaining 38%, “moderate” MA users, were lower-dose (<300 mg), bimonthly intranasal users. Ninety-three percent (15 of 16) had used MA at least once in the 30 d before the study, and only one case had used MA for <3 years at the time of the study. Cocaine use was prevalent in the sample based on hair data. Urine toxicology at the time of the screening (>14 d before the scan) indicated that 5 of 12 MA users who tested positive for cocaine in hair recently used cocaine. Self-report indicated that the average use of cocaine in the last 30 d corresponded to 1.75 d.
Table 3.
Methamphetamine use patterns
| Methamphetamine usea | |
|---|---|
| Years of MA use | 5.1 ± 2.7; 2–11; 4 (16) |
| Days since last use | 18.5 ± 20.5; 6–90; 14 (16) |
| Typical frequency (days/week) | 2.1 ± 1.1; 1–5; 2 (16) |
| *Binge in the last 30 db | 5.1 ± 2.7; 0–10; 5.5 (16) |
| Route | 8 (16) intravenous, smoke; 8 (16) nasal, oral |
| Estimated dose (mg) | 325 ± 167; 100–500; 300 (12) |
aData are mean ± SD; range; median (n).
bPeriod of 2–3 d of use.
PET [11C]-(+)-PHNO BPND
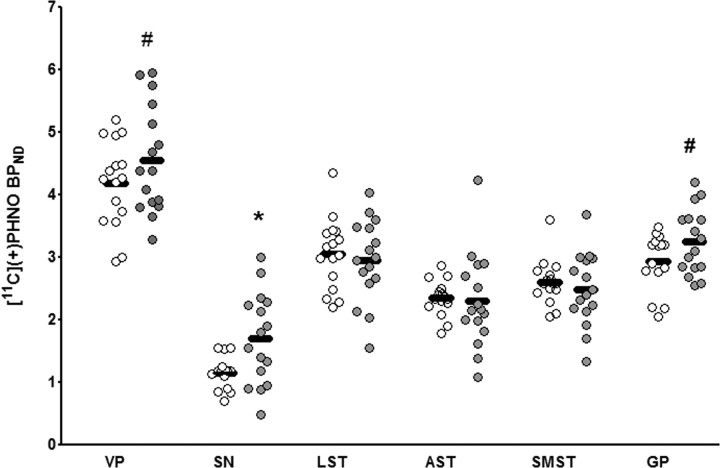
An ANOVA investigating regional differences in [11C]-(+)-PHNO binding across groups (with age as a covariate, as age related changes in D2 (Rinne et al., 1993) and D3 (Graff-Guerrero et al., 2009) binding have been suggested) yielded a significant Group × ROI interaction (F(5,135) = 3.35; p = 0.02). Pairwise contrasts revealed that MA use was associated with significantly higher [11C]-(+)-PHNO BPND in D3-rich SN (46%; corrected p = 0.02), with trends in mixed D3/D2 regions including the GP (11%; corrected p = 0.06) and VP (9%; corrected p = 0.1). Accounting for use of nicotine and cannabis did not change this finding (ANOVA with cannabis as covariate: F(5,135) = 1.98, p = 0.04; ANOVA with nicotine as covariate: F(5,130) = 1.78, p = 0.05). In subcompartments of the D2-rich dorsal striatum, only a small nonsignificant difference in BPND was observed (∼4%) (Fig. 1). However, a separate ANOVA entering severity of MA use as a grouping variable and age as a covariate revealed that heavy but not moderate MA use was associated with significantly lower [11C]-(+)-PHNO binding (F(10,130) = 3.89; p = 0.001). Relative to controls, decreased [11C]-(+)-PHNO binding was maximal in the SMST (−11.4%; p = 0.04, uncorrected for one-tailed comparison) but also occurred in the whole dorsal striatum [one-way ANOVA; F(2,30) = 2.87; p = 0.04 (−11%; p = 0.03)]. This effect was not significant in the LST (p > 0.05). The pairwise comparison also revealed that the increase in D3 receptor binding in the midbrain SN was driven by severity of use since heavy but not moderate use was associated with above control [11C]-(+)-PHNO BPND (F(10,130) = 3.89; p = 0.001; p = 0.04). Investigating differences in D3/D2 ratios (SN/SMST [11C]-(+)-PHNO BPND) between groups, MA users had a 55% greater D3 to D2 binding fraction when compared with controls (p = 0.004), with heavy (72%; p = 0.001) but not moderate (26%; p = 0.2) users having ratios significantly different from controls. Standard uptake values for cerebellum did not differ between MA and controls (p > 0.05). [11C]-(+)-PHNO mass injected was not significantly different between groups (p > 0.3), and no significant correlation between mass and [11C]-(+)-PHNO BPND was observed in any region investigated (p > 0.1).
Figure 1.
Regional [11C]-(+)-PHNO BPND in MA users (n = 16; gray circles) and in control subjects (n = 16; white circles). *p < 0.05, corrected; #p < 0.1, corrected.
To investigate the functional significance of differences in [11C]-(+)-PHNO binding, we tested for relationships between regional BPND and self-report measures taken at baseline and after a priming dose of amphetamine. In the MA group, lower regional [11C]-(+)-PHNO binding in dorsal striatum was associated with greater severity of MA use (years of use: AST, r = −0.6, p = 0.01; SMST, r = −0.5, p = 0.01; severity rank: AST, ρ = −0.6, p = 0.01; SMST, ρ = −0.8, p < 0.001) and daily nicotine use (SMST, r = −0.5, p = 0.01). On the other hand, greater [11C]-(+)-PHNO binding in dorsal striatum was associated with higher (negative) subjective responses to the low-dose amphetamine challenge (VAS “mind racing”: AST, r = 0.6, p = 0.01; SMST, r = 0.6, p = 0.01). This effect was also observed in the overall sample after covarying out the effect of Group (partial correlation with group as a covariate) such that, in the dorsal striatum, higher binding was associated with greater drug-related (negative) feelings (VAS mind racing: AST, r = 0.6, p = 0.005; SMST, r = 0.7, p = 0.001). Higher [11C]-(+)-PHNO BPND in the D3-rich SN predicted motivation to use methamphetamine and, to a lesser extent, to amphetamine-induced “rush” in MA users (VAS “drug wanting”: r = 0.8, p = 0.001; VAS “rush”: r = 0.4, p = 0.06) and in the sample overall (VAS drug wanting: r = 0.6, p = 0.001; VAS rush: r = 0.5, p = 0.01).
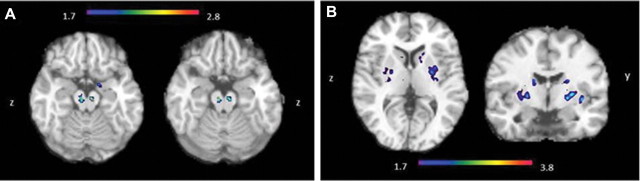
In the voxel-wise statistical analyses (SPM8), we identified small clusters of greater [11C]-(+)-PHNO BPND in MA users relative to controls in an area corresponding to the midbrain (SN/VTA) (Fig. 2A). Small, noncontiguous clusters of increased binding were also detected in the basal forebrain (around VP); however, those were below the significance threshold (data not shown). No clusters of significantly lower [11C]-(+)-PHNO BPND survived correction for multiple comparisons; however, using a more liberal threshold, differences between groups were identified at discrete sites within the dorsal striatum, with the most significant cluster of lower [11C]-(+)-PHNO BPND in the MA group occurring in the postcommissural putamen (SMST) and the caudate (AST) (Fig. 2B).
Figure 2.
A, Voxel-wise group comparison illustrating higher [11C]-(+)-PHNO BPND in the midbrain of MA-abusing than control subjects (tmax = 2.79; p = 0.01; MNI coordinates: 8, −10, −12) and cluster above the p < 0.05 significance threshold when using the random field FWE correction for multiple comparisons within a small volume (10-mm-radius sphere; MNI coordinates: 10, −18, −12). B, Voxel-wise group comparison illustrating lower [11C]-(+)-PHNO BPND in the striatum of MA-abusing than control subjects (tmax = 3.90; p = 0.04, uncorrected; kE = 21; MNI coordinates: 24, 2, 0). The image is thresholded at a probability uncorrected for multiple comparisons.
Discussion
To our knowledge, this is the first in vivo brain imaging study of the D3 receptor in drug-abusing humans. We found evidence for greater DA D3 receptor binding in brain of MA users. As the D3 receptor has been implicated in drug-taking behavior, this finding is relevant to proposed therapeutic strategies targeting D3-selective antagonism.
Our findings are discussed below in the context of assessing [11C]-(+)-PHNO binding in a region-dependent manner, with binding in dorsal striatum more likely reflecting D2 receptor availability and binding in SN reflecting predominantly D3 availability (Tziortzi et al., 2011).
[11C]-(+)-PHNO binding in D2-rich striatum is slightly decreased
PET studies have shown that addictive disorders in humans are associated with low striatal D2/3 receptor binding (Volkow et al., 2001; Martinez et al., 2004; Lee et al., 2009). In our sample, which included heavy and moderate MA users, we detected only minimal nonsignificant decreases in D2/3 receptor binding in D2-rich dorsal striatum; however, in line with previous reports (Lee et al., 2009), MA abuse severity and chronicity were predictive of binding, such that heavy, but not moderate, use of MA was associated with significantly lower binding in dorsal striatum. The regional extent of the finding as indicated by both the ROI and voxel-wise approach, was comparable to that in previous PET studies in MA users (Lee et al., 2009), showing maximal effect in dorsal striatum (including SMST and AST) versus LST.
Overall, the differences in D2/3 receptor binding in dorsal striatum were slightly below values reported in the literature. Although lower cumulative exposure to MA in our sample can reasonably explain our marginal finding, intrinsic properties of [11C]-(+)-PHNO could also partly account for the smaller magnitude of effect. As animal studies show that repeated exposure to DA-elevating drugs increases D3 receptor population in areas previously almost entirely devoid of D3 receptors [i.e., the dorsal striatum (Bordet et al., 1997)], an ectopic upregulation of the D3 receptor in dorsal striatum could have masked potential loss of D2 receptor binding. Alternatively, differences in DA levels (Kd) across groups might have confounded measurement of receptor density (Bmax). As [11C]-(+)-PHNO is a high-affinity agonist ligand, it is more sensitive (vs [11C]raclopride) to modulation by DA (Willeit et al., 2008; Shotbolt et al., 2012). It is therefore possible that low levels of DA in brain of MA users (Wilson et al., 1996; Moszczynska et al., 2004) could lead to greater available receptor sites for binding, hence increasing BPND and masking the presumed loss.
The functional significance of low D2 receptor binding in addiction is unclear, and, concurring with other studies (Martinez et al., 2004), we did not find that decreased D2 receptor density was related to drug-craving or positive effects of amphetamine. However, partly in line with the notion that greater D2 DA stimulation is associated with negative effects of stimulants (Volkow et al., 1999), which could protect against further drug use, higher striatal D2 receptor binding was related to “racing thoughts,” a negative effect of amphetamine associated with anxiety (and hypomanic state). Thus, in MA users, lower aversive side effects of amphetamine, presumably mediated by decreased D2 stimulation, could contribute to MA abuse.
[11C]-(+)-PHNO binding in D3-rich compartments is increased
In contrast to the D2 findings, our data suggest that brain D3 receptor density in the D3-rich SN, but also in the mixed D2/D3 GP/VP, might be higher in psychostimulant users. Although lower DA levels (Moszczynska et al., 2004) might, in principle (see Shotbolt et al., 2012), explain increased [11C]-(+)-PHNO binding in the MA users, this possibility is less likely in view of the results of some (though not all; see Richtand et al., 2001) preclinical studies showing increased D3 receptor levels and mRNA after stimulant exposure in nucleus accumbens (where D3 predominates) and extrastriatal areas (SN, VP, and GP) (Morissette et al., 1998; Quik et al., 2000), as well as postmortem brain investigations reporting that D3 receptor binding is higher in cocaine overdose fatalities (Staley and Mash, 1996; Mash, 1997; Segal et al., 1997; Sokoloff et al., 2001). Increased [11C]-(+)-PHNO binding may be a consequence of D3 receptor upregulation in GABAergic neurons containing substance P and dynorphin (Frankel et al., 2008), as concentrations of dynorphin in brain and plasma brain-derived neurotrophic factor, the latter considered to regulate D3 expression (Guillin et al., 2001), are elevated in human stimulant users (Kim et al., 2005; Frankel et al., 2007, 2008).
Study limitations
Currently, use of [11C]-(+)-PHNO is the only method available to quantify D3 receptors in vivo. In this regard, study limitations include use of a radioligand lacking absolute specificity for the DA D3 receptor and problematic interpretation of binding data in areas that contain both D2 and D3 receptors. Recent studies, however, have suggested that the [11C]-(+)-PHNO signal can be regionally divided into a “relatively pure” D3 component (the SN and hypothalamus, in which 100% of [11C]-(+)-PHNO binding is to D3) and a D2 component [the dorsal striatum, where 100% of the binding is to the D2 (Tziortzi et al., 2011)]. This characterization of the signal makes it possible to draw some conclusions, albeit highly region dependent, from our findings.
Our failure to find group differences in D2/3 receptor binding in the LST, a region where the relative fraction of D3 to D2 receptor (26%) is larger than that in the dorsal striatum (<6%), could, for example, be attributed to the above-mentioned limitation. Specifically, the possibility that coexisting D2 and D3 receptor systems have opposing functional responses to DA-elevating drugs (Levesque et al., 1995) could have canceled out an effect in either direction. An alternative explanation is the fact that the smaller LST is more prone to partial volume effects and higher variability of binding values, which together increase noise and limit measurements in this area.
A practical issue also to be considered is the fact that at the doses of [11C]-(+)-PHNO used in the current study and in studies of other groups (Searle et al., 2010; Tziortzi et al., 2011), injected mass may lead to receptor occupancies higher than tracer doses, which could result in an underestimation of BPND in both groups and possibly decrease ability to detect a difference. Importantly, however, there were no significant differences in mass injected between groups or correlations between BPND and mass injected, making it unlikely that the finding of a group difference is attributable to a mass effect (see Shotbolt et al., 2012, their supplementary information). A methodological caveat of using [11C]-(+)-PHNO that needs to be mentioned is the possibility that the result is biased by differential specific binding in the cerebellum. This potential bias is unlikely to explain our finding since cerebellar standard uptake values were not significantly different between groups; furthermore, the region selected as cerebellar input function excludes areas reported to contain D3 receptors (vermis, lobules IX and X) (Murray et al., 1994). Another issue for consideration is the potential generic confound of other drugs used on D3 receptor binding. Although subjects reported MA as the primary drug of abuse, drug hair analysis and self-report data showed, not unexpectedly, that drugs other than MA (nicotine, cocaine, and opiates) were used (and sometimes not reported) by subjects, which might well have influenced DA receptor expression. However, in light of findings that low D2 receptor binding is a feature of different classes of abused drugs (Volkow et al., 2009), it could be argued that high brain D3 might also be a characteristic across different drugs of abuse, a possibility that could be addressed in future investigations. Finally, we acknowledge that although animal studies suggest that heightened D3 expression in MA users could reasonably be caused by sustained MA-induced dopaminergic stimulation, this difference could have predated drug use.
Possible functional implication of increased D3 and conclusion
Notwithstanding the above limitations, our brain imaging findings do suggest that the DA D3 receptor might be upregulated in polydrug MA users. The brain area involved includes at least the D3-rich SN but might also involve GP/VP brain regions and striatum in which a D3 increase might have been masked by a D2 reduction. Although D3 receptors are both reciprocal autoreceptors and heteroreceptors (Sokoloff et al., 1990), evidence of D3 receptor mRNA induction and increased D3 receptor binding in animals pretreated with DA-elevating drugs has suggested that the newly synthesized receptors are likely to occur in medium-sized spiny neurons containing D1 receptors, dynorphin, and substance P (vs D2 receptors and enkephalin), since their appearance coincided with increased prodynorphin mRNA (Bordet et al., 1997, 2000). However, this finding does not exclude the possibility of some increase in D3 receptor occurring on SN DA cells for which there is, as yet, no known physiological role (Davila et al., 2003). The clinical implications of the increase (presumably on striatonigral projections) depend on the actual function of the D3 receptor, still to be determined in mammalian brain, but might be related to a hypersensitive DAergic response to DA stimulation. Thus, studies investigating the effects of D3 receptor induction find that increased D3 receptor mRNA parallels the appearance of locomotor sensitization to a DA-elevating challenge (an animal model of addiction), possibly through increased inhibition of GABAergic neurons via stimulation of D3 receptors in SN pars reticulata (Bordet et al., 1997, 2000; Guillin et al., 2001; Le Foll et al., 2003). Overall, increased D3 receptor function in areas of the SN, VP, and GP, which receive afferent ventral striatum projections (Haber et al., 2000), could modify the functional system responsible for the output of the limbic striatum and therefore modulate motivation to use drugs (Sokoloff et al., 2001). Indeed, across MA users, we found a robust relationship between [11C]-(+)-PHNO binding in midbrain SN and self-reported drug wanting after a priming dose of amphetamine, suggesting that D3 receptor activation could contribute to craving (and relapse). The finding is consistent with attenuation of drug seeking, self-administration, and cue- and stress-induced reinstatement after highly selective D3 receptor antagonists (for review, see Heidbreder et al., 2005), together suggesting that a D3 receptor increase might contribute to the addicted state in humans.
To summarize, our brain imaging data suggest that the D3 receptor, unlike the D2 receptor, might be upregulated in brain of MA users. Preclinical findings suggest that D3 upregulation might contribute to the addicted state, but pharmacological studies in the human using D3-specific antagonists and agonists are needed to establish the clinical significance of our observations.
Footnotes
This work was supported in part by National Institutes of Health (NIH)–NIDA Grant R01DA025096 (S.J.K. and I.B.). I.B. was supported by an Investigator Award from the Ontario Mental Health Foundation and by the Canadian Institute of Health Research (CIHR) and Parkinson's Society of Canada. T.P.G. was supported by grants/contracts from Pfizer, the National Institutes of Health, the Canada Foundation for Innovation, the Canadian Institutes of Health Research, and the Ontario Mental Health Foundation. P.S. was supported by grants/contracts from Health Canada, Smoke Free Ontario, Ontario Ministry of Health Promotion, Canadian Tobacco Control Research Initiative, CIHR, Alberta Health Services (formerly Alberta Cancer Board),Vancouver Coastal Authority, Pfizer, Ontario Lung Association, NIDA, and Canadian Cardiovascular Society and by speakers bureau/honoraria from Schering Canada, Johnson & Johnson Consumer Health Care Canada, Pfizer Inc. Canada, Pfizer Global, Sanofi-Synthelabo, Canada, GSK Canada, Genpharm and Prempharm Canada, and NABI Pharmaceuticals. M.Z. was supported by the Ontario Problem Gambling Research Centre. A.A.W. was supported by the Ontario Mental Health Foundation. S.H. was supported by the Canada Foundation for Innovation and Ontario Research Funds. D.W. was supported by the NIH–National Cancer Institute and NIH–NIDA, McNeil Consumer Products, and Partnership for a Clean Competition in Sports. We thank Alvina Ng, Jeannie Fong, Armando Garcia, Winston Stableford, and Min Wong for excellent technical assistance.
S.J.K. received a fee to provide expert witness testimony regarding the adverse effects of methamphetamine. T.P.G. received consulting fees from Pfizer Janssen, Astra Zeneca, Prempharm, Bristol Myers Squibb, Sepracor, and Eli Lilly. P.S. received consulting fees from Schering Canada, Johnson & Johnson Consumer Health Care Canada, Pfizer Inc. Canada, Pfizer Global, Sanofi-Synthelabo Canada, GSK Canada, Genpharm and Prempharm Canada, NABI Pharmaceuticals, V-CC Systems Inc., E-Health Behavior Change Software Co., and Astra Zeneca Canada Inc. D.P., A.B., P.M.R., J.T., T.M., and Y.F. report no financial relationships with commercial interests.
References
- Boileau I, Rusjan P, Houle S, Wilkins D, Tong J, Selby P, Guttman M, Saint-Cyr JA, Wilson AA, Kish SJ. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2000;12:2117–2123. doi: 10.1046/j.1460-9568.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci. 2003;23:5693–5697. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ. The application of factor analysis to the study of personality: a reply. Br J Psychol. 1953;44:169–172. doi: 10.1111/j.2044-8295.1953.tb01193.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders–patient edition (SCID-IP, version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Brain levels of neuropeptides in human chronic methamphetamine users. Neuropharmacology. 2007;53:447–454. doi: 10.1016/j.neuropharm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D(2/3) agonist [(11)C]-(+)-PHNO and the D(2/3) antagonist [(11)C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Shammi CM, Uchida H, Kapur S, Mamo D. Age-dependent decline in dopamine D2 receptors but not D3 receptors in healthy volunteers: a [11C]-(+)-PHNO and [11C]-raclopride PET study. Biol Psychiatry Abstr. 2009;64:9S. [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Roh S, Kim Y, Yoon SJ, Lee HK, Han CS, Kim YK. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci Lett. 2005;388:112–115. doi: 10.1016/j.neulet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178:1679–1682. doi: 10.1503/cmaj.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Levesque D, Martres MP, Diaz J, Griffon N, Lammers CH, Sokoloff P, Schwartz JC. A paradoxical regulation of the dopamine D3 receptor expression suggests the involvement of an anterograde factor from dopamine neurons. Proc Natl Acad Sci U S A. 1995;92:1719–1723. doi: 10.1073/pnas.92.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankel WG, Cooper T, Kieber HD, Fischman MW, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC. D3 receptor binding in human brain during cocaine overdose. Mol Psychiatry. 1997;2:5–6. [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman L. Manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, Service EaIT. EITS manual for profile of mood states. San Diego: 1992. [Google Scholar]
- Morissette M, Goulet M, Grondin R, Blanchet P, Bédard PJ, Di Paolo T, Lévesque D. Associative and limbic regions of monkey striatum express high levels of dopamine D3 receptors: effects of MPTP and dopamine agonist replacement therapies. Eur J Neurosci. 1998;10:2565. doi: 10.1046/j.1460-9568.1998.00264.x. [DOI] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci U S A. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Police S, He L, Di Monte DA, Langston JW. Expression of D(3) receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience. 2000;98:263–273. doi: 10.1016/s0306-4522(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Goldsmith RJ, Nolan JE, Berger SP. The D3 dopamine receptor and substance dependence. J Addict Dis. 2001;20:19–32. doi: 10.1300/J069v20n03_03. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Hietala J, Ruotsalainen U, Säkö E, Laihinen A, Någren K, Lehikoinen P, Oikonen V, Syvälahti E. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13:310–314. doi: 10.1038/jcbfm.1993.39. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rusjan P. Kinetics of [11C]-(+)-PHNO binding in the globus pallidus. Neuroimage. 2008;41:T137. [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, Tetsuya S, Houle S, Kapur S. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo-Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M. Imaging dopamine D(3) receptors in the human brain with positron emission tomography, [(11)C]PHNO, and a selective D(3) receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Le Foll B, Perachon S, Bordet R, Ridray S, Schwartz JC. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3:433–441. doi: 10.1007/BF03033202. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: a [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]