Abstract
Given the manifold ways that depression impairs Darwinian fitness, the persistence in the human genome of risk alleles for the disorder remains a much debated mystery. Evolutionary theories that view depressive symptoms as adaptive fail to provide parsimonious explanations for why even mild depressive symptoms impair fitness-relevant social functioning, whereas theories that suggest that depression is maladaptive fail to account for the high prevalence of depression risk alleles in human populations. These limitations warrant novel explanations for the origin and persistence of depression risk alleles. Accordingly, studies on risk alleles for depression were identified using PubMed and Ovid MEDLINE to examine data supporting the hypothesis that risk alleles for depression originated and have been retained in the human genome because these alleles promote pathogen host defense, which includes an integrated suite of immunological and behavioral responses to infection. Depression risk alleles identified by both candidate gene and genome-wide association study (GWAS) methodologies were found to be regularly associated with immune responses to infection that were likely to enhance survival in the ancestral environment. Moreover, data support the role of specific depressive symptoms in pathogen host defense including hyperthermia, reduced bodily iron stores, conservation/withdrawal behavior, hypervigilance and anorexia. By shifting the adaptive context of depression risk alleles from relations with conspecifics to relations with the microbial world, the Pathogen Host Defense (PATHOS-D) hypothesis provides a novel explanation for how depression can be nonadaptive in the social realm, whereas its risk alleles are nonetheless represented at prevalence rates that bespeak an adaptive function.
Supplementary information
The online version of this article (doi:10.1038/mp.2012.2) contains supplementary material, which is available to authorized users.
Keywords: major depression, evolution, immune, inflammation, infection, genetic
Subject terms: Evolution, Innate immunity, Pathogens, Depression
Introduction
Major depression is so detrimental to survival and reproduction that it is hard to understand why allelic variants that promote the disorder have not been culled from the human genome, why in fact—far from being culled—genes that promote depression are so common and numerous and appear to have actually increased in prevalence during recent human evolution.1 To address this issue, we have developed a novel theoretical framework positing that risk alleles for depression originated and have been largely retained in the human genome because these alleles encode for an integrated suite of immunological and behavioral responses that promote host defense against pathogens. This enhanced pathogen defense is accomplished primarily via heightened innate immune system activation, which results in reduced death from infectious causes,2, 3, 4, 5 especially in infancy when selection pressure from infection is strongest,6 and the adaptive immune system is not yet fully operational.6, 7, 8, 9 A vast literature has associated depressive symptoms and/or major depressive disorder (MDD) with increased innate immune inflammatory responses,10 with meta-analyses reporting the most consistent findings for increased plasma concentrations of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), C-reactive protein and haptoglobin.11, 12, 13 Recent longitudinal studies extend these cross-sectional observations by reporting that increased inflammatory markers in nondepressed individuals predict the later development of depression.14, 15, 16 Because infection has been the primary cause of early mortality and hence reproductive failure across human evolution,9, 17, 18, 19, 20, 21 it would be expected that if depressive symptoms were an integral part of a heightened immunological response, allelic variants that support this response would have undergone strong positive selection pressure and thus would be both numerous and prevalent, as they appear to be. However, because the survival benefits of inflammatory processes are tempered by their costs in terms of increased mortality from septic shock,22, 23 pathogen manipulation,21, 24 long-term tissue damage and chronic disease,10 these alleles would not be predicted to go to fixation (that is, 100% prevalence) but would be expected to manifest an intermediate prevalence reflecting the benefit of enhanced host defense in any given environment minus attendant costs. Again, this is consistent with current findings in the genetics of depression.
It should be noted that this Pathogen Host Defense (PAThos HOSt Defense=PATHOS-D) hypothesis is not the first theory to associate depression with protection from infection. Indeed, similar to PATHOS-D, at least one previous hypothesis has envisioned depression as a behavioral response that helps the immune system combat existing infections while avoiding additional pathogen exposure.25 However, prior theoretical articulations have envisioned depressive symptoms as adaptive primarily because they compensate for various types of immune system vulnerabilities.25 PATHOS-D suggests something qualitatively different and more far-reaching; specifically that depressive symptoms were integral components of immune-mediated host defense against pathogens in the ancestral environment. In this model, depressive symptoms are inextricably intertwined with—and generated by—physiological responses to infection that—on average—have been selected as a result of reducing infectious mortality across mammalian evolution (Figure 1). Thus, it is proposed that the alleles for depression, rather than having coevolved with immunological alleles that support pathogen defense, are in fact one in the same as those alleles, and therefore genes associated with depression would be predicted to be the same genes that are associated with successful host immune responses.
Figure 1.
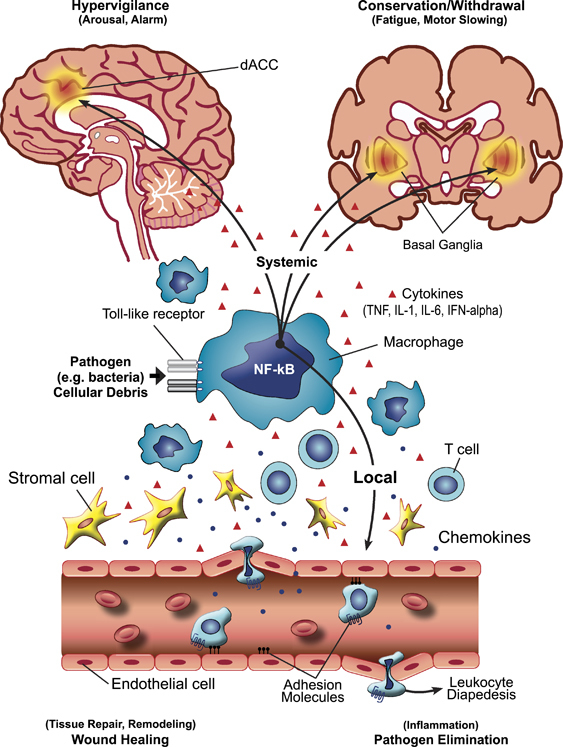
The integrated suite of immunological and behavioral responses to infection and wounding that comprise pathogen host defense. Upon encountering a pathogen or cellular debris from tissue damage or destruction, the body reacts with an orchestrated local and systemic response that recruits both immunological and nervous system elements. The response is initiated by interaction of pathogens and/or cellular debris with pattern recognition receptors such as Toll-like receptors on relevant immune cells including macrophages that in turn are linked to inflammatory signaling pathways such as nuclear factor-κB (NF-κB), a lynchpin transcription factor in the host defense cascade. Release of cytokines (including tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6 and interferon-α (IFN-α)) and chemokines as well as the induction of adhesion molecules attracts and activates cells such as T cells at the site of infection/wounding, leading to the cardinal signs of inflammation (redness, heat, swelling and pain) and ultimately promoting local pathogen elimination and wound healing. Cytokines and cells in the peripheral circulation mediate the systemic host response that engages neurocircuits in the brain that mediate hypervigilance (dorsal anterior cingulate cortex (dACC)) to avoid further wounding and pathogen exposure and conservation/withdrawal (basal ganglia), which promotes the shunting of energy resources to pathogen elimination and wound healing.
To fully elaborate this hypothesis, this article is structured to evaluate the foundations of the PATHOS-D theory (Table 1) by first examining the immune relevance of previously identified depression risk alleles, followed by an exploration of relationships among environmental risk factors for depression, inflammation and pathogen host defense. The role of depression-associated immune changes in promoting survival during infection is reviewed next, followed by an examination of the potential utility of depressive symptoms in host defense. We conclude with a consideration of the potential limitations of—and challenges to— the PATHOS-D theory.
Table 1.
Pathogen Host Defense (PATHOS-D) theory of depression: foundational hypotheses
| (1) Depression should be associated with increased inflammation and inflammatory activation should induce depression. |
| (2) Allelic variants that increase the risk for major depressive disorder (MDD) should enhance host defense mechanisms in general and innate immune inflammatory responses in particular. |
| (3) Environmental risk factors for MDD should be associated with increased risk of infection and attendant inflammatory activation. |
| (4) On the whole, patterns of increased immune activity associated with MDD should have decreased mortality from infection in ancestral environments. |
| (5) Depressive symptoms should enhance survival in the context of acute infection and in situations in which risk of infection from wounding is high. |
Risk alleles for MDD and host defense
The failed promise of genome-wide association studies (GWASs) to unambiguously identify genetic risk variants for MDD has led increasingly to the suggestion that depression and other major psychiatric conditions arise not from common allelic variants with small effect sizes, but rather from an array of highly nonadaptive genetic variants too rare to be identified by GWASs that nonetheless have large effect sizes.26, 27 Confirmation of this would effectively preclude the possibility that depressive risk alleles conferred any selective advantage during human evolution.28 However, an alternative possibility is that differences in common allelic variants between depressed and nondepressed individuals might be more apparent/consistent if the unit of analysis was extended from single genes to groupings of genes that form functional units. In the context of the PATHOS-D theory, this suggests that small allelic differences between depressed and nondepressed groups should not be randomly distributed across the genome, but rather should be largely localized to genes with host defense functions, and that the effect sizes for differences in individual host defense alleles should be additive (that is, positive epistasis), so that large effect size differences should emerge when functionally related host defense-enhancing alleles are evaluated as a unit. Support for this possibility comes from a recent network analysis of candidate genes for MDD. Although this analysis only interpreted findings in terms of potential central nervous system (CNS) effects,29 from a PATHOS-D perspective, it is striking that pathways identified as central to the best-supported MDD gene networks all have well-documented inflammatory and/or anti-inflammatory effects.
To be fully consistent with the PATHOS-D theory, allelic risk variants should meet three criteria. They should (1) be located in genes with known immune effects; (2) increase signaling in inflammatory/host defense pathways; and (3) increase survival in the context of infection. Although a number of candidate gene studies have identified depression risk alleles that are associated with inflammatory processes,30, 31, 32, 33, 34 to evaluate in the most conservative manner whether putative risk alleles meet the three criteria above, we have limited our examination to candidate genes confirmed either by GWASs or meta-analysis and to alleles identified in meta-analyses of GWAS data.
Candidate genes confirmed by GWASs
Currently, only two candidate single-nucleotide polymorphisms (SNPs; rs12520799 in DCNP1 (dendritic cell nuclear protein-1) and rs16139 in NPY (neuropeptide Y)) and one candidate gene for MDD (TNF-α), smallest P-value for rs76917) have been confirmed by GWASs.35 It is striking that each of these genes plays an important role in processes central to host defense, including proinflammatory cytokine signaling (TNF), antigen presentation (DCNP1) and T helper type 1 cell differentiation and function (NPY). Of these SNPs, functionality has only been established for rs16139 in NPY. Although NPY has numerous and contrasting effects on innate and adaptive immune functioning, its primary actions appear to be anti-inflammatory in both the brain and periphery.36, 37, 38 Given this, the PATHOS-D theory predicts that MDD should be characterized by reduced NPY activity and that the depression risk T allele at rs16139 should be associated with reduced NPY production. Significant data support both predictions.39, 40, 41, 42, 43
Unlike NPY, the functionality of rs76917 in TNF is currently unknown. A clear prediction of PATHOS-D theory is that this SNP should be associated with increased TNF-α production, given that TNF-α is increased in MDD and appears to be especially relevant to enhanced survival from infection in the types of pathogen-dense environments that were normative during human evolution. A separate SNP (−308G/A) in the promoter region for TNF is worthy of comment in this regard. Although not found to be significant by GWASs,35 several studies have associated the high-production A allele at −308 (ref. 44) with depression and related states such as anger.33, 34, 45 As predicted by PATHOS-D theory, the −308A allele has also been associated with reduced risk for infection with a number of pathogens, including Mycobacterium tuberculosis, parvovirus B19 and hepatitis B virus (HBV),46, 47, 48 and with an increased likelihood of survival in critically ill hospitalized patients.49 On a population level, Canadian First Peoples who are highly susceptible to tuberculosis have a markedly reduced prevalence of the A allele compared with Caucasians.50
DCNP1 was initially considered to be unique to dendritic cells,51 although it has subsequently been identified in neurons.52 The rs12520799 T allele, which is associated with MDD, codes for a truncated version of the protein. No data are available regarding the effect of this allele on either inflammatory signaling or infection outcomes, but given strong patterns of comorbidity between asthma/atopy and MDD, it is intriguing that the allele has been associated with increased levels of immunoglobulin E for common specific antigens in individuals with asthma.53
Candidate genes confirmed by meta-analysis
Although findings on candidate genes for depression have proven remarkably difficult to replicate,35 a recent meta-analysis provides at least some additional support for several allelic variants being risk factors for MDD, including GNB3 825T, MTHFR 677T, APOE ɛ2, SLC6A3 40 bpVNTR 9/10 genotype and SLC6A4 44 bp ins/del short allele.54 Although not traditionally considered as primarily immune related, each of these genes has well-documented immunological effects and hence meets the first of the three criteria for consistency with the PATHOS-D theory. In addition, each to a varying degree has some evidence consistent with either the second or third criterion.
GNB3 825T produces a shortened splice variant of the guanine nucleotide-binding protein subunit β-3 (GNB3) that has enhanced signal transduction properties.55 Also, 825T has been reported to enhance in vitro cellular immune responses to recall antigens and IL-2 stimulation, to increase neutrophil chemotaxis in response to IL-8 and to increase both lymphocyte chemotaxis and the number of circulating CD4+ T cells.55, 56 These immune-enhancing effects come at the price of increased rates of microalbunemia, hypertension and cardiovascular disease in T allele carriers.57, 58 However, as predicted by the PATHOS-D theory, these effects also appear to translate into improved host defense, given associations between the T allele and reduced death from infection in infancy and evidence of positive selection for the T allele in geographical areas with high rates of infectious pathology.59, 60 Also consistent with enhanced host defense responses, the T allele is associated with improved antiviral responses following interferon-α (IFN-α) treatment for hepatitis C virus and highly active retroviral treatment for human immunodeficiency virus.61, 62, 63 In addition, following HBV booster vaccination, the T allele increases in vitro lymphocyte proliferative responses to HBV surface antigen.64
The MTHFR 677T allele produces a version of the methylenetetrahydrofolate reductase (MTHFR) enzyme with reduced activity,65 leading to elevations in plasma concentrations of homocysteine and other markers of inflammation.66, 67, 68, 69, 70, 71, 72 Animal and human data suggest that this reduced MTHFR activity and concomitant increase in inflammatory tone may enhance host defense in at least some situations. For example, in a mouse model, MTHFR deficiency protects against cytomegalovirus infection,65 and in pregnant females, increased MTHF is associated with the presence of a sexually transmitted disease and bacterial vaginosis.73 Directly supporting a protective role for the T allele are data demonstrating that the allele protects against HBV infection in African populations.72 Moreover, the hyperhomocysteinemia associated with reduced MTHFR activity has been posited as protective against malaria and has been suggested as a selection factor for the T allele in sub-Saharan Africa.74 Interestingly, however, the prevalence of the T allele is actually far lower in sub-Saharan populations than in other ethnic/geographical groups despite these potential benefits, likely because homozygosity for the allele is lethal in situations of low folate availability such as pertain throughout much of the region.72 On the other hand, given the array of disease states that has been associated with MTHFR 677T,75, 76, 77, 78, 79, 80 as well as reduced fertility,81 its increased prevalence in environments of ready folate availability may reflect more substantial benefits for host defense than are currently recognized.
Apolipoprotein E (APOE), a glycoprotein central to lipid transport and metabolism, has been implicated as a risk and/or protective factor in a wide range of illnesses. The APOE gene has three primary alleles, termed ɛ2, ɛ3 and ɛ4, with ɛ3 being the most common worldwide, but with significant data suggesting that ɛ4 is the ancestral human allele.82, 83, 84, 85 APOE affects immune functioning in complex and apparently contradictory ways, with both immune-enhancing and immune-suppressing effects reported. The depression-protective ɛ2 allele does not appear to be associated with reduced inflammation per se, as PATHOS-D theory would predict, but may meet the third criteria required by PATHOS-D by being a risk factor for diseases known to have exerted significant selection pressure on humans, including tuberculosis and malaria.86 Conversely, the ɛ4 allele, which increases the risk for MDD when compared with ɛ2, is associated with increases in many measures of inflammation and related processes such as oxidative stress,82, 83, 84, 85 and has been reported to protect against the development of childhood diarrhea in high-pathogen environments.
Dopamine and serotonin are pivotal neurotransmitters in mood regulation, and yet like other factors linked to depression, these monoamines both affect, and are affected by, the immune system. The bulk of available evidence suggests that MDD is best characterized as a condition of low dopamine availability, at least in CNS regions linked to motivation and reward.87, 88, 89, 90 The possibility that reduced dopamine availability in MDD may serve host defense purposes is suggested by animal studies showing that hyperdopaminemia is associated with reductions in both innate and adaptive T helper 1-type cellular immunity, with resultant increased susceptibility to infection.91, 92 That dopamine transporter activity in particular may be important for host defense in humans is suggested by findings from two recent genome-wide linkage analyses of risk factors for tuberculosis in geographic areas in which the disease is endemic. Both studies localized a genetic protective factor to a locus of chromosome 15.93, 94 Fine mapping of this locus identified a SNP (rs250682) within the dopamine transporter gene (SLC6A3) as conferring the strongest protective effect.93 The G allele of rs250682 was found to be associated with reduced skin reactions to the tuberculin test, which predicts reduced risk of later active disease in endemic areas.93 However, no data were found indicating that rs250682 is in linkage disequilibrium with the SLC6A3 40 bpVNTR that has been associated with MDD. Nor do any data address whether the 9 repeat allele of the VNTR has immunological effects that would enhance host defense. Indeed, even the question of whether this putative depression risk allele is a gain-of-function or loss-of-function variant for the dopamine transporter remains to be definitively clarified.95, 96
The SLC6A4 44 bp ins/del polymorphism (often referred to as 5HTTLPR) is by far the most extensively studied, and debated, genetic risk factor for MDD. Significant data suggest that the ‘short’ allele of this serotonin transporter polymorphism (which is less efficient in the reuptake of serotonin) increases the risk for developing depression in response to psychosocial adversity, both during development and in adulthood. Less well known, but consistent with PATHOS-D predictions, the short allele has also been shown to protect against sudden infant death syndrome, a condition often associated with unrecognized infectious morbidity.97, 98, 99, 100 Given the PATHOS-D prediction that stress should activate inflammation as a prepotent protection against the risk of wounding (see below), it is intriguing that the 5HTTLPR short allele is associated with an increase in the ratio of circulating proinflammatory to anti-inflammatory cytokines (for example, IL-6/IL-10) following a psychosocial stressor.101 Further supporting a role for SLC6A4 in host defense is the recent finding that the gene might account for 10% of the correlation between depressive symptoms and circulating levels of IL-6 in a group of medically healthy adults.102 Finally, the prevalence of the short allele in cultures around the world is strongly correlated with historical burden of disease-causing pathogens in these cultures,103 consistent with the possibility that the short allele has undergone positive selection as a result of enhancing host defense.104
Alleles identified by meta-analyses of GWAS data
Far less is known about the general functionality of alleles identified in GWASs, let alone which physiological effects may be relevant to MDD. Therefore, it should not be surprising that limited data are available regarding whether these potentially depressogenic SNPs affect immunity to enhance host defense. On the other hand, it is intriguing that associations with immune/inflammatory function or other aspects of host defense against pathogens have been demonstrated for 8 of the top 10 genes (or their very close homologs) identified in the largest GWAS meta-analysis of MDD conducted to date (Table 2).105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140 Many other depression-relevant genes identified in earlier large GWASs (as well as meta-analyses of these studies), including PBRM1, GNL3, ATP6V1B2, SP4, AK294384, LY86, KSP37, SMG7, NFKB1, LOC654346, LAMC2, ATG7, CUGBP1, NFE2L3, LOC647167, VCAN, NLGN1, BBOX1, ATF3, RORA, EIF3F, CDH13, ITGB1 and GRM8, have also been linked to immune system and/or host defense functions (see Supplementary Table S1 for additional information/relevant references).
Table 2.
Immune/host defense functions of single-nucleotide polymorphisms (SNPs) associated with major depression based on the largest meta-analysis of genome-wide association studies (GWASs) conducted to date for major depression (MDD)
| Gene ID | Gene name | SNP with minimum P-value | Immune/host defense function of gene |
|---|---|---|---|
| SEL1L2 | Sel-1 suppressor of lin-12-like 2 | rs17226852 | No specific immune or host defense functions identified for SEL1L2. However, the other member of the sel1 gene family, SEL1L, has been shown to be important for quality control of IgM,100 and the infectious capacity of several viruses,101 including human cytomegalovirus,102 a microsatellite polymorphism of SEL1L, is associated with autoimmune thyroid diseases103 |
| ADCY3 | Adenylate cyclase 3 | rs2384061 | ADCY3 is integral to a rapid, NF-κB-independent, signaling cascade initiated by microbial stimulation of TLR4.104 ADCY3 also regulates crosstalk between FP prostanoid and prostaglandin E2 receptors.105 This crosstalk regulates expression of SAT1 gene, which has been reported to be underexpressed in prefrontal cortex of suicide completers.106 |
| UNC93A | Unc-93 homolog A | rs2076008 | No specific immune or host defense functions identified for UNC93A, but a closely related homolog, UNC93B, plays a crucial role in antigen presentation and TLR functioning, and deficiency in its expression reduces TNF-α production and increases vulnerability to a number of infections.107, 108, 109 Blockade of UNC93B may protect against autoimmunity.110 |
| TEX10 | Testis expressed 10 | rs1930243 | No specific immune or host defense functions identified. |
| TTLL2 | Tubulin tyrosine ligase-like family, member 2 | No specific immune or host defense functions identified for TTLL2; however, other TTLL family members have been shown to be essential for proper cilliary structure and function and with this ability to clear pathogens and other harmful substances from the airway.111 | |
| GAL | Galanin | rs2156464 | Signaling through either type 1 or type 2 receptors, GAL has numerous anti-inflammatory effects;112, 113, 114, 115 Consistent with PATHOS-D, multiple lines of evidence indicate GAL signaling is reduced in MDD;116, 117, 118 GMAP, which is produced by cleavage of the same precursor as galanin, has direct antifungal activity119 |
| PDK4 | Pyruvate dehydrogenase kinase, isozyme 4 | rs11531570 | PDK4 gene expression is upregulated by IFN-α and by LPS and contributes to muscle glycogen breakdown and lactate accumulation;120, 121 conversely, PDK4 is inhibited by TNF-α via p38 MAPK and NF-κB signaling, leading to increased glucose oxidation;122 anti-inflammatory omega-3 fatty acids increase PDK4 in immature dendritic cells via enhanced PPAR-γ signaling123 |
| NPM1 | Nucleophosmin | rs11134697 | Functions as an endogenous ‘alarmin’ that activates proinflammatory cytokines;124, 125, 126 identified as a host virulence factor in viral infection;127, 128 may aid in HIV and HSV1 virus dispersal within cells;129, 130 complexes with, and inhibits, PKR, which has important antiviral properties131 |
| USP3 | Ubiquitin-specific peptidase 3 | rs7183892 | Embedding of USP genes in the copy number variable β-defensin cluster on chromosome 8p23.1 suggests a close tie with innate immunity;132 USP3 is activated by IL-4 and IL-6 and has antiproliferative and apoptotic properties;133 highly homologous USP17 necessary for type I IFN production in response to virus infection;134 |
| ASB4 | Ankyrin repeat and SOCS box-containing 4 | rs11531570 | No specific immune or host defense functions identified. |
Abbreviations: FP, prostaglandin F; GMAP, galanin message associated peptide; HSV1, herpes simplex virus type 1; IFN-α, interferon-α; IgM, immunoglobulin M; IL, interleukin; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MDD, major depressive disorder; NF-κB, nuclear factor-κβ; PATHOS-D, Pathogen Host Defense; PKR, protein kinase R; PPAR-γ, peroxisome proliferator-activated receptor-γ; TLR, Toll-like receptor; TNF-α, tumor necrosis factor-α.
Reference numbers in Table 2 correspond to reference numbers in paper bibliography.
An exception to the general lack of knowledge regarding GWAS-identified depression risk alleles is provided by the rs1006737 SNP in the CACNA1C gene, which codes for the α1 subunit of the L-type voltage-gated calcium channel (Cav1.2).29 CACNA1C has been identified as a potential depression risk gene in several GWASs,105, 141, 142 and convergent validity for its role in depression is provided by data demonstrating that carriers of the risk A allele have changes in brain function and morphology relevant to MDD.143, 144, 145
An examination of the immune effects of CACNA1C highlights both the promise and complexities of a PATHOS-D perspective. Calcium signaling pathways play central and essential roles in multiple aspects of immune function, and the Cav1.2 channel in particular contributes to the function of a variety of immune cell types, including dendritic cells, CD4+ and CD8+ T cells, mast cells and macrophages.146, 147, 148, 149, 150, 151, 152, 153, 154 Consistent with an overall proinflammatory effect for Cav1.2, agents that block this calcium channel have been repeatedly observed to have anti-inflammatory properties.155 Given these findings, the PATHOS-D theory predicts that the depressogenic A allele at rs1006737 should be a gain-of-function variant with an overall proinflammatory effect. In support of this, the A allele has been associated with reduced activation of the anti-inflammatory intracellular messenger Akt,156 which is known from in vitro studies to downregulate TNF-α and inducible nitric oxide synthase production in response to challenge with bacterial endotoxin.157 Moreover, if Cav1.2 activation promotes host defense via activation of inflammatory processes, one would predict that the A allele should be associated with increased CACNA1C protein production. Although this has yet to be confirmed, data from post-mortem brain tissue indicate that carriers of the A allele have increased CACNA1C mRNA production in the CNS.144
These data suggest that the A allele of CACNA1C meets the first two criteria for consistency with a PATHOS-D perspective (that is, located in a gene with known immune effects and associated with increased signaling in inflammatory/host defense pathways). The finding that Cav1.2 activation is necessary for T-cell defense against Leishmania major infection is consistent with the third criteria,148 given that the A allele appears to be a gain-of-function variant. However, other lines of circumstantial evidence undermine any straightforward association between allelic variants that increase Cav1.2 function and enhanced host defense. In fact, the opposite appears to be the case, given that Timothy Syndrome, caused by a rare gain-of-function variant in CACNA1C,158 is associated with a strikingly increased risk of infection.154 Similarly, activation of Cav1.2 channels appears to actually impede host defense against M. tuberculosis by reducing the bactericidal activity of dendritic cell-activated T cells.149 These results appear paradoxical given that calcium influx into immune cells is essential for eradication of M. tuberculosis, and significant data indicate that L-type voltage-gated channels play an important role in this regard.150 However, conflicting data suggest that L-type calcium channels may actually downregulate overall calcium influx, given that blocking these channels increased calcium signaling and bactericidal activity in M. tuberculosis-infected macrophages.149 These findings are consistent with the observation that bacterial endotoxin acutely downregulates L-type calcium channel mRNA, as would be expected if Cav1.2 has an anti-inflammatory function.159
These considerations introduce a critically important complication into our discussion of the immune effects of depressogenic gene variants. Up to this point we have proceeded as though pathogen host defense is a monolithic process, which is a simplification exposed by the bivalent effects of L-type intracellular calcium signaling on infectious outcomes. Because calcium signaling activates multiple facets of the immune system, it is not surprising that this signaling has been shown to contribute to the antipathogen capacities of a variety of cell types. For example, macrophages rely on L-type calcium channel activation in response to Chlamydia pneumonia lipopolysaccharide to kill the microorganism.160 However, other microbes have evolved to manipulate this host defense system to their own benefit, such as Legionella pneumophila, which requires L-type calcium signaling to replicate within infected host cells.161 These examples demonstrate that the same physiological process can enhance host defense to one pathogen, while simultaneously increasing vulnerability to another.
Infection, inflammation and environmental risk factors for MDD
If depressogenic alleles contribute to protection against pathogen invasion, the circumstances in which such invasion was likely or already a fait accompli should be especially potent activators of these genes, and hence especially likely to induce depression. Moreover, if these alleles heighten host defense in large part by increasing inflammation, inflammatory mediators released in response to environments rife with pathogen danger would be expected to induce depressive symptoms. These predictions are borne out by many studies demonstrating the depressogenic effects of inflammatory mediators,10, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173 as well as the remarkably diverse array of conditions that activate inflammatory processes and also increase the risk for depression.174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221
Psychosocial stress may be especially relevant in this regard. Stress is a universal and powerful risk for the development of depression both during development and adulthood.222, 223, 224, 225 This squares nicely with social theories of depression, and at first glance appears to challenge host defense perspectives. But if we consider that the vast majority of stressors in mammals over evolutionary time boiled down to risks inherent in hunting, being hunted or fighting conspecifics in dominance hierarchies for reproductive access/status, it is not surprising that these states are also circumstances in which the risk of pathogen invasion—and subsequent death from infection—was greatly increased as a result of traumatic opening of the protective skin barrier from wounding.226 Such wounding is common in social species and was a significant source of morbidity and mortality among humans in the ancestral environment, and indeed well into the historical period.227, 228, 229 Given this, it is not surprising that—to quote Firdaus Dhabhar—‘stress perception by the brain may serve as an early warning signal to activate the immune system in preparation for a markedly increased likelihood of subsequent infection’.230 And although chronic stress is best known to suppress immune function,231 the types of acute and/or psychosocial stressors most likely to be associated with immediate risk of wounding and hence infection activate both innate and adaptive immunity.232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242 And while suppressing certain measures of adaptive immunity, chronic stress (whether experienced during childhood or as an adult) has been repeatedly associated with increased peripheral inflammatory biomarkers.233, 243, 244, 245, 246, 247, 248
From a PATHOS-D perspective, then, psychosocial stress may increase the risk for depression, at least in part, because it activates host defense mechanisms that reliably induce depressive symptoms. In ancestral environments, the association between stress perception and risk of subsequent wounding was reliable enough that evolution, operating by the so-called ‘smoke detector’ principle,249 favored organisms that prepotently activated inflammatory systems in response to a wide array of environmental threats and challenges (including psychosocial stressors), even if this activation was often in vain. This perspective provides a parsimonious explanation for why psychosocial stressors reliably induce depression, even though depressive reactions to stressors often appear so patently maladaptive. Across evolutionary time, the benefit that depressive symptoms (and their underlying physiology) conferred in terms of host defense in situations of high infectious danger (including most psychosocial adversities) outweighed their cost in terms of any social impairment they incurred in these same contexts. A clear prediction of this hypothesis is that genes promoting inflammatory responses to psychosocial stress should decrease in prevalence over time in human societies in which the association between stressors and subsequent infection has been weakened by factors such as modern health practices. Consistent with this possibility, the prevalence of the short allele of the serotonin transporter gene, which has been associated with increased inflammatory responses to psychosocial stress, is lower in societies with reduced rates of historical infectious mortality.104
In addition to providing a novel explanation for why stress is a primary risk factor for developing depression, the PATHOS-D theory offers a unifying perspective on why many other facets of modern life are also depressogenic, a perspective not readily provided by theories focused more exclusively on the social realm. Indeed, if the adaptive value of depression is to be found primarily in its effects on social functioning, it is hard to understand why so many risks for depression, including obesity, sedentary lifestyle, dietary factors, diminished sleep and smoking, are at least partially nonsocial in nature. On the other hand, these conditions are all associated with increased inflammation (for reviews see refs. 207, and 250, 251, 252), suggesting that they may be depressogenic because they tap into pathways that initially evolved to fight infection.
Patterns of immune activation in MDD and protection from infectious mortality in ancestral environments
The hypothesis that patterns of immune activity associated with MDD should have decreased mortality from infection in ancestral environments appears to face a challenge from data indicating that depression worsens outcome in a number of infectious processes253, 254, 255, 256, 257, 258, 259, 260 and is associated with impairments in adaptive immune mechanisms important for protection against both viruses and bacteria.13, 261, 262 To address this challenge, we have first to inquire whether innate immune inflammatory processes that are increased in MDD produce the patterns of infectious vulnerability and adaptive immune impairment that are apparent in depression. Surprisingly, the answer is yes.263, 264 Although essential for activating adaptive immunity in response to pathogen invasion, chronic inflammation can actually suppress T- and B-cell function through various mechanisms.263, 264, 265, 266, 267, 268, 269, 270 Consistent with this, rates of infection are often increased—not decreased—in autoimmune conditions characterized by chronic inflammation.271 However, PATHOS-D theory requires only that across evolutionary time the survival benefits of enhanced inflammatory activity characteristic of depression outweighed any costs imposed by associated reductions in other aspects of immune functioning. Several lines of evidence support this possibility.
One such line of evidence comes from Ghana, a country in which some regions rely for drinking water on heavily contaminated rivers and other regions obtain clean water from boreholes. As would be expected, death rates from infection are higher in river-drinking regions than in areas where borehole-obtained water is available. Consistent with the prediction that increased inflammatory signaling is protective in the type of high-infection environments common during human evolution (and especially common since the origin of agriculture and the rise of cities),272 a haplotype of the IL-10 gene associated with increased inflammation was found to be significantly more prevalent in populations that relied on river water than in populations that drank from boreholes—suggesting positive selection driven by enhanced pathogen protection.2 Consistent with this possibility, during a 5-year follow-up period, the high-inflammation IL-10 haplotype was associated with increased survival in populations that drank from rivers, but reduced survival in individuals who drank from boreholes.2 These results are consistent with the observation that cytokine-stimulated production of TNF-α declines with age in the Netherlands, a country with a low infectious burden, but does not decline with age in Ghana, a country with high rates of infection (that is, 85% of Ghanan study participants were infected with malaria),273 again suggesting that increased proinflammatory cytokine production—as observed in MDD—promotes survival under conditions of high pathogen burden.274
Multiple facets of modernity have reconfigured our relationship with the microbial/parasitic world in ways that have reduced the benefits of inflammation and increased its costs.274, 275 Nonetheless, even in an environment so different from that in which humans evolved, multiple studies have identified associations between patterns of increased inflammation observed in MDD and improved outcome in the context of infection,3, 4, 5, 50, 276, 277, 278, 279, 280, 281, 282, 283, 284, 285, 286, 287, 288, 289, 290 as shown in Table 3.
Table 3.
Inflammation and infectious outcomes in industrialized societies
| Biological factor | Immune effect | Host defense findings |
|---|---|---|
| Plasma concentration of interleukin (IL)-10; ratio of plasma IL-10 to tumor necrosis factor-α (TNF-α; IL-10/TNF-α) | IL-10 is a powerful anti-inflammatory cytokine that suppresses innate immunity and T helper type I (Th1) immune responses; TNF-α is an innate immune potent proinflammatory cytokine that produces sickness behavior and acutely plays important role in facilitating activation of the adaptive immune system. |
In a large study of consecutive patients admitted to hospital with fever, elevations in plasma concentrations of the anti-inflammatory cytokine IL-10—as well as the ratio of IL-10/TNF-α—were associated with increased mortality;3 Increased IL-10 production late in the disease course predicted reduced survival following infection with the pandemic A/H1N1/2009 influenza virus.4 |
| Plasma concentration of C-reactive protein (CRP) via high-sensitivity assay | CRP is an acute-phase reactant primarily stimulated by IL-6 shown in multiple studies to predict the development of multiple illness associated with chronic inflammation | CRP has been associated with increased survival in children with meningococcal sepsis.276 |
| Stimulated production of pro- and anti-inflammatory cytokines | In vitro measure of ability of immune cells to produce/release cytokines in response to an immune stimulus. | Families with a member who died from meningococcal disease were characterized by increased production of IL-10 and reduced production of TNF-α when compared with families with a member who had bacterial meningitis but survived.5 |
| Th1 and Th2 cytokine concentrations in the supernatant of cultured peripheral blood mononuclear cells (PBMCs) | In vitro measure of ability of PBMCs to promote either Th1 or Th2 immunity | Reduced PBMC production of IFN-γ and IL-12 is associated with increased severity of respiratory syncytial virus symptoms in infants under 1 year of age.277 |
| Increased activity alleles of the IL-10 gene (i.e., −1082G) | Associated with higher levels of IL-10 |
−1082G allele is associated with increased symptom severity and mortality in the context of community-acquired pneumonia,278 −1082G allele is associated with reduced antibody responses to tetanus, influenza and hepatitis B virus (HBV) vaccines.279, 280, 281 |
| Increased activity alleles of the interferon-γ (IFN-γ) gene (i.e., +874T) | The +874T allele increases Th1 immunity via increased IFN-γ production as a result of enhanced binding of nuclear factor-κβ (NF-κB)283, 284 |
+874T allele has been associated with protection against Mediterranean Spotted fever.282 Multiple studies and a large meta-analysis find that the +874T allele is associated with protection against the development of tuberculosis at the individual285 and population levels.50 In individuals with active tuberculosis, the T allele is associated with reduced severity and risk of disseminated disease.286 +874T allele has been associated with reduced risk of leprosy,287 severe acute respiratory syndrome (SARS),288 and Chagas disease.289 |
| Reduced activity alleles of the IFN-γ gene (i.e., +874A) | An allele at +874 is associated with reduced IFN-γ production and consequent reductions in Th1 activity | +874 A predisposes for hepatitis B and C persistence and negatively affects clinical course of these diseases.290 |
Reference numbers in Table 3 correspond to reference numbers in paper bibliography.
Survival-promoting elements of depressive/ sickness symptoms in response to infection
Microbial activation of the mammalian inflammatory response produces a highly regulated suite of symptoms known as sickness behavior that bears a striking resemblance to behavioral changes induced by stress in laboratory animals, as well as to the symptoms of MDD in humans.10, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173 Many of these symptoms can be ameliorated by antidepressants in animal models,291, 292, 293, 294, 295 further suggesting that cytokine-induced behavioral changes are either closely aligned with, or identical to, MDD in humans. Studies report that 20–70% of patients undergoing chronic immune activation as a result of treatment with the cytokine IFN-α meet symptom criteria for MDD, providing additional evidence in this regard.164, 296 Moreover, IFN-α-induced depression shares symptom homology with idiopathic MDD,297 and responds to treatment with antidepressants.164, 298, 299, 300, 301 In addition to a remarkable symptom overlap, sickness and depression during cytokine exposure also appear to be causally linked, given the strong association between sickness in the first week of treatment with IFN-α and the development of cognitive/emotional symptoms of depression over the ensuing 6 months of therapy.302 Finally, peripheral inflammatory activation induces many—if not all—of the most replicated CNS and neuroendocrine abnormalities observed in MDD (Figure 1).303, 304, 305, 306, 307, 308, 309, 310
The PATHOS-D theory asserts that depressogenic alleles are common not because depression is adaptive in managing social negotiations, but because these alleles promote symptoms and behaviors that decreased mortality from infectious causes across mammalian evolution. However, from an evolutionary perspective, there is no a priori reason why these antipathogen effects should overlap with the depressogenic effects of these risk alleles. That they do so is powerful evidence, we would suggest, for the primacy of immune defense in the pathogenesis of depression, regardless of the environmental adversity that initiates the disorder in individual cases. In keeping with this perspective is the possibility that some of the symptoms of depression promote survival in response to infection.
Fever and hypoferremia
Although once viewed as a maladaptive consequence of immune activation,311 several decades of research have produced a consensus that sickness behavior is an adaptive central motivational state evolved to promote survival and necessitated to a large degree by the metabolic costs of mounting a fever.163, 169, 311, 312, 313, 314 Fever, in turn, has been shown to enhance resistance to both viral and bacterial pathogens, over and above other antipathogen effects of the inflammatory mechanisms by which fever is induced. In addition to retarding pathogen replication/spread,315, 316, 317, 318 febrile range temperatures have multiple stimulatory effects on the immune system relevant to host defense.319, 320, 321, 322, 323, 324, 325
Because these effects are enhanced in conditions of low iron availability, it should not be surprising that in addition to causing fever, inflammatory cytokines deplete bodily iron stores.326 Nor should it be surprising that sickness is associated with hypoferremia,327, 328 which—after fever—is probably the feature of sickness that has been best established as of adaptive value.329, 330, 331 For example, low bodily iron stores protect against infection in children in the developing world,332 and multiple studies suggest that iron supplementation worsens an array of infection-related health outcomes and increases infectious mortality.333, 334, 335, 336, 337
If depressive symptoms aid in pathogen defense and if fever and hypoferremia are important in this regard, one would expect that MDD should be associated with elevated body temperature and reduced bodily iron stores, even in individuals with no evidence of an infectious process. In this regard, it is surprising, given the centrality of fever to the adaptive function of sickness behavior,163, 169, 315 that so little attention has been paid to the fact that MDD appears to be reliably characterized by an elevation in body temperature into the range known to be maximally protective in the context of infection.338, 339, 340, 341, 342, 343, 344, 345 As with elevated body temperature, a number of studies have reported that depressive symptoms are associated with reductions in various measures of bodily iron stores.346, 347, 348, 349, 350
Because fever and hypoferremia are central to the adaptive purposes of sickness, their presence in depression is mandated from a PATHOS-D perspective, and their absence would strongly argue against the validity of this approach. On the other hand, their presence in depression is not parsimoniously explained by theories that focus on potential social benefits of depression. Similarly, if depression is simply a nonadaptive phenomenon, why would such ancient, highly conserved and highly complex physiological responses be a hallmark of the disorder?
Conservation-withdrawal
Proinflammatory cytokines induce a behavioral state of conservation-withdrawal,351 characterized by depressed mood, anhedonia, psychomotor retardation, fatigue, social avoidance and anorexia.163, 252, 352, 353 This state is an integral component of depressive disorders and has been widely considered to develop in the context of infection and/or tissue injury as a means of marshalling limited metabolic resources for the expensive tasks of immune activation, fever generation and tissue repair.311 In addition to energy allocation, conservation-withdrawal symptoms may have also proved adaptive by reducing interpersonal contact and thereby limiting infectious exposure.25 Because ancestral humans typically lived in small coalitional groups of genetically related individuals, the logic of inclusive fitness suggests that social withdrawal might have been adaptive for an individual's genes by reducing the risk of infection in kin, even if such withdrawal limited the provision of much needed care from others and thus reduced individual survival.354, 355 As would be predicted from this line of reasoning, acute exposure to an inflammatory mediator has been shown to induce feelings of social isolation/withdrawal in humans,356 and increased neural sensitivity to social rejection (indexed by changes in activity in dorsal anterior cingulate and anterior insula cortices) is associated with increased inflammatory responses to psychosocial stress.357 However, in addition to potential benefits related to kin selection, significant data demonstrate that viral infections promote aggressive immune responses to bacterial superinfections that can greatly increase mortality;358, 359, 360, 361, 362, 363, 364 therefore, any decrement in survival from loss of social aid might have been more than offset by reduced risk of exposure to other pathogens while in a vulnerable state due to a pre-existing infection. Moreover, social withdrawal and reduced environmental exploration might also have promoted individual survival by limiting a sick person's contact with immunologically dissimilar out-group members who potentially harbored pathogens against which the sick person would have had reduced immunity compared with pathogens endemic in the home group.354, 365
Hypervigilance
Although withdrawal-conservation-type behavior is prominent in MDD, depressed individuals also often manifest metabolically expensive symptoms more consistent with behavioral activation, including anxiety/agitation and insomnia.366, 367, 368, 369, 370 By siphoning energy away from immune activity, these symptoms would be expected to impair host defense and hence to argue against a PATHOS-D perspective. However, sickness behavior—although of benefit for surviving infection—carries its own survival and reproduction costs as a result of increased risk for predation and reduced ability to care for one's young, as well as potential loss of status in a social species and/or loss of breeding territory.371 Therefore, evolutionary logic dictates that inflammatory processes—especially when chronic—might promote hypervigilant behavior that, while shunting energy away from fighting infection, would nonetheless serve adaptive purposes by protecting against environmental dangers engendered by sickness. In fact, significant data demonstrate that chronic cytokine activation reliably produces hypervigilant behaviors/symptoms, including anxiety/agitation, insomnia and anger/irritability.305, 353, 372 Neurobiological substrates for the mixture of withdrawal-conservation and behavioral activation/hypervigilance symptoms that is common to chronic inflammation/medical illness and MDD have been recently identified, including the effects of cytokines on both the basal ganglia (withdrawal-conservation) and the dorsal anterior cingulate cortex (hypervigilance) (Figure 1).303, 309, 357, 373, 374, 375, 376
Anorexia
As suggested above, anorexia may enhance survival during infection by redirecting energy away from food procurement to the metabolic demands of immune activation/fever, while also limiting the exposure to food-borne pathogens. But the metabolic requirements of fighting infection make the anorexic response a paradox in need of a more robust adaptive explanation. Although it remains unclear whether food restriction protects against the development of infection,377 animal data indicate that force feeding rodents once they are infected increases mortality.378 Similarly, the provision of total parenteral nutrition in animal models and to critically ill patients has been associated with increased risk for infection and subsequent mortality.379, 380, 381, 382 Interestingly, rats injected with lipopolysaccharide consume proportionately more carbohydrates—as do depressed individuals with hyperphagia383—even though more energy is available from ingesting lipids. This suggests that lipid consumption may be counterproductive during an infection. Several observations are consistent with this possibility. For example, preclinical data demonstrate that lipid consumption increases infectious mortality,384 and a meta-analysis of total parenteral nutrition use found that infected patients provided lipids in their feedings had higher complication rates than those receiving total parenteral nutrition without lipids.385 Finally, omega-3 fatty acids have been shown to activate peroxisome proliferator-activated receptor-γ signaling in dendritic cells, with a resultant downregulation of CD1a receptor expression.129 These receptors play an essential role in activating T-cell responses to pathogens, as demonstrated by the ability of Leishmania donovani to survive in host cells by downregulating these receptors.386 Moreover, CD1a expression in dendritic cells is also crucial for the presentation of M. tuberculosis antigens to cells of the adaptive immune system.387
Potential limitations of, and challenges to, PATHOS-D theory
In this article we have focused our analyses on allelic variants associated with phenotypic variability. Many genetic features contributing to MDD may have swept to fixation over evolutionary history and by becoming nonpolymorphic remain invisible to genetic association studies. It is possible that such sequences are preferentially associated with species-typical social, rather than immunological, factors. Were this to be the case, our analyses may have overestimated immune risk factors for depression at the cost of universal depressogenic risk alleles maintained as a ‘price of being human’.
It should also be noted that inflammatory biomarkers are not elevated in all individuals with MDD. Whether patients with increased inflammation represent a biologically and evolutionary distinct subset of MDD is an area of active research.388 If this turns out to be the case, it may be that selection for enhanced host pathogen defense is relevant primarily to these individuals (and their allelic variants) and is thus only one adaptive factor driving the persistence of depressogenic alleles. Prior theorists have posited a variety of potentially fitness-enhancing psychosocial effects of low mood and/or depression not obviously related to host defense functions (that is, abandoning unattainable goals, yielding in dominance struggles and so on),389, 390, 391 and it may be that these types of psychosocial benefits are promoted by allelic variants retained in the human genome independently of variants maintained as a result of conferring pathogen host defense benefits. If both immune and nonimmune etiological pathways contribute to MDD, the next question is how they combine. One hypothesis consistent with the general absence of documented epistatic interactions among MDD risk alleles is that inflammation/immune alleles provide one hit and social/stress factors provide a second (biologically distinct) hit, which together sum to exceed an MDD symptom threshold. If distinct social and immune-related genetic risk factors were identified, statistical analysis of epistasis could help distinguish intrinsic interactions between pathways from a simple additive model.
On the other hand, findings from patients undergoing treatment with IFN-α suggest a more inclusive scenario for the role of pathogen defense in the evolution/persistence of depressogenic alleles. Specifically, although standardized dosages of IFN-α are employed, a wide range of behavioral responses are observed during treatment, from mild neurovegetative/sickness symptoms, such as fatigue, to completed suicide in response to catastrophic major depression. Individuals who develop significant depressive symptoms evince changes in CNS and neuroendocrine functioning that are also observed in idiopathic MDD,305, 306, 307, 308, 309, 310 but that are not observed to a significant degree in patients on IFN-α who do not develop depression. These findings raise the possibility that depression reflects a state of immune response element amplification, such that for any given amount of inflammatory input, depressed individuals react with enhanced downstream CNS/neuroendocrine activity. If so, depressogenic alleles that do not promote an increase in inflammatory biomarkers may nonetheless have undergone positive selection because they enhanced host pathogen defense via sensitization of downstream CNS/neuroendocrine pathways that themselves promote survival during infection. Some evidence for this hypothesis comes from the finding, noted above, that individuals who develop depression during IFN-α manifest enhanced sickness behavior at the start of treatment, which may aid in acutely clearing pathogens from the body during infection.302 Moreover, a clear prediction of PATHOS-D theory is that changes in CNS/neuroendocrine function that typically accompany MDD should enhance survival in the context of acute infection. To date, few data support this possibility, although it is intriguing to note that glucocorticoid resistance, which is common in MDD392 and is associated with the development of depressive symptoms during IFN-α treatment (Raison et al., unpublished observations), has been associated with improved T-cell function in HIV infection,393 and that enrichment paradigms known to enhance glucocorticoid sensitivity in animal models increase mortality in response to Escherichia coli infection.394
These two possibilities (that is, distinct additive social and immunological risks vs inflammatory mediation of social risk) might be genetically discriminated based on their contrasting implications for the functional relationship between social–environmental precipitants and immune-related genetic risks for MDD. In the former model, one would expect to find largely additive effects of social risk factors and immune-related genetic risk alleles, whereas the meditational model would suggest a product-term interaction (that is, a social stimulus shows larger depressogenic ‘gain’ in the context of a sensitizing genotype). This approach could be extended to use an instrumental variables analysis (for example, a Mendelian randomization study) to determine whether inflammatory signals function as mediators of, moderators of, or functionally independent additional additive influences with respect to, social precipitants of MDD.
Thus far, we have focused on the possibility that risk alleles promote depressive symptoms primarily as a result of increasing activity in inflammatory and/or immune-relevant downstream physiological pathways (that is, gene → immune effects → depression). However, many of the associations cited in this review could be equally well accounted for by the hypothesis that alleles directly influence CNS functioning to increase MDD risk, and that MDD subsequently affects immune function (that is, gene → depression → immune effects). This possibility is especially likely for genes such as NPY, which we have described in immune terms, but that also has well-documented effects on CNS functioning relevant to depression. In addition to downstream immune effects, such genes may also have enhanced host defense in ancestral environments by promoting behavioral patterns likely to reduce the risk of becoming infected, spreading infection to kin or of dying once an infection had commenced.395, 396 Just such effects have been proposed for the short allele of the serotonin transporter, which has been associated with collectivistic social behavior relevant to host defense.104
Immune changes associated with MDD are not only specific but also occur in other severe mental disorders, including bipolar disorder and schizophrenia. Although the high prevalence of depression in these conditions is consistent with a PATHOS-D perspective, it is hard to imagine that other behavioral states associated with these diseases, including mania and psychosis, are adaptive for pathogen host defense. Indeed, the impaired decision-making characteristic of both states and the social isolation/reduced access to resources that is common in psychosis would be expected to increase vulnerability to pathogen exposure. Given overlapping genetic risk factors for these conditions and MDD, it is possible that they are best understood as purely maladaptive states supported at relatively low levels in the human population, at least in part, because their genetic antecedents enhanced host defense in carriers of immune-relevant risk alleles who responded to infectious challenges with enhanced immune activation and sickness behavior/depression without developing the full disease phenotype. Another possibility is that very severe disorders such as bipolar disorder and schizophrenia have been maintained in the human genome because immune benefits accrued to afflicted individuals that counteracted the fitness-reducing behavioral profiles (including increased risk of infection) associated with these diseases. This scenario would suggest that immune changes seen in schizophrenia and bipolar disorder should be more robust than those seen in depression, because they would have to be large enough to offset behavioral costs not present in depression. Although not entirely consistent,397 some data support this possibility.398, 399, 400
Summary
By shifting the adaptive context of depressogenic alleles from any purported benefit of depressive symptoms on relations with conspecifics to the potential benefits of sickness behavior (and its attendant physiology) on relations with the microbial world, the PATHOS-D hypothesis provides a straightforward explanation for how depression can be nonadaptive in the social realm, whereas its risk alleles are nonetheless represented at prevalence rates suggesting an adaptive function. Across vertebrate evolution, innate immune inflammatory responses were essential for effective host defense against pathogens. In humans, these responses are especially relevant during the first several years of life when infectious mortality was highest and adaptive immunity was not yet fully functional. Given these considerations, it is not surprising that the immune system alterations most frequently observed in MDD are proinflammatory in nature, and that the best characterized MDD risk alleles appear to generally produce a proinflammatory phenotype. However, we should not infer from this that any given depressogenic allele will uniformly increase innate immune function or enhance host defense against all microbes. Rather, what PATHOS-D suggests is that depressogenic alleles—and the physiological processes they promote—can be understood as reflecting a summation of the most successful pathogen defense mechanisms against the wide array of pathogens encountered during human evolution, with all the imperfections and tradeoffs this has entailed. Moreover, knowing the effects of depressogenic alleles on outcomes following infection with specific pathogens may cast light on the relative importance of each pathogen for driving human evolution, because the high price imposed by depressogenic alleles mandates a compensatory high payoff in terms of pathogen defense. If confirmed in future studies, this perspective raises the intriguing possibility that gaining a better understanding of how genes promote MDD may significantly advance the field of immunology and that—conversely—a better understanding of the ongoing evolutionary ‘arms race’ between pathogens and their human hosts may suggest novel theoretical paradigms and treatment strategies for MDD.
Supplementary information
Acknowledgements
This work was supported in part by grants from the National Institute of Mental Health to CLR (Award Numbers R01AT004698 and R01MH75102) and AHM (Award Numbers R01MH087604, R01MH083746 and R01MH075102) as well as PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources. We acknowledge the significant role played by three anonymous reviewers in strengthening this manuscript and extending its scientific implications.
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, National Center for Complementary and Alternative Medicine or the National Institutes of Health.
PowerPoint slides
Competing interests
The authors declare no conflict of interest. Charles L Raison serves as a consultant for Pamlab, Biolex Therapeutics and Bristol Myers Squibb. Andrew H Miller has served as a consultant for Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Lundbeck Research USA, F. Hoffmann-La Roche, Schering-Plough Research Institute and Wyeth/Pfizer and has received research support from Centocor, GlaxoSmithKline and Schering-Plough Research Institute.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website
References
- 1.Crespi B, Summers K, Dorus S. Adaptive evolution of genes underlying schizophrenia. Proc Biol Sci. 2007;274:2801–2810. doi: 10.1098/rspb.2007.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuningas M, May L, Tamm R, van Bodegom D, van den Biggelaar AH, Meij JJ. Selection for genetic variation inducing pro-inflammatory responses under adverse environmental conditions in a Ghanaian population. PLoS One. 2009;4:e7795. doi: 10.1371/journal.pone.0007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 4.Bermejo-Martin JF, Martin-Loeches I, Rello J, Anton A, Almansa R, Xu L. Host adaptive immunity deficiency in severe pandemic influenza. Crit Care. 2010;14:R167. doi: 10.1186/cc9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/S0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 6.McDade Thomas W. Life history theory and the immune system: Steps toward a human ecological immunology. American Journal of Physical Anthropology. 2003;122(S37):100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- 7.Pedron B, Guerin V, Cordeiro DJ, Masmoudi S, Dalle JH, Sterkers G. Development of cytomegalovirus and adenovirus-specific memory CD4 T-cell functions from birth to adulthood. Pediatr Res. 2011;69:106–111. doi: 10.1203/PDR.0b013e318204e469. [DOI] [PubMed] [Google Scholar]
- 8.Nwachuku N, Gerba CP. Health risks of enteric viral infections in children. Rev Environ Contam Toxicol. 2006;186:1–56. doi: 10.1007/0-387-32883-1_1. [DOI] [PubMed] [Google Scholar]
- 9.Chen LC, Rahman M, Sarder AM. Epidemiology and causes of death among children in a rural area of Bangladesh. Int J Epidemiol. 1980;9:25–33. doi: 10.1093/ije/9.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 12.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 14.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2008;39:1–11. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 17.Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson AP, Carper ER. Infectious diseases and human population history. Biosci. 1996;46:115–126. doi: 10.2307/1312814. [DOI] [Google Scholar]
- 19.Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovel H. Targeted interventions and infant mortality. Trans R Soc Trop Med Hyg. 1989;83:10–18. doi: 10.1016/0035-9203(89)90689-5. [DOI] [PubMed] [Google Scholar]
- 21.Kavaliers M, Colwell DD, Choleris E. Parasites and behavior: an ethopharmacological analysis and biomedical implications. Neurosci Biobehav Rev. 1999;23:1037–1045. doi: 10.1016/S0149-7634(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves GM, Zamboni DS, Camara NO. The role of innate immunity in septic acute kidney injuries. Shock. 2010;34(Suppl 1):22–26. doi: 10.1097/SHK.0b013e3181e7e69e. [DOI] [PubMed] [Google Scholar]
- 23.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 24.Klein SL. Parasite manipulation of the proximate mechanisms that mediate social behavior in vertebrates. Physiol Behav. 2003;79:441–449. doi: 10.1016/S0031-9384(03)00163-X. [DOI] [PubMed] [Google Scholar]
- 25.Kinney DK, Tanaka M. An evolutionary hypothesis of depression and its symptoms, adaptive value, and risk factors. J Nerv Ment Dis. 2009;197:561–567. doi: 10.1097/NMD.0b013e3181b05fa8. [DOI] [PubMed] [Google Scholar]
- 26.Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–256. doi: 10.1176/appi.ajp.2010.10091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licinio J, Dong C, Wong ML. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry. 2009;66:488–497. doi: 10.1001/archgenpsychiatry.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? Behav Brain Sci. 2006;29:385–404. doi: 10.1017/S0140525X06009095. [DOI] [PubMed] [Google Scholar]
- 29.Detera-Wadleigh SD, Akula N. A systems approach to the biology of mood disorders through network analysis of candidate genes. Pharmacopsychiatry. 2011;44(Suppl 1):S35–S42. doi: 10.1055/s-0031-1275275. [DOI] [PubMed] [Google Scholar]
- 30.Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerri AP, Arosio B, Viazzoli C, Confalonieri R, Vergani C, Annoni G. The −308 (G/A) single nucleotide polymorphism in the TNF-alpha gene and the risk of major depression in the elderly. Int J Geriatr Psychiatry. 2010;25:219–223. doi: 10.1002/gps.2323. [DOI] [PubMed] [Google Scholar]
- 32.Clerici M, Arosio B, Mundo E, Cattaneo E, Pozzoli S, Dell'osso B. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectr. 2009;14:419–425. doi: 10.1017/S1092852900020393. [DOI] [PubMed] [Google Scholar]
- 33.Jun TY, Pae CU, Hoon H, Chae JH, Bahk WM, Kim KS. Possible association between -G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population. Psychiatr Genet. 2003;13:179–181. doi: 10.1097/00041444-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Pae CU, Lee KU, Han H, Serretti A, Jun TY. Tumor necrosis factor alpha gene-G308A polymorphism associated with bipolar I disorder in the Korean population. Psychiatry Res. 2004;125:65–68. doi: 10.1016/j.psychres.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol Psychiatry. 2011;16:516–532. doi: 10.1038/mp.2010.38. [DOI] [PubMed] [Google Scholar]
- 36.Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem. 2007;7:1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira R, Xapelli S, Santos T, Silva AP, Cristovao A, Cortes L. Neuropeptide Y modulation of interleukin-1{beta} (IL-1{beta})-induced nitric oxide production in microglia. J Biol Chem. 2010;285:41921–41934. doi: 10.1074/jbc.M110.164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides. 2007;28:453–458. doi: 10.1016/j.peptides.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res. 2004;38:113–121. doi: 10.1016/S0022-3956(03)00101-8. [DOI] [PubMed] [Google Scholar]
- 40.Sjoholm LK, Melas PA, Forsell Y, Lavebratt C. PreproNPY Pro7 protects against depression despite exposure to environmental risk factors. J Affect Disord. 2009;118:124–130. doi: 10.1016/j.jad.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011;68:158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 43.Kallio J, Pesonen U, Kaipio K, Karvonen MK, Jaakkola U, Heinonen OJ. Altered intracellular processing and release of neuropeptide Y due to leucine 7 to proline 7 polymorphism in the signal peptide of preproneuropeptide Y in humans. FASEB J. 2001;15:1242–1244. doi: 10.1096/fj.00-0436fje. [DOI] [PubMed] [Google Scholar]
- 44.Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 45.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon Alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clin Neuropharmacol. 2010;33:191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correa PA, Gomez LM, Cadena J, Anaya JM. Autoimmunity and tuberculosis. Opposite association with TNF polymorphism. J Rheumatol. 2005;32:219–224. [PubMed] [Google Scholar]
- 47.Kerr JR, McCoy M, Burke B, Mattey DL, Pravica V, Hutchinson IV. Cytokine gene polymorphisms associated with symptomatic parvovirus B19 infection. J Clin Pathol. 2003;56:725–727. doi: 10.1136/jcp.56.10.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen DQ, Zeng Y, Zhou J, Yang L, Jiang S, Huang JD. Association of candidate susceptible loci with chronic infection with hepatitis B virus in a Chinese population. J Med Virol. 2010;82:371–378. doi: 10.1002/jmv.21716. [DOI] [PubMed] [Google Scholar]
- 49.Surbatovic M, Grujic K, Cikota B, Jevtic M, Filipovic N, Romic P. Polymorphisms of genes encoding tumor necrosis factor-alpha, interleukin-10, cluster of differentiation-14 and interleukin-1ra in critically ill patients. J Crit Care. 2010;25:542. doi: 10.1016/j.jcrc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Larcombe LA, Orr PH, Lodge AM, Brown JS, Dembinski IJ, Milligan LC. Functional gene polymorphisms in Canadian aboriginal populations with high rates of tuberculosis. J Infect Dis. 2008;198:1175–1179. doi: 10.1086/592049. [DOI] [PubMed] [Google Scholar]
- 51.Masuda M, Senju S, Fujii Si S, Terasaki Y, Takeya M, Hashimoto Si S. Identification and immunocytochemical analysis of DCNP1, a dendritic cell-associated nuclear protein. Biochem Biophys Res Commun. 2002;290:1022–1029. doi: 10.1006/bbrc.2001.6202. [DOI] [PubMed] [Google Scholar]
- 52.Zhou T, Wang S, Ren H, Qi XR, Luchetti S, Kamphuis W. Dendritic cell nuclear protein-1, a novel depression-related protein, upregulates corticotropin-releasing hormone expression. Brain. 2010;133:3069–3079. doi: 10.1093/brain/awq207. [DOI] [PubMed] [Google Scholar]
- 53.Kim Y, Park CS, Shin HD, Choi JW, Cheong HS, Park BL. A promoter nucleotide variant of the dendritic cell-specific DCNP1 associates with serum IgE levels specific for dust mite allergens among the Korean asthmatics. Genes Immun. 2007;8:369–378. doi: 10.1038/sj.gene.6364394. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Leon S, Janssens AC, Hofman A, Claes S, Breteler MM, Tiemeier H. No association between the angiotensin-converting enzyme gene and major depression: a case-control study and meta-analysis. Psychiatr Genet. 2006;16:225–226. doi: 10.1097/01.ypg.0000242191.51397.3d. [DOI] [PubMed] [Google Scholar]
- 55.Virchow S, Ansorge N, Rosskopf D, Rubben H, Siffert W. The G protein beta3 subunit splice variant Gbeta3-s causes enhanced chemotaxis of human neutrophils in response to interleukin-8. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:27–32. doi: 10.1007/s002109900040. [DOI] [PubMed] [Google Scholar]
- 56.Lindemann M, Virchow S, Ramann F, Barsegian V, Kreuzfelder E, Siffert W. The G protein beta3 subunit 825T allele is a genetic marker for enhanced T cell response. FEBS Lett. 2001;495:82–86. doi: 10.1016/S0014-5793(01)02339-0. [DOI] [PubMed] [Google Scholar]
- 57.Romundstad S, Melien O, Holmen J. The G protein beta3 subunit C825T polymorphism is associated with microalbuminuria in hypertensive women and cardiovascular disease in hypertensive men. Am J Hypertens. 2010;23:1114–1120. doi: 10.1038/ajh.2010.131. [DOI] [PubMed] [Google Scholar]
- 58.Holmen OL, Romundstad S, Melien O. Association between the G protein beta3 subunit C825T polymorphism and the occurrence of cardiovascular disease in hypertensives: The Nord-Trondelag Health Study (HUNT) Am J Hypertens. 2010;23:1121–1127. doi: 10.1038/ajh.2010.121. [DOI] [PubMed] [Google Scholar]
- 59.Hauge Opdal S, Melien O, Rootwelt H, Vege A, Arnestad M, Ole Rognum T. The G protein beta3 subunit 825C allele is associated with sudden infant death due to infection. Acta Paediatr. 2006;95:1129–1132. doi: 10.1080/08035250600580529. [DOI] [PubMed] [Google Scholar]
- 60.Bagos PG, Elefsinioti AL, Nikolopoulos GK, Hamodrakas SJ. The GNB3 C825T polymorphism and essential hypertension: a meta-analysis of 34 studies including 14,094 cases and 17,760 controls. J Hypertens. 2007;25:487–500. doi: 10.1097/HJH.0b013e328011db24. [DOI] [PubMed] [Google Scholar]
- 61.Ahlenstiel G, Nischalke HD, Bueren K, Berg T, Vogel M, Biermer M. The GNB3 C825T polymorphism affects response to HCV therapy with pegylated interferon in HCV/HIV co-infected but not in HCV mono-infected patients. J Hepatol. 2007;47:348–355. doi: 10.1016/j.jhep.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Sarrazin C, Berg T, Weich V, Mueller T, Frey UH, Zeuzem S. GNB3 C825T polymorphism and response to interferon-alfa/ribavirin treatment in patients with hepatitis C virus genotype 1 (HCV-1) infection. J Hepatol. 2005;43:388–393. doi: 10.1016/j.jhep.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Brockmeyer NH, Potthoff A, Kasper A, Nabring C, Jockel KH, Siffert W. GNB3 C825T polymorphism and response to anti-retroviral combination therapy in HIV-1-infected patients--a pilot study. Eur J Med Res. 2005;10:489–494. [PubMed] [Google Scholar]
- 64.Lindemann M, Barsegian V, Siffert W, Ferencik S, Roggendorf M, Grosse-Wilde H. Role of G protein beta3 subunit C825T and HLA class II polymorphisms in the immune response after HBV vaccination. Virology. 2002;297:245–252. doi: 10.1006/viro.2002.1467. [DOI] [PubMed] [Google Scholar]
- 65.Fodil-Cornu N, Kozij N, Wu Q, Rozen R, Vidal SM. Methylenetetrahydrofolate reductase (MTHFR) deficiency enhances resistance against cytomegalovirus infection. Genes Immun. 2009;10:662–666. doi: 10.1038/gene.2009.50. [DOI] [PubMed] [Google Scholar]
- 66.Imamura A, Murakami R, Takahashi R, Cheng XW, Numaguchi Y, Murohara T. Low folate levels may be an atherogenic factor regardless of homocysteine levels in young healthy nonsmokers. Metabolism. 2010;59:728–733. doi: 10.1016/j.metabol.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Fujimaki C, Hayashi H, Tsuboi S, Matsuyama T, Kosuge K, Yamada H. Plasma total homocysteine level and methylenetetrahydrofolate reductase 677C>T genetic polymorphism in Japanese patients with rheumatoid arthritis. Biomarkers. 2009;14:49–54. doi: 10.1080/13547500902730664. [DOI] [PubMed] [Google Scholar]
- 68.Dedoussis GV, Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Choumerianou D. An association between the methylenetetrahydrofolate reductase (MTHFR) C677T mutation and inflammation markers related to cardiovascular disease. Int J Cardiol. 2005;100:409–414. doi: 10.1016/j.ijcard.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 69.Chen AR, Zhang HG, Wang ZP, Fu SJ, Yang PQ, Ren JG. C-reactive protein, vitamin B12 and C677T polymorphism of N-5,10-methylenetetrahydrofolate reductase gene are related to insulin resistance and risk factors for metabolic syndrome in Chinese population. Clin Invest Med. 2010;33:E290–E297. doi: 10.25011/cim.v33i5.14354. [DOI] [PubMed] [Google Scholar]
- 70.Hammons AL, Summers CM, Woodside JV, McNulty H, Strain JJ, Young IS. Folate/homocysteine phenotypes and MTHFR 677C>T genotypes are associated with serum levels of monocyte chemoattractant protein-1. Clin Immunol. 2009;133:132–137. doi: 10.1016/j.clim.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu ZY, Morales M, Khartulyari S, Mei M, Murphy KM, Stanislawska-Sachadyn A. Genetic and biochemical determinants of serum concentrations of monocyte chemoattractant protein-1, a potential neural tube defect risk factor. Birth Defects Res A Clin Mol Teratol. 2008;82:736–741. doi: 10.1002/bdra.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bronowicki JP, Abdelmouttaleb I, Peyrin-Biroulet L, Venard V, Khiri H, Chabi N. Methylenetetrahydrofolate reductase 677T allele protects against persistent HBV infection in West Africa. J Hepatol. 2008;48:532–539. doi: 10.1016/j.jhep.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 73.Simhan HN, Himes KP, Venkataramanan R, Bodnar LM. Maternal serum folate species in early pregnancy and lower genital tract inflammatory milieu. Am J Obstet Gynecol. 2011;205:61. e1–e7. doi: 10.1016/j.ajog.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chillemi R, Zappacosta B, Simpore J, Persichilli S, Musumeci M, Musumeci S. Hyperhomocysteinemia in acute Plasmodium falciparum malaria: an effect of host-parasite interaction. Clin Chim Acta. 2004;348:113–120. doi: 10.1016/j.cccn.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Maeda M, Yamamoto I, Fukuda M, Motomura T, Nishida M, Nonen S. MTHFR gene polymorphism is susceptible to diabetic retinopathy but not to diabetic nephropathy in Japanese type 2 diabetic patients. J Diabetes Complications. 2008;22:119–125. doi: 10.1016/j.jdiacomp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Khandanpour N, Willis G, Meyer FJ, Armon MP, Loke YK, Wright AJ. Peripheral arterial disease and methylenetetrahydrofolate reductase (MTHFR) C677T mutations: a case-control study and meta-analysis. J Vasc Surg. 2009;49:711–718. doi: 10.1016/j.jvs.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Ferrara F, Novo S, Grimaudo S, Raimondi F, Meli F, Amato C. Methylenetetrahydrofolate reductase mutation in subjects with abdominal aortic aneurysm subdivided for age. Clin Hemorheol Microcirc. 2006;34:421–426. [PubMed] [Google Scholar]
- 78.Pollex RL, Mamakeesick M, Zinman B, Harris SB, Hanley AJ, Hegele RA. Methylenetetrahydrofolate reductase polymorphism 677C>T is associated with peripheral arterial disease in type 2 diabetes. Cardiovasc Diabetol. 2005;4:17. doi: 10.1186/1475-2840-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J, Ma J, Stampfer MJ, Palomeque C, Selhub J, Hunter DJ. Linkage disequilibrium between the 677C>T and 1298A>C polymorphisms in human methylenetetrahydrofolate reductase gene and their contributions to risk of colorectal cancer. Pharmacogenetics. 2002;12:339–342. doi: 10.1097/00008571-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Movva S, Alluri RV, Venkatasubramanian S, Vedicherla B, Vattam KK, Ahuja YR. Association of methylene tetrahydrofolate reductase C677T genotype with type 2 diabetes mellitus patients with and without renal complications. Genet Test Mol Biomarkers. 2011;15:257–261. doi: 10.1089/gtmb.2010.0118. [DOI] [PubMed] [Google Scholar]
- 81.Reyes-Engel A, Munoz E, Gaitan MJ, Fabre E, Gallo M, Dieguez JL. Implications on human fertility of the 677C → T and 1298A → C polymorphisms of the MTHFR gene: consequences of a possible genetic selection. Mol Hum Reprod. 2002;8:952–957. doi: 10.1093/molehr/8.10.952. [DOI] [PubMed] [Google Scholar]
- 82.Gerdes LU. The common polymorphism of apolipoprotein E: geographical aspects and new pathophysiological relations. Clin Chem Lab Med. 2003;41:628–631. doi: 10.1515/CCLM.2003.094. [DOI] [PubMed] [Google Scholar]
- 83.Urosevic N, Martins RN. Infection and Alzheimer's disease: the APOE epsilon4 connection and lipid metabolism. J Alzheimers Dis. 2008;13:421–435. doi: 10.3233/JAD-2008-13407. [DOI] [PubMed] [Google Scholar]
- 84.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 85.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 86.Wozniak MA, Maude RJ, Innes JA, Hawkey PM, Itzhaki RF. Apolipoprotein E-epsilon2 confers risk of pulmonary tuberculosis in women from the Indian subcontinent--a preliminary study. J Infect. 2009;59:219–222. doi: 10.1016/j.jinf.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine metabolism among patients with depression who attempt suicide. Arch Gen Psychiatry. 1992;49:447–450. doi: 10.1001/archpsyc.1992.01820060027004. [DOI] [PubMed] [Google Scholar]
- 88.Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A. Lower dopamine transporter binding potential in striatum during depression. NeuroReport. 2001;12:4121–4125. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- 89.Lambert G, Johansson M, Agren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- 90.Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry. 2002;52:740–748. doi: 10.1016/S0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- 91.Kavelaars A, Cobelens PM, Teunis MA, Heijnen CJ. Changes in innate and acquired immune responses in mice with targeted deletion of the dopamine transporter gene. J Neuroimmunol. 2005;161:162–168. doi: 10.1016/j.jneuroim.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 92.Alaniz RC, Thomas SA, Perez-Melgosa M, Mueller K, Farr AG, Palmiter RD. Dopamine beta-hydroxylase deficiency impairs cellular immunity. Proc Natl Acad Sci USA. 1999;96:2274–2278. doi: 10.1073/pnas.96.5.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cobat A, Gallant CJ, Simkin L, Black GF, Stanley K, Hughes J. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med. 2009;206:2583–2591. doi: 10.1084/jem.20090892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stein CM, Zalwango S, Malone LL, Won S, Mayanja-Kizza H, Mugerwa RD. Genome scan of M. tuberculosis infection and disease in Ugandans. PLoS One. 2008;3:e4094. doi: 10.1371/journal.pone.0004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 96.van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- 97.Opdal SH, Vege A, Rognum TO. Serotonin transporter gene variation in sudden infant death syndrome. Acta Paediatr. 2008;97:861–865. doi: 10.1111/j.1651-2227.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- 98.Narita N, Narita M, Takashima S, Nakayama M, Nagai T, Okado N. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 2001;107:690–692. doi: 10.1542/peds.107.4.690. [DOI] [PubMed] [Google Scholar]
- 99.Weese-Mayer DE, Berry-Kravis EM, Maher BS, Silvestri JM, Curran ME, Marazita ML. Sudden infant death syndrome: association with a promoter polymorphism of the serotonin transporter gene. Am J Med Genet A. 2003;117A:268–274. doi: 10.1002/ajmg.a.20005. [DOI] [PubMed] [Google Scholar]
- 100.Prandota J. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther. 2004;11:517–546. doi: 10.1097/01.mjt.0000140648.30948.bd. [DOI] [PubMed] [Google Scholar]
- 101.Fredericks CA, Drabant EM, Edge MD, Tillie JM, Hallmayer J, Ramel W. Healthy young women with serotonin transporter SS polymorphism show a pro-inflammatory bias under resting and stress conditions. Brain Behav Immun. 2010;24:350–357. doi: 10.1016/j.bbi.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su S, Zhao J, Bremner JD, Miller AH, Tang W, Bouzyk M. Serotonin transporter gene, depressive symptoms, and interleukin-6. Circ Cardiovasc Genet. 2009;2:614–620. doi: 10.1161/CIRCGENETICS.109.870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murray DR, Schaller M. Historical prevalence of infectious diseases within 230 geopolitical regions: a tool for investigating origins of culture. J Cross Cult Psychol. 2010;41:99–108. doi: 10.1177/0022022109349510. [DOI] [Google Scholar]
- 104.Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc Biol Sci. 2010;277:529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wray NR, Pergadia ML, Blackwood DH, Penninx BW, Gordon SD, Nyholt DR. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry. 2012;17:36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cattaneo M, Otsu M, Fagioli C, Martino S, Lotti LV, Sitia R. SEL1L and HRD1 are involved in the degradation of unassembled secretory Ig-mu chains. J Cell Physiol. 2008;215:794–802. doi: 10.1002/jcp.21364. [DOI] [PubMed] [Google Scholar]
- 107.Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131:516–529. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 108.Oresic K, Ng CL, Tortorella D. TRAM1 participates in human cytomegalovirus US2- and US11-mediated dislocation of an endoplasmic reticulum membrane glycoprotein. J Biol Chem. 2009;284:5905–5914. doi: 10.1074/jbc.M807568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ban Y, Taniyama M, Tozaki T, Yanagawa T, Tomita M. SEL1L microsatellite polymorphism in Japanese patients with autoimmune thyroid diseases. Thyroid. 2001;11:335–338. doi: 10.1089/10507250152039064. [DOI] [PubMed] [Google Scholar]
- 110.Song J, Duncan MJ, Li G, Chan C, Grady R, Stapleton A. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. Plos Pathog. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abera AB, Sales KJ, Catalano RD, Katz AA, Jabbour HN. EP2 receptor mediated cAMP release is augmented by PGF 2 alpha activation of the FP receptor via the calcium-calmodulin pathway. Cell Signal. 2010;22:71–79. doi: 10.1016/j.cellsig.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, Canetti L. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:934–943. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- 113.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 114.Koehn J, Huesken D, Jaritz M, Rot A, Zurini M, Dwertmann A. Assessing the function of human UNC-93B in Toll-like receptor signaling and major histocompatibility complex II response. Hum Immunol. 2007;68:871–878. doi: 10.1016/j.humimm.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 115.Pifer R, Benson A, Sturge CR, Yarovinsky F. UNC93B1 is essential for TLR11 activation and IL-12-dependent host resistance to Toxoplasma gondii. J Biol Chem. 2011;286:3307–3314. doi: 10.1074/jbc.M110.171025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crozat K, Vivier E, Dalod M. Crosstalk between components of the innate immune system: promoting anti-microbial defenses and avoiding immunopathologies. Immunol Rev. 2009;227:129–149. doi: 10.1111/j.1600-065X.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 117.Lang R, Kofler B. The galanin peptide family in inflammation. Neuropeptides. 2011;45:1–8. doi: 10.1016/j.npep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Matkowskyj KA, Danilkovich A, Marrero J, Savkovic SD, Hecht G, Benya RV. Galanin-1 receptor up-regulation mediates the excess colonic fluid production caused by infection with enteric pathogens. Nat Med. 2000;6:1048–1051. doi: 10.1038/79563. [DOI] [PubMed] [Google Scholar]
- 119.McDonald AC, Schuijers JA, Gundlach AL, Grills BL. Galanin treatment offsets the inhibition of bone formation and downregulates the increase in mouse calvarial expression of TNFalpha and GalR2 mRNA induced by chronic daily injections of an injurious vehicle. Bone. 2007;40:895–903. doi: 10.1016/j.bone.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 120.Su Y, Ganea D, Peng X, Jonakait GM. Galanin down-regulates microglial tumor necrosis factor-alpha production by a post-transcriptional mechanism. J Neuroimmunol. 2003;134:52–60. doi: 10.1016/S0165-5728(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 121.Christiansen SH, Olesen MV, Wortwein G, Woldbye DP. Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav Brain Res. 2011;216:585–591. doi: 10.1016/j.bbr.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 122.Wardi Le Maitre T, Xia S, Le Maitre E, Dun XP, Lu J, Theodorsson E. Galanin receptor 2 overexpressing mice display an antidepressive-like phenotype: possible involvement of the subiculum. Neurosci. 2011;190:270–288. doi: 10.1016/j.neuroscience.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 123.Davidson S, Lear M, Shanley L, Hing B, Baizan-Edge A, Herwig A. Differential activity by polymorphic variants of a remote enhancer that supports galanin expression in the hypothalamus and amygdala: implications for obesity, depression and alcoholism. Neuropsychopharmacology. 2011;36:2211–2221. doi: 10.1038/npp.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rauch I, Lundstrom L, Hell M, Sperl W, Kofler B. Galanin message-associated peptide suppresses growth and the budded-to-hyphal-form transition of Candida albicans. Antimicrob Agents Chemother. 2007;51:4167–4170. doi: 10.1128/AAC.00166-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci USA. 2010;107:10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen C, Han YH, Yang Z, Rodrigues AD. Effect of interferon-alpha2b on the expression of various drug-metabolizing enzymes and transporters in co-cultures of freshly prepared human primary hepatocytes. Xenobiotica. 2011;41:476–485. doi: 10.3109/00498254.2011.560971. [DOI] [PubMed] [Google Scholar]
- 127.Crossland H, Constantin-Teodosiu D, Greenhaff PL, Gardiner SM. Low-dose dexamethasone prevents endotoxaemia-induced muscle protein loss and impairment of carbohydrate oxidation in rat skeletal muscle. J Physiol. 2010;588:1333–1347. doi: 10.1113/jphysiol.2009.183699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palomer X, Alvarez-Guardia D, Rodriguez-Calvo R, Coll T, Laguna JC, Davidson MM. TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res. 2009;81:703–712. doi: 10.1093/cvr/cvn327. [DOI] [PubMed] [Google Scholar]
- 129.Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol. 2008;84:1172–1182. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]
- 130.Nawa Y, Kawahara K, Tancharoen S, Meng X, Sameshima H, Ito T. Nucleophosmin may act as an alarmin: implications for severe sepsis. J Leukoc Biol. 2009;86:645–653. doi: 10.1189/jlb.1008644. [DOI] [PubMed] [Google Scholar]
- 131.Sarek G, Jarviluoma A, Moore HM, Tojkander S, Vartia S, Biberfeld P. Nucleophosmin phosphorylation by v-cyclin-CDK6 controls KSHV latency. Plos Pathog. 2010;6:e1000818. doi: 10.1371/journal.ppat.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson JS, Samulski RJ. Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus. J Virol. 2009;83:2632–2644. doi: 10.1128/JVI.02309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee JJ, Seah JB, Chow VT, Poh CL, Tan EL. Comparative proteome analyses of host protein expression in response to Enterovirus 71 and Coxsackievirus A16 infections. J Proteomics. 2011;74:2018–2024. doi: 10.1016/j.jprot.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 134.Zeng Y, Ye L, Zhu S, Zheng H, Zhao P, Cai W. The nucleocapsid protein of SARS-associated coronavirus inhibits B23 phosphorylation. Biochem Biophys Res Commun. 2008;369:287–291. doi: 10.1016/j.bbrc.2008.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fankhauser C, Izaurralde E, Adachi Y, Wingfield P, Laemmli UK. Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: dissociation by the Rev response element. Mol Cell Biol. 1991;11:2567–2575. doi: 10.1128/MCB.11.5.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lymberopoulos MH, Bourget A, Abdeljelil NB, Pearson A. Involvement of the UL24 protein in herpes simplex virus 1-induced dispersal of B23 and in nuclear egress. Virology. 2011;412:341–348. doi: 10.1016/j.virol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 137.Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 138.Burrows JF, McGrattan MJ, Johnston JA. The DUB/USP17 deubiquitinating enzymes, a multigene family within a tandemly repeated sequence. Genomics. 2005;85:524–529. doi: 10.1016/j.ygeno.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 139.Burrows JF, McGrattan MJ, Rascle A, Humbert M, Baek KH, Johnston JA. DUB-3, a cytokine-inducible deubiquitinating enzyme that blocks proliferation. J Biol Chem. 2004;279:13993–14000. doi: 10.1074/jbc.M311291200. [DOI] [PubMed] [Google Scholar]
- 140.Chen R, Zhang L, Zhong B, Tan B, Liu Y, Shu HB. The ubiquitin-specific protease 17 is involved in virus-triggered type I IFN signaling. Cell Res. 2010;20:802–811. doi: 10.1038/cr.2010.41. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry. 2011;16:2–4. doi: 10.1038/mp.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P. Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry. 2010;67:803–811. doi: 10.1001/archgenpsychiatry.2010.94. [DOI] [PubMed] [Google Scholar]
- 144.Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Perrier E, Pompei F, Ruberto G, Vassos E, Collier D, Frangou S. Initial evidence for the role of CACNA1C on subcortical brain morphology in patients with bipolar disorder. Eur Psychiatry. 2011;26:135–137. doi: 10.1016/j.eurpsy.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 146.Suzuki Y, Inoue T, Ra C. L-type Ca2+ channels: a new player in the regulation of Ca2+ signaling, cell activation and cell survival in immune cells. Mol Immunol. 2010;47:640–648. doi: 10.1016/j.molimm.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 147.Badou A, Jha MK, Matza D, Mehal WZ, Freichel M, Flockerzi V. Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci USA. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matza D, Badou A, Kobayashi KS, Goldsmith-Pestana K, Masuda Y, Komuro A. A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation. Immunity. 2008;28:64–74. doi: 10.1016/j.immuni.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta S, Salam N, Srivastava V, Singla R, Behera D, Khayyam KU. Voltage gated calcium channels negatively regulate protective immunity to Mycobacterium tuberculosis. PLoS One. 2009;4:e5305. doi: 10.1371/journal.pone.0005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Radermacher AN, Crabtree GR. Monster protein controls calcium entry and fights infection. Immunity. 2008;28:13–14. doi: 10.1016/j.immuni.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 151.Suzuki Y, Yoshimaru T, Inoue T, Ra C. Ca v 1.2L-type Ca2+ channel protects mast cells against activation-induced cell death by preventing mitochondrial integrity disruption. Mol Immunol. 2009;46:2370–2380. doi: 10.1016/j.molimm.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 152.Das R, Burke T, Van Wagoner DR, Plow EF. L-type calcium channel blockers exert an antiinflammatory effect by suppressing expression of plasminogen receptors on macrophages. Circ Res. 2009;105:167–175. doi: 10.1161/CIRCRESAHA.109.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matza D, Badou A, Jha MK, Willinger T, Antov A, Sanjabi S. Requirement for AHNAK1-mediated calcium signaling during T lymphocyte cytolysis. Proc Natl Acad Sci USA. 2009;106:9785–9790. doi: 10.1073/pnas.0902844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liao P, Soong TW. CaV1.2 channelopathies: from arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch. 2010;460:353–359. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- 155.Das R, Plow EF. A new function for old drugs. Cell Cycle. 2010;9:638–639. doi: 10.4161/cc.9.4.11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balog Z, Kiss I, Keri S. CACNA1C risk allele for psychotic disorders is related to the activation of the AKT-pathway. Am J Psychiatry. 2010;167:1276–1277. doi: 10.1176/appi.ajp.2010.10040635. [DOI] [PubMed] [Google Scholar]
- 157.Li XQ, Cao W, Li T, Zeng AG, Hao LL, Zhang XN. Amlodipine inhibits TNF-alpha production and attenuates cardiac dysfunction induced by lipopolysaccharide involving PI3K/Akt pathway. Int Immunopharmacol. 2009;9:1032–1041. doi: 10.1016/j.intimp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 158.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 159.Okazaki R, Iwasaki YK, Miyauchi Y, Hirayama Y, Kobayashi Y, Katoh T. Lipopolysaccharide induces atrial arrhythmogenesis via down-regulation of L-type Ca2+ channel genes in rats. Int Heart J. 2009;50:353–363. doi: 10.1536/ihj.50.353. [DOI] [PubMed] [Google Scholar]
- 160.Azenabor AA, Chaudhry AU. Effective macrophage redox defense against Chlamydia pneumoniae depends on L-type Ca2+ channel activation. Med Microbiol Immunol. 2003;192:99–106. doi: 10.1007/s00430-002-0164-8. [DOI] [PubMed] [Google Scholar]
- 161.Wieland H, Hechtel N, Faigle M, Neumeister B. Efficient intracellular multiplication of Legionella pneumophila in human monocytes requires functional host cell L-type calcium channels. FEMS Immunol Med Microbiol. 2006;47:296–301. doi: 10.1111/j.1574-695X.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 162.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 163.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS. Paroxetine for the prevention of depression induced by high-dose interferon alfa. New Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 165.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/JCP.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kraus MR, Schafer A, Csef H, Scheurlen M. Psychiatric side effects of pegylated interferon alfa-2b as compared to conventional interferon alfa-2b in patients with chronic hepatitis C. World J Gastroenterol. 2005;11:1769–1774. doi: 10.3748/wjg.v11.i12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19(Suppl 3):S174–S178. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- 168.Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun. 2007;21:147–152. doi: 10.1016/j.bbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 169.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295X.105.1.83. [DOI] [PubMed] [Google Scholar]
- 170.Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exper Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 171.Hayley S, Merali Z, Anisman H. Stress and cytokine-elicited neuroendocrine and neurotransmitter sensitization: implications for depressive illness. Stress. 2003;6:19–32. doi: 10.1080/1025389031000091167. [DOI] [PubMed] [Google Scholar]
- 172.Gibb J, Audet MC, Hayley S, Anisman H. Neurochemical and behavioral responses to inflammatory immune stressors. Front Biosci (Schol Ed) 2009;1:275–295. doi: 10.2741/s26. [DOI] [PubMed] [Google Scholar]
- 173.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 174.Simon GE, Ludman EJ, Linde JA, Operskalski BH, Ichikawa L, Rohde P. Association between obesity and depression in middle-aged women. Gen Hosp Psychiatry. 2008;30:32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–285. doi: 10.1016/S0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 177.Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156:759. doi: 10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Int Med. 2007;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 179.Kloiber S, Ising M, Reppermund S, Horstmann S, Dose T, Majer M. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62:321–326. doi: 10.1016/j.biopsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 180.Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Network. 2006;17:196–201. [PubMed] [Google Scholar]
- 181.Suarez EC. C-reactive protein is associated with psychological risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med. 2004;66:684–691. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 182.Douglas KM, Taylor AJ, O'Malley PG. Relationship between depression and C-reactive protein in a screening population. Psychosom Med. 2004;66:679–683. doi: 10.1097/01.psy.0000138132.66332.85. [DOI] [PubMed] [Google Scholar]
- 183.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 184.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 185.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 186.Katz JR, Taylor NF, Goodrick S, Perry L, Yudkin JS, Coppack SW. Central obesity, depression and the hypothalamo-pituitary-adrenal axis in men and postmenopausal women. Int J Obesity Related Metab Disord. 2000;24:246–251. doi: 10.1038/sj.ijo.0801122. [DOI] [PubMed] [Google Scholar]
- 187.Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kuhn M, Schuld A. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res. 1999;33:407–418. doi: 10.1016/S0022-3956(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 188.Vieira VJ, Valentine RJ, McAuley E, Evans E, Woods JA. Independent relationship between heart rate recovery and C-reactive protein in older adults. J Am Geriat Soc. 2007;55:747–751. doi: 10.1111/j.1532-5415.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 189.Moyna NM, Bodnar JD, Goldberg HR, Shurin MS, Robertson RJ, Rabin BS. Relation between aerobic fitness level and stress induced alterations in neuroendocrine and immune function. Int J Sports Med. 1999;20:136–141. doi: 10.1055/s-2007-971107. [DOI] [PubMed] [Google Scholar]
- 190.Kohut ML, McCann DA, Russell DW, Konopka DN, Cunnick JE, Franke WD. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 191.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2010;20:608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 192.Ghosh S, Khazaei M, Moien-Afshari F, Ang LS, Granville DJ, Verchere CB. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol. 2009;296:F700–F708. doi: 10.1152/ajprenal.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anton SD, Newton RL, Jr, Sothern M, Martin CK, Stewart TM, Williamson DA. Association of depression with body mass index, sedentary behavior, and maladaptive eating attitudes and behaviors in 11 to 13-year old children. Eat Weight Disord. 2006;11:e102–e108. doi: 10.1007/BF03327566. [DOI] [PubMed] [Google Scholar]
- 194.Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31:S663–S667. doi: 10.1097/00005768-199911001-00026. [DOI] [PubMed] [Google Scholar]
- 195.Rajala U, Uusimaki A, Keinanen-Kiukaanniemi S, Kivela SL. Prevalence of depression in a 55-year-old Finnish population. Soc Psychiatry Psychiatr Epidemiol. 1994;29:126–130. doi: 10.1007/BF00796492. [DOI] [PubMed] [Google Scholar]
- 196.Tanskanen A, Hibbeln JR, Tuomilehto J, Uutela A, Haukkala A, Viinamaki H. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52:529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 197.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/S0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 198.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 199.Williams LL, Kiecolt-Glaser JK, Horrocks LA, Hillhouse JT, Glaser R. Quantitative association between altered plasma esterified omega-6 fatty acid proportions and psychological stress. Prostaglandins Leukot Essent Fatty Acids. 1992;47:165–170. doi: 10.1016/0952-3278(92)90155-C. [DOI] [PubMed] [Google Scholar]
- 200.Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L. Adherence to the mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–124. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 202.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 203.Westover AN, Marangell LB. A cross-national relationship between sugar consumption and major depression? Depress Anxiety. 2002;16:118–120. doi: 10.1002/da.10054. [DOI] [PubMed] [Google Scholar]
- 204.O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 205.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–684. doi: 10.1093/ajcn/82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee O, Bruce WR, Dong Q, Bruce J, Mehta R, O'Brien PJ. Fructose and carbonyl metabolites as endogenous toxins. Chem Biol Interact. 2009;178:332–339. doi: 10.1016/j.cbi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 207.O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.1989.03430110069030. [DOI] [PubMed] [Google Scholar]
- 209.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Brit J Gen Pract. 1993;43:445–448. [PMC free article] [PubMed] [Google Scholar]
- 210.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 211.Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr Scand Suppl. 1998;393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- 212.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 214.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Int Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 215.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 216.Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, Davidson RJ. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci USA. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–360. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 218.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metab Clin Exper. 2002;51:887–892. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 219.Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 220.Saules KK, Pomerleau CS, Snedecor SM, Mehringer AM, Shadle MB, Kurth C. Relationship of onset of cigarette smoking during college to alcohol use, dieting concerns, and depressed mood: results from the Young Women's Health Survey. Addict Behav. 2004;29:893–899. doi: 10.1016/j.addbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 221.Lenz BK. Tobacco, depression, and lifestyle choices in the pivotal early college years. J Am Coll Health. 2004;52:213–219. doi: 10.3200/JACH.52.5.213-220. [DOI] [PubMed] [Google Scholar]
- 222.Handwerker WP. Cultural diversity, stress, and depression: working women in the Americas. J Women Health Gend Based Med. 1999;8:1303–1311. doi: 10.1089/jwh.1.1999.8.1303. [DOI] [PubMed] [Google Scholar]
- 223.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 224.Brown GW, Harris TO, Hepworth C. Life events and endogenous depression. A puzzle reexamined. Arch Gen Psychiat. 1994;51:525–534. doi: 10.1001/archpsyc.1994.03950070017006. [DOI] [PubMed] [Google Scholar]
- 225.Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 226.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. doi: 10.1097/00024382-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 227.Ross SR, Bloomsmith MA, Bettinger TL, Wagner KE. The influence of captive adolescent male chimpanzees on wounding: management and welfare implications. Zoo Biol. 2009;28:623–634. doi: 10.1002/zoo.20220. [DOI] [PubMed] [Google Scholar]
- 228.Eshed V, Gopher A, Pinhasi R, Hershkovitz I. Paleopathology and the origin of agriculture in the Levant. Am J Phys Anthropol. 2010;143:121–133. doi: 10.1002/ajpa.21301. [DOI] [PubMed] [Google Scholar]
- 229.Pinker S. The Better Angels of Our Nature: Why Violence Has Declined. Viking Adult: New York, NY; 2011. [Google Scholar]
- 230.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 232.Bailey MT, Kinsey SG, Padgett DA, Sheridan JF, Leblebicioglu B. Social stress enhances IL-1beta and TNF-alpha production by Porphyromonas gingivalis lipopolysaccharide-stimulated CD11b+ cells. Physiol Behav. 2009;98:351–358. doi: 10.1016/j.physbeh.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 234.Powell ND, Mays JW, Bailey MT, Hanke ML, Sheridan JF. Immunogenic dendritic cells primed by social defeat enhance adaptive immunity to influenza A virus. Brain Behav Immun. 2011;25:46–52. doi: 10.1016/j.bbi.2010.07.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J Immunol. 2010;184:2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Steptoe A, Hamer M, Chida Y. The effect of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;7:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 237.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19:311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 239.Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/S0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 240.Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am. 2009;91:2783–2794. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Joachim RA, Handjiski B, Blois SM, Hagen E, Paus R, Arck PC. Stress-induced neurogenic inflammation in murine skin skews dendritic cells towards maturation and migration: key role of intercellular adhesion molecule-1/leukocyte function-associated antigen interactions. Am J Pathol. 2008;173:1379–1388. doi: 10.2353/ajpath.2008.080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–1069. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- 243.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nesse RM. The smoke detector principle. Natural selection and the regulation of defensive responses. Ann NY Acad Sci. 2001;935:75–85. doi: 10.1111/j.1749-6632.2001.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 250.Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacol: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Leutscher PD, Lagging M, Buhl MR, Pedersen C, Norkrans G, Langeland N. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology. 2010;52:430–435. doi: 10.1002/hep.23699. [DOI] [PubMed] [Google Scholar]
- 254.Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Woolwine BJ, Jacobson IM. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav Immun. 2005;19:23–27. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 255.Doering LV, Martinez-Maza O, Vredevoe DL, Cowan MJ. Relation of depression, natural killer cell function, and infections after coronary artery bypass in women. Eur J Cardiovasc Nurs. 2008;7:52–58. doi: 10.1016/j.ejcnurse.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Faulkner S, Smith A. A longitudinal study of the relationship between psychological distress and recurrence of upper respiratory tract infections in chronic fatigue syndrome. Br J Health Psychol. 2008;13:177–186. doi: 10.1348/135910706X171469. [DOI] [PubMed] [Google Scholar]
- 257.Cruess DG, Petitto JM, Leserman J, Douglas SD, Gettes DR, Ten Have TR. Depression and HIV infection: impact on immune function and disease progression. CNS Spectr. 2003;8:52–58. doi: 10.1017/S1092852900023452. [DOI] [PubMed] [Google Scholar]
- 258.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 259.Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS. Association of depression with viral load, CD8T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002;159:1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- 260.Zorrilla EP, McKay JR, Luborsky L, Schmidt K. Relation of stressors and depressive symptoms to clinical progression of viral illness. Am J Psychiatry. 1996;153:626–635. doi: 10.1176/ajp.153.5.626. [DOI] [PubMed] [Google Scholar]
- 261.Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychol Bull. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- 262.Castilla-Cortazar I, Castilla A, Gurpegui M. Opioid peptides and immunodysfunction in patients with major depression and anxiety disorders. J Physiol Biochem. 1998;54:203–215. [PubMed] [Google Scholar]
- 263.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vaknin I, Blinder L, Wang L, Gazit R, Shapira E, Genina O. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008;111:1437–1447. doi: 10.1182/blood-2007-07-100404. [DOI] [PubMed] [Google Scholar]
- 265.Moraska A, Campisi J, Nguyen KT, Maier SF, Watkins LR, Fleshner M. Elevated IL-1beta contributes to antibody suppression produced by stress. J Appl Physiol. 2002;93:207–215. doi: 10.1152/japplphysiol.01151.2001. [DOI] [PubMed] [Google Scholar]
- 266.Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997;185:1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cope AP. Exploring the reciprocal relationship between immunity and inflammation in chronic inflammatory arthritis. Rheumatology (Oxford) 2003;42:716–731. doi: 10.1093/rheumatology/keg262. [DOI] [PubMed] [Google Scholar]
- 268.Eleftheriadis T, Kartsios C, Yiannaki E, Antoniadi G, Kazila P, Pliakos K. Decreased CD3+CD16+ natural killer-like T-cell percentage and zeta-chain expression accompany chronic inflammation in haemodialysis patients. Nephrology (Carlton) 2009;14:471–475. doi: 10.1111/j.1440-1797.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 269.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA., III Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Clark J, Vagenas P, Panesar M, Cope AP. What does tumour necrosis factor excess do to the immune system long term? Ann Rheum Dis. 2005;64(Suppl 4):iv70–iv76. doi: 10.1136/ard.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 272.Armelagos GJ, Brown PJ, Turner B. Evolutionary, historical and political economic perspectives on health and disease. Soc Sci Med. 2005;61:755–765. doi: 10.1016/j.socscimed.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 273.May L, van den Biggelaar AH, van Bodegom D, Meij HJ, de Craen AJ, Amankwa J. Adverse environmental conditions influence age-related innate immune responsiveness. Immun Ageing. 2009;6:7. doi: 10.1186/1742-4933-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Drenos F, Westendorp RG, Kirkwood TB. Trade-off mediated effects on the genetics of human survival caused by increasingly benign living conditions. Biogerontology. 2006;7:287–295. doi: 10.1007/s10522-006-9027-9. [DOI] [PubMed] [Google Scholar]
- 275.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Joosten KF, de Kleijn ED, Westerterp M, de Hoog M, Eijck FC, Hop WCJ. Endocrine and metabolic responses in children with meningoccocal sepsis: striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab. 2000;85:3746–3753. doi: 10.1210/jcem.85.10.6901. [DOI] [PubMed] [Google Scholar]
- 277.Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Diaz PV. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117:e878–e886. doi: 10.1542/peds.2005-2119. [DOI] [PubMed] [Google Scholar]
- 278.Gallagher PM, Lowe G, Fitzgerald T, Bella A, Greene CM, McElvaney NG. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58:154–156. doi: 10.1136/thorax.58.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Li J, Cowden LG, King JD, Briles DA, Schroeder HW, Jr, Stevens AB. Effects of chronic stress and interleukin-10 gene polymorphisms on antibody response to tetanus vaccine in family caregivers of patients with Alzheimer's disease. Psychosom Med. 2007;69:551–559. doi: 10.1097/PSY.0b013e3180cc2c61. [DOI] [PubMed] [Google Scholar]
- 280.Hohler T, Reuss E, Freitag CM, Schneider PM. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology. 2005;42:72–76. doi: 10.1002/hep.20740. [DOI] [PubMed] [Google Scholar]
- 281.Corsini E, Vismara L, Lucchi L, Viviani B, Govoni S, Galli CL. High interleukin-10 production is associated with low antibody response to influenza vaccination in the elderly. J Leukoc Biol. 2006;80:376–382. doi: 10.1189/jlb.0306190. [DOI] [PubMed] [Google Scholar]
- 282.Forte GI, Scola L, Misiano G, Milano S, Mansueto P, Vitale G. Relevance of gamma interferon, tumor necrosis factor alpha, and interleukin-10 gene polymorphisms to susceptibility to Mediterranean spotted fever. Clin Vaccine Immunol. 2009;16:811–815. doi: 10.1128/CVI.00121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV. In vitro production of IFN-gamma correlates with CA repeat polymorphism in the human IFN-gamma gene. Eur J Immunogenet. 1999;26:1–3. doi: 10.1046/j.1365-2370.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- 284.Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-gamma gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-gamma production. Hum Immunol. 2000;61:863–866. doi: 10.1016/S0198-8859(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 285.Pacheco AG, Cardoso CC, Moraes MO. IFNG +874T/A, IL10 −1082G/A and TNF −308G/A polymorphisms in association with tuberculosis susceptibility: a meta-analysis study. Hum Genet. 2008;123:477–484. doi: 10.1007/s00439-008-0497-5. [DOI] [PubMed] [Google Scholar]
- 286.Ansari A, Talat N, Jamil B, Hasan Z, Razzaki T, Dawood G. Cytokine gene polymorphisms across tuberculosis clinical spectrum in Pakistani patients. PLoS One. 2009;4:e4778. doi: 10.1371/journal.pone.0004778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cardoso CC, Pereira AC, Brito-de-Souza VN, Dias-Baptista IM, Maniero VC, Venturini J. IFNG +874 T>A single nucleotide polymorphism is associated with leprosy among Brazilians. Hum Genet. 2010;128:481–490. doi: 10.1007/s00439-010-0872-x. [DOI] [PubMed] [Google Scholar]
- 288.Chong WP, Ip WK, Tso GH, Ng MW, Wong WH, Law HK. The interferon gamma gene polymorphism +874 A/T is associated with severe acute respiratory syndrome. BMC Infect Dis. 2006;6:82. doi: 10.1186/1471-2334-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Torres OA, Calzada JE, Beraun Y, Morillo CA, Gonzalez A, Gonzalez CI. Role of the IFNG +874T/A polymorphism in Chagas disease in a Colombian population. Infect Genet Evol. 2010;10:682–685. doi: 10.1016/j.meegid.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gao QJ, Liu DW, Zhang SY, Jia M, Wang LM, Wu LH. Polymorphisms of some cytokines and chronic hepatitis B and C virus infection. World J Gastroenterol. 2009;15:5610–5619. doi: 10.3748/wjg.15.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 292.Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology (Berl) 2003;165:413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- 293.Dunn AJ, Swiergiel AH. The reductions in sweetened milk intake induced by interleukin-1 and endotoxin are not prevented by chronic antidepressant treatment. Neuroimmunomodulation. 2001;9:163–169. doi: 10.1159/000049021. [DOI] [PubMed] [Google Scholar]
- 294.Castanon N, Bluthe RM, Dantzer R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology. 2001;154:50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- 295.Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE. Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci. 1999;65:1773–1786. doi: 10.1016/S0024-3205(99)00430-0. [DOI] [PubMed] [Google Scholar]
- 296.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharm Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 299.Kraus MR, Schafer A, Al-Taie O, Scheurlen M. Prophylactic SSRI during interferon alpha re-therapy in patients with chronic hepatitis C and a history of interferon-induced depression. J Viral Hepatitis. 2005;12:96–100. doi: 10.1111/j.1365-2893.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 300.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Paroxetine for the treatment of interferon-alpha-induced depression in chronic hepatitis C. Aliment Pharm Ther. 2002;16:1091–1099. doi: 10.1046/j.1365-2036.2002.01265.x. [DOI] [PubMed] [Google Scholar]
- 301.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 302.Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol Med. 2005;35:433–441. doi: 10.1017/S0033291704003526. [DOI] [PubMed] [Google Scholar]
- 303.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with Hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine BJ. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 310.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hanff TC, Furst SJ, Minor TR. Biochemical and anatomical substrates of depression and sickness behavior. Isr J Psychiatry Relat Sci. 2010;47:64–71. [PubMed] [Google Scholar]
- 312.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/S0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 313.Hart BL. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci Biobehav Rev. 1990;14:273–294. doi: 10.1016/S0149-7634(05)80038-7. [DOI] [PubMed] [Google Scholar]
- 314.Kluger MJ. Phylogeny of fever. Fed Proc. 1979;38:30–34. [PubMed] [Google Scholar]
- 315.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann NY Acad Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- 316.Sweet C, Cavanagh D, Collie MH, Smith H. Sensitivity to pyrexial temperatures: a factor contributing to virulence differences between two clones of influenza virus. Br J Exp Pathol. 1978;59:373–380. [PMC free article] [PubMed] [Google Scholar]
- 317.Dixon G, Booth C, Price E, Westran R, Turner M, Klein N. Fever as nature's engine. Part of beneficial host response? BMJ. 2010;340:c450. doi: 10.1136/bmj.c450. [DOI] [PubMed] [Google Scholar]
- 318.Tyrrell D, Barrow I, Arthur J. Local hyperthermia benefits natural and experimental common colds. BMJ. 1989;298:1280–1283. doi: 10.1136/bmj.298.6683.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukoc Biol. 2000;68:815–820. [PubMed] [Google Scholar]
- 320.Jiang Q, Detolla L, Singh IS, Gatdula L, Fitzgerald B, van Rooijen N. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653–R1660. doi: 10.1152/ajpcell.1999.276.1.C152. [DOI] [PubMed] [Google Scholar]
- 321.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82:1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 322.Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother. 2006;55:292–298. doi: 10.1007/s00262-005-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97:2727–2733. doi: 10.1182/blood.V97.9.2727. [DOI] [PubMed] [Google Scholar]
- 324.Swenson BR, Hedrick TL, Popovsky K, Pruett TL, Sawyer RG. Is fever protective in surgical patients with bloodstream infection? J Am Coll Surg. 2007;204:815–821. doi: 10.1016/j.jamcollsurg.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 325.Mizushima Y, Ueno M, Idoguchi K, Ishikawa K, Matsuoka T. Fever in trauma patients: friend or foe? J Trauma. 2009;67:1062–1065. doi: 10.1097/TA.0b013e3181b848fc. [DOI] [PubMed] [Google Scholar]
- 326.Ohsugi Y. Recent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol Pharm Bull. 2007;30:2001–2006. doi: 10.1248/bpb.30.2001. [DOI] [PubMed] [Google Scholar]
- 327.Kung'u JK, Wright VJ, Haji HJ, Ramsan M, Goodman D, Tielsch JM. Adjusting for the acute phase response is essential to interpret iron status indicators among young Zanzibari children prone to chronic malaria and helminth infections. J Nutr. 2009;139:2124–2131. doi: 10.3945/jn.108.104026. [DOI] [PubMed] [Google Scholar]
- 328.Cartwright GE, Lauritsen MA. The anemia of infection; hypoferremia, hypercupremia, and alterations in porphyrin metabolism in patients. J Clin Invest. 1946;25:65–80. doi: 10.1172/JCI101690. [DOI] [PubMed] [Google Scholar]
- 329.Kochan I, Wagner SK, Wasynczuk J. Effect of iron on antibacterial immunity in vaccinated mice. Infect Immun. 1984;43:543–548. doi: 10.1128/iai.43.2.543-548.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Weinberg ED. Survival advantage of the hemochromatosis C282Y mutation. Perspect Biol Med. 2008;51:98–102. doi: 10.1353/pbm.2008.0001. [DOI] [PubMed] [Google Scholar]
- 331.Foster SL, Richardson SH, Failla ML. Elevated iron status increases bacterial invasion and survival and alters cytokine/chemokine mRNA expression in Caco-2 human intestinal cells. J Nutr. 2001;131:1452–1458. doi: 10.1093/jn/131.5.1452. [DOI] [PubMed] [Google Scholar]
- 332.Wander K, Shell-Duncan B, McDade TW. Evaluation of iron deficiency as a nutritional adaptation to infectious disease: an evolutionary medicine perspective. Am J Hum Biol. 2009;21:172–179. doi: 10.1002/ajhb.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Mitra AK, Akramuzzaman SM, Fuchs GJ, Rahman MM, Mahalanabis D. Long-term oral supplementation with iron is not harmful for young children in a poor community of Bangladesh. J Nutr. 1997;127:1451–1455. doi: 10.1093/jn/127.8.1451. [DOI] [PubMed] [Google Scholar]
- 334.Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. The effects on malaria of treatment of iron-deficiency anaemia with oral iron in Gambian children. Ann Trop Paediatr. 1989;9:17–23. doi: 10.1080/02724936.1989.11748589. [DOI] [PubMed] [Google Scholar]
- 335.van den Hombergh J, Dalderop E, Smit Y. Does iron therapy benefit children with severe malaria-associated anaemia? A clinical trial with 12 weeks supplementation of oral iron in young children from the Turiani Division, Tanzania. J Trop Pediatr. 1996;42:220–227. doi: 10.1093/tropej/42.4.220. [DOI] [PubMed] [Google Scholar]
- 336.Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R. Effect of routine prophylactic supplementation with iron and folic acid on preschool child mortality in southern Nepal: community-based, cluster-randomised, placebo-controlled trial. Lancet. 2006;367:144–152. doi: 10.1016/S0140-6736(06)67963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 338.McEnany GW, Lee KA. Effects of light therapy on sleep, mood, and temperature in women with nonseasonal major depression. Issues Ment Health Nurs. 2005;26:781–794. doi: 10.1080/01612840591008410. [DOI] [PubMed] [Google Scholar]
- 339.Rausch JL, Johnson ME, Corley KM, Hobby HM, Shendarkar N, Fei Y. Depressed patients have higher body temperature: 5-HT transporter long promoter region effects. Neuropsychobiology. 2003;47:120–127. doi: 10.1159/000070579. [DOI] [PubMed] [Google Scholar]
- 340.Szuba MP, Guze BH, Baxter LR., Jr Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry. 1997;42:1130–1137. doi: 10.1016/S0006-3223(97)00046-2. [DOI] [PubMed] [Google Scholar]
- 341.Daimon K, Yamada N, Tsujimoto T, Takahashi S. Circadian rhythm abnormalities of deep body temperature in depressive disorders. J Affect Disord. 1992;26:191–198. doi: 10.1016/0165-0327(92)90015-X. [DOI] [PubMed] [Google Scholar]
- 342.Avery DH, Shah SH, Eder DN, Wildschiodtz G. Nocturnal sweating and temperature in depression. Acta Psychiatr Scand. 1999;100:295–301. doi: 10.1111/j.1600-0447.1999.tb10864.x. [DOI] [PubMed] [Google Scholar]
- 343.Avery DH, Wildschiodtz G, Smallwood RG, Martin D, Rafaelsen OJ. REM latency and core temperature relationships in primary depression. Acta Psychiatr Scand. 1986;74:269–280. doi: 10.1111/j.1600-0447.1986.tb06244.x. [DOI] [PubMed] [Google Scholar]
- 344.Avery DH, Wildschiodtz G, Rafaelsen OJ. Nocturnal temperature in affective disorder. J Affect Disord. 1982;4:61–71. doi: 10.1016/0165-0327(82)90020-9. [DOI] [PubMed] [Google Scholar]
- 345.Sugahara H, Akamine M, Kondo T, Fujisawa K, Yoshimasu K, Tokunaga S. Somatic symptoms most often associated with depression in an urban hospital medical setting in Japan. Psychiatry Res. 2004;128:305–311. doi: 10.1016/j.psychres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 346.Rangan AM, Blight GD, Binns CW. Iron status and non-specific symptoms of female students. J Am Coll Nutr. 1998;17:351–355. doi: 10.1080/07315724.1998.10718774. [DOI] [PubMed] [Google Scholar]
- 347.Maes M, Van de Vyvere J, Vandoolaeghe E, Bril T, Demedts P, Wauters A. Alterations in iron metabolism and the erythron in major depression: further evidence for a chronic inflammatory process. J Affect Disord. 1996;40:23–33. doi: 10.1016/0165-0327(96)00038-9. [DOI] [PubMed] [Google Scholar]
- 348.Maes M, Vandewoude M, Scharpe S, De Clercq L, Stevens W, Lepoutre L. Anthropometric and biochemical assessment of the nutritional state in depression: evidence for lower visceral protein plasma levels in depression. J Affect Disord. 1991;23:25–33. doi: 10.1016/0165-0327(91)90032-N. [DOI] [PubMed] [Google Scholar]
- 349.Maes M, Scharpe S, Bosmans E, Vandewoude M, Suy E, Uyttenbroeck W. Disturbances in acute phase plasma proteins during melancholia: additional evidence for the presence of an inflammatory process during that illness. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:501–515. doi: 10.1016/0278-5846(92)90056-K. [DOI] [PubMed] [Google Scholar]
- 350.Albacar G, Sans T, Martin-Santos R, Garcia-Esteve L, Guillamat R, Sanjuan J. An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. J Affect Disord. 2011;131:136–142. doi: 10.1016/j.jad.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 351.Engel GL, Schmale AH. Conservation-withdrawal: a primary regulatory process for organismic homeostasis. Ciba Found Symp. 1972;8:57–75. doi: 10.1002/9780470719916.ch5. [DOI] [PubMed] [Google Scholar]
- 352.Majer M, Wellberg LAM, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;25:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 354.Schaller M, Murray DR. Pathogens, personality, and culture: disease prevalence predicts worldwide variability in sociosexuality, extraversion, and openness to experience. J Pers Soc Psychol. 2008;95:212–221. doi: 10.1037/0022-3514.95.1.212. [DOI] [PubMed] [Google Scholar]
- 355.Cole SW. Complex Systems Science in Biomedicine. Springer: New York, NY; 2006. The complexity of dynamic host networks; pp. 605–629. [Google Scholar]
- 356.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci USA. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Nguyen KB, Biron CA. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J Immunol. 1999;162:5238–5246. [PubMed] [Google Scholar]
- 359.Jakab GJ, Dick EC. Synergistic effect in viral-bacterial infection: combined infection of the murine respiratory tract with Sendai virus and Pasteurella pneumotropica. Infect Immun. 1973;8:762–768. doi: 10.1128/iai.8.5.762-768.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Degre M, Glasgow LA. Synergistic effect in viral-bacterial infection. I. Combined infection of the respiratory tract in mice with parainfluenza virus and Hemophilus influenza. J Infect Dis. 1968;118:449–462. doi: 10.1093/infdis/118.5.449. [DOI] [PubMed] [Google Scholar]
- 361.Jones WT, Menna JH, Wennerstrom DE. Lethal synergism induced in mice by influenza type A virus and type Ia group B streptococci. Infect Immun. 1983;41:618–623. doi: 10.1128/iai.41.2.618-623.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004;17:185–191. doi: 10.1097/00001432-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 363.Molyneux EM, Tembo M, Kayira K, Bwanaisa L, Mweneychanya J, Njobvu A. The effect of HIV infection on paediatric bacterial meningitis in Blantyre, Malawi. Arch Dis Child. 2003;88:1112–1118. doi: 10.1136/adc.88.12.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202:1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Thornhill R, Fincher CL, Aran D. Parasites, democratization, and the liberalization of values across contemporary countries. Biol Rev Camb Philos Soc. 2009;84:113–131. doi: 10.1111/j.1469-185X.2008.00062.x. [DOI] [PubMed] [Google Scholar]
- 366.Diagnostic and Statistical Manual of Mental Disorders, 4th edn Text Revision: DSM-IV--4--TR. American Psychiatric Association: Washington, DC, 2000.
- 367.Schmidt WD, O'Connor PJ, Cochrane JB, Cantwell M. Resting metabolic rate is influenced by anxiety in college men. J Appl Physiol. 1996;80:638–642. doi: 10.1152/jappl.1996.80.2.638. [DOI] [PubMed] [Google Scholar]
- 368.Blaza SE, Garrow JS. The effect of anxiety on metabolic rate. Proc Nutr Soc. 1980;39:13A. doi: 10.1079/PNS19800003. [DOI] [PubMed] [Google Scholar]
- 369.Bonnet MH, Arand DL. Insomnia, metabolic rate and sleep restoration. J Intern Med. 2003;254:23–31. doi: 10.1046/j.1365-2796.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 370.Bonnet MH, Arand DL. 24-h metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 371.Miller GE, Cohen S. Human Psychoneuroimmunology. Oxford University Press: Oxford, UK; 2005. Infectious disease and psychoneuroimmunology; pp. 219–242. [Google Scholar]
- 372.Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/JCP.v66n0814. [DOI] [PubMed] [Google Scholar]
- 373.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 374.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 375.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wing EJ, Barczynski LK, Boehmer SM. Effect of acute nutritional deprivation on immune function in mice. I. Macrophages. Immunol. 1983;48:543–550. [PMC free article] [PubMed] [Google Scholar]
- 378.Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutr. 1979;32:593–596. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- 379.Heuer JG, Bailey DL, Sharma GR, Zhang T, Ding C, Ford A. Cecal ligation and puncture with total parenteral nutrition: a clinically relevant model of the metabolic, hormonal, and inflammatory dysfunction associated with critical illness. J Surg Res. 2004;121:178–186. doi: 10.1016/j.jss.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 380.Sena MJ, Utter GH, Cuschieri J, Maier RV, Tompkins RG, Harbrecht BG. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg. 2008;207:459–467. doi: 10.1016/j.jamcollsurg.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Heyland DK. Parenteral nutrition in the critically-ill patient: more harm than good? Proc Nutr Soc. 2000;59:457–466. doi: 10.1017/S002966510000063X. [DOI] [PubMed] [Google Scholar]
- 382.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011;365:506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 383.Carter JD, Joyce PR, Mulder RT, Luty SE, McKenzie J. Gender differences in the presentation of depressed outpatients: a comparison of descriptive variables. J Affect Disord. 2000;61:59–67. doi: 10.1016/S0165-0327(00)00151-8. [DOI] [PubMed] [Google Scholar]
- 384.Adamo SA, Fidler TL, Forestell CA. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav Immun. 2007;21:292–300. doi: 10.1016/j.bbi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 385.Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA. 1998;280:2013–2019. doi: 10.1001/jama.280.23.2013. [DOI] [PubMed] [Google Scholar]
- 386.Amprey JL, Spath GF, Porcelli SA. Inhibition of CD1 expression in human dendritic cells during intracellular infection with Leishmania donovani. Infect Immun. 2004;72:589–592. doi: 10.1128/IAI.72.1.589-592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Roura-Mir C, Wang L, Cheng TY, Matsunaga I, Dascher CC, Peng SL. Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J Immunol. 2005;175:1758–1766. doi: 10.4049/jimmunol.175.3.1758. [DOI] [PubMed] [Google Scholar]
- 388.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Nesse RM. Is depression an adaptation? Arch Gen Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- 390.Sloman L, Gilbert P, Hasey G. Evolved mechanisms in depression: the role and interaction of attachment and social rank in depression. J Affect Disord. 2003;74:107–121. doi: 10.1016/S0165-0327(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 391.Andrews PW, Thomson JA., Jr The bright side of being blue: depression as an adaptation for analyzing complex problems. Psychol Rev. 2009;116:620–654. doi: 10.1037/a0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 393.Norbiato G, Bevilacqua M, Vago T, Taddei A, Clerici M. Glucocorticoids and the immune function in the human immunodeficiency virus infection: a study in hypercortisolemic and cortisol-resistant patients. J Clin Endocrinol Metab. 1997;82:3260–3263. doi: 10.1210/jcem.82.10.4304. [DOI] [PubMed] [Google Scholar]
- 394.Huff GR, Huff WE, Balog JM, Rath NC. The effects of behavior and environmental enrichment on disease resistance of turkeys. Brain Behav Immun. 2003;17:339–349. doi: 10.1016/S0889-1591(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 395.Schaller M. The behavioural immune system and the psychology of human sociality. Philos Trans R Soc London B Biol Sci. 2011;366:3418–3426. doi: 10.1098/rstb.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Fincher CL, Thornhill R, Murray DR, Schaller M. Pathogen prevalence predicts human cross-cultural variability in individualism/collectivism. Proc Biol Sci. 2008;275:1279–1285. doi: 10.1098/rspb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Kim YK, Suh IB, Kim H, Han CS, Lim CS, Choi SH. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. 2002;7:1107–1114. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 398.Foster R, Kandanearatchi A, Beasley C, Williams B, Khan N, Fagerhol MK. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: evidence for an inflammatory process? Eur J Neurosci. 2006;24:3561–3566. doi: 10.1111/j.1460-9568.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- 399.Weigelt K, Carvalho LA, Drexhage RC, Wijkhuijs A, de Wit H, van Beveren NJ. TREM-1 and DAP12 expression in monocytes of patients with severe psychiatric disorders. EGR3, ATF3 and PU.1 as important transcription factors. Brain Behav Immun. 2011;25:1162–1169. doi: 10.1016/j.bbi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 400.Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/S0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


