Abstract
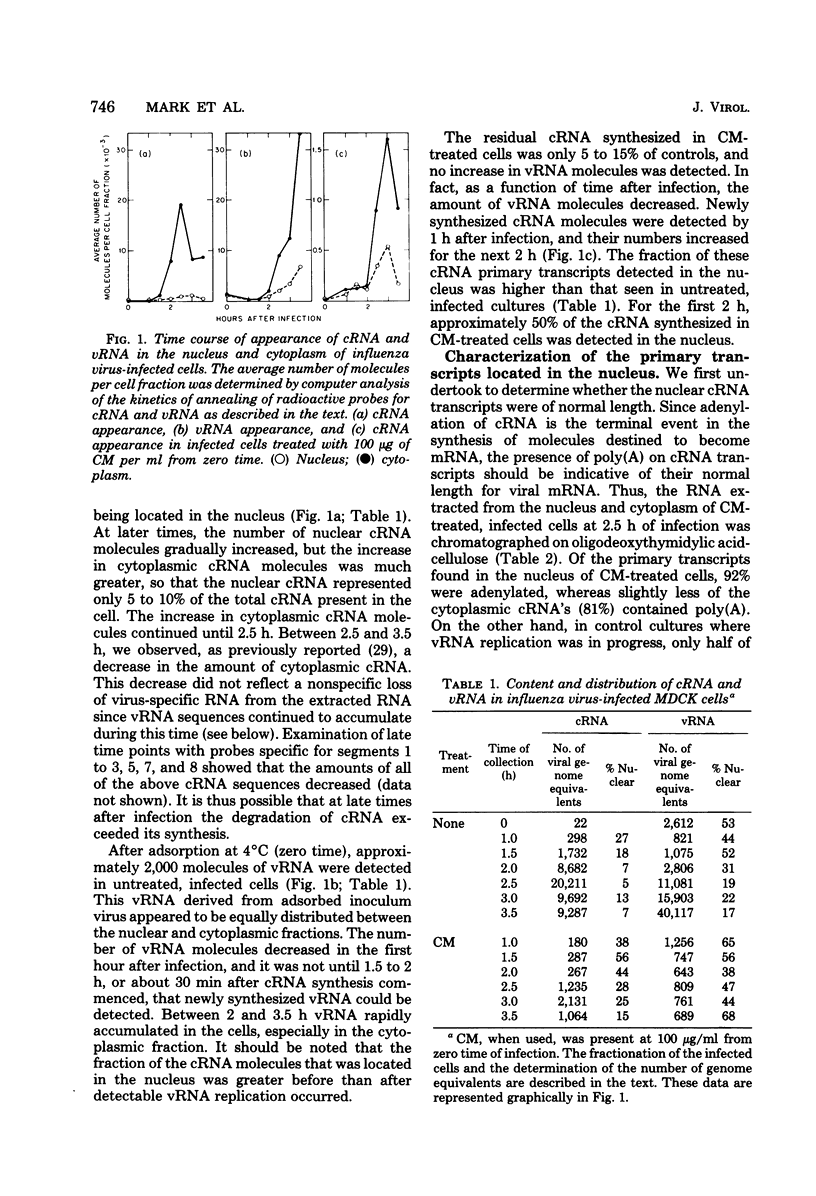
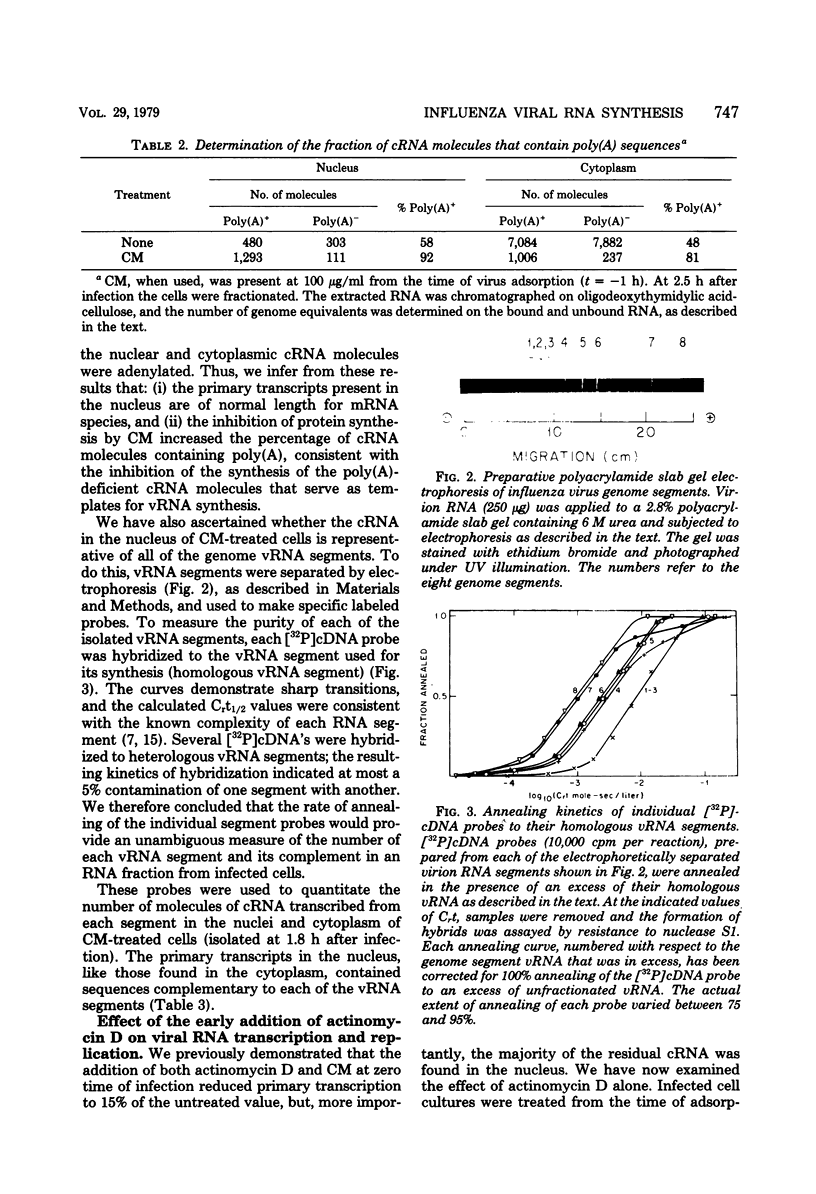
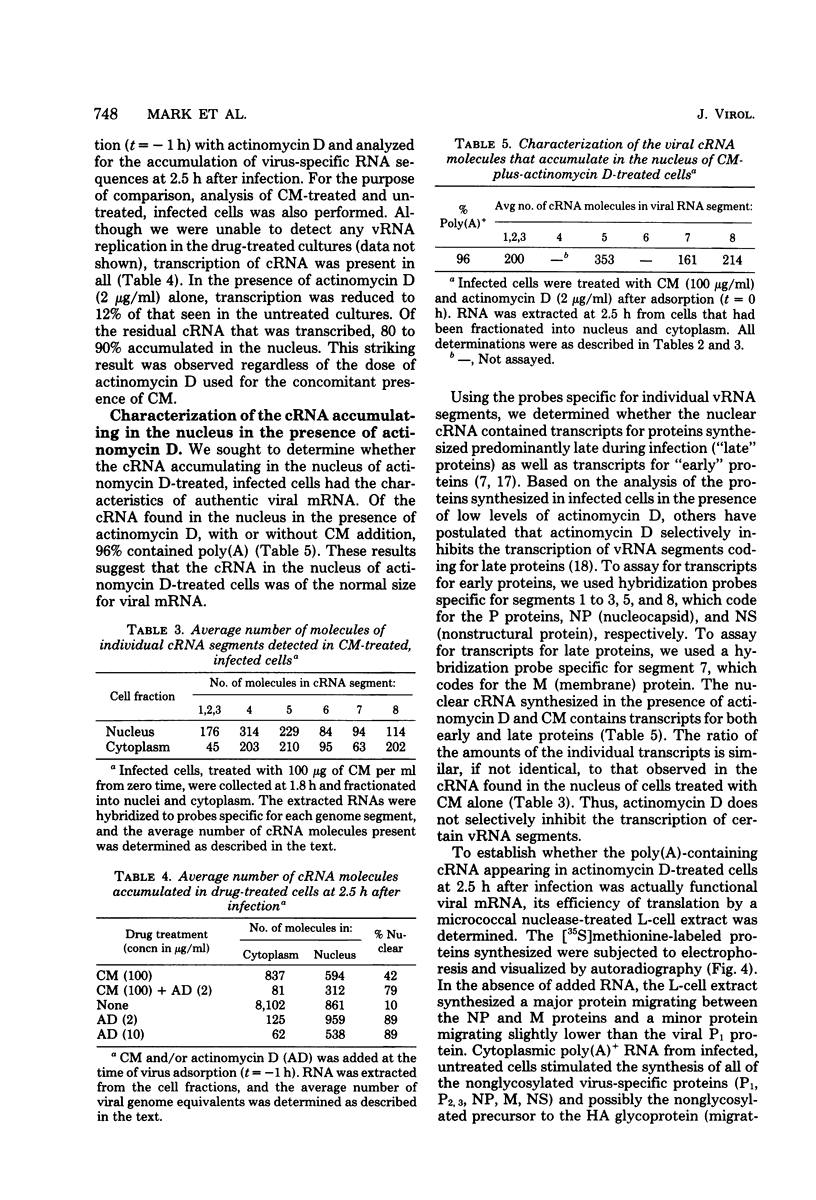
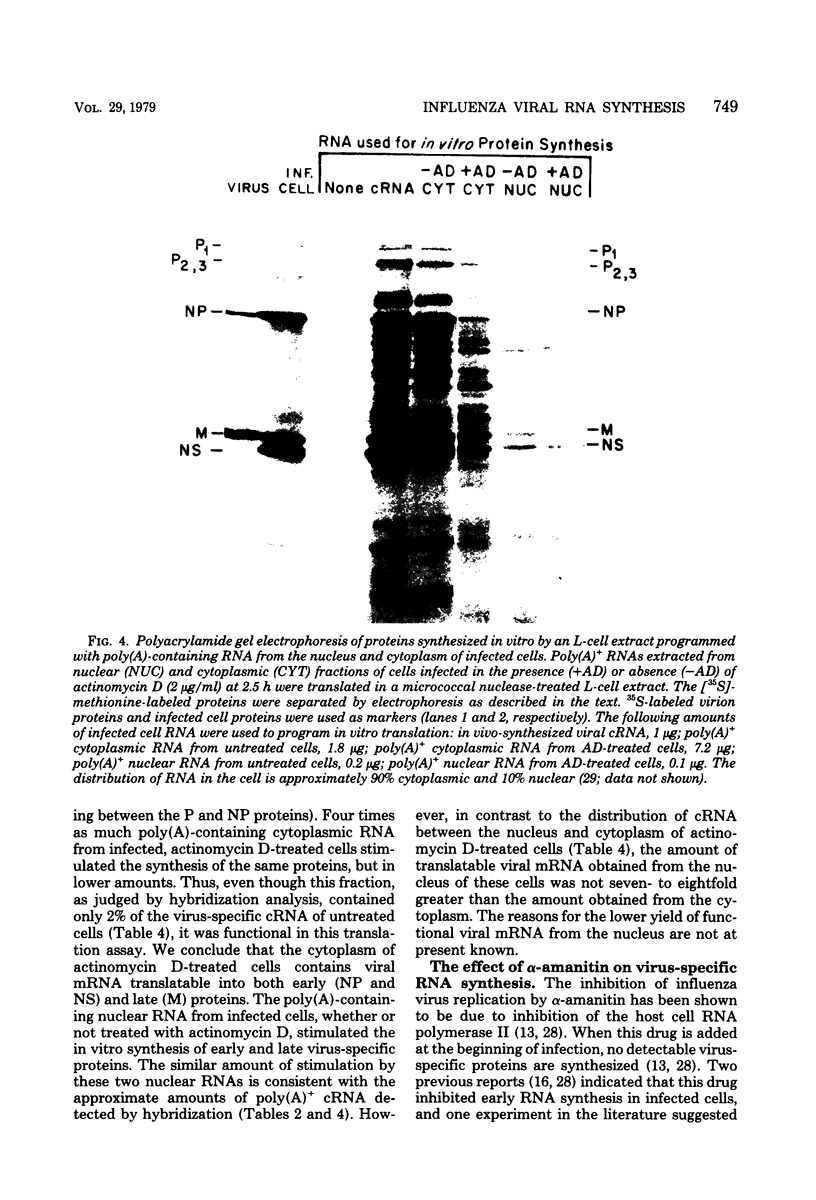
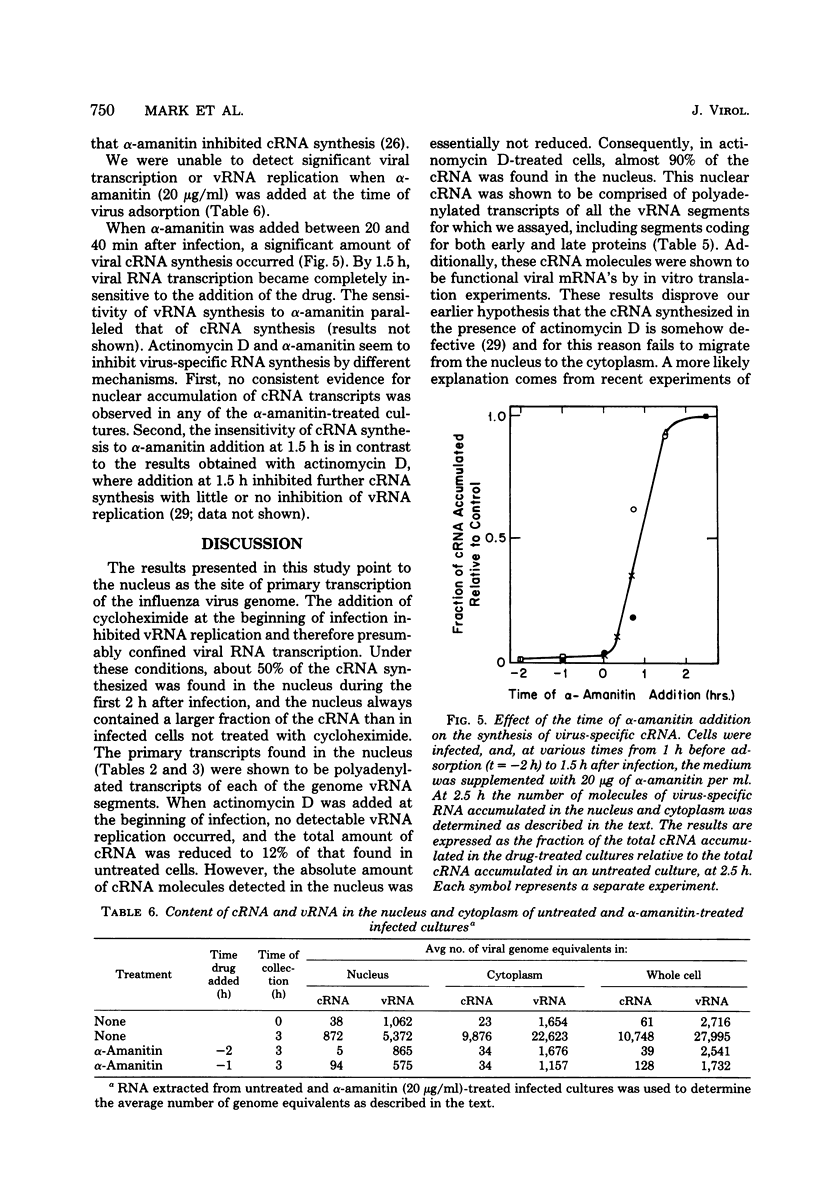
The use of virus-specific 32P-labeled complementary DNA and 125I-labeled virion RNA as hybridization probes has allowed us to quantitate the number of molecules of complementary RNA (cRNA) and progeny virion RNA in MDCK cells infected with influenza virus. We compared the distribution of cRNA between the nucleus and the cytoplasm in cycloheximide-treated cells to that found in untreated cells, beginning 1 h after infection. A greater percentage of the total cRNA was detected in the nucleus of the drug-treated cells at all times investigated. For the first 2 h after infection about 50% of the cRNA synthesized in the cycloheximide-treated cells was found in the nucleus. These nuclear cRNA molecules were characterized and shown to be polyadenylated transcripts of each of the genome virion RNA segments. Viral cRNA synthesis was not completely inhibited by the addition of actinomycin D at the beginning of infection, with or without the concomitant addition of cycloheximide. A large fraction (about 90%) of these cRNA sequences were detected in the nucleus. Characterization of these nuclear cRNA molecules showed that they contained polyadenylic acid and represented transcripts of both those segments coding for proteins synthesized predominantly early after infection (“early” proteins) and those virion RNA segments coding for “late” proteins. Also, in vitro translation of these cRNA molecules showed that they were functional virus mRNA's. In contrast to actinomycin D, α-amanitin completely inhibited cRNA synthesis when added at the beginning of infection, and addition of this drug after 1.5 h had no effect on further cRNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Abraham G., Skehel J. J., Smith J. C., Fellner P. Influenza virus messenger RNAs are incomplete transcripts of the genome RNAs. Nucleic Acids Res. 1977 Dec;4(12):4197–4209. doi: 10.1093/nar/4.12.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Avery R. J., Dimmock N. J. Failure of an influenza virus to initiate infection in enucleate BHK cells. J Virol. 1974 Jun;13(6):1155–1161. doi: 10.1128/jvi.13.6.1155-1161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M. Cytoplasmic and nucleoplasmic viral RNPs in influenza virus-infected MDCK cells. Virology. 1972 Oct;50(1):103–113. doi: 10.1016/0042-6822(72)90350-9. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus polypeptides in cells resistant to alpha-amanitin: evidence for the involvement of cellular RNA polymerase II in virus replication. J Virol. 1977 Sep;23(3):816–819. doi: 10.1128/jvi.23.3.816-819.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Penman S. The metabolism of poly (A)+ and poly(A)-hnRNA in cultured Drosophila cells studied with a rapid uridine pulse-chase. Cell. 1977 May;11(1):105–113. doi: 10.1016/0092-8674(77)90321-x. [DOI] [PubMed] [Google Scholar]
- Mahy B. W., Hastie N. D., Armstrong S. J. Inhibition of influenza virus replication by -amanitin: mode of action. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1421–1424. doi: 10.1073/pnas.69.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert H., Compans R. W. Time course of synthesis and assembly of influenza virus proteins. J Virol. 1974 Nov;14(5):1083–1091. doi: 10.1128/jvi.14.5.1083-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Dimmock N. J. Inhibition of synthesis of influenza virus proteins: evidence of two host-cell-dependent events during multiplication. Virology. 1975 Sep;67(1):114–123. doi: 10.1016/0042-6822(75)90409-2. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Influenza virion transcriptase: synthesis in vitro of large, polyadenylic acid-containing complementary RNA. J Virol. 1977 Jan;21(1):24–34. doi: 10.1128/jvi.21.1.24-34.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. A reexamination of influenza single-and double-stranded RNAs by gel electrophoresis. Virology. 1976 Feb;69(2):789–792. doi: 10.1016/0042-6822(76)90508-0. [DOI] [PubMed] [Google Scholar]
- Pons M. W. Studies on the replication of influenza virus RNA. Virology. 1972 Mar;47(3):823–832. doi: 10.1016/0042-6822(72)90574-0. [DOI] [PubMed] [Google Scholar]
- Rott R., Scholtissek C. Specific inhibition of influenza replication by alpha-amanitin. Nature. 1970 Oct 3;228(5266):56–56. doi: 10.1038/228056a0. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner L. L., Barry R. D. Participation of DNA-dependent RNA polymerase II in replication of influenza viruses. Nature. 1977 Aug 18;268(5621):650–652. doi: 10.1038/268650a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Litwin S., Herring L., Broni B., Krug R. M. Use of specific radioactive probes to study transcription and replication of the influenza virus genome. J Virol. 1977 Feb;21(2):530–540. doi: 10.1128/jvi.21.2.530-540.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]