Abstract
Background
The purpose of this study was to determine the efficacy of sorafenib in preventing and treating tumor recurrence after liver transplantation in patients with primary hepatic carcinoma exceeding the Milan criteria.
Methods
Thirty patients with primary hepatic carcinoma exceeding the Milan criteria underwent liver transplantation at our hospital between March 2008 and June 2010. Matched for age and gender, the patients were randomized to treatment with sorafenib 400 mg bid or capecitabine (control group, 1500 mg bid, administered for 2 weeks followed by a 2-week rest interval in each cycle). Treatments were discontinued 18 months after transplantation if no recurrence occurred. Patients who experienced tumor recurrence continued their allocated treatment until they were deemed no longer suitable for the medication. Sorafenib and capecitabine were stopped or their dose was reduced in patients with severe adverse reactions.
Results
The one-year recurrence rates were 53.3% and 86.6% in patients treated with sorafenib and capecitabine, respectively (χ2 = 3.968, P < 0.05), and the one-year survival rates were 93.3% and 46.6%, respectively (χ2 = 7.777, P < 0.05). Mean survival time was significantly longer in the sorafenib group (24.6 ± 1.7 [range 7–28] months) than in the capecitabine group (16.4 ± 2.7 [range 5–34], months (χ2 = 7.154, P < 0.05). Most treatment-emergent adverse reactions in both treatment groups were of grade 1 or 2 in severity. The incidence of diarrhea and hand-foot syndrome tended to be higher in the sorafenib group.
Conclusion
For patients with primary hepatic carcinoma exceeding the Milan criteria, sorafenib may reduce or delay tumor recurrence after liver transplantation and prolong patient survival, with tolerable side effects.
Keywords: follow up, recurrence, targeted therapy, chemotherapy, survival rate, side effect
Introduction
In 1996, Mazzaferro et al introduced the Milan criteria for liver transplantation, defined as a solitary tumor < 5 cm in diameter or up to three lesions each <3 cm in diameter without evidence of vascular invasion or lymph node/extrahepatic spread. When the Milan criteria are strictly applied, the tumor recurrence rate after liver transplantation is often below 10% and the 5-year survival rate is higher than 75%.1 China has a high incidence of hepatic carcinoma, with most patients being at the mid to late stage and therefore exceeding the Milan criteria at the time of diagnosis, and liver transplant offers the only possibility for cure in such circumstances. Meanwhile, the donor organ supply system in China has not yet been well established, and liver transplantation surgery is also not yet covered by medical insurance. As such, some patients who have an indication for surgery could still undergo liver transplant surgery with self-pay even though their tumor exceeds Milan criteria. Therefore, the high recurrence rate remains a problem that greatly reduces the long-term survival of these patients. Sorafenib has shown good efficacy in treating advanced liver cancer. Therefore, in this preliminary study, we explored the potential role of sorafenib in preventing and treating tumor recurrence after liver transplantation in patients with primary hepatic carcinoma exceeding the Milan criteria.
Materials and methods
Patients
A total of 97 patients underwent liver transplantation at our department between March 2008 and June 2010. Of these, 30 patients had primary hepatic carcinoma exceeding the Milan criteria, which was pathologically confirmed to be hepatocellular carcinoma. The 30 patients were randomized into two age-matched and gender-matched groups for treatment with sorafenib (12 men and 3 women; mean age 52.3 ± 7.7 [range 40–64] years) or capecitabine (control group, 13 men and 2 women; mean age 49.2 ± 8.9 [range 31–64] years).
Surgery
Both groups included two cases with tumor recurrence following hepatic tumor resection, while the remaining cases had previously untreated new tumors. All of the patients had undergone regular radionuclide bone scanning and lung computed tomography to rule out extrahepatic spread. All the livers are from voluntary donations after cardiac death. The donor’s blood type matched that of the recipient. Liver grafts were obtained using a rapid en bloc technique for the liver and kidney, and cold-stored in University of Wisconsin solution. All procedures were performed using conventional orthotopic liver transplantation methods without venovenous bypass. The “tumor-free operation” principle was followed during surgery. The following measures were taken to prevent tumor spread: excessive mechanical pressure on the tumor or repeated movement of the liver was avoided, and tumors located on the liver surface were covered with gauze; if the tumor adhered to the surrounding diaphragm, abdominal wall or omentum majus, the adhered local tissues were resected; if the tumor was located close to the first porta hepatis, at least 2 cm of the hepatic portal vein was resected, or the main portal vein was to be ligated in advance; during surgery, 5-fluorouracil 1 g was slowly infused via the peripheral vein, while epirubicin 20 mg was infused intravenously during the anhepatic period in patients with portal vein tumor thrombi; during dissociation of the liver arteries, the lymph nodes were dissected near the common hepatic artery and inside the hepatoduodenal ligament; and during the anhepatic period and before closure of the abdomen, the abdominal cavity was rinsed thoroughly with 2000 mL of warm, sterile distilled water.
Tumor staging
Hepatocellular carcinoma stage was determined based on intraoperative findings and postoperative pathology using the International Union Against Cancer tumor-node-metastasis (TNM) staging criteria. Both groups included 13 patients classified as T4 N0M0 and 2 patients classified as T4 N1M0. All patients had stage IVa disease.
Postoperative follow-up and treatment
After surgery, the patients were treated with an immunosuppression regimen consisting of tacrolimus, mycophenolate mofetil, and prednisone. The dose of tacrolimus was adjusted according to the blood drug concentration and indices of liver function. Mycophenolate mofetil was administered at a dose of 1 g/day for 6 months after surgery and then reduced to 0.5 g/day or discontinued when the leucocyte count was <3 × 1012/L. Prednisone was discontinued in all patients within one month after surgery. All patients also received combined hepatitis B immunoglobulin and lamivudine/entecavir therapy to prevent hepatitis B virus reinfection after liver transplantation.
Sorafenib 400 mg bid orally was started one month after surgery. When intolerable toxicity occurred, the dose was reduced to 200 mg bid or was discontinued for 2 weeks until symptoms were resolved. In the control group, capecitabine 1500 mg bid orally was started one month after surgery. Capecitabine was administered for 2 weeks, followed by a 2-week rest interval in each cycle. If no severe adverse effects were noted, the next treatment cycle was started. Both drugs were discontinued 18 months after surgery if no recurrence occurred. Patients who experienced tumor recurrence continued their allocated treatment at the scheduled dose. Both drugs were stopped or reduced in patients with severe adverse reactions.
The following parameters were assessed at regular outpatient visits: routine blood tests, liver and kidney function, blood concentration of tacrolimus, and hepatitis B surface antibody titer measurement. Serum alfa-fetoprotein was measured every month. Lung and contrast-enhanced abdominal computed tomography was performed every 2–3 months. All patients were followed until their death or until June 30, 2011.
Statistical analysis
Continuous data are expressed as the mean ± standard deviation. Categorical data were compared between the two groups using χ2 tests. Survival curves were plotted using the Kaplan–Meier method and compared using the log-rank test. All data were analyzed using the Statistical Package for the Social Sciences software version 11.5 (SPSS Inc, Chicago, IL).
Results
There were no perioperative deaths. All patients were followed for 6–34 months. Hepatocellular carcinoma recurred within one year after surgery in 21 patients, including 8 patients treated with sorafenib and 13 patients treated with capecitabine. Of these, 1 patient treated with sorafenib and 8 patients treated with capecitabine died within one year after surgery. As a result, the survival rate was significantly different between the two groups (Table 1).
Table 1.
Recurrence and survival rates at one year
| Treatment | Sorafenib group | Capecitabine group |
|---|---|---|
| One-year recurrence rate (%) | 53.3 | 86.6* |
| One-year survival rate (%) | 93.3 | 46.6** |
Notes:
χ2 = 3.968, P = 0.046 versus sorafenib;
χ2 = 7.777, P = 0.005 versus sorafenib.
As of June 30, 2011, 23 patients had experienced tumor recurrence (9 sorafenib-treated patients and 14 capecitabine-treated patients). Of these, 15 had died (3 sorafenib-treated patients and 12 capecitabine-treated patients) and 8 (6 sorafenib-treated patients and 2 capecitabine-treated patients) were living and had a tumor at the last follow-up. The first sites of recurrence included pulmonary metastasis (n = 14), metastatic peritoneal implants (n = 7), abdominal lymph node metastasis (n = 1), and metastatic tumor in a liver graft (n = 1). In addition to treatment with sorafenib or capecitabine, all of the patients with recurrence received local treatment, including gamma knife surgery for pulmonary metastases (n = 24), peritoneal metastases (n = 6) or for liver metastases (n = 2), resection (n = 4), or tumor ablation (n = 2).
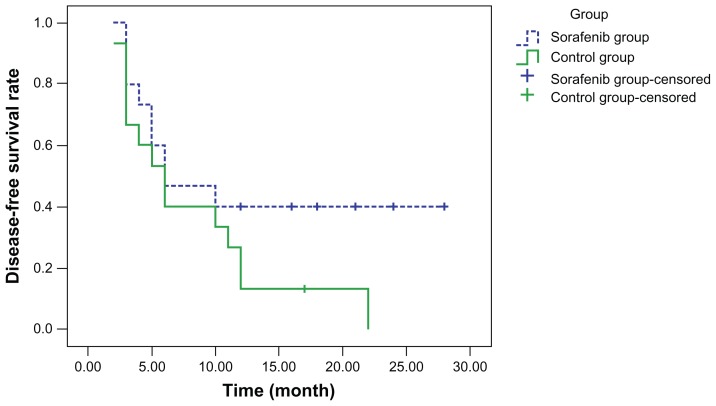
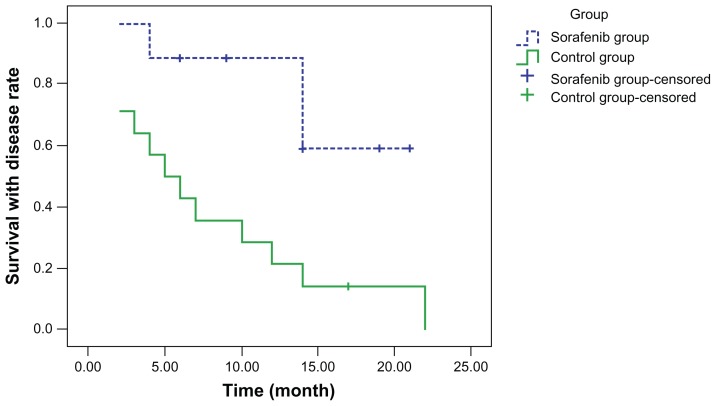
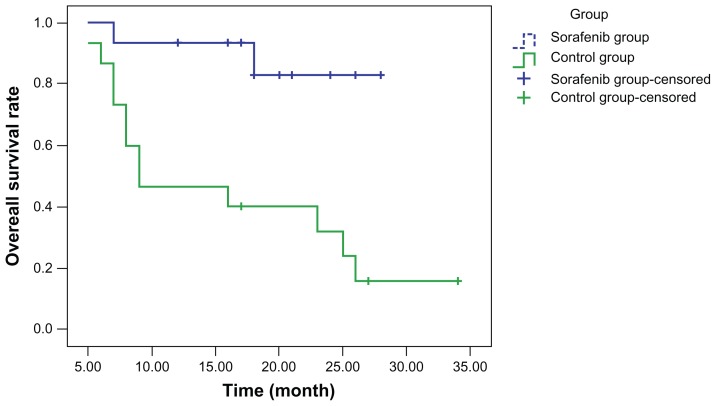
Curves for disease-free survival (Figure 1), survival with disease (Figure 2), and overall survival (Figure 3) were drawn using the Kaplan–Meier method and compared using log-rank tests. Although disease-free survival was longer in the sorafenib group, this was not significantly different compared with the capecitabine group (χ2 = 2.444, P = 0.118). On the other hand, survival with disease (χ2 = 6.901, P = 0.009) and overall survival (χ2 = 7.154, P = 0.007) were significantly longer in the sorafenib group than in the capecitabine group.
Figure 1.
Disease-free survival.
Figure 2.
Survival with disease.
Figure 3.
Overall survival.
Most treatment-emergent adverse reactions in both study groups were grade 1 or 2 in severity, and resolved after symptom management and required no dose reduction. 3 patients treated with sorafenib experienced grade 3 hand-foot syndrome. In 2 of these patients, the sorafenib dose was reduced to 200 mg bid, and the symptoms resolved after symptom management. In the other patient, sorafenib was discontinued until the toxicity had decreased to grade 1 after 3 weeks. Sorafenib was then resumed at a dose of 200 mg bid, and no further episodes of grade 3 hand-foot syndrome occurred. The incidence of diarrhea and hand-foot syndrome was significantly higher and that of rash and hypertension was slightly higher, although not significantly, in the sorafenib group than in the capecitabine group. The incidences of fatigue/weakness, nausea, anorexia, neutropenia, and alopecia were similar in both groups (Table 2).
Table 2.
Treatment-emergent adverse reactions
| Group | Sorafenib group | Capecitabine group | χ2 | P |
|---|---|---|---|---|
| Diarrhea | 5 | 1 | 4.565 | 0.033 |
| Hand-foot syndrome | 6 | 0 | 7.500 | 0.006 |
| Rash | 3 | 1 | 1.154 | 0.283 |
| Hypertension | 3 | 1 | 1.154 | 0.283 |
| Alopecia | 4 | 4 | 0 | 1 |
| Fatigue weakness | 3 | 2 | 0.240 | 0.624 |
| Neutropenia | 1 | 2 | 0.371 | 0.543 |
| Nausea/anorexia | 4 | 5 | 0.159 | 0.690 |
Discussion
Many patients with advanced hepatocellular carcinoma exceeding the Milan criteria have disease that is unresectable at diagnosis, meaning liver transplantation is their only option for long-term survival. Nevertheless, postoperative tumor recurrence remains the leading cause of death among these patients. Considering that liver transplantation dramatically reduces the tumor burden, the prophylactic use of antitumor drugs after surgery is expected to help prevent or delay tumor recurrence. Accordingly, effective antitumor drugs may improve the prognosis of patients with hepatocellular carcinoma exceeding the Milan criteria.
Sorafenib is an oral multitargeted kinase inhibitor, with multiple mechanisms. First, sorafenib suppresses the proliferation of tumor cells by inhibiting the RAS/RAF/MEK/ERK signal pathway. Second, it inhibits the tyrosine kinase activity of receptors for vascular endothelial growth factor-2 and platelet-derived growth factor, thus blocking tumor angiogenesis and further suppressing tumor growth.2–4 Sorafenib has inhibitory effects on multiple tumors, including kidney cancer, liver cancer, non-small cell lung cancer, and melanoma.5–7 In an international, multicenter, randomized, controlled Phase III trial in patients with advanced hepatocellular carcinoma, overall survival was 10.7 months in sorafenib-treated patients compared with 7.9 months in the placebo group (P < 0.05), while median time to tumor progression was 5.5 months and 2.8 months, respectively (P < 0.05), suggesting that sorafenib can prolong survival in patients with advanced hepatocellular carcinoma.7–9 A Phase III, randomized controlled trial conducted in the Republic of Korea, China, and Taiwan showed that sorafenib prolonged the survival of Asian patients with advanced hepatocellular carcinoma.10
All of these trials demonstrated that sorafenib has a good therapeutic effect for unresectable advanced liver cancer. There have been limited data reported on the recurrence rate of post liver transplant surgery in patients with high-risk hepatic carcinoma treated by sorafenib.11,12 Our current study investigated whether sorafenib can also help to prevent and/or treat tumor recurrence after liver transplantation. These preliminary results show a one-year recurrence rate of 53.3% in the sorafenib group, which is significantly lower than that in the capecitabine group (86.6%, P < 0.05). Meanwhile, the one-year survival rate was significantly higher in the sorafenib group (93.3%) than in the capecitabine group (46.6%). In our previous study, most tumor recurrences occurred within 6–14 months after transplantation, with relatively few cases later than 18 months after surgery.13 We also considered patients who did not experience recurrence within 2 years after surgery as being clinically cured.13 Patients in the current study are still undergoing follow-up, and some new recurrent cases may be identified in the sorafenib group over time. Nevertheless, the current results show that administration of sorafenib to patients with liver cancer exceeding the Milan criteria may reduce or delay tumor recurrence after liver transplantation.
Interestingly, the mean survival duration was 24.6 ± 1.7 (range 7–28) months in the sorafenib group and 16.4 ± 2.7 (range 5–34) months in the capecitabine group. The disease and overall survival curves (drawn using the Kaplan–Meier method) show that sorafenib conferred significantly longer survival than did capecitabine. Therefore, administration of sorafenib for some time after transplantation may improve the prognosis in patients with liver cancer exceeding the Milan criteria, with efficacy superior to that of capecitabine. Patients with tumor recurrence should continue to take sorafenib to suppress tumor growth and prolong their survival.
The sites of recurrence in both groups mainly included pulmonary metastasis, metastatic peritoneal implants, and abdominal lymph node metastasis, similar to the patterns of recurrence in our earlier study.13 It is believed that, in addition to the prophylactic use of effective antitumor drugs after surgery, early detection of the site of recurrence and appropriate local treatment (eg, gamma knife surgery, tumor resection, tumor ablation) are helpful and essential to suppress tumor progression. Patients with postoperative pathology showing abdominal lymph node metastasis and/or intravascular cancer emboli in the hepatic vein system are more likely to experience tumor relapse and their prognosis is likely to be poor. For these patients, sorafenib should be administered as early as possible. The main adverse reactions in the sorafenib group included diarrhea and hand-foot syndrome, which resolved after reducing the sorafenib dose. The incidence of severe adverse reactions (grade 3 or above) was very low. Sorafenib may also be discontinued, if necessary, and then resumed at a lower dose (eg, 200 mg bid) once the toxicities have been resolved. In such settings, subsequent severe toxic effects are rare.
In summary, the results of this preliminary study indicate that, for patients with advanced liver cancer exceeding the Milan criteria, prophylactic use of sorafenib after liver transplantation can improve prognosis and prolong survival, with an acceptable toxicity profile. Further studies are needed to confirm the present findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 3.Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M. Molecular targeted therapy for hepatocellular carcinoma. J Gastroenterol. 2009;44:136–141. doi: 10.1007/s00535-008-2252-z. [DOI] [PubMed] [Google Scholar]
- 5.Dallago L, Dhondt V, Awada A. Selected combination therapy with sorafenib a review of clinical data and perspectives in advanced solid tumors. Oncologist. 2008;13:845–848. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 6.Kelley RK, Venook AP. Sorafenib in hepatocellular carcinoma: separating the hype from the hope. J Clin Oncol. 2008;26:5845–5848. doi: 10.1200/JCO.2008.19.7996. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Rimassal L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther. 2009;9:739–745. doi: 10.1586/era.09.41. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 11.Saab S, McTigue M, Finn RS, et al. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and efficacy. Exp Clin Transplant. 2010;8:307–313. [PubMed] [Google Scholar]
- 12.Saidi RF, Shah SA, Rawson AP, et al. Treating hepatocellular carcinoma with sorafenib in liver transplant patients: an initial experience. Transplant Proc. 2010;42:4582–4584. doi: 10.1016/j.transproceed.2010.09.147. [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Zhu JY, Li GM, et al. Postoperative recurrence of liver malignant tumor after liver transplantation. Chinese Journal of General Surgery. 2009;24:984–987. [Google Scholar]