Abstract
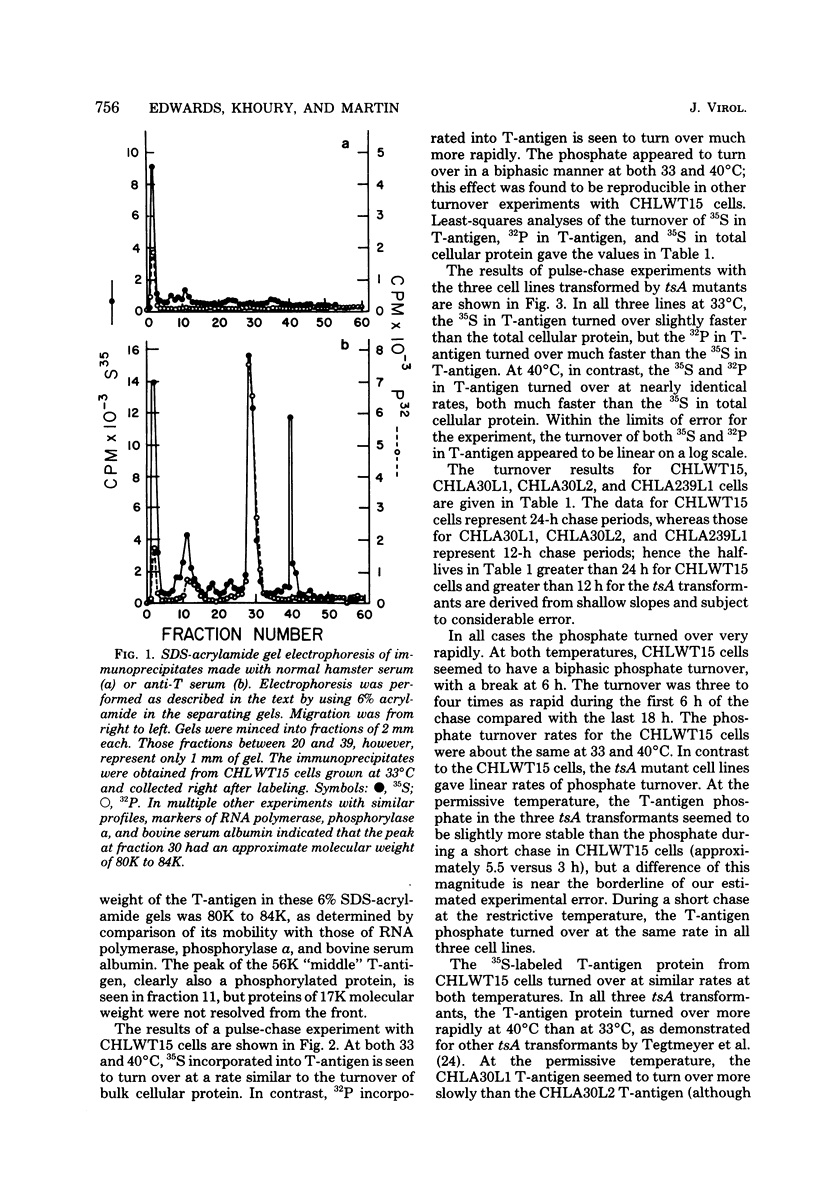
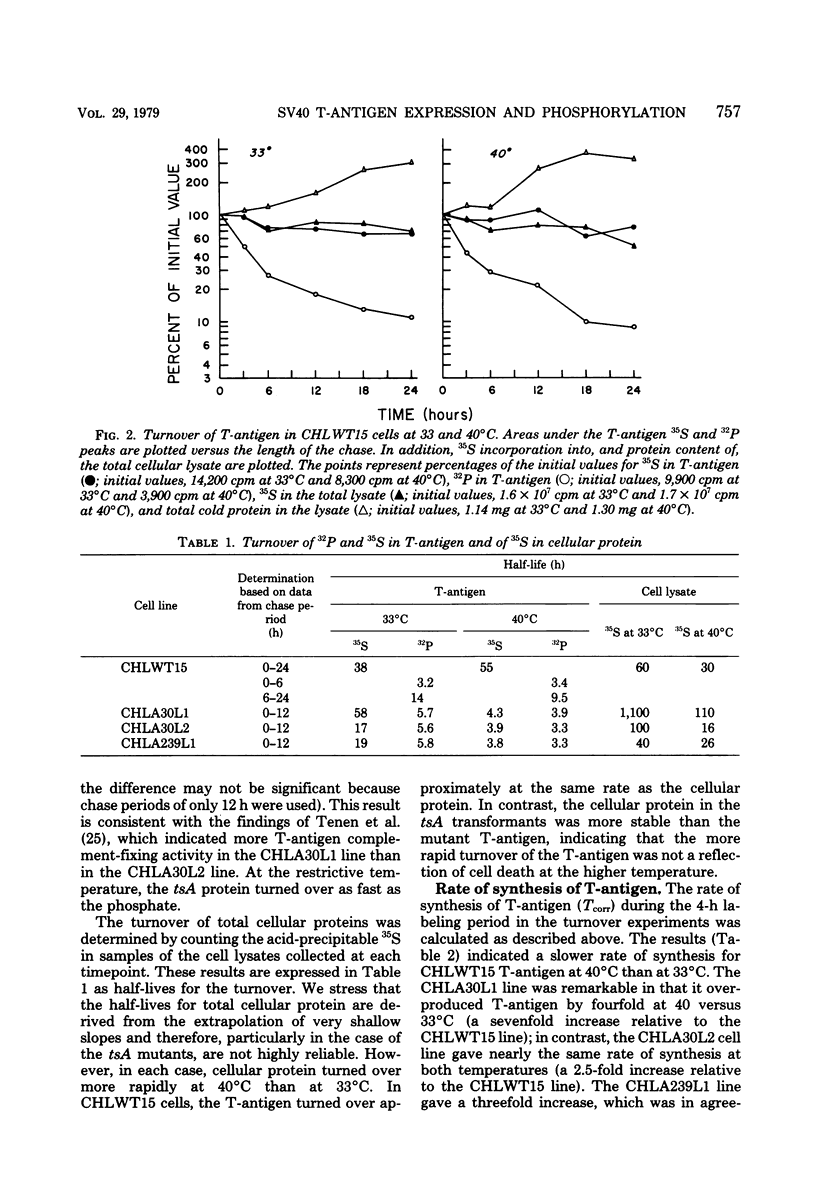
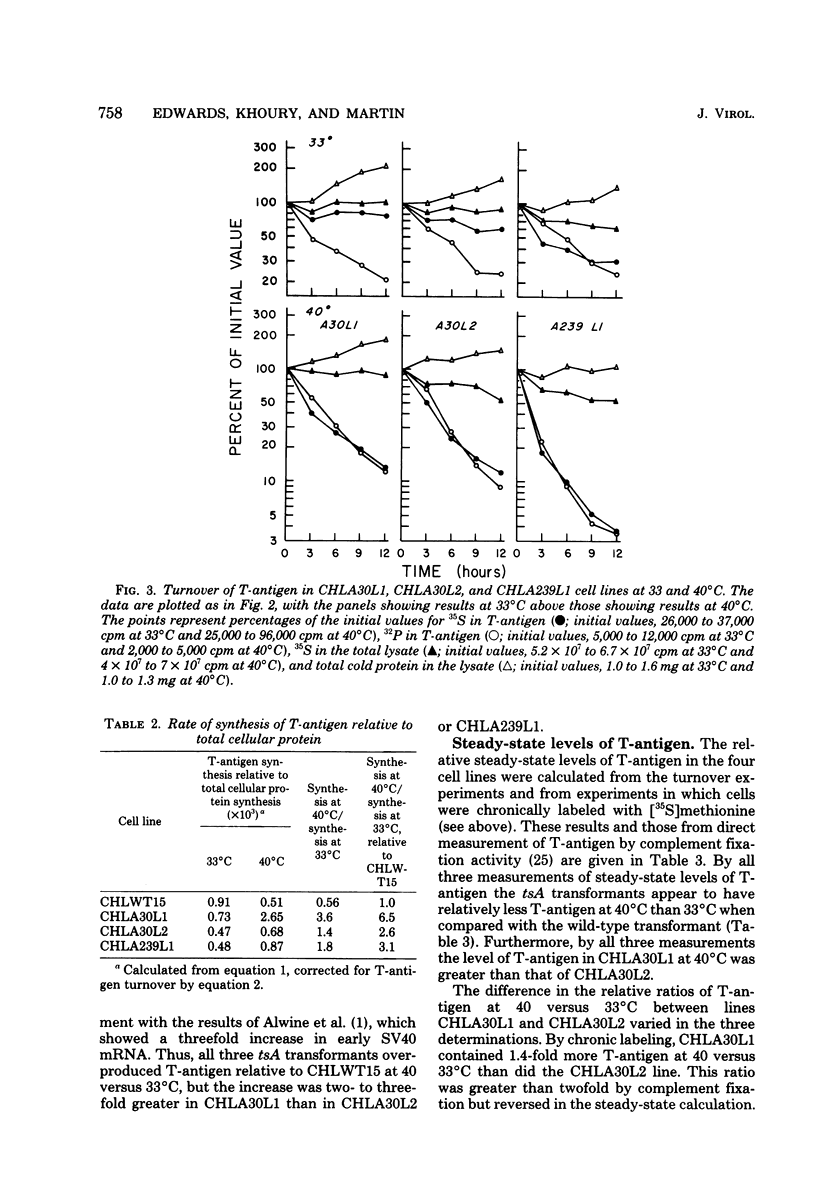
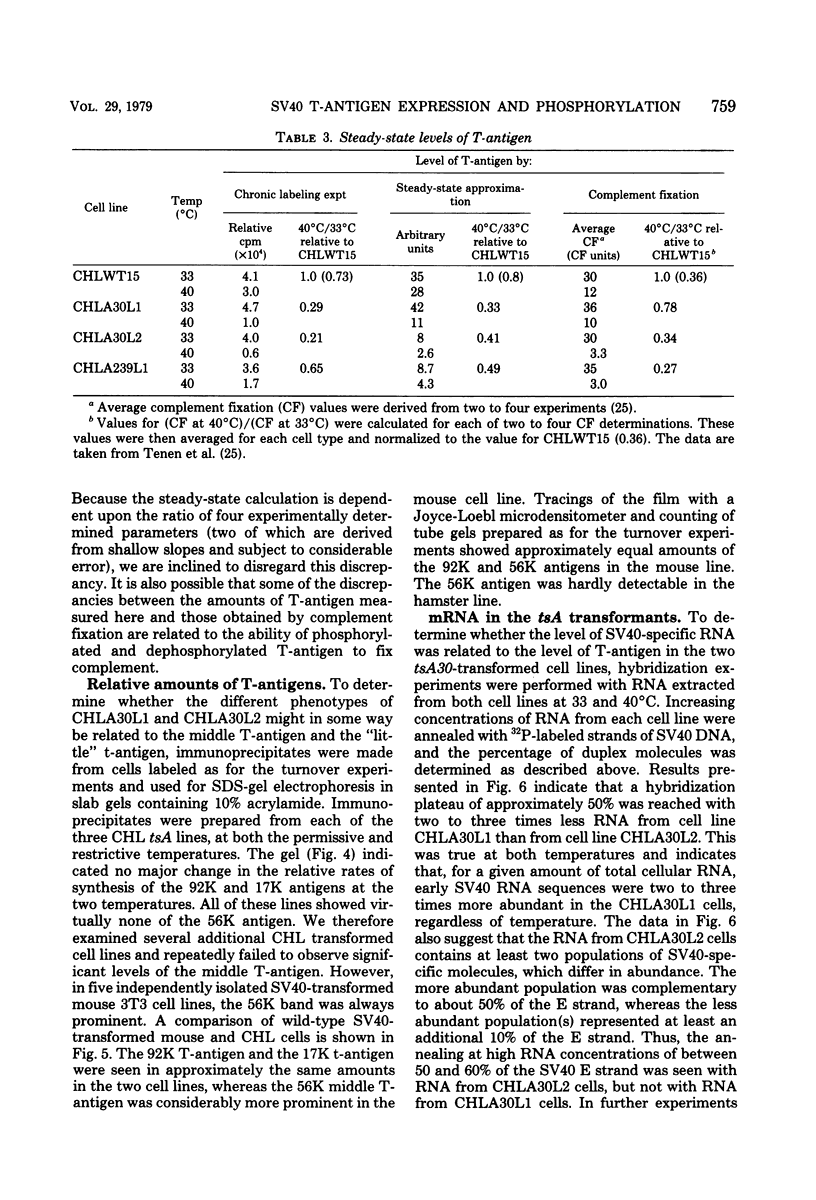
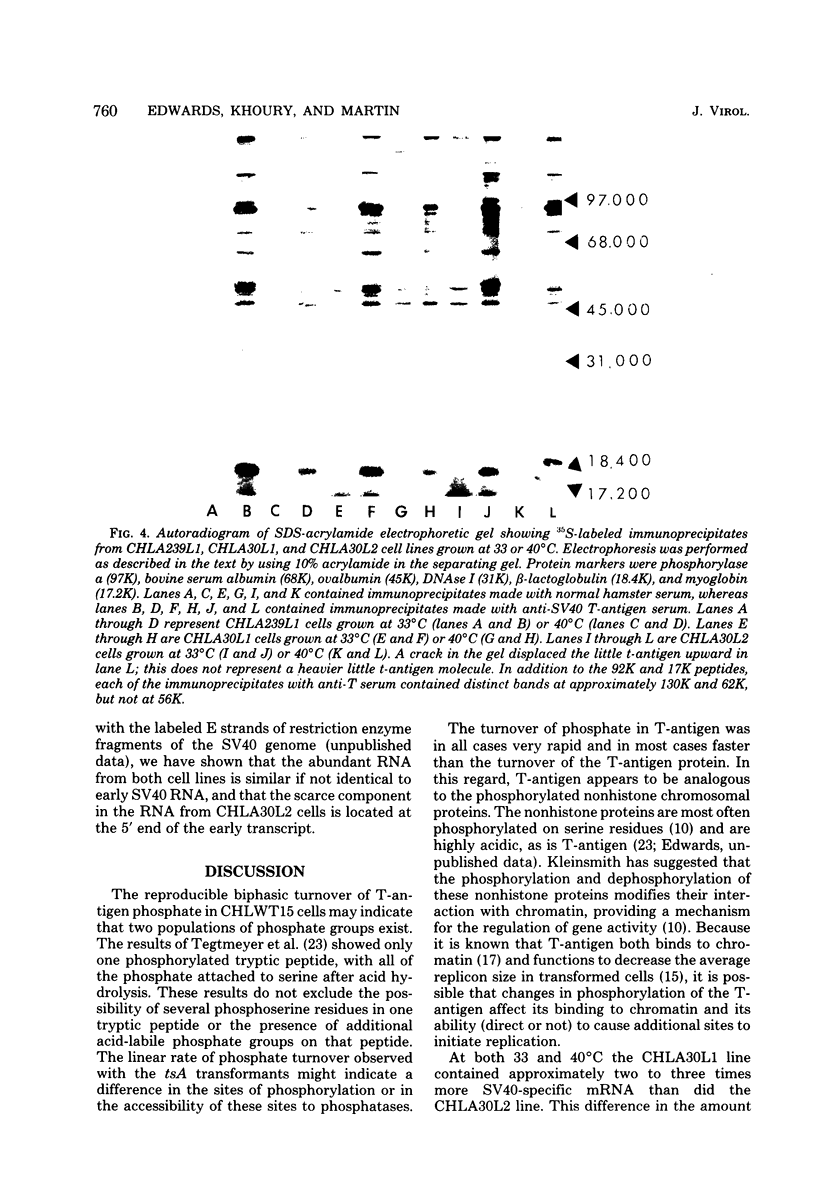
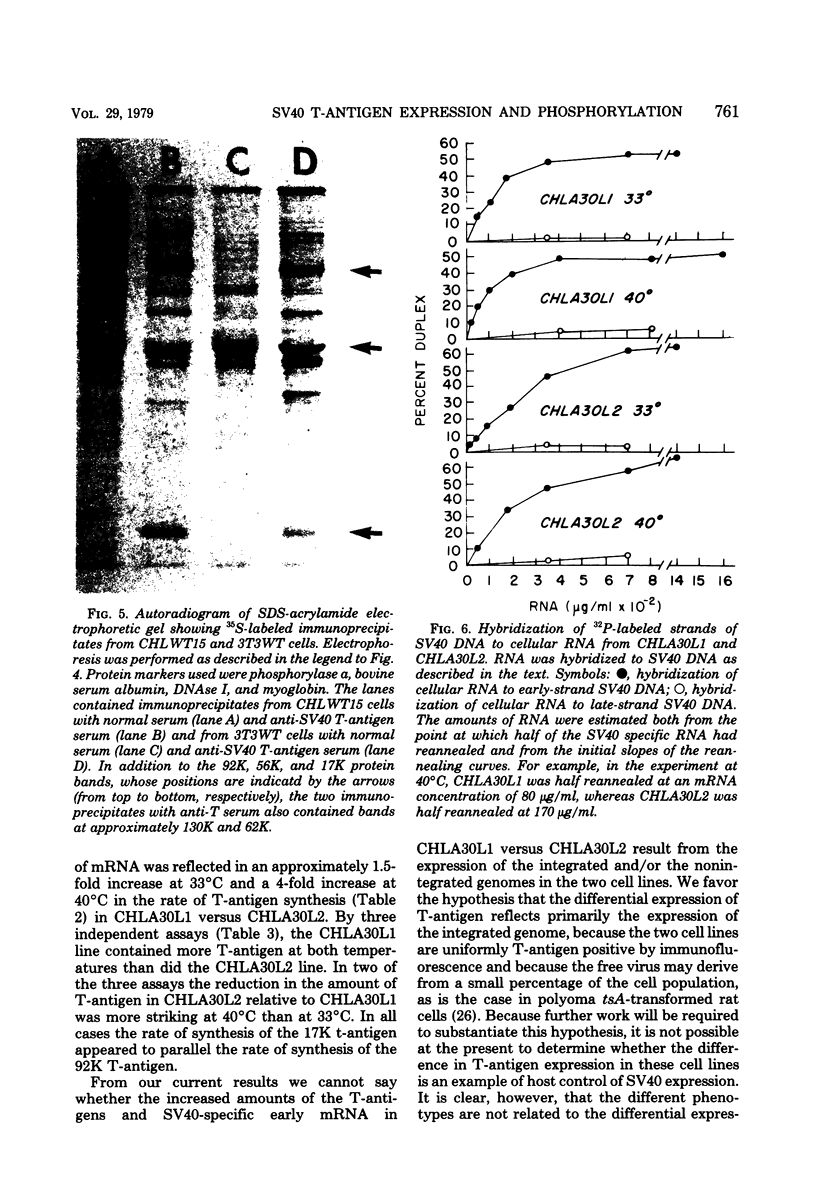
Chinese hamster lung (CHL) cells transformed by wild-type simian virus 40 (cell line CHLWT15) or transformed by the simian virus 40 mutants tsA30 (cell lines CHLA30L1 and CHLA30L2) or tsA239 (cell line CHLA239L1) were used to determine the rates of turnover and synthesis of the T-antigen protein and the rate of turnover of the phosphate group(s) attached to the T-antigen at both the permissive and restrictive temperatures. The phosphate group turned over several times within the lifetime of the protein to which it was attached, with the exception of the phosphate group in the tsA transformants at 40 degrees C, which turned over at the same rate as the T-antigen protein. The steady-state levels of the T-antigens (molecular weights, 92,000 [92K] and 17K) and the amount of simian virus 40-specific RNA was also determined in each of the lines. The CHLA30L1 line contained two to three times more early simian virus 40 RNA than the CHLA30L2 line; although neither line formed colonies in agar at 40 degrees C, CHLA30L1 overgrew a normal monolayer at 40 degrees C. The rate of 92K-T-antigen synthesis was 1.5 times faster in CHLA30L1 than in CHLA30L2 at 33 degrees C and 4 times faster at 40 degrees C. The different phenotype of these two presumably isogenic cell lines seem to be related to the levels of the T-antigens. The ratios of the 92K T-antigen to the 17K T-antigens were similar in the two lines. Transformed CHL cell lines, unlike transformed mouse 3T3 cell lines, were found to contain very small amounts of the 56K T-antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Martin R. G., Chang C., Mora P. T. Tumor-specific transplantation antigen is expressed during SV40 lytic infection with wild-type and tsA mutant viruses. Virology. 1977 Jan;76(1):254–262. doi: 10.1016/0042-6822(77)90301-4. [DOI] [PubMed] [Google Scholar]
- Basilico C., Zouzias D. Regulation of viral transciption and tumor antigen expression in cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1931–1935. doi: 10.1073/pnas.73.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Howley P., Brown M., Martin M. The detection and quantitation of SV40 nucleic acid sequences using single-stranded SV40 DNA probes. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):147–152. doi: 10.1101/sqb.1974.039.01.020. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J. Phosphorylation of non-histone proteins in the regulation of chromosome structure and function. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):459–475. doi: 10.1002/jcp.1040850412. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y., Avila J., Saral R. The semiautonomous replicon: a molecular model for the oncogenicity of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):17–24. doi: 10.1101/sqb.1974.039.01.005. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Oppenheim A. Initiation points for DNA replication in nontransformed and simian virus 40-transformed Chinese hamster lung cells. Cell. 1977 Aug;11(4):859–869. doi: 10.1016/0092-8674(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Persico-DiLauro M., Martin R. G., Livingston D. M. Interaction of Simian Virus 40 chromatin with Simian Virus 40 T-antigen. J Virol. 1977 Nov;24(2):451–460. doi: 10.1128/jvi.24.2.451-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Martin R. G., Anderson J., Livingston D. M. Biological and biochemical studies of cells transformed by simian virus 40 temperature-sensitive gene A mutants and A mutant revertants. J Virol. 1977 Apr;22(1):210–218. doi: 10.1128/jvi.22.1.210-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouzias D., Prasad I., Basilico C. State of the viral DNA in rat cells transformed by polyma virus. II. Identification of the cells containing nonintegrated viral DNA and the effect of viral mutations. J Virol. 1977 Oct;24(1):142–150. doi: 10.1128/jvi.24.1.142-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]