Abstract
Background
The anaerobic spirochaete Brachyspira pilosicoli causes enteric disease in avian, porcine and human hosts, amongst others. To date, the only available genome sequence of B. pilosicoli is that of strain 95/1000, a porcine isolate. In the first intra-species genome comparison within the Brachyspira genus, we report the whole genome sequence of B. pilosicoli B2904, an avian isolate, the incomplete genome sequence of B. pilosicoli WesB, a human isolate, and the comparisons with B. pilosicoli 95/1000. We also draw on incomplete genome sequences from three other Brachyspira species. Finally we report the first application of the high-throughput Biolog phenotype screening tool on the B. pilosicoli strains for detailed comparisons between genotype and phenotype.
Results
Feature and sequence genome comparisons revealed a high degree of similarity between the three B. pilosicoli strains, although the genomes of B2904 and WesB were larger than that of 95/1000 (~2,765, 2.890 and 2.596 Mb, respectively). Genome rearrangements were observed which correlated largely with the positions of mobile genetic elements. Through comparison of the B2904 and WesB genomes with the 95/1000 genome, features that we propose are non-essential due to their absence from 95/1000 include a peptidase, glycine reductase complex components and transposases. Novel bacteriophages were detected in the newly-sequenced genomes, which appeared to have involvement in intra- and inter-species horizontal gene transfer. Phenotypic differences predicted from genome analysis, such as the lack of genes for glucuronate catabolism in 95/1000, were confirmed by phenotyping.
Conclusions
The availability of multiple B. pilosicoli genome sequences has allowed us to demonstrate the substantial genomic variation that exists between these strains, and provides an insight into genetic events that are shaping the species. In addition, phenotype screening allowed determination of how genotypic differences translated to phenotype. Further application of such comparisons will improve understanding of the metabolic capabilities of Brachyspira species.
Keywords: Brachyspira pilosicoli, Spirochaete, Colitis, Zoonosis, Whole genome sequencing, Genome comparison, Horizontal gene transfer, Phenotype MicroArray™
Background
Spirochaetes represent a monophyletic lineage and a major branch in eubacterial evolution; Brachyspira is the sole genus of the family Brachyspiraceae within the order Spirochaetales, which belongs to the spirochaete phylum [1]. Brachyspira are Gram-negative, loosely coiled, aerotolerant anaerobes that colonise the lower gastrointestinal (GI) tract of mammals and birds, but vary in pathogenicity. There are seven Brachyspira species that are currently officially recognised: B. aalborgi, a potential human pathogen [2]; the porcine pathogen, B. hyodysenteriae[3]; the avian pathogens, B. alvinipulli[4] and B. intermedia[5]; the avian, porcine and human pathogen, B. pilosicoli[6]; non-pathogenic B. innocens[7] and B. murdochii, which is of uncertain pathogenic potential [5]. In addition, there are a number of proposed species including “B. canis” [8], “B. pulli” [9] and “B. suanatina” [10] amongst others. The classification of the genus is still immature and the often used descriptors of certain Brachyspira as pathogenic, intermediate pathogenic or non-pathogenic is subject to debate.
B. pilosicoli is the only species considered to be a pathogen of birds, pigs and humans. The species is quite diverse, and it seems unlikely that there are barriers to cross-species and zoonotic transmission [11]. B. pilosicoli is an aetiological agent of colitis and occasional spirochaetaemia in humans [12], and a cause of porcine intestinal spirochaetosis (PIS) and avian intestinal spirochaetosis (AIS) [13]. It may also cause disease in other species [8]. B. pilosicoli is commonly found in humans living in densely populated areas with poor hygienic conditions [14-17], and in homosexual males [18]. B. pilosicoli infections are highly prevalent in intensively farmed swine and poultry, inducing inflammation in the colon and caeca, diarrhoea and reducing growth and productivity [13]. Chemotaxis and motility are deemed important virulence factors, and, as with B. hyodysenteriae, B. pilosicoli has a chemoattraction to mucin that facilitates penetration of the mucus and association with the underlying intestinal epithelial surface [19,20]. The intimate contact with the epithelia induces a mucus outpouring and epithelial sloughing [21]. An unusual feature of B. pilosicoli infection, and shared only by B. aalborgi, is the ability to insert one cell end into the luminal surface of enterocytes in the large intestine, forming a pit-like structure, with arrays of such attached spirochaetes giving the appearance of a “false brush-border” [22,23]. This unusual form of attachment of B. pilosicoli also occurs in Caco-2 cells in vitro, resulting in apoptosis, actin rearrangement and elevated interleukin expression [24].
The paucity of genomic information and absence of tools for genetic manipulation are responsible, at least partly, for the lack of knowledge regarding the adaptations that Brachyspira have undergone to colonise the lower GI tract, and for the pathogenic species to induce disease. Brachyspira whole genome sequences have only recently been made available for the following species: B. hyodysenteriae[25], B. intermedia[26], B. murdochii[27] and B. pilosicoli[28]. The four published sequences showed substantial genetic diversity, and their availability has facilitated research on the corresponding species. However, the availability of only one genome sequence per species has limited the conclusions that can be drawn from the genome as a representation for the species as a whole, and does not allow analysis of intra-species genomic variation. Here, we report the whole genome sequence of B. pilosicoli B2904, isolated from a chicken exhibiting clinical symptoms of AIS in the UK, and the partial genome sequence of B. pilosicoli WesB, isolated from an Australian Aboriginal child with diarrhoea. Experimentally, the latter strain has been shown to colonise and cause disease in pigs [22]. Although the strains were originally isolated from different host species, it is unlikely that the differences that were found between them were related to their host species of origin [11]. The genomes are presented alongside the whole genome sequence of B. pilosicoli 95/1000, isolated from a pig with PIS in Australia, and which has been confirmed to be virulent in experimental infection studies in pigs [22]. We employed the Biolog Phenotype MicroArray™ (PM) technology [29,30] to assess carbon utilisation in these strains. These studies facilitated the validation of differences observed in genotype and permitted detailed correlation between genotype and phenotype.
Methods
Bacterial strains and growth conditions
B. pilosicoli B2904 was isolated from a chicken displaying clinical symptoms of AIS in the UK; WesB was isolated from an Australian child with diarrhoea [14] and 95/1000 from the diarrhoeic faeces of a pig with PIS, in Australia [22]. The strains were cultured on fastidious anaerobe blood agar (FABA) [31] in an anaerobic atmosphere (10% H2 and 10% CO2 in N2) at 37°C or 42°C for 5 days, for phenotypic studies. For genomic DNA extraction, strains were grown in pre-reduced anaerobic broth [32] at 37°C and a cell pellet was prepared from mid-log phase broth growth.
Genomic DNA preparation, library construction and sequencing
Cetyltrimethylammonium bromide (CTAB) extraction was used to purify high molecular weight genomic DNA [33]. The B. pilosicoli B2904 and WesB genomes were sequenced on a Roche 454 FLX platform, using a standard preparation for a 3 Kb and 8 Kb library, respectively.
For the B2904 genome, a de novo assembly of the sequence reads into contiguous sequences was generated using Newbler Assembler software. The reads were assembled into one scaffold of 173 contigs with an average coverage of × 20. Remaining gaps were closed by PCR walking between unlinked, contiguous sequences [33], followed by Sanger sequencing. In total, 170 Sanger reads were incorporated into the assembly.
For the WesB genome, sequence data were initially assembled with Short Oligonucleotide Alignment Program (SOAP) [34] and subsequently Newbler Assembler software was used to create a combined assembly with Illumina reads. Iterative Mapping and Assembly for Gap Elimination (IMAGE) [35] improved genome assemblies by targeted re-assembly of Illumina reads to span gaps within scaffolds. To check for indels (insertion/deletions) and single nucleotide polymorphisms (SNP), Iterative Correction of Reference Nucleotides (iCORN) [36] was applied to the genome and appropriate corrections were made. All repeats over 100 bp were checked to ensure that they were confirmed by at least two spanning read pairs. The incomplete WesB genome was sequenced within one scaffold, with an average coverage of × 34.
Sequence analysis and annotation
The complete nucleotide sequence and annotation of B. pilosicoli B2904 (accession number: CP003490 Project ID: 80999) and partial nucleotide sequence and annotation of B. pilosicoli WesB B2904 (accession number HE793032; Project ID: 89437) have been deposited in GenBank. Scaffold sequences for unpublished genomes B. alvinipulli C1T and B. intermedia HB60 can be accessed from the authors via e-mail request. The draft genome scaffolds for B. aalborgii are available at the MetaHit website (http://www.sanger.ac.uk/resources/downloads/bacteria/metahit/).
Sequence and protein analysis and annotation (including rRNA and tRNA prediction) for the complete B. pilosicoli B2904 and partial B. pilosicoli WesB genomes was as previously described for B. hyodysenteriae WA1 [25] and B. pilosicoli 95/1000 [28] unless otherwise stated.
The Multi Locus Sequence Typing (MLST) dendrogram of six Brachyspira strains that have undergone genome sequencing, and three that are currently within unpublished genome sequencing projects being undertaken by the authors was calculated and constructed from the concatenation of 7 gene nucleotide sequences (adh, pgm, est, glp, gdh, thi, alp) [37]. These concatenated sequences were aligned by ClustalW [38] and the maximum likelihood dendrogram was generated via MEGA5 [39]. The condensed bootstrap maximum likelihood dendrogram was constructed from the General Time Reversible (GTR) model with a Gamma of 2.83 (+G) and an assumption that a fraction of sites (0.27) are evolutionarily invariable (+I).
The open source utility ‘Freckle’ was used for sequence dot plotting (code.google.com/p/freckle/). Gene prediction and gene and protein sequence extraction was achieved using prodigal 2.50 (prodigal.ornl.gov/).
Initial coding region annotation was completed with an in-house updated compilation of the annotation pipeline AutoFACT 3.4 [40]. Resulting annotations were manually checked and edited where appropriate to be consistent with previous Brachyspira genome annotation methodologies for comparative purposes [25,26,28]. Final annotations were assessed with the NCBI Microbial Genome Submission Tool (preview.ncbi.nlm.nih.gov/genomes/frameshifts/frameshifts.cgi).
Protein cluster analysis
Protein reciprocal blast similarity searches with a threshold maximum expected value 1e-20 were conducted with BlastlineMCL, which is an implementation of the Markov clustering algorithm (MCL) for graphs (http://www.micans.org/mcl/). The granularity of the output cluster was set with an inflation value of 2.5.
Biolog phenotype MicroArray™
B. pilosicoli 95/1000, B2904 and WesB were analysed using the Biolog PM™ technology [29] for high throughput substrate utilisation screening, which included 191 unique carbon sources (PM1 and PM2). PM panels and reagents were supplied by Biolog and used according to the manufacturer’s instructions. Briefly, under anaerobic conditions, bacterial cells were aseptically picked from the FABA agar surface with a sterile cotton swab and suspended in 10 ml of Biolog inoculating fluid (IF-0) until a cell density of 40% transmittance was reached on a Biolog turbidimeter. Before the addition to PM microtitre plates, bacterial suspensions were further diluted into 12 ml of IF-0 (per plate) in sterile water. PM microtitre plates were pre-incubated with two AGELESS® oxygen absorbers (Mitsubishi) 48 h prior to inoculation, at ambient temperature. The resuspended bacterial cells were pipetted into the 96-well plates at a volume of 100 μl/well. Prior to removal from the anaerobic chamber, one AGELESS® oxygen absorber and one CO2GEN compact sachet (Oxoid) were attached per PM panel, which were then placed into 4 oz Whirl-Pak® Long-Term Sample Retention Bags (Nasco) with the open end heat-sealed.
Substrate utilisation was measured via the reduction of a tetrazolium dye (clear yellow) to formazan (purple), indicative of cellular respiration at 37°C. Experiments were also run at 42°C, using bacteria cultured at this temperature. Formazan formation was monitored at 15 min intervals for 120 h in OmniLog apparatus. Kinetic data were analyzed with OmniLog-PM software. Each experiment was performed at least twice per strain. It was noted that although tetrazolium dye reduction is indicative of cellular respiration, it can occur independent of cell growth [29,30].
Blank PM1 and PM2 controls were run, whereby IF-0 was added in place of the bacterial cell suspension, to assess for abiotic reactions that occur in the anaerobic atmosphere across the 120 h monitoring period. The following compounds were omitted from analysis due to the nature of the abiotic reactions that occurred in wells containing these compounds, under the conditions of the study: D-arabinose and L-arabinose, dihydroxyacetone, D-glucosamine, 5-keto-D-gluconate, L-lyxose, palatinose, D-ribose, 2-deoxy-D-ribose, sorbate, D-tagatose and D-xylose.
Results and discussion
Comparison of general genome features
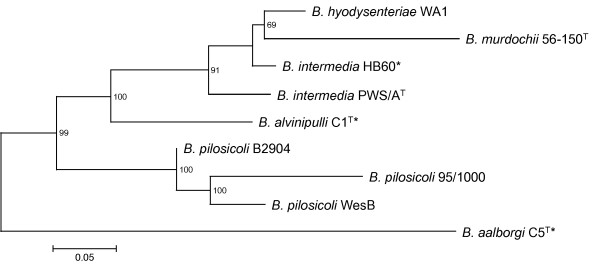
A dendrogram based on the MLST data for nine Brachyspira strains highlighted the close relationship between the three B. pilosicoli strains, with B. aalborgi being distinct, but most closely related to B. pilosicoli and distantly related to B. hyodysenteriae (Figure 1), concordant with previous findings [26,28]. The two B. intermedia strains appeared less closely related than might be expected, supporting reports of extensive diversity in this species based on results of pulse-field gel electrophoresis (PFGE) [41], and a previous MLST study which indicated that these two strains belong to distinct groups [42]. It has been suggested that not all isolates with the B. intermedia phenotype should be assigned to this species [26].
Figure 1.
Dendrogram showing relationships among nine Brachyspira strains, representing six of the seven known species. Analysis was based on concatenated DNA sequences of seven MLST loci [37]. The genome sequences of the strains used in the analysis have either been completed or are currently within a genome sequencing project (*). The tree was constructed using the maximum likelihood method. Bootstrap values (%) are shown for stable nodes. The length of the scale bar is equivalent.
The general genome features of the three sequenced B. pilosicoli genomes are compared in Table 1. The G + C content of the B. pilosicoli genomes were very similar to each other (27.44% to 27.9%), and to that of other chromosomes in the genus, which range from 27.1% to 27.9% [26]. The complete genome sequence of B. pilosicoli B2904 consisted of a 2,765,477 bp circular chromosome (Figure 2), whereas the incomplete WesB genome was larger, at 2,889,522 bp. The 2,586,443 bp genome of strain 95/1000 was the smallest of the three genomes. Not only did the B. pilosicoli genomes show size variability, but also they were smaller than the genomes of the other sequenced species, apart from B. aalborgi 513T which our preliminary studies suggest is ~2.5 Mb (unpublished data). The relatively small size of the B. pilosicoli genomes is most likely due to them being members of a more specialised species that has undergone a high degree of reductive genome evolution. If this is the case, then B. pilosicoli is likely to be an older pathogen than other Brachyspira species such as B. hyodysenteriae[26]. Such a reductive genome evolution may have allowed improved energy efficiency, and enhanced pathogenic potential. Reductive genome evolution is particularly evident in obligate, intracellular bacterial pathogens [43] and consistent with this, of the Brachyspira species, only B. pilosicoli and B. aalborgi show long-term intimate associations with the surface of enterocytes, into which they interdigitate one of their cell ends. In addition to their small genomes, the sequenced B. pilosicoli strains lacked plasmids, whereas the genomes of the other fully sequenced Brachyspira species have included plasmids [26].
Table 1.
General genome feature comparison for B. pilosicoli strains of different host origin
| Genome features | 95/1000 | B2904 | WesBa |
|---|---|---|---|
| Genome size (bp) |
2586443 |
2765477 |
2889522 |
| G+C content |
27.90% |
27.79% |
27.45% |
| Total predicted ORFs |
2339 |
2696 |
2690 |
| Non-significant PID and coverage ORFs |
3 |
23 |
101 |
| Significant PID and/or coverage ORFs |
2336 |
2673 |
2589 |
| rRNA genes |
3 |
3 |
3 |
| tRNA genes |
34 |
34 |
34 |
| tmRNA genes |
1 |
1 |
1 |
| hypothetical/conserved hypothetical proteins |
657 |
590 |
545 |
| genes with function prediction |
1641 |
2045 |
2006 |
| Genes assigned to COGb |
1201 |
1196 |
1276 |
| Genes assigned a KO numberbc |
1048 |
1082 |
1128 |
| Genes assigned E.C. numbersb |
523 |
567 |
563 |
| Genes with signal peptide |
244 |
322 |
316 |
| Genes with transmembrane helices |
48 |
61 |
68 |
| Mobile genetic elements (MGE) |
4 |
61 |
31 |
| insertion sequence elements (ISE) |
0 |
15 |
17 |
| integrases |
0 |
43 |
10 |
| transposases |
2 |
1 |
2 |
| recombinases |
2 |
2 |
2 |
| Suspected truncated proteins |
55 |
130 |
64 |
| Suspected protein frameshift/deletions | 4 | 223 | 50 |
The comparison includes strains 95/1000 (porcine), B2904 (avian) and WesB (human).
a The incomplete WesB strain genome was within one scaffold.
b Those genes with significant PID and/or query/target coverage hits; significance equals blastx/blastp PID of at least 25% and/or 75% query or target coverage.
c Assigned to KO via KEGG Automatic Annotation Server (KAAS)
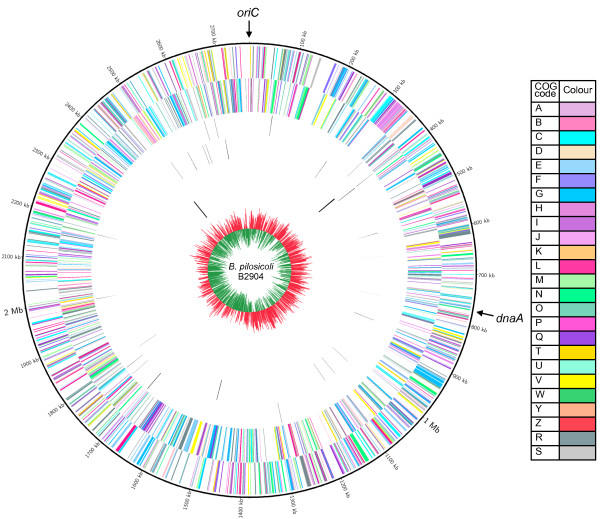
Figure 2.
Circos circular representation of the complete B. pilosicoli B2904 genome with annotated genes. The genome is orientated from the oriC and also displays the location of dnaA. Circles range from 1 (outer circle) to 7 (inner circle). Circle 1, COG-coded forward strand genes; circle 2, COG-coded reverse strand genes; circle 3, forward strand tRNA; circle 4, reverse strand tRNA; circle 5, forward strand rRNA; circle 6, reverse strand rRNA; circle 7, GC skew ((G-C)/(G + C); red indicates values >0; green indicates values <0). All genes are colour-coded according to Cluster of Orthologous Group (COG) functions shown in the key table; A, RNA processing and modification; B, chromatin structure and dynamics; C, energy production and conversion; D, cell cycle control, cell division and chromosome partitioning; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G, carbohydrate transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; J, translation, ribosomal structure and biogenesis; K, transcription; L, replication, recombination and repair; M, cell wall, membrane and envelope biogenesis; N, cell motility and secretion; O, posttranslational modification, protein turnover and chaperones; P, inorganic ion transport and metabolism; Q, secondary metabolite biosynthesis, transport and catabolism; T, signal transduction mechanisms; U, intracellular trafficking, secretion and vesicular transport; V, defence mechanisms; W, extracellular structures; Y, nuclear structure wheat for cell division and chromosome partitioning; Z, cytoskeleton; R, general function prediction only; S, function unknown.
The disparity between the number of open reading frames (ORF) and genome size between the B2904 and WesB strains and the high number of non-significant percentage identity (PID) and coverage ORFs in the WesB genome may be an artefact of the incomplete nature of this genome, which is the largest of the three strains. In 95/1000, 44.8% of ORFs were assigned a KEGG Orthology (KO), whereas only 40.5% and 43.6% of ORFs were assigned in B2904 and WesB, respectively. A lower proportion of ORFs were matched in COG database for B2904 and WesB compared to 95/1000.
All three B. pilosicoli strains harboured the same number of transfer RNA (tRNA), rRNA and transfer-messenger (tmRNA) genes (Table 1). The tRNA genes represented all 20 amino acids and there were single copies of the 5S, 16S and 23S rRNA genes. The rrf (5S) and rrl (23S) genes were co-located in all three B. pilosicoli genomes, with the rrs (16S) gene located approximately 645 Kb, 679 Kb and 773 Kb from the other rRNA genes in the 95/1000, B2904 and WesB genomes, respectively. This rRNA gene organisation has been considered a distinguishing feature of Brachyspira species [44], since other spirochaetes typically have differing copy numbers and organisations [45,46]; however, similar arrangements to Brachyspira have been detected in the spirochaete Borrelia burgdorferi[47]. Situated between the rrs gene and rrfrrl cluster, which are either side of the oriC, was the tmRNA (ssrA, 10Sa RNA) gene and nine of the total 34 tRNAs that were otherwise dispersed throughout the genome (Figure 2).
The origin of replication of the B. pilosicoli genomes was set according to the position of the oriC and GC-skew pattern, as previously suggested [26]; this was supported by the Ori-Finder program [48]. The origin of replication was originally considered to be adjacent to the dnaA gene [25,28], however there was no association between the oriC and dnaA genes in the B. pilosicoli B2904 genome (Figure 2), as found in other Brachyspira genomes [26]. The arrangement of genes surrounding the dnaA gene was consistent between the B. pilosicoli strains, as with the other sequenced Brachyspira genomes [28]. The genes at the oriC, although consistent between the B. pilosicoli strains, appear to vary extensively between the species.
B. pilosicoli genome architecture
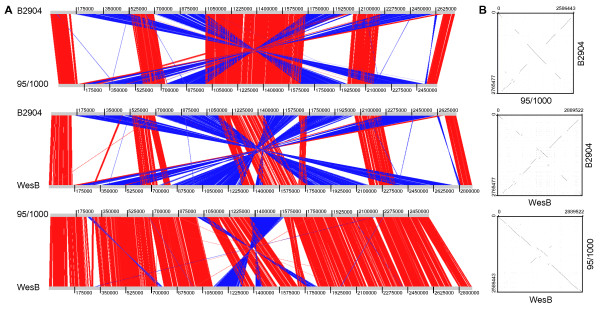
On comparing B2904 with 95/1000, four major genome rearrangement events appeared to have occurred, whereas two rearrangements were evident when comparing WesB to 95/1000 (Figure 3A). Mobile genetic elements (MGE) were found adjacent to or within close proximity of the sites where recombination events appear to have occurred in the B2904 and WesB genomes. Sixty-one and 31 MGEs, including insertion sequence elements (ISE), integrases, recombinases and transposases were identified in the B2904 and WesB genomes, respectively, compared to just four in the 95/1000 genome (Table 1). The proportion of these features therefore seems to correlate with the extent of rearrangement within the genome. Furthermore, multiple copies of an integrase gene that was absent from the 95/1000 genome were identified in the genomes of B2904 (n = 43) and WesB (n = 7) (Additional file 1). The lower number of copies in WesB may be an artefact of the genes not assembling in the incomplete genome. MGEs have been implicated in chromosomal rearrangements, gene disruptions resulting in pseudogenes, and eventual loss of genes, which may contribute to reductive genome evolution [49]. Species and strains that are undergoing or have recently undergone reductive genome evolution, and hence become more specialised pathogens, typically harbour large numbers of MGEs [50-52].
Figure 3.
Pairwise genome alignments and dot matrix plots comparing the genomes of B. pilosicoli strains 95/1000, B2904 and WesB. The Artemis Comparison Tool (ACT) was used to compare the three genome sequences against each other (A). Genome sequences were aligned from the predicted oriC and visualised in ACT with a cut-off set to blast scores >500. Red and blue bars indicate regions of similarity in the same orientation (red) and inverted (blue). Dot matrix plots of the genome sequences linearised at the oriC were generated using Freckle (B). The incomplete WesB strain genome was within one scaffold. The output displays a two-dimensional plot, whereby the dots represent matched regions between the genomes. The minimum size of matched sequences was set to 20 bp.
There were fewest suspected pseudogenes (gene truncation or frameshift) found in 95/1000 and most in B2904 (Table 1), a finding that correlates to the number of MGEs and degree of genome rearrangements in these strains. Of the total number of pseudogenes in each strain, 91.5%, 84.5% and 81.3% were in a cluster with orthologs in the other two strains in 95/1000, B2904 and WesB, respectively. Most strikingly, all shared clusters included either multiple B2904 and/or WesB pseudogenes with a complete 95/1000 gene.
Differences in the number of MGEs in the three B. pilosicoli genomes may relate to their different stages of reductive genome evolution. Strain 95/1000, which had the smallest genome, also had the fewest MGEs and this could be interpreted as indicating that the MGEs that induced the genome reduction in this strain have become lost. Alternatively, MGE expansion may not have occurred in 95/1000 to the same degree as in B2904 and WesB, as MGEs are generally lost in a fragmentary manner by pseudogenisation. Thus, it is unlikely that the 95/1000 genome, which has the fewest pseudogenes has been reduced in this way. On the other hand, the greater number of pseudogenes in the larger B2904 and WesB genomes does suggest that they may be undergoing genome reduction. A possible explanation would be that these strains are in the initial stages of genome reduction, at the point at which MGE expansion occurs [49,52]. Genome reduction and MGE expansion is often associated with niche specialisation or host restriction [53,54], however B. pilosicoli are not considered host-restricted, and WesB, of human origin, has been shown also to infect chickens and pigs [22,55]. B. pilosicoli is a highly recombinant species [56], and despite differences in genome arrangement and the number of pseudogenes, part of the difference in genome sizes simply reflects the carriage of different subsets of the pan-genome.
A dot plot comparison of the three B. pilosicoli genomes revealed that the chromosomal rearrangements were arranged symmetrically around the origin or terminus of replication, highlighted by the X-patterns in the alignments (Figure 3B). It has been postulated that symmetrical rearrangements occur because recombination events are determined by the replication forks that are approximately equal distance from the oriC during bidirectional replication [57]. It has also been argued that non-symmetrical rearrangements can be disadvantageous, and so genome rearrangements such as those found in the B. pilosicoli strains are a product of selection [58].
Despite the significant chromosomal rearrangements, genome alignments showed that the majority of genome sequence was shared between the three strains, with the larger B2904 and WesB genomes possessing the greatest proportion of unique sequences (Figure 3). Furthermore, a 26 Kb region, likely to have involvement in horizontal gene transfer (HGT), and that is partially conserved in all previously reported Brachyspira genomes as well as Enterococcus faecalis and Escherichia coli[59], was identified in the B. pilosicoli B2904 (B2904_orf2096 – B2904_orf2111) and WesB (wesB2037 – wesB2051) genomes.
Functional genome comparisons
Functional classifications assigned to each of the protein-coding genes of the three B. pilosicoli strains using the COG database showed that the general distribution of features into categories was similar for the three strains (Table 2), and this highlighted their close relationship. Despite having the smallest genome, B. pilosicoli 95/1000 possessed the greatest number of features in six categories. B2904 contained the most features in eight categories, and WesB in one category. A striking difference between the strains was in the carbohydrate (G), amino acid (E) and nucleotide (F) transport and metabolism categories, with the larger WesB genome containing considerably more features than the B2904 and 95/1000 genomes. In addition, compared to other Brachyspira species the B. pilosicoli strains had a reduced number of features associated with inorganic ion transport and metabolism (P) [26,28].
Table 2.
Distribution of Cluster of Orthologous Genes (COG) categories in B. pilosicoli strains 95/1000, B2904 and WesBa
| Function (COG category) | 95/1000 | % | B2904 | % | WesBc | % |
|---|---|---|---|---|---|---|
|
Cellular Processes | ||||||
| Translation, ribosomal structure and biogenesis (J) |
122 |
5.22 |
119 |
4.45 |
125 |
4.83 |
| Transcription (K) |
51 |
2.18 |
49 |
1.83 |
61 |
2.36 |
| Replication, recombination and repair (L) |
51 |
2.18 |
56 |
2.10 |
61 |
2.36 |
|
Cellular Processes and Signalling | ||||||
| Cell cycle control, cell division and chromosome partitioning (D) |
10 |
0.43 |
8 |
0.30 |
9 |
0.35 |
| Defence mechanisms (V) |
35 |
1.50 |
33 |
1.23 |
35 |
1.35 |
| Signal transduction mechanisms (T) |
16 |
0.68 |
15 |
0.56 |
15 |
0.58 |
| Cell wall, membrane and envelope biogenesis (M) |
74 |
3.17 |
72 |
2.69 |
79 |
3.05 |
| Cell motility (N) |
40 |
1.71 |
39 |
1.46 |
40 |
1.54 |
| Intracellular trafficking, secretion and vesicular transport (U) |
11 |
0.47 |
7 |
0.26 |
9 |
0.35 |
| Posttranslational modification, protein turnover and chaperones (O) |
40 |
1.71 |
36 |
1.35 |
39 |
1.51 |
|
Metabolism | ||||||
| Energy production and conservation (C) |
89 |
3.81 |
84 |
3.14 |
84 |
3.24 |
| Carbohydrate transport and metabolism (G) |
101 |
4.32 |
123 |
4.60 |
139 |
5.37 |
| Amino acid transport and metabolism (E) |
141 |
6.03 |
138 |
5.16 |
149 |
5.76 |
| Nucleotide transport and metabolism (F) |
49 |
2.10 |
54 |
2.02 |
57 |
2.20 |
| Coenzyme transport and metabolism (H) |
47 |
2.01 |
44 |
1.65 |
48 |
1.85 |
| Lipid transport and metabolism (I) |
41 |
1.75 |
33 |
1.23 |
34 |
1.31 |
| Inorganic ion transport and metabolism (P) |
53 |
2.27 |
53 |
1.98 |
49 |
1.89 |
| Secondary metabolites biosynthesis, transport and catabolism (Q) |
9 |
0.38 |
10 |
0.37 |
9 |
0.35 |
|
Poorly characterised | ||||||
| General function prediction only (R) |
149 |
6.37 |
147 |
5.50 |
157 |
6.06 |
| Function unknown (S) |
72 |
3.08 |
76 |
2.84 |
77 |
2.97 |
|
Unassigned | ||||||
| Not in COG (X) |
1137 |
48.63 |
1477 |
55.26 |
1313 |
50.71 |
| TOTAL | 2338 | 100 | 2673 | 100 | 2589 | 100 |
aThe number and percentage of the total genes within each of the genomes, assigned to each functional group are shownb.
bThose genes with significant PID and/or query/target coverage hits; significance equals blastx/blastp PID of at least 25% and/or 75% query or target coverage.
c The incomplete WesB strain genome was within one scaffold.
Global genome feature comparisons
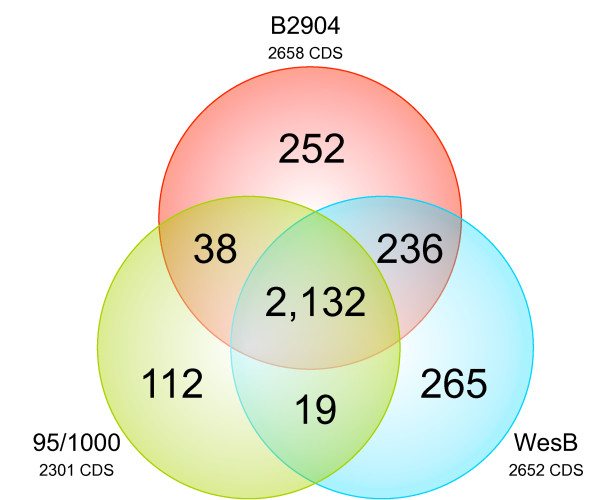
The three B. pilosicoli strains contained 2,132 conserved genes, and these contribute to defining the B. pilosicoli pan-genome (Figure 4); this related to 92.6%, 80.2% and 80.4% of the total genes of the 95/1000, B2904 and WesB genomes, respectively. As expected, there was a greater number of core genes between the strains of B. pilosicoli than between strains of different species; substantially fewer core genes (1,087) were identified for B. hyodysenteriae WA1, B. pilosicoli 95/1000 and B. murdochii 56-150T[28]. B. pilosicoli WesB harboured the greatest number of unique genes, with 10.0% of its genes being absent from the other genomes; B2904 had a similar proportion (9.5%), whereas 95/1000 had considerably fewer (4.9%). B. pilosicoli B2904 and WesB shared the greatest proportion of genes (~8.9%) while B2904 shared a greater percentage of its genes with 95/1000 (1.4%) than with WesB (0.7%).
Figure 4.
Venn diagram of genes unique to and shared between B. pilosicoli strains 95/1000, B2904 and WesB. The Venn diagram was resolved via BLASTlineMCL protein clustering. Each circle represents the total number of protein-coding genes in the genome, whereby overlapping regions indicate the number of genes shared between the respective genomes.
Global genome feature comparisons against other Brachyspira species
Complete genome sequences of B. hyodysenteriae WA1, B. intermedia PWS/AT, B. pilosicoli 95/1000 and B. murdochii 56-150T have previously undergone comparative analysis [26,28]. Genome sequences of B. pilosicoli B2904 and WesB can now be added to these comparisons, giving the first opportunity for a Brachyspira intra-species genome comparison. A protein blastmatrix comparison was performed on the four previously sequenced genomes, the two newly-sequenced B. pilosicoli genomes and the draft genome scaffolds of B. aalborgi 513T, B. alvinipulli C1T and B. intermedia HB60 (unpublished) (Table 3). Not unexpectedly, the analysis revealed that the B. pilosicoli strains shared the greatest proportion of proteins (54.9-68.4%). Of B. pilosicoli strains, B2904 had the greatest proportion of protein repeats relating to paralogs (2.7%), despite not having the largest genome. Overall, the non-pathogenic B. murdochii had the greatest proportion of protein repeats (5.3%), perhaps relating to its large genome. High proportions of shared proteins highlighted the close relationships of B. hyodysenteriae with B. intermedia (>46.7%) and B. murdochii (33.7%) (Figure 1). B. aalborgi shared the lowest percentage of proteins with other Brachyspira species, consistent with evidence that this is the most divergent species (Figure 1).
Table 3.
Protein blastmatrix analysis of nine Brachyspira genomes
|
B. aalborgi |
B. alvinipulli |
B. hyodysenteriae |
B. intermedia |
B. intermedia |
B. murdochii |
B. pilosicoli |
B. pilosicoli |
B. pilosicoli |
|
|---|---|---|---|---|---|---|---|---|---|
|
513Ta |
C1Ta |
WA1 |
PWS/AT |
HB60a |
56-150T |
B2904 |
95/1000 |
WesBb |
|
| 2257 CDS | 3228 CDS | 2613 CDS | 2890 CDS | 3392 CDS | 2809 CDS | 2658 CDS | 2301 CDS | 2652 CDS | |
|
WesBb |
21.36% |
21.65% |
22.73% |
24.95% |
19.84% |
25.95% |
68.43% |
65.32% |
1.73% |
|
95/1000 |
17.94% |
18.56% |
20.78% |
20.90% |
16.77% |
22.11% |
54.93% |
0.74% |
|
|
B2904 |
20.47% |
21.25% |
21.74% |
23.60% |
19.25% |
25.35% |
2.71% |
|
|
|
56-150T |
22.37% |
31.51% |
33.68% |
36.40% |
29.33% |
5.30% |
|
|
|
|
HB60a |
20.65% |
31.23% |
46.77% |
57.65% |
1.77% |
|
|
|
|
|
PWS/AT |
21.05% |
32.03% |
50.33% |
1.56% |
|
|
|
|
|
|
WA1 |
17.72% |
27.48% |
1.11% |
|
|
|
|
|
|
|
C1Ta |
23.48% |
2.54% |
|
|
|
|
|
|
|
| 513Ta | 1.11% |
The percentage of the total CDS that were identified in other genomes (green) and the proportion of protein repeats within the genome (red), is shown. A cut-off e-value of 1e-05 was used.
a Incomplete genome currently within a genome sequencing project.
b The incomplete WesB strain genome was within one scaffold.
A protein Markov clustering analysis of the six published Brachyspira genomes, identified 1,647 protein clusters shared by all six strains (Additional file 2), the encoding genes of which may be used to define a Brachyspira species pan-genome. This analysis revealed B. intermedia PWS/AT harboured the greatest number of clusters not found in the other sequenced Brachyspira genomes (n = 277) and it has the largest genome. The greatest number of clusters shared only between two strains was with B. intermedia PWS/AT and B. hyodysenteriae WA1 (n = 61), consistent with the close relationship of these species (Figure 1). Of the B. pilosicoli strains, B2904 and WesB shared the most unique protein clusters (n = 47), and WesB also shared the greatest number of clusters with a non-B. pilosicoli strain, having 36 clusters in common with B. intermedia and 16 with B. murdochii. The B. pilosicoli strains collectively shared the most clusters with B. murdochii 56-150T (n = 58), and fewest with B. hyodysenteriae WA1 (n = 4), as noted previously [26]. Non-B. pilosicoli strains shared 173 clusters, whereas the B. pilosicoli strains shared 110 clusters, reflecting gene loss and genome reduction.
Unique to the three strains of B. pilosicoli
Of 110 protein clusters present only in the B. pilosicoli genomes (Additional file 2), 54.6% were hypothetical or unclassified. The majority of protein clusters were metabolic features, including an α-galactosidase (BP951000_0276; B2904_orf1586; wesB_1069), the activity of which is a distinguishing feature of the species [60,61]. Although it was suggested that B. pilosicoli had lost many transport-related genes during reductive evolution [26], 13 clusters were for transport proteins. Sialidase family-like protein genes unique to B. pilosicoli 95/1000 (BP951000_2021, BP951000_2022 and BP951000_2023) [26,28] were also present in B2904 (B2904_orf1812, B2904_orf1813 and B2904_orf1814) and WesB (wesB_0922, wesB_0923 and wesB_0924); the products of such genes are produced by a variety of mucosal pathogens may play a role in colonisation or inducing tissue damage [62-64]. Clusters for an α-1,2-fucosyl transferase (BP951000_1232; B2904_orf14; wesB_0014), two membrane proteins (BP951000_1751; B2904_orf2268; wesB_0587) (BP951000_1752; B2904_orf2267; wesB_0586) and two glycosyltransferases (BP951000_0003; B2904_orf1276; wesB_1428) (BP951000_2338; B2904_orf1277 and B2904_orf1282; wesB_1429) were unique to B. pilosicoli and may contribute to host cell adherence. Other B. pilosicoli-specific clusters were for an ankyrin repeat protein (BP951000_0080; B2904_orf1369; wesB_1511), a β-lactamase (BP95100_1338; B2904_orf2576; wesB_0148), two peptidases (BP951000_1129; B2904_orf205; wesB_2479) (BP951000_1260; B2904_orf40; wesB_0047) and phage proteins (BP951000_1211; B2904_orf2686; wesB_2642) (BP951000_1258; B2904_orf39; wesB_0046).
Unique and shared by two strains of B. pilosicoli
Of the B. pilosicoli strains, B2904 and WesB shared most unique clusters (Additional file 2). Fewer clusters were shared with 95/1000, but of twelve clusters unique to 95/1000 and B2904, all but N-acetyl mannosamine-6-phosphate 2-epimerase (BP951000_2135; B2904_orf1689) were hypothetical. Six clusters were unique to 95/1000 and WesB, all lacking a specified function. Of 47 clusters unique to B2904 and WesB, 51.1% were hypothetical; notable clusters shared between these strains were for a further sialidase-like protein (B2904_orf1811; wesB_0925) and a peptidase (B2904_orf863; wesB_1557). The glycine reductase complex locus of 95/1000 (BP951000_1852 – BP951000_1860) and B. murdochii 56-150T (Bmur_2720 – Bmur_2728) [28] was identified in B2904 (B2904_orf665 – B2904_orf673) and WesB (wesB_0746 – wesB_0754), but with an additional ATP-binding cassette (ABC)-type glycine betaine transport component in a separate locus (B2904_orf1065; wesB_1632). Moreover, a cluster for a transposase unique to B2904 (n = 47) and WesB (n = 7) was detected. Genes that were shared only by the larger B2904 and WesB genomes and were absent from 95/1000, without apparent detriment, presumably have some specialised function that is not essential for survival. These features may have been lost from 95/1000, as they are not essential, or acquired in B2904 and WesB, perhaps by HGT.
Unique to one strain of B. pilosicoli
B. pilosicoli 95/1000 harboured the fewest and WesB the most unique features (Additional file 2), correlating with their genome size. As discussed above, the 95/1000 strain may have become more specialised, having lost non-essential features through reductive evolution [43]; alternatively, the absence of orthologs in other strains or species may suggest that these features have been acquired via HGT. Of the strain-unique clusters, 77.7%, 65.9% and 68.1% were for hypothetical proteins in 95/1000, B2904 and WesB, respectively. In 95/1000, unique clusters included a sodium/pantothenate symporter and an outer membrane lipoprotein (BP951000_0731) with a potential role in host cell adherence (BP951000_0634). In B2904, unique clusters included putative phage proteins (B2904_orf136, B2904_orf143 and B2904_orf816), additional glycine reductase complex proteins (B2904_orf2051 and B2904_orf2052) and proteins involved in ascorbate metabolism (B2904_orf1019, B2904_orf1020 and B2904_orf1024) and mannitol metabolism (B2904_orf2446 and B2904_orf2447). In WesB, unique features included mannose/sorbose-specific phosphotransferase system (PTS) components (wesB_1270, wesB_1271 and wesB_1272), fructose-specific PTS components (wesB_2317 and wesB_2318) and a D-allose kinase (wesB_1174). Six unique phage-related features and an integrase were identified at two loci in the WesB genome (wesB_0297, wesB_0298, wesB_2528, wesB_2540, wesB_2545, wesB_2550 and wesB_2567). Interestingly, each of the strains harboured unique genes for ankyrin proteins (BP951000_0037; B2904_orf892 and B2904_orf1944; wesB_0903).
Comparison of potential virulence features
Virulence factor screening in Brachyspira genomes was performed as described previously [25,28], but with the analysis encompassing a greater array of genes, particularly in categories relating to adhesion and/or surface proteins and MGEs (Table 4). The greatest number of potential virulence features was in B2904, however additional features may be identified in the WesB genome once it is completed.
Table 4.
The number of genes with potential roles in pathogenesis and virulence in the three B. pilosicoli genomes
| Role of putative gene | 95/1000 | B2904 | WesBa |
|---|---|---|---|
| Core genes involved in lipopolysaccharide biosynthesisb |
27 |
30 |
32 |
| Chemotaxis | |||
| putative methyl-accepting chemotaxis protein |
7 |
7 |
10 |
| methyl-accepting chemotaxis protein A (mcpA) |
2 |
0 |
2 |
| methyl-accepting chemotaxis protein B (mcpB) |
8 |
11 |
11 |
| chemotaxis protein |
15 |
15 |
15 |
| Flagella |
42 |
42 |
42 |
| Adhesion and membrane protein | |||
| lipoprotein |
21 |
31 |
29 |
| variable surface protein |
3 |
4 |
4 |
| integral membrane protein |
1 |
1 |
1 |
| outer membrane protein |
25 |
25 |
23 |
| periplasmic protein |
25 |
25 |
28 |
| inner membrane protein |
75 |
83 |
83 |
| Host tissue degradation | |||
| haemolysis |
12 |
12 |
12 |
| phospholipase |
2 |
3 |
2 |
| peptidase |
44 |
48 |
48 |
| protease |
19 |
19 |
17 |
| Oxidative stress |
7 |
7 |
7 |
| Ankyrin-like protein |
31 |
34 |
35 |
| Phage and other mobile genetic elements |
46 |
109 |
100 |
| Total | 412 | 506 | 501 |
The analysis categorised the genes from the genomes of B. pilosicoli 95/1000, B2904 and WesB.
a The incomplete WesB strain genome was within one scaffold.
b Core lipooligosaccharide (LOS) biosynthesis genes.
Lipooligosaccharides
An rfbBADC cluster, encoding proteins for nucleotide sugar biosynthesis and with a suggested role in O-antigen assimilation in bacteria such as Salmonella[65,66], was identified on the B. hyodysenteriae WA1 plasmid [25]. Although lacking this cluster, the three B. pilosicoli strains possessed rfbA (BP951000_1687; B2904_orf2229; wesB_0523) and rfbB (BP951000_1148; B2904_orf2569; wesB_2572), but rfbC was noted only in B2904 (n = 1) and WesB (n = 2) (B2904_orf117; wesB_0130 and wesB_0131). Genes inferred to be involved in the biosynthesis of 3,5-dideoxyhexose, an O-antigen component of lipopolysaccharide (LPS) [67], were found adjacent to the rfbC gene(s) in B2904 and WesB; both strains contained rfbF (B2904_orf115; wesB_0127) and rfbG (B2904_orf116; wesB_0128), but rfbH was present only in WesB (wesB_0129). The absence of such genes in the pathogenic strain 95/1000 suggests that they have a limited impact on virulence.
Chemotaxis and motility
As with 95/1000, the two other B. pilosicoli strains possessed fewer chemotaxis genes than B. hyodysenteriae and B. murdochii (Table 4) [28]. No mcpC genes were found in the three B. pilosicoli strains, despite their detection in the genomes of the other fully sequenced Brachyspira species. The inter-species differences in the number and complement of chemotaxis-related genes may account for differences in their attraction to mucins and affinity to local host niches [19]. No mcpA genes were identified in B2904, but two copies were found in the other B. pilosicoli strains. The same complement of chemosensory transducer genes was identified in all three strains, as was the previously described cluster of seven such genes [28]. Differences in the number of chemotaxis-related genes between the three strains may translate from differences in genome size. This may denote a redundancy of features that can be lost without apparent detriment to long-term survival. The same flagella genes were shared by all three B. pilosicoli strains.
Adhesion and membrane proteins
End-on attachment of the spirochaete to the luminal surface of the lower intestinal tract epithelia is characteristic of B. pilosicoli and B. aalborgi colonisation [2,68], and hence surface-associated proteins or lipoproteins are potential candidates for virulence. All lipoprotein genes in 95/1000 were found in B2904 and WesB, but these strains also had a predicted secreted lipoprotein (B2904_orf1676; wesB_1576) and a lipoprotein carrier protein, LolA (B2904_orf608; wesB_0637), which anchors lipoproteins to the outer membrane [69]. The same complement of genes encoding variable surface proteins found in 95/1000 [28] and the putative integral membrane virulence factor, MviN (B2904_orf469; wesB_2218) were noted in B2904 and WesB. Genes for outer membrane proteins with a potential role in virulence were identified, including BspA antigens, which may bind fibronectin and initiate a serological response [70], OmpA proteins, similar to proteins implicated in Leptospira virulence [71], and Tia invasion determinants. Genes encoding TolC were identified in all three B. pilosicoli strains, and this protein has been implicated in host invasion, virulence gene expression, and as an outer membrane component of efflux pumps [72-74]. The periplasmic proteins identified were predicted to be primarily associated with other membrane proteins, and constitute ABC transporters with putative roles in virulence [75]. Gene duplications were largely responsible for the greater number of inner membrane virulence factors in B2904 and WesB, but since they were absent for 95/1000 they were unlikely to have significant impact on virulence. WesB harboured two additional genes encoding OppA, which has suggested involvement in spirochaete-host interactions in Treponema denticola[76]. Genes encoding P-type ATPase components, such as cadA and zntA, were noted in the three strains and these have been implicated in the ability of pathogens to sense and adapt to intracellular environments through heavy metal ion regulation [77,78], in addition to Trk potassium transport components, required for invasion and intracellular growth of Salmonella[79]. Genes encoding outer, periplasmic and inner membrane proteins that constitute transport systems implicated in bacterial virulence mechanisms were detected, such as polyamine ABC-type transport, which is important for Streptococcus pneumoniae pathogenesis [80], TonB-dependant iron transport, which is related to Shigella dysenteriae virulence [81], and PTS systems implicated in the virulence of Mycobacterium tuberculosis and E. coli[82,83]. Genes were found encoding components of the AcrAB-TolC complex, which confers antibiotic resistance and survival in the GI tract [84], a ferrous iron transporter, feoB, for iron acquisition, gut colonisation and intracellular survival of multiple enteropathogens [85,86], and a glutamine transporter gene, glnQ, which has been implicated in Streptococcus adherence and virulence [87]. In the B. pilosicoli strains, an mgl operon similar to one with a proposed role in virulence expression in Treponema pallidum[88] was noted. Multidrug efflux features were found in all three strains, which aside from drug resistance, are attributed with a range of roles in pathogenesis [89]. Genes for the Sec pathway described in 95/1000 [28], with no needle-associated genes were also noted in B2904 and WesB, with an additional secA-like gene in WesB (wesB_0869).
Host tissue degradation
The complement of haemolysis-related genes was identical between the three strains. Compared to previous analysis, other genes were detected including a haemolysin, previously undetected in 95/1000 (BP951000_1925) and three streptolysin genes, sagB (BP951000_0919; B2904_orf445; wesB_2241), sagC (BP951000_0918; B2904_orf446; wesB_2240) and sagD (BP951000_0917; B2904_orf447; wesB_2239), involved in β-haemolysis and virulence in streptococci [90,91]. A putative phospholipase/carboxylesterase (B2904_orf1218) was found only in B2904. The three strains contained similar numbers of peptidases and proteases, which may participate in local degradation of host tissues, however 95/1000 lacked peptidase E, which had no effect on protein degradation in Salmonella Typhimurium [92], and hence this non-essential enzyme may have been lost through reductive evolution.
Oxidative stress
Genes related to oxidative stress were shared by the three strains. A partial BatI (Bacteroides aerotolerance) operon [93] was noted in all strains, in close proximity to one of the nox genes and consisted of batB (BP951000_0196; B2904_orf1493; wesB_1155), batC (BP951000_0195; B2904_orf1492; wesB_1156), batD (BP951000_0194; B2904_orf1491; wesB_1157) and batE (BP951000_0193; B2904_orf1490; wesB_1158). The batA gene was in a distinct locus in the three strains (BP951000_1387; B2904_orf2546; wesB_0200).
Ankyrin-like protein
There was little difference in the number of genes encoding ankyrin-like proteins between the B. pilosicoli strains, which may be involved in host cell interactions through their ability to bind host chromatin as in Orientia[94]. B. pilosicoli had consistently fewer of these genes than B. hyodysenteriae[28].
Phage and other mobile genetic elements
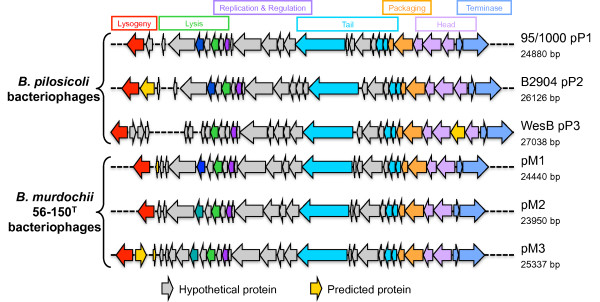
Outside of bacteriophage regions, four, 61 and 31 MGEs were identified in 95/1000, B2904 and WesB, respectively, correlating with the extent of genomic rearrangements. The types and copy number of all MGEs in the B. pilosicoli genomes are detailed in Additional file 1. The region encoding genes related to the VSH-1 prophage-like gene transfer agent (GTA) in 95/1000 [28], was identified in B2904 (B2904_orf2669 – B2904_orf2692) and WesB (wesB_2625 – wesB_2648). This region was ~15 Kb in 95/1000 compared to ~21 Kb in B2904 and WesB due to an insertion between genes encoding OrfE and Hvp53, containing genes for a monosaccharide-transporting ATPase (B2904_orf2671; wesB_2628), an ABC transporter-related protein (B2904_orf2672; wesB_2629), a ROK family protein (B2904_orf2674; wesB_2631), an integrase in B2904 only (B2904_orf2675), and a periplasmic binding protein/LacI transcriptional regulator (B2904_orf2673; wesB_2627 and wesB_2630). Generally, these features had high homology with those in Clostridium carboxidivorans (e-value < 1e-74), consistent with the finding that Brachyspira share a high degree of gene similarity with Clostridium[25], and supporting the notion that the bacteriophages exchange genetic material between species [26]. In WesB, an additional cluster of VSH-1-associated genes, flanked by a phage terminase, was detected (wesB_2527 – wesB_2553); the different genes in this region shared highest homology with C. carboxidivorans, B. hyodysenteriae, B. intermedia, B. pilosicoli and B. murdochii, suggesting that the GTA had involvement in intra- and inter-species gene transfer. The bacteriophage that was identified in B. pilosicoli 95/1000 (pP1), and in B. murdochii 56-150T (pM1, pM2 and pM3) [26,28], was also found in B2904 (pP2; B2904_orf1942 – B2904_orf1970) and WesB (pP3; wesB_0739 – wesB_0708) (Figure 5). In B. pilosicoli, the bacteriophage size was proportional to genome size. Hypothetical proteins encoded in this region were shared between 95/1000 and B2904, however WesB contained four unique hypothetical genes. The B2904 pP2 bacteriophage possessed a unique ankyrin repeat protein (B2904_orf1943). An adenine-specific DNA methyltransferase gene was present only in the WesB pP3 bacteriophage (wesB_0711), adjacent to the DNA methylase gene found in B. pilosicoli bacteriophages (BP951000_1480; B2904_orf1968; wesB_0710), but absent from those of B. murdochii 56-150T. Two separate novel bacteriophages regions were found in B2904 (pP4) and WesB (pP5). The ~29 Kb pP4 bacteriophage contained seven phage proteins (B2904_orf133 – B2904_orf180), six predicted proteins with homology to sequences of other Brachyspira species, and 35 unique hypothetical genes. The ~28 Kb pP5 bacteriophage (wesB_0301 – wesB_0341) shared all the components of the pI1 bacteriophage of B. intermedia PWS/AT, suggesting transfer of the bacteriophage in an inter-species HGT event. Interestingly, pP5 was flanked by VSH-1 components (wesB_0297, wesB_0298 and wesB_0343), and hence the VSH-1 GTA may be responsible for mediating the HGT event. Two nuclease genes (wesB_0306 and wesB_0308) and a number of unique hypothetical genes in pP5 were not identified in pI1. Clustered regularly interspaced short palindromic repeats (CRISPR), which provide bacteria with acquired resistance to bacteriophages [95], were only identified in the non-pathogenic B. murdochii 56-150T, which suggests a role for bacteriophages in Brachyspira pathogenicity. B. pilosicoli B2904 and B. intermedia PWS/AT did however possess a bacteriophage resistance protein (B2904_orf2624; Bint_2390) which has been implicated in protecting against bacteriophages [96].
Figure 5.
Comparison of the organisation of the bacteriophages in the three B. pilosicoli genomes. A comparison on bacteriophages pP1 in 95/1000, pP2 in B2904 and pP3 in WesB and also the three bacteriophages found in B. murdochii 56-150T; pM1, pM2 and pM3. Genes encoding hypothetical proteins (grey) and genes with predicted protein function (yellow) are indicated.
Central metabolism and correlation with phenotype
Analysis of the genomes of B. hyodysenteriae and B. pilosicoli has revealed that these species share many metabolic capabilities [25,28]. In the current study the analysis of central metabolic pathway detection in Brachyspira genomes was extended by application of Biolog PM™ technology for phenotypic determination of carbon source utilisation of the B. pilosicoli strains. The utilisation of 178 unique carbon compounds (Additional file 3) by the strains was screened, and their metabolic capabilities were found to be highly conserved. Differences were found in the utilisation of just seven carbon sources, which were correlated with genotypic variations (Table 5).
Table 5.
Correlation between differences in carbon source utilisation and genotype of B. pilosicoli 95/1000, B2904 and WesB
| Unique carbon source compound tested | 95/1000 | B2904 | WesB | Possible explanation |
|---|---|---|---|---|
| D-Mannose |
- |
- |
+ |
WesB is the only strain with the mannose/sorbose-specific PTS system IIABCD components (wesB_1269, wesB_1270, wesB_1271 and wesB_1272) for uptake and phosphorylation of D-mannose. |
| D-Glucuronic acid |
- |
+ |
+ |
95/1000 lacks the pfkB carbohydrate kinase, 2-dehydro-3-deoxygluconate kinase, which links D-glucuronic acid metabolism to glycolysis. This enzyme is found in both B2904 (B2904_orf899 and B2904_orf900) and WesB (wesB_1781). |
| D-Mannitol |
- |
+ |
- |
B2904 is the only strain with the D-mannitol PTS system IIABC components (B2904_orf2447) and also a mannitol-1-phosphate 5-dehydrogenase (B2904_orf2446) for D-mannitol, uptake, phosphorylation and catabolism. |
| Glucuronamide |
- |
+ |
+ |
95/1000 lacks the pfkB carbohydrate kinase, 2-dehydro-3-deoxygluconate kinase, which links glucuronate and related compound metabolism to glycolysis. This enzyme is found in both B2904 (B2904_orf899 and B2904_orf899 and B2904_orf900) and WesB (wesB_1781). |
| β-D-Allose |
- |
- |
+ |
WesB is the only strains with D-allose ABC transporter components (wesB_1171, wesB_1172 and wesB_1175) and D-allose kinase (wesB_0259 and wesB_1174) for uptake and metabolism of D-allose. |
| β-Methyl-D-glucuronic acid |
- |
+ |
+ |
95/1000 lacks the pfkB carbohydrate kinase, 2-dehydro-3-deoxygluconate kinase, which links glucuronate and related compound metabolism to glycolysis. This enzyme is found in both B2904 (B2904_orf899 and B2904_orf900) and WesB (wesB_1781). |
| L-Sorbose | - | - | + | WesB is the only strain with the mannose/sorbose-specific PTS system IIABCD (wesB_1269, wesB_1270, wesB_1271, wesB_1272) components for uptake and phosphorylation of L-sorbose. |
Possible explanations for the differences in phenotype relate to differences in genomic features.
Carbohydrate metabolism
High proportions of the B. pilosicoli genomes were associated with carbohydrate transport and metabolism (Table 2), and from metabolic pathway reconstructions it is evident that glycolysis constitutes a major backbone of energy production [28]. Collectively the B. pilosicoli strains utilised 51.9% of carbohydrate compounds tested, and more specifically 69.4% of hexose sugars (Additional file 3). Genes for enzymes involved in converting glucose-6-phosphate to ribulose-5-phosphate that were identified in B. hyodysenteriae WA1 [25], were found in the B. pilosicoli genomes. These features are likely to direct carbohydrate oxidation towards the non-oxidative pentose phosphate pathway, to generate reducing power required for biosynthetic pathways. B. pilosicoli is characterised by an absence of β-glucosidase activity [60], however a novel system for metabolising β-glucosides found in 95/1000 [28] was also present in B2904 and WesB, which, alongside specific PTS systems, is likely to be involved in the utilisation of D-cellobiose and arbutin as carbon sources. Despite lacking β-glucosidase, metabolism of β-glucosides may be important to B. pilosicoli virulence as this phenotype is associated with growth, adhesion and colonisation in other bacteria [97]. Of the disaccharides tested, 64.3% were utilised by the B. pilosicoli strains, whereas, of the oligosaccharides only dextrin was utilised, which is likely to be attributed to α-glucosidase activity (BP951000_1130; B2904_orf204; wesB_2480).
Amino acid metabolism
Of the COG categories related to metabolism, the greatest proportion of the genome was related to amino acid transport and metabolism (Table 2). Phenotypic studies revealed that despite the high number of genes for amino acid/ oligopeptide transporters found in the genomes, only five of the tested amino acids were able to support B. pilosicoli as a sole carbon source (Additional file 3). Genes encoding enzymes to direct these amino acids towards pyruvate metabolism and hence energy production were identified, including alanine dehydrogenase (BP951000_0036; B2904_orf1321; wesB_1465), threonine aldolase (BP951000_1568; B2904_orf2409; wesB_0396), glycine hydroxymethyltransferase (BP951000_1528; B2904_orf2450; wesB_0361) and L-serine dehydratase (BP951000_0452 and BP951000_0453; B2904_orf939 and B2904_orf940; wesB_1746 and wesB_1747). Moreover, a glycine reductase complex found in the B. pilosicoli strains, which catalyses the reductive deamination of glycine, forming ATP, would be involved in the utilisation of glycine. A high proportion of amino acid metabolic features in B. pilosicoli were related to biosynthesis and potentially maintaining intermediates of the partial tricarboxylic acid (TCA) cycle identified in this species [28], rather than catabolism to produce energy. L-glutamate and L-glutamine were insufficient to sustain B. pilosicoli as a sole carbon source; these amino acids are primary products of ammonia assimilation used in peptidoglycan, LOS and outer membrane protein biosynthesis [98], hence their metabolism is redirected to energy yielding pathways. The B. pilosicoli strains possessed genes for glutamate dehydrogenase (BP951000_1312; B2904_orf93; wesB_0103), which catalyses the reversible synthesis of glutamate from α-ketoglutarate and ammonium. Since α-ketoglutarate was able to sustain B. pilosicoli, the presence of a transporter for α-ketoglutarate and not glutamate may explain this phenotype. The ability to utilise certain amino acids as an energy source may have become redundant in Brachyspira, which typically occupy the nutrient-rich lower GI tract, and thus associated features may have been lost through reductive evolution.
Nucleotide metabolism
The B. pilosicoli strains were able to utilise three purine and two pyrimidine nucleosides tested as a sole carbon source (Additional file 3). The enzymes suggested to complete a metabolic link between nucleoside and central metabolism in B. hyodysenteriae WA1 [25] were identified in the B. pilosicoli strains.
Lipid metabolism
Despite the presence of enzymes involved in the β-oxidation of fatty acids, including a long chain fatty acid-CoA ligase (BP951000_0887; B2904_orf479; wesB_2210), no long chain fatty acids tested were utilised by B. pilosicoli as a carbon source; however, the short chain fatty acids, butyric acid and propionic acid, were utilised (Additional file 3). Glycerol was utilised as a carbon source, and genes for its metabolism were detected including those for a glycerol uptake facilitator (BP951000_0799; B2904_orf2190; wesB_2118), glycerol kinase (BP951000_0800; B2904_orf2191; wesB_2119) and glycerol-3-phosphate dehydrogenase (BP951000_1696; B2904_orf2220; wesB_0532). The gene set required for fatty acid biosynthesis was incomplete in B2904 and WesB, as it was in 95/1000 [28].
Conclusions
In this study, we report the genome of B. pilosicoli strain B2904 and the near complete genome of strain WesB. Together with the previously reported 95/1000 genome, this allowed the first intra-species genome comparison within the genus Brachyspira. Our feature-based analysis revealed a high level of similarity between the three strains and identified genes that we suggest different strains of the spirochaete may have lost in a process of reductive genome evolution. Sequence-based comparisons showed the majority of sequence was shared between the strains, with few unique regions; however, genome rearrangements were observed around the oriC. MGEs were found associated to areas of rearrangements, and these features may be a factor that has driven or is driving reductive evolution. Novel bacteriophages were identified in the newly-sequenced genomes, which displayed evidence of intra- and inter-species HGT, and these may have key practical applications for use in genetic manipulation. This is the first analysis of the spirochaete in a high-throughput phenotype screening tool, allowing correlation between genotype and phenotype. Future work will focus on the application of this technology to a wider range of Brachyspira species to validate genome differences, potentially providing a means by which these phenotypes can be used for rapid screening to infer genotypes and improve current diagnostic methods. With the increasing availability of Brachyspira genome sequences, such technology should facilitate the validation of metabolic models based on genome sequence.
Abbreviations
A: Adenine; AIS: Avian intestinal spirochaetosis; ABC: ATP-binding cassette; ACT: Artemis comparison tool; ATP: Adenosine triphosphate; bp: Base pair; C: Cytosine; CDS: Coding DNA sequence; COG: Cluster of Orthologous Genes; CRISPR: Clustered regularly interspaced short palindromic repeats; CTAB: Cetyltrimethylammonium bromide; DNA: Deoxyribonucleic acid; FABA: Fastidious anaerobe blood agar; G: Guanine; GI: Gastrointestinal; GTA: Gene transfer agent; GTR: General time reversible; HGT: Horizontal gene transfer; iCORN: Iterative correction of reference nucleotides; IMAGE: Iterative mapping and assembly for gap elimination; Indels: Insertions/deletions; ISE: Insertion sequence element; KAAS: KEGG automatic annotation server; Kb: Kilobase; KEGG: Kyoto encyclopedia of genes and genomes; KO: KEGG orthology; LOS: Lipooligosaccharide; LPS: Lipopolysaccharide; Mb: Megabase; MCL: Markov clustering algorithm; MGE: Mobile genetic element; MLST: Multi locus sequence typing; NCBI: National center for biotechnology information; ORF: Open reading frame; PCR: Polymerase chain reaction; PFGE: Pulse-field gel electrophoresis; PID: Percentage identity; PIS: Porcine intestinal spirochaetosis; PM: Phenotype MicroArrayTM; PTS: Phosphotransferase system; RNA: Ribonucleic acid; rRNA: Ribosomal RNA; SNP: Single nucleotide polymorphisms; SOAP: Short oligonucleotide alignment program; T: Thymine; TCA: Tricarboxylic acid; tmRNA: Transfer-messenger RNA; tRNA: Transfer RNA.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LJM, DJH and RML conceived the study. LJM and ACD performed the genome sequencing and gap closure of B. pilosicoli B2904. JP and AKT were involved in genome sequencing of the WesB. DJH, TL and NDP were involved in the genome sequencing of B. aalborgi 513T, B. alvinipulli C1T and B. intermedia HB60. LJM, MLB, DJH, MIB and ACD conceived and designed comparative genomic studies. LJM and MLB performed comparative genomic analysis and analysed the data. LJM, MA, MJW and RML conceived and designed phenotyping experiments. LJM and MA conducted phenotyping experiments. LJM wrote the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Type and copy number of mobile genetic elements (MGE) in the genomes ofB. pilosicoli 95/1000, B2904 and WesB. A combination of protein markov cluster analysis and reciprocal blast searches against the conserved domain database (CDD) was used to determine the copy number of each type of MGE across the three B. pilosicoli genomes, using a cut-off e-value of 1e-20. The ORF number, position, and size of all MGEs identified in each of the B. pilosicoli genomes is displayed.
Conserved and shared protein clusters between the six genome-sequencedBrachyspira strains. B. hyodysenteriae WA1 (H), B. intermedia PWS/AT (I), B. murdochii 56-150T (M) and B. pilosicoli 95/1000 (Pa), B2904 (Pb) and WesB (Pc)a strains we included in the protein cluster analysis. A cut-off e-value of 1e-20 was used.
Comparison of the utilisation of unique carbon sources byB. pilosicoli95/1000, B2904 and WesB. Biolog Phenotype MicroArray™ (PM) technology was employed for these studies and OmniLog apparatus was used to detect formazan formation and hence, respiration due to utilisation of the carbon source; +, able to utilise the compound; -, unable to utilise the compound.
Contributor Information
Luke J Mappley, Email: l.mappley@reading.ac.uk.
Michael L Black, Email: M.Black@murdoch.edu.au.
Manal AbuOun, Email: Manal.AbuOun@ahvla.gsi.gov.uk.
Alistair C Darby, Email: Alistair.Darby@liverpool.ac.uk.
Martin J Woodward, Email: m.j.woodward@reading.ac.uk.
Julian Parkhill, Email: parkhill@sanger.ac.uk.
A Keith Turner, Email: Keith.turner@discuva.com.
Matthew I Bellgard, Email: mbellgard@ccg.murdoch.edu.au.
Tom La, Email: T.La@murdoch.edu.au.
Nyree D Phillips, Email: N.Phillips@murdoch.edu.au.
Roberto M La Ragione, Email: r.laragione@surrey.ac.uk.
David J Hampson, Email: D.Hampson@murdoch.edu.au.
Acknowledgments
The authors acknowledge receipt of funding for this study from the British Egg Marketing Board Research and Education trust, the EU grant MetaHIT (HEALTH-F4-2007-201052), the Royal College of Veterinary Surgeons (RCVS), and Murdoch University. We are grateful to Richard Rance, David Harris, Ruth Gilderthorp, Nicola Corton, and other members of the Sequencing and Genome Improvement Teams at the Wellcome Trust Sanger Institute for their assistance in the preparation of the incomplete genome sequence of B. pilosicoli WesB.
References
- Ludwig W, Euzeby J, Whitman WB. Draft taxonomic outline of the Bacteroidetes, Planctomycetes, Chlamydiae, Spirochaetes, Fibrobacteres, Fusobacteria, Acidobacteria, Verrucomicrobia, Dictyoglomi and Gemmatimonadetes. 2008. http://www.bergeys.org/outlines/Bergeys_Vol_4_Outline.pdf01/05/12.
- Hovind-Hougen K, Birch-Andersen A, Henrik-Nielsen R, Orholm M, Pedersen JO, Teglbjaerg PS, Thaysen EH. Intestinal spirochetosis: morphological characterization and cultivation of the spirochete Brachyspira aalborgi gen. nov., sp. nov. J Clin Microbiol. 1982;16:1127–1136. doi: 10.1128/jcm.16.6.1127-1136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DL, Glock RD, Christensen CR, Kinyon JM. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction of the disease. Vet Med Small Anim Clin. 1972;67:61–64. [PubMed] [Google Scholar]
- Stanton TB, Postic D, Jensen NS. Serpulina alvinipulli sp. nov., a new Serpulina species that is enteropathogenic for chickens. Int J Syst Bacteriol. 1998;48:669–676. doi: 10.1099/00207713-48-3-669. [DOI] [PubMed] [Google Scholar]
- Stanton TB, Fournie-Amazouz E, Postic D, Trott DJ, Grimont PA, Baranton G, Hampson DJ, Saint Girons I. Recognition of two new species of intestinal spirochetes: Serpulina intermedia sp. nov. and Serpulina murdochii sp. nov. Int J Syst Bacteriol. 1997;47:1007–1012. doi: 10.1099/00207713-47-4-1007. [DOI] [PubMed] [Google Scholar]
- Trott DJ, Stanton TB, Jensen NS, Duhamel GE, Johnson JL, Hampson DJ. Serpulina pilosicoli sp. nov., the agent of porcine intestinal spirochetosis. Int J Syst Bacteriol. 1996;46:206–215. doi: 10.1099/00207713-46-1-206. [DOI] [PubMed] [Google Scholar]
- Kinyon JM, Harris DL. Treponema innocens, a new species of intestinal bacteria, and emended description of the type strain of Treponema hyodysenteriae. Int J Syst Bacteriol. 1979;29:102–109. doi: 10.1099/00207713-29-2-102. [DOI] [Google Scholar]
- Duhamel GE, Trott DJ, Muniappa N, Mathiesen MR, Tarasiuk K, Lee JI, Hampson DJ. Canine intestinal spirochetes consist of Serpulina pilosicoli and a newly identified group provisionally designated "Serpulina canis" sp. nov. J Clin Microbiol. 1998;36:2264–2270. doi: 10.1128/jcm.36.8.2264-2270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens CP, Hampson DJ. Prevalence and disease association of intestinal spirochaetes in chickens in Eastern Australia. Avian Pathol. 1999;28:447–454. doi: 10.1080/03079459994461. [DOI] [PubMed] [Google Scholar]
- Rasback T, Jansson DS, Johansson KE, Fellstrom C. A novel enteropathogenic, strongly haemolytic spirochaete isolated from pig and mallard, provisionally designated 'Brachyspira suanatina' sp. nov. Environ Microbiol. 2007;9:983–991. doi: 10.1111/j.1462-2920.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- Hampson DJ, Oxberry SL, La T. Potential for zoonotic transmission of Brachyspira pilosicoli. Emerg Infect Dis. 2006;12:869–870. doi: 10.3201/eid1205.051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsinganou E, Gebbers JO. Human intestinal spirochetosis--a review. Ger Med Sci. 2010;8:Doc01. doi: 10.3205/000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL. Colonic spirochetosis in animals and humans. J Food Prot. 2005;68:1525–1534. doi: 10.4315/0362-028x-68.7.1525. [DOI] [PubMed] [Google Scholar]
- Lee JI, Hampson DJ. Intestinal spirochaetes colonizing aborigines from communities in the remote north of Western Australia. Epidemiol Infect. 1992;109:133–141. [PMC free article] [PubMed] [Google Scholar]
- Trott DJ, Combs BG, Mikosza AS, Oxberry SL, Robertson ID, Passey M, Taime J, Sehuko R, Alpers MP, Hampson DJ. The prevalence of Serpulina pilosicoli in humans and domestic animals in the Eastern Highlands of Papua New Guinea. Epidemiol Infect. 1997;119:369–379. doi: 10.1017/S0950268897008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margawani KR, Robertson ID, Brooke CJ, Hampson DJ. Prevalence, risk factors and molecular epidemiology of Brachyspira pilosicoli in humans on the island of Bali, Indonesia. J Med Microbiol. 2004;53:325–332. doi: 10.1099/jmm.0.05415-0. [DOI] [PubMed] [Google Scholar]
- Munshi MA, Traub RJ, Robertson ID, Mikosza AS, Hampson DJ. Colonization and risk factors for Brachyspira aalborgi and Brachyspira pilosicoli in humans and dogs on tea estates in Assam, India. Epidemiol Infect. 2004;132:137–144. doi: 10.1017/S095026880300116X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivett-Moore NL, Gilbert GL, Law CL, Trott DJ, Hampson DJ. Isolation of Serpulina pilosicoli from rectal biopsy specimens showing evidence of intestinal spirochetosis. J Clin Microbiol. 1998;36:261–265. doi: 10.1128/jcm.36.1.261-265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh R, Hampson DJ. Attraction of Brachyspira pilosicoli to mucin. Microbiology. 2010;156:191–197. doi: 10.1099/mic.0.030262-0. [DOI] [PubMed] [Google Scholar]
- Milner JA, Sellwood R. Chemotactic response to mucin by Serpulina hyodysenteriae and other porcine spirochetes: potential role in intestinal colonization. Infect Immun. 1994;62:4095–4099. doi: 10.1128/iai.62.9.4095-4099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Rosnick DK, Ulrich RG, Yancey RJ Jr. Association of Treponema hyodysenteriae with porcine intestinal mucosa. J Gen Microbiol. 1988;134:1565–1576. doi: 10.1099/00221287-134-6-1565. [DOI] [PubMed] [Google Scholar]
- Trott DJ, Huxtable CR, Hampson DJ. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect Immun. 1996;64:4648–4654. doi: 10.1128/iai.64.11.4648-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland WA, Lee FD. Intestinal spirochaetosis. Br Med J. 1967;3:718–719. doi: 10.1136/bmj.3.5567.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh R, Song Y, Hampson DJ. The intestinal spirochete Brachyspira pilosicoli attaches to cultured Caco-2 cells and induces pathological changes. PLoS One. 2009;4:e8352. doi: 10.1371/journal.pone.0008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgard MI, Wanchanthuek P, La T, Ryan K, Moolhuijzen P, Albertyn Z, Shaban B, Motro Y, Dunn DS, Schibeci D. et al. Genome sequence of the pathogenic intestinal spirochete Brachyspira hyodysenteriae reveals adaptations to its lifestyle in the porcine large intestine. PLoS One. 2009;4:e4641. doi: 10.1371/journal.pone.0004641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom T, Jansson DS, Segerman B. Complete genome sequence of Brachyspira intermedia reveals unique genomic features in Brachyspira species and phage-mediated horizontal gene transfer. BMC Genomics. 2011;12:395. doi: 10.1186/1471-2164-12-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati A, Sikorski J, Gronow S, Munk C, Lapidus A, Copeland A, Glavina Del Tio T, Nolan M, Lucas S, Chen F. et al. Complete genome sequence of Brachyspira murdochii type strain (56-150T) Stand Genomic Sci. 2010;2:260–269. doi: 10.4056/sigs.831993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchanthuek P, Bellgard MI, La T, Ryan K, Moolhuijzen P, Chapman B, Black M, Schibeci D, Hunter A, Barrero R. et al. The complete genome sequence of the pathogenic intestinal spirochete Brachyspira pilosicoli and comparison with other Brachyspira genomes. PLoS One. 2010;5:e11455. doi: 10.1371/journal.pone.0011455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR, Gadzinski P, Panomitros E. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 2001;11:1246–1255. doi: 10.1101/gr.186501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR. Global phenotypic characterization of bacteria. FEMS Microbiol Rev. 2009;33:191–205. doi: 10.1111/j.1574-6976.2008.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasback T, Fellstrom C, Bergsjo B, Cizek A, Collin K, Gunnarsson A, Jensen SM, Mars A, Thomson J, Vyt P. et al. Assessment of diagnostics and antimicrobial susceptibility testing of Brachyspira species using a ring test. Vet Microbiol. 2005;109:229–243. doi: 10.1016/j.vetmic.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kunkle RA, Harris DL, Kinyon JM. Autoclaved liquid medium for propagation of Treponema hyodysenteriae. J Clin Microbiol. 1986;24:669–671. doi: 10.1128/jcm.24.4.669-671.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. In: Current Protocols in Molecular Biology. Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Struhl K, editor. New York: John Wiley & Sons; 1990. Preparation of genomic DNA from bacteria; pp. 241–242. [Google Scholar]
- Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–714. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- Tsai IJ, Otto TD, Berriman M. Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol. 2010;11:R41. doi: 10.1186/gb-2010-11-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto TD, Sanders M, Berriman M, Newbold C. Iterative Correction of Reference Nucleotides (iCORN) using second generation sequencing technology. Bioinformatics. 2010;26:1704–1707. doi: 10.1093/bioinformatics/btq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasback T, Johansson KE, Jansson DS, Fellstrom C, Alikhani MY, La T, Dunn DS, Hampson DJ. Development of a multilocus sequence typing scheme for intestinal spirochaetes within the genus Brachyspira. Microbiology. 2007;153:4074–4087. doi: 10.1099/mic.0.2007/008540-0. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski LB, Gray MW, Lang BF, Burger G. AutoFACT: an automatic functional annotation and classification tool. BMC Bioinforma. 2005;6:151. doi: 10.1186/1471-2105-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellstrom C, Rasback T, Johansson KE, Olofsson T, Aspan A. Identification and genetic fingerprinting of Brachyspira species. J Microbiol Methods. 2008;72:133–140. doi: 10.1016/j.mimet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Phillips ND, La T, Amin MM, Hampson DJ. Brachyspira intermedia strain diversity and relationships to the other indole-positive Brachyspira species. Vet Microbiol. 2010;143:246–254. doi: 10.1016/j.vetmic.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Andersson SG, Kurland CG. Reductive evolution of resident genomes. Trends Microbiol. 1998;6:263–268. doi: 10.1016/S0966-842X(98)01312-2. [DOI] [PubMed] [Google Scholar]
- Zuerner RL, Stanton TB. Physical and genetic map of the Serpulina hyodysenteriae B78T chromosome. J Bacteriol. 1994;176:1087–1092. doi: 10.1128/jb.176.4.1087-1092.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Okuzako N, Mifuchi I, Arimitsu Y, Seki M. Organization of the ribosomal RNA genes in Treponema phagedenis and Treponema pallidum. Microbiol Immunol. 1992;36:161–167. doi: 10.1111/j.1348-0421.1992.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Mifuchi I, Yanagihara Y, Okuzako N. Comparison of flanking regions of the 5S ribosomal ribonucleic acid genes in Leptospira biflexa and Leptospira interrogans. Chem Pharm Bull (Tokyo) 1992;40:544–546. doi: 10.1248/cpb.40.544. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Yanagihara Y, Sohnaka M. The 23S/5S ribosomal RNA genes (rrl/rrf) are separate from the 16S ribosomal RNA gene (rrs) in Borrelia burgdorferi, the aetiological agent of Lyme disease. J Gen Microbiol. 1992;138:871–877. doi: 10.1099/00221287-138-5-871. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhang CT. Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinforma. 2008;9:79. doi: 10.1186/1471-2105-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Plague GR. Genomic changes following host restriction in bacteria. Curr Opin Genet Dev. 2004;14:627–633. doi: 10.1016/j.gde.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Schmitz-Esser S, Penz T, Spang A, Horn M. A bacterial genome in transition–an exceptional enrichment of IS elements but lack of evidence for recent transposition in the symbiont Amoebophilus asiaticus. BMC Evol Biol. 2011;11:270. doi: 10.1186/1471-2148-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plague GR, Dunbar HE, Tran PL, Moran NA. Extensive proliferation of transposable elements in heritable bacterial symbionts. J Bacteriol. 2008;190:777–779. doi: 10.1128/JB.01082-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hwang J, Yi H, Ulrich RL, Yu Y, Nierman WC, Kim HS. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog. 2010;6:e1000922. doi: 10.1371/journal.ppat.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MT, Churcher CM, Bentley SD, Mungall KL. et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MT, Prentice MB, Sebaihia M, James KD, Churcher C, Mungall KL. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- Trott DJ, McLaren AJ, Hampson DJ. Pathogenicity of human and porcine intestinal spirochetes in one-day-old specific-pathogen-free chicks: an animal model of intestinal spirochetosis. Infect Immun. 1995;63:3705–3710. doi: 10.1128/iai.63.9.3705-3710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DJ, Mikosza AS, Combs BG, Oxberry SL, Hampson DJ. Population genetic analysis of Serpulina pilosicoli and its molecular epidemiology in villages in the eastern Highlands of Papua New Guinea. Int J Syst Bacteriol. 1998;48:659–668. doi: 10.1099/00207713-48-3-659. [DOI] [PubMed] [Google Scholar]
- Tillier ER, Collins RA. Genome rearrangement by replication-directed translocation. Nat Genet. 2000;26:195–197. doi: 10.1038/79918. [DOI] [PubMed] [Google Scholar]
- Mackiewicz P, Mackiewicz D, Kowalczuk M, Cebrat S. Flip-flop around the origin and terminus of replication in prokaryotic genomes. Gen Biol. 2001;2:INTERACTIONS1004. doi: 10.1186/gb-2001-2-12-interactions1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motro Y, Dunn DS, La T, Phillips ND, Hampson DJ, Bellgard MI. Intestinal spirochaetes of the genus Brachyspira share a partially conserved 26 kilobase genomic region with Enterococcus faecalis and Escherichia coli. Microbiol Insights. 2008;1:3–11. [Google Scholar]
- Fellstrom C, Gunnarsson A. Phenotypical characterisation of intestinal spirochaetes isolated from pigs. Res Vet Sci. 1995;59:1–4. doi: 10.1016/0034-5288(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Fellstrom C, Pettersson B, Thomson J, Gunnarsson A, Persson M, Johansson KE. Identification of Serpulina species associated with porcine colitis by biochemical analysis and PCR. J Clin Microbiol. 1997;35:462–467. doi: 10.1128/jcm.35.2.462-467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtensteiger CA, Vimr ER. Neuraminidase (sialidase) activity of Haemophilus parasuis. FEMS Microbiol Lett. 1997;152:269–274. doi: 10.1111/j.1574-6968.1997.tb10438.x. [DOI] [PubMed] [Google Scholar]
- Soong G, Muir A, Gomez MI, Waks J, Reddy B, Planet P, Singh PK, Kaneko Y, Wolfgang MC, Hsiao YS. et al. Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J Clin Invest. 2006;116:2297–2305. doi: 10.1172/JCI27920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. Biosynthesis of lipopolysaccharide O antigens. Trends Microbiol. 1995;3:178–185. doi: 10.1016/S0966-842X(00)88917-9. [DOI] [PubMed] [Google Scholar]
- Wildschutte H, Wolfe DM, Tamewitz A, Lawrence JG. Protozoan predation, diversifying selection, and the evolution of antigenic diversity in Salmonella. Proc Natl Acad Sci U S A. 2004;101:10644–10649. doi: 10.1073/pnas.0404028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler AC, Haase A, Reeves PR. Molecular analysis of the 3,6-dideoxyhexose pathway genes of Yersinia pseudotuberculosis serogroup IIA. J Bacteriol. 1993;175:1412–1422. doi: 10.1128/jb.175.5.1412-1422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren AJ, Trott DJ, Swayne DE, Oxberry SL, Hampson DJ. Genetic and phenotypic characterization of intestinal spirochetes colonizing chickens and allocation of known pathogenic isolates to three distinct genetic groups. J Clin Microbiol. 1997;35:412–417. doi: 10.1128/jcm.35.2.412-417.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Miyatake H, Yokota N, Matsuyama S, Tokuda H, Miki K. Crystal structures of bacterial lipoprotein localization factors, LolA and LolB. EMBO J. 2003;22:3199–3209. doi: 10.1093/emboj/cdg324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sojar HT, Glurich I, Honma K, Kuramitsu HK, Genco RJ. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect Immun. 1998;66:5703–5710. doi: 10.1128/iai.66.12.5703-5710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow P, Bourhy P, da Cruz McBride FW, Figueira CP, Huerre M, Ave P, Girons IS, Ko AI, Picardeau M. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 2007;3:e97. doi: 10.1371/journal.ppat.0030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhat M, Atlan D, Vianney A, Lazzaroni JC, Doublet P, Gilbert C. The TolC protein of Legionella pneumophila plays a major role in multi-drug resistance and the early steps of host invasion. PLoS One. 2009;4:e7732. doi: 10.1371/journal.pone.0007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgurskaya HI, Krishnamoorthy G, Ntreh A, Lu S. Mechanism and Function of the Outer Membrane Channel TolC in Multidrug Resistance and Physiology of Enterobacteria. Front Microbiol. 2011;2:189. doi: 10.3389/fmicb.2011.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minato Y, Siefken RL, Hase CC. TolC affects virulence gene expression in Vibrio cholerae. J Bacteriol. 2011;193:5850–5852. doi: 10.1128/JB.05222-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AL, Dassa E, Orelle C, Chen J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev. 2008;72:317–364. doi: 10.1128/MMBR.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno JC, Tamura M, Hannam PM, Wong GW, Chan RA, McBride BC. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect Immun. 2000;68:1884–1892. doi: 10.1128/IAI.68.4.1884-1892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MS, Thomas CJ. The Listeria monocytogenes gene ctpA encodes a putative P-type ATPase involved in copper transport. Mol Gen Genet. 1997;253:484–491. doi: 10.1007/s004380050347. [DOI] [PubMed] [Google Scholar]
- Silver S, Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992;56:195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Gong H, Lai J, Main A, Lu S. The potassium transporter Trk and external potassium modulate Salmonella enterica protein secretion and virulence. Infect Immun. 2009;77:667–675. doi: 10.1128/IAI.01027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Romero DG, Swiatlo E. Role of polyamine transport in Streptococcus pneumoniae response to physiological stress and murine septicemia. Microb Pathog. 2008;45:167–172. doi: 10.1016/j.micpath.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Reeves SA, Torres AG, Payne SM. TonB is required for intracellular growth and virulence of Shigella dysenteriae. Infect Immun. 2000;68:6329–6336. doi: 10.1128/IAI.68.11.6329-6336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs P, Lefevre P, Boarbi S, Wang XM, Denis O, Braibant M, Pethe K, Locht C, Huygen K, Content J. Mycobacterium tuberculosis with disruption in genes encoding the phosphate binding proteins PstS1 and PstS2 is deficient in phosphate uptake and demonstrates reduced in vivo virulence. Infect Immun. 2005;73:1898–1902. doi: 10.1128/IAI.73.3.1898-1902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R 3rd, Dubreuil JD, Harel J. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun. 2005;73:4138–4145. doi: 10.1128/IAI.73.7.4138-4145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Poza M, Fernandez A, Del Carmen Fernandez M, Mallo S, Merino M, Rumbo-Feal S, Cabral MP, Bou G. Involvement of the AcrAB-TolC Efflux Pump in the Resistance, Fitness, and Virulence of Enterobacter cloacae. Antimicrob Agents Chemother. 2012;56:2084–2090. doi: 10.1128/AAC.05509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A. Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect Immun. 2006;74:5433–5444. doi: 10.1128/IAI.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan J, Hughes NJ, McColm AA, Bagshaw J, Clayton CL, Andrews SC, Kelly DJ. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol Microbiol. 2000;37:274–286. doi: 10.1046/j.1365-2958.2000.01987.x. [DOI] [PubMed] [Google Scholar]
- Tamura GS, Nittayajarn A, Schoentag DL. A glutamine transport gene, glnQ, is required for fibronectin adherence and virulence of group B streptococci. Infect Immun. 2002;70:2877–2885. doi: 10.1128/IAI.70.6.2877-2885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcella SF, Popova TG, Hagman KE, Penn CW, Radolf JD, Norgard MV. A mgl-like operon in Treponema pallidum, the syphilis spirochete. Gene. 1996;177:115–121. doi: 10.1016/0378-1119(96)00286-7. [DOI] [PubMed] [Google Scholar]
- Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- Betschel SD, Borgia SM, Barg NL, Low DE, De Azavedo JC. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect Immun. 1998;66:1671–1679. doi: 10.1128/iai.66.4.1671-1679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierig G, Cywes C, Wessels MR, Ashbaugh CD. Cytotoxic effects of streptolysin o and streptolysin s enhance the virulence of poorly encapsulated group a streptococci. Infect Immun. 2003;71:446–455. doi: 10.1128/IAI.71.1.446-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TH, Miller CG. Aspartate-specific peptidases in Salmonella typhimurium: mutants deficient in peptidase E. J Bacteriol. 1984;159:453–459. doi: 10.1128/jb.159.2.453-459.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Dallas MM, Malamy MH. Characterization of the Batl (Bacteroides aerotolerance) operon in Bacteroides fragilis: isolation of a B. fragilis mutant with reduced aerotolerance and impaired growth in in vivo model systems. Mol Microbiol. 1999;32:139–149. doi: 10.1046/j.1365-2958.1999.01337.x. [DOI] [PubMed] [Google Scholar]
- Cho NH, Kim JM, Kwon EK, Kim SY, Han SH, Chu H, Lee JH, Choi MS, Kim IS. Molecular characterization of a group of proteins containing ankyrin repeats in Orientia tsutsugamushi. Ann N Y Acad Sci. 2005;1063:100–101. doi: 10.1196/annals.1355.016. [DOI] [PubMed] [Google Scholar]
- Sorek R, Kunin V, Hugenholtz P. CRISPR–a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol. 2008;6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, Salmond GP. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc Natl Acad Sci U S A. 2009;106:894–899. doi: 10.1073/pnas.0808832106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic AO, Tao L, Zhang Y, Lei Y, Khammanivong A, Herzberg MC. Involvement of Streptococcus gordonii beta-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J Bacteriol. 2004;186:4246–4253. doi: 10.1128/JB.186.13.4246-4253.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MJ, Edwards RA. Nitrogen control in bacteria. Microbiol Rev. 1995;59:604–622. doi: 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Type and copy number of mobile genetic elements (MGE) in the genomes ofB. pilosicoli 95/1000, B2904 and WesB. A combination of protein markov cluster analysis and reciprocal blast searches against the conserved domain database (CDD) was used to determine the copy number of each type of MGE across the three B. pilosicoli genomes, using a cut-off e-value of 1e-20. The ORF number, position, and size of all MGEs identified in each of the B. pilosicoli genomes is displayed.
Conserved and shared protein clusters between the six genome-sequencedBrachyspira strains. B. hyodysenteriae WA1 (H), B. intermedia PWS/AT (I), B. murdochii 56-150T (M) and B. pilosicoli 95/1000 (Pa), B2904 (Pb) and WesB (Pc)a strains we included in the protein cluster analysis. A cut-off e-value of 1e-20 was used.
Comparison of the utilisation of unique carbon sources byB. pilosicoli95/1000, B2904 and WesB. Biolog Phenotype MicroArray™ (PM) technology was employed for these studies and OmniLog apparatus was used to detect formazan formation and hence, respiration due to utilisation of the carbon source; +, able to utilise the compound; -, unable to utilise the compound.