Abstract
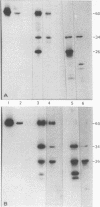
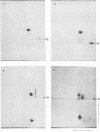
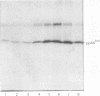
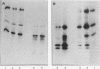
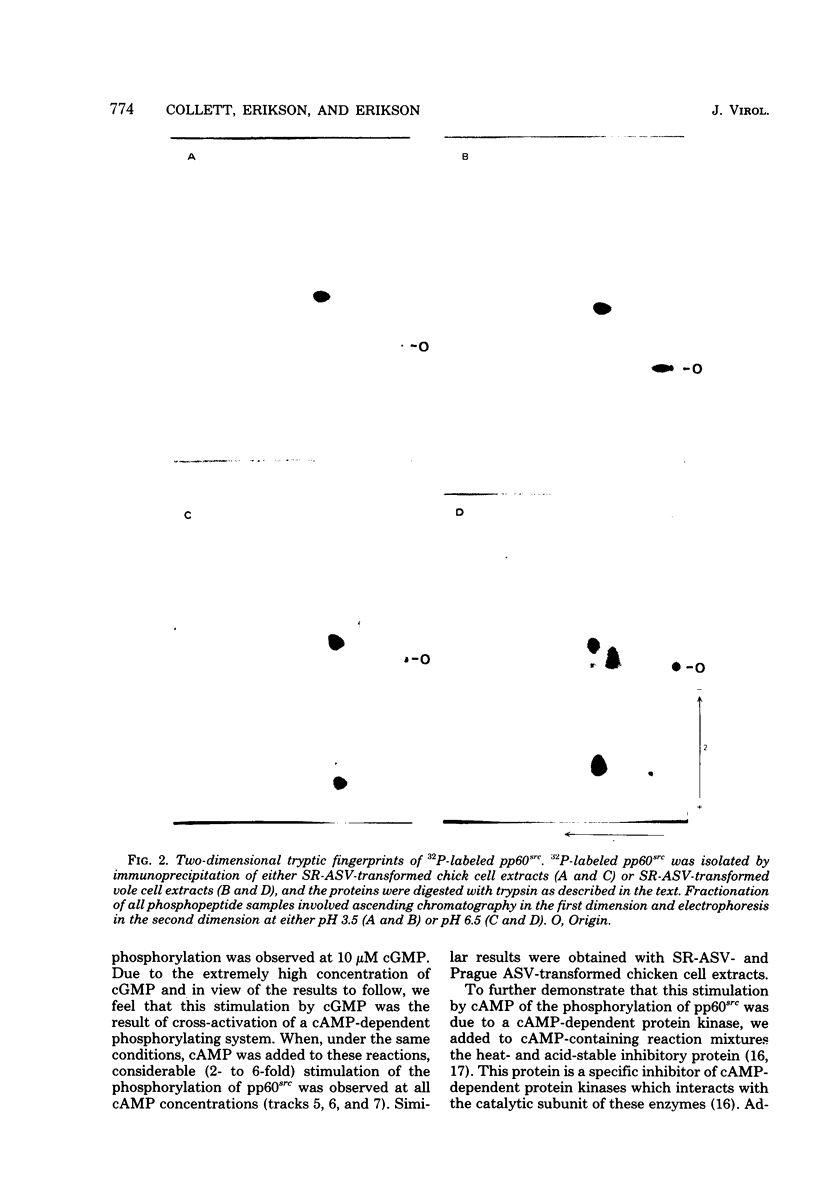
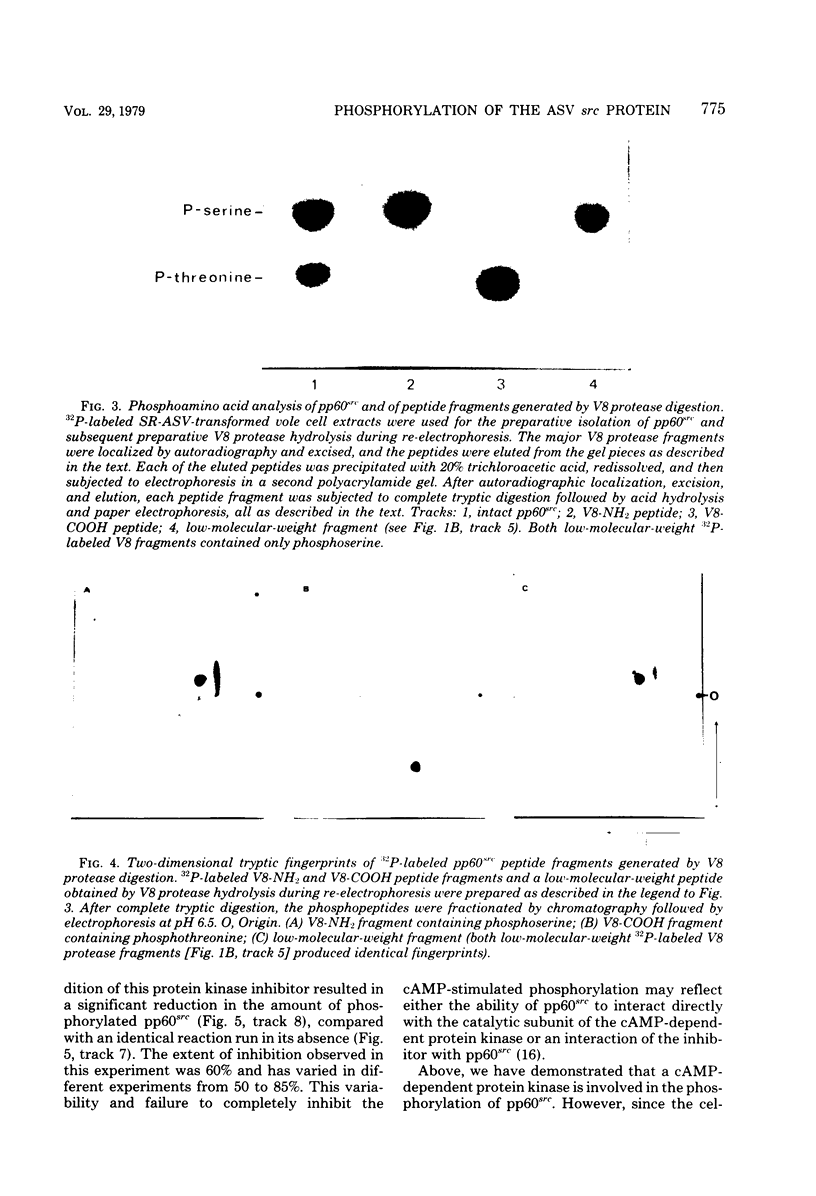
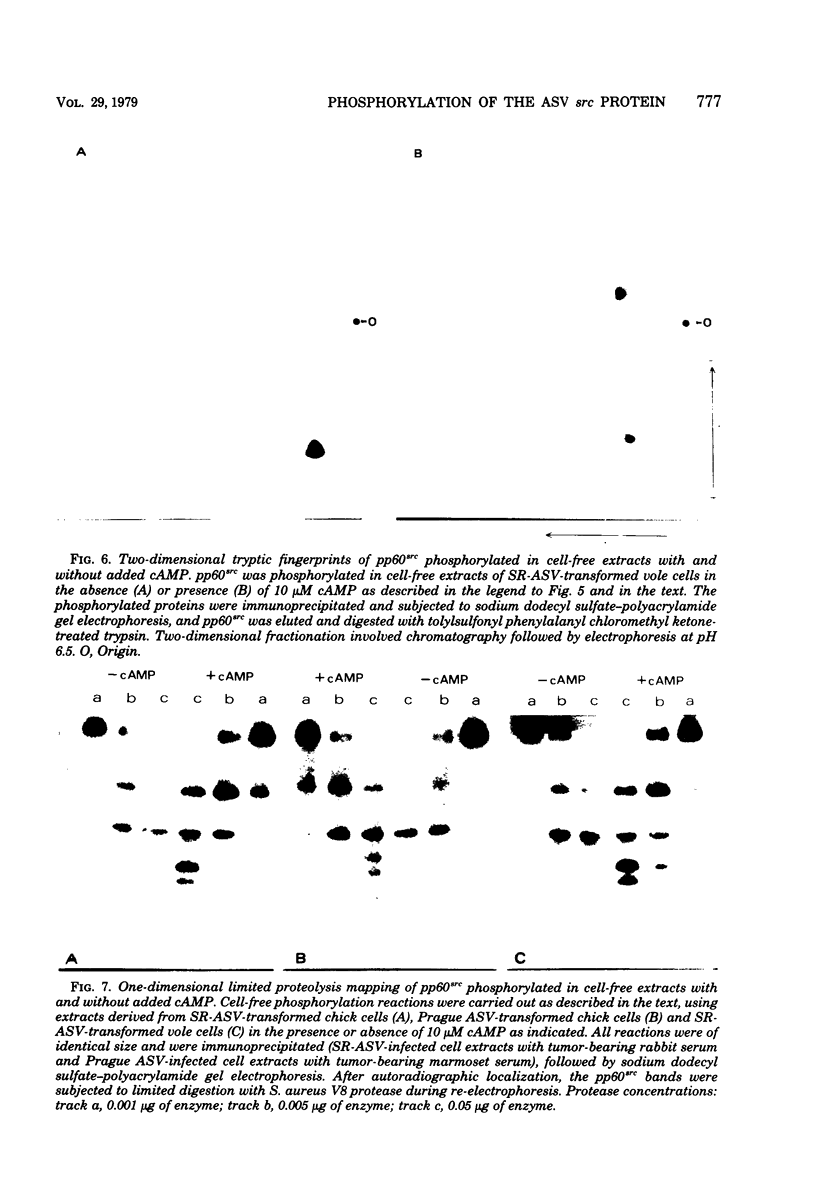
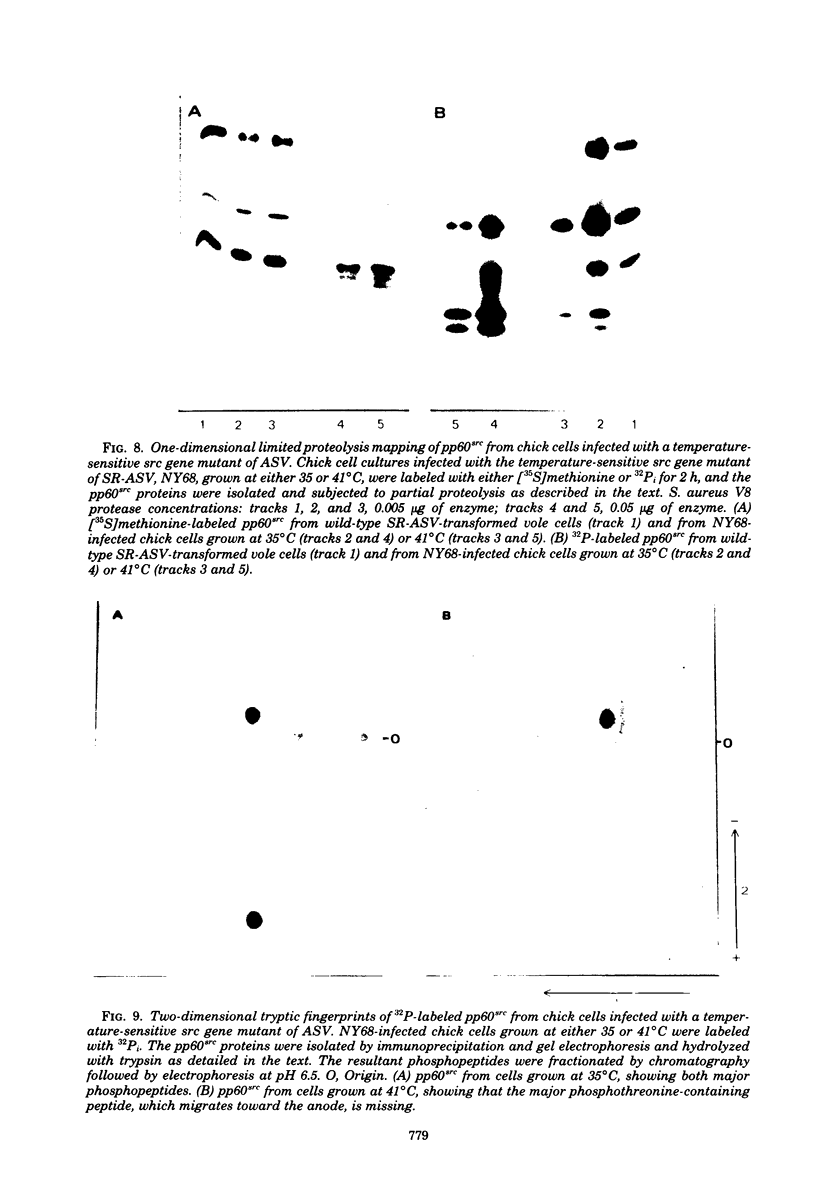
The avian sarcoma virus (ASV) protein responsible for cellular transformation in vitro and sarcomagenesis in animals was studied structurally with special reference to the sites of phosphorylation on the polypeptide. The product of the ASV src gene, pp60src, is a phosphoprotein of 60,000 daltons. We found that pp60src contained two major sites of phosphorylation, one involving phosphoserine and the other involving phosphothreonine and possible addtional minor sites of phosphorylation. By using N-formyl[35S]methionyl-tRNAf as a radiolabeled precursor in the cell-free synthesis of the src protein in conjunction with partial proteolysis mapping, we determined that the major phosphoserine residue was located on the amino-terminal two-thirds of the molecule and that the phosphothreonine was located on the carboxy-terminal third. We further determined that the phosphorylation of pp60src in cell extracts involved at least two protein kinases, the one that phosphorylated the major serine site being cyclic AMP dependent and the other, acting on the threonine residue, being a cyclic nucleotide-independnet phosphotransferase. Finally, analysis of the pp60src isolated from cells infected with a temperature-sensitive src gene mutant of ASV revealed that phosphorylation of the major threonine residue was severely reduced when infected cells were grown at the nonpermissive temperature, whereas a phosphorylation pattern characteristic of the wild-type pp60src was observed at the permissive temperature. As pp60src has an associated protein kinase activity, the possible involvement of phosphorylation-dephosphorylation reactions in the functional regulation of ASV transforming protein enzymatic activity is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Collett M. S., Erikson R. L. In vitro synthesis of a functional avian sarcoma virus transforming-gene product. Nature. 1978 Aug 31;274(5674):919–921. doi: 10.1038/274919a0. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Pastan I., Johnson G. S. Cyclic AMP and the transformation of fibroblasts. Adv Cancer Res. 1974;19(0):303–329. doi: 10.1016/s0065-230x(08)60057-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Rangel-Aldao R., Erlichman J. Soluble cyclic AMP-dependent protein kinases: review of the enzyme isolated from bovine cardiac muscle. Curr Top Cell Regul. 1977;12:39–74. [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Schofield P., Zamecnik P. C. Cupric ion catalysis in hydrolysis of aminoacyl-tRNA. Biochim Biophys Acta. 1968 Feb 26;155(2):410–416. doi: 10.1016/0005-2787(68)90185-8. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. Posttranslational covalent modification of proteins. Science. 1977 Dec 2;198(4320):890–896. doi: 10.1126/science.337487. [DOI] [PubMed] [Google Scholar]