Abstract
In eukaryotic cells, protein trafficking plays an essential role in biogenesis of proteins that belong to the endomembrane compartments. In this process, an important step is the sorting of organellar proteins depending on their final destinations. For vacuolar proteins, vacuolar sorting receptors (VSRs) and receptor homology-transmembrane-RING H2 domain proteins (RMRs) are thought to be responsible. Arabidopsis (Arabidopsis thaliana) contains seven VSRs. Among them, VSR1, VSR3, and VSR4 are involved in sorting storage proteins targeted to the protein storage vacuole (PSV) in seeds. However, the identity of VSRs for soluble proteins of the lytic vacuole in vegetative cells remains controversial. Here, we provide evidence that VSR1, VSR3, and VSR4 are involved in sorting soluble lytic vacuolar and PSV proteins in vegetative cells. In protoplasts from leaf tissues of vsr1vsr3 and vsr1vsr4 but not vsr5vsr6, and rmr1rmr2 and rmr3rmr4 double mutants, soluble lytic vacuolar (Arabidopsis aleurain-like protein:green fluorescent protein [GFP] and carboxypeptidase Y:GFP and PSV (phaseolin) proteins, but not the vacuolar membrane protein Arabidopsis βFructosidase4:GFP, exhibited defects in their trafficking; they accumulated to the endoplasmic reticulum with an increased secretion into medium. The trafficking defects in vsr1vsr4 protoplasts were rescued by VSR1 or VSR4 but not VSR5 or AtRMR1. Furthermore, of the luminal domain swapping mutants between VSR1 and VSR5, the mutant with the luminal domain of VSR1, but not that of VSR5, rescued the trafficking defects of Arabidopsis aleurain-like protein:GFP and phaseolin in vsr1vsr4 protoplasts. Based on these results, we propose that VSR1, VSR3, and VSR4, but not other VSRs, are involved in sorting soluble lytic vacuolar and PSV proteins for their trafficking to the vacuoles in vegetative cells.
Two different types of vacuoles have been identified in plant cells. One of them is the lytic vacuole (LV) that is present in vegetative cells, and the other is the protein storage vacuole (PSV) that is present in seed cells (Frigerio et al., 2008; Zouhar and Rojo, 2009; De Marcos Lousa et al., 2012). These two types of vacuoles have different functions. The LV carries out various functions such as osmotic pressure regulation, various hydrolytic activities, detoxification, and homeostasis of calcium and sodium ions. For some of these aspects LV is analogous to the vacuole in yeast (Saccharomyces cerevisiae) or lysosomes in animal cells. In contrast, the PSV is unique in plants and stores a large amount of proteins and minerals that are necessary for seed germination. To perform these functions, vacuoles need a large number of proteins.
The organellar proteins destined for vacuoles have to be transported from the endoplasmic reticulum (ER) via a process called protein trafficking. This has been extensively studied in many different eukaryotic cell types, including plant cells. In general, proteins that belong to various endomembrane compartments are cotranslationally translocated into the ER and then transported through the Golgi apparatus and other intermediate compartments depending on their final destinations (Jurgens, 2004; Jolliffe et al., 2005; Sato and Nakano, 2007; Hwang and Robinson, 2009; Reyes et al., 2011). Vesicles are used to transport proteins from one compartment to another. Another important aspect is the specific targeting of organellar proteins. For this, organellar proteins carry a specific sorting or targeting signal that can be a sequence motif generated intrinsically or added posttranslationally (Hadlington and Denecke, 2000; Robinson et al., 2005; Hwang, 2008). The sequence motifs are recognized specifically by sorting receptors localized at the organelles that serve as donor compartments in trafficking pathways (Bassham and Raikhel, 2000; De Marcos Lousa et al., 2012).
Two different types of sorting receptors, receptor homology-transmembrane-RING H2 domain proteins (RMRs) and vacuolar sorting receptors (VSRs), have been shown to be involved in the trafficking of vacuolar proteins. It has been proposed that RMRs function as a sorting receptor for storage proteins (Park et al., 2005; Hinz et al., 2007; Wang et al., 2011a). RMRs are type I membrane proteins and those in the luminal domain specifically interact with the C-terminal vacuolar sorting sequence (ctVSS) of storage proteins (Park et al., 2005; Shen et al., 2011). In addition, overexpression of an AtRMR1 deletion mutant inhibits the trafficking of phaseolin to the PSV, but not the protein trafficking to the LV, in protoplasts from leaf cells (Park et al., 2005). VSRs have been identified from various plant species and shown to specifically interact with the sorting motif of vacuolar proteins, which is known as the sequence-specific vacuolar sorting signal (ssVSS) or N-terminal propeptide (Ahmed et al., 1997; Hadlington and Denecke, 2000; Masclaux et al., 2005; Robinson et al., 2005; Hwang, 2008). In plant cells, the majority of VSRs localize to the prevacuolar compartment (PVC), which is the intermediate organelle between the trans-Golgi network (TGN) and vacuole (Tse et al., 2004; daSilva et al., 2005; Miao et al., 2006). In addition, a minor portion of VSR1 localizes to the TGN in plant cells, which supports the notion that VSRs recycle to the TGN from the PVC for sorting of their cargo proteins (Kim et al., 2010). Recent studies in plant cells questioned this concept and proposed other mechanisms for sorting vacuolar proteins. In the alternative proposal, sorting of vacuolar proteins may occur at the ER, and the VSRs may recycle from the TGN to the ER (Castelli and Vitale, 2005; Niemes et al., 2010). VSRs that were once thought to function as sorting receptors at the TGN for the LV proteins (daSilva et al., 2005; Foresti et al., 2010; Kim et al., 2010) have an additional function in the protein trafficking to the PSV in seed cells (Shimada et al., 2003; Zouhar et al., 2010). By using a genetic approach, it has been shown that among seven Arabidopsis (Arabidopsis thaliana) VSRs, VSR1, VSR3, and VSR4 play a role in trafficking of 12S globulins and 2S albumins in seed cells.
The VSR isoforms involved in the protein trafficking to the PSV also exist in vegetative tissues (Laval et al., 1999; Kim et al., 2010; Zouhar et al., 2010). Mutations in both VSR1 and VSR4 cause secretion of AtAleurain, but not other LV proteins, into the apoplasts. Thus, it is not clearly understood what is the physiological role of AtVSRs in vegetative tissues (except for their role in vacuolar trafficking of AtAleurain), and what are the VSRs of other vacuolar proteins. In previous studies, it was demonstrated that overexpression of mutant forms of VSR1, VSR2, or BP80 of pea (Pisum sativum), a close homolog of VSR3 and VSR4, in protoplasts from wild-type plants affects trafficking of proteins to the LV (daSilva et al., 2005; Foresti et al., 2010; Kim et al., 2010). In this study, we utilized various VSR and RMR mutant plants and examined the effect of these mutations on the trafficking of LV and PSV proteins in protoplasts. These studies demonstrated that VSR1, VSR3, and VSR4, but not other VSRs and RMRs, are involved in trafficking of soluble LV and PSV proteins in vegetative cells. Further, the luminal domain but not the cytosolic tail of VSRs contains the determinant for the sorting specificity.
RESULTS
Loss-of-Function Mutation in VSR1, VSR3, or VSR4 Causes a Minor Defect in Trafficking of Both LV and PSV Proteins in Protoplasts from Vegetative Tissues
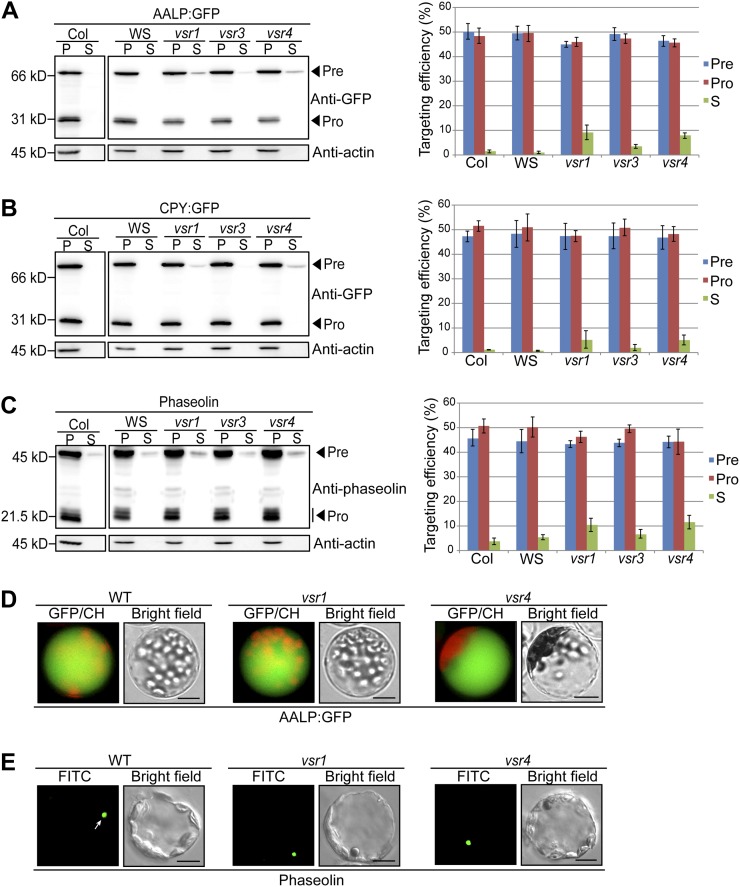
In previous studies, the role of VSRs in vacuolar trafficking was examined by employing an approach of overexpression of wild-type or mutant forms of VSRs in wild-type protoplasts (daSilva et al., 2005; Foresti et al., 2010; Kim et al., 2010). Based on the expression data in Genevestigator (Hruz et al., 2008), Arabidopsis VSR1, VSR3, VSR4, and VSR7 are expressed in multiple tissues and show relatively high expression in mesophyll cell protoplast (Supplemental Figure S1). Thus, to gain further insight into the role of VSRs in vegetative tissues, we used the loss-of-function mutant of VSR1, VSR3, and VSR4 and examined the trafficking of vacuolar luminal proteins, AALP:GFP and CPY:GFP, in protoplasts from leaf tissues of vsr single mutant plants. A previous study showed that the vsr1 mutant plants exhibit a defect in protein trafficking to the PSV in seed cells (Shimada et al., 2003). AALP:GFP is a C-terminal GFP fusion of AALP (for Arabidopsis aleurain-like protein) that has both an N-terminal ssVSS and ctVSS (Sohn et al., 2003). In protoplasts, AALP:GFP is transported to the central vacuole where it is processed proteolytically into a smaller form (Sohn et al., 2003). CPY:GFP is a C-terminal GFP fusion protein of AtCPY (for Arabidopsis carboxypeptidase Y) that has an N-terminal vacuolar sorting signal and is targeted to the lumen of the LV (Rojo et al., 2003). Protoplasts from wild-type and vsr single mutant plants were transformed with AALP:GFP or CPY:GFP, and protein extracts from transformed protoplasts were analyzed by western blotting using anti-GFP antibody. When the vacuolar trafficking is inhibited, vacuolar proteins are secreted into the incubation medium (Sohn et al., 2003; daSilva et al., 2005). Therefore, proteins from protoplast incubation medium were included in the analysis. At 24 h after transformation, AALP:GFP was processed to a smaller form that accumulated to 48% to 49% of total proteins in both Columbia-0 (Col-0) and Wassilewskija wild-type protoplasts. In vsr1, vsr3, and vsr4 mutant protoplasts, although the degree of AALP:GFP secretion varies depending on these vsr mutants, a small proportion (3%–9%) of AALP:GFP was secreted into the incubation medium and the amount of the processed form was slightly reduced (Fig. 1A). CPY:GFP, another luminal vacuolar protein, also produced similar results with AALP:GFP: the amount of the secreted protein was increased to 5% in these vsr single mutant protoplasts and concomitantly the amount of the processed form was slightly reduced (Fig. 1B), indicating that vsr1, vsr3, and vsr4 mutants exhibit a minor defect in protein trafficking to the LV. In a previous study (Zouhar et al., 2010), both AALP (AtAleurain) and AtCPY were not secreted into the apoplasts in vsr1 and vsr4 mutants. One possible explanation for the discrepancy is that both AALP:GFP and AtCPY:GFP were expressed at higher levels in protoplasts than in leaf tissues and the reduced capacity of the vacuolar trafficking pathway resulted from the mutation cannot handle the higher levels of these proteins for vacuolar trafficking.
Figure 1.
Trafficking of vacuolar soluble cargoes is partially inhibited in vsr single mutants. A to C, Western-blot analysis of AALP:GFP, CPY:GFP, or phaseolin trafficking in vsr1, vsr3, and vsr4 mutants. Protoplasts from the wild type and vsr single mutants were transformed with AALP:GFP (A), CPY:GFP (B), or phaseolin (C). After 24 h of incubation, protein extracts were prepared from the protoplasts (P) and from the incubation medium (S). Twenty-five percent of protein extracts from the protoplasts and incubation medium were used for western-blot analysis. To measure the targeting efficiency, the amount of precursor (Pre), processed (Pro), and secreted (S) protein was quantified using multigauge software and expressed as a value relative to the amount of total protein (proteins in protoplasts and incubation medium). Actin detected with antiactin antibody was used as a control for protein leakage from protoplasts and as a loading control. Three independent transformation experiments were performed to obtain an average targeting efficiency with sd. Error bars indicate sd (n = 3). WS, Wassilewskija wild type. D and E, Subcellular localization of AALP:GFP (D) and phaseolin (E) in vsr1 and vsr4 mutant protoplasts. Protoplasts derived from wild-type and vsr1 and vsr4 mutant plants were transformed with AALP:GFP and phaseolin, respectively. At 24 h after transformation, localization of these cargo proteins was examined under the fluorescent microscope. The fluorescent signal of AALP:GFP and CPY:GFP was observed directly. Localization of phaseolin was examined by laser-scanning confocal microscopy. Transformed protoplasts were immunostained with an antiphaseolin antibody followed by an FITC-labeled secondary antibody. Arrow indicates the localization of phaseolin. CH, Chloroplast autofluorescence; WT, Col-0 wild type. Bars = 20 μm.
VSR1 is involved in sorting of PSV proteins (Shimada et al., 2003). Accordingly, we examined the trafficking of phaseolin, a storage protein of Phaseolus vulgaris (common bean) that contains a ctVSS, in vsr1, vsr3, and vsr4 mutant protoplasts. Phaseolin was introduced into protoplasts from leaf tissues of wild-type and vsr single mutant plants, and protein extracts from protoplasts and incubation medium were analyzed by western blotting using antiphaseolin antibody. When phaseolin is targeted to the vacuole in tobacco (Nicotiana tabacum) leaf tissues or Arabidopsis protoplasts derived from leaf tissues, it is processed proteolytically into multiple smaller protein species (Frigerio et al., 1998; Park et al., 2004, 2005). As reported previously, approximately 50% of total expressed phaseolin was processed into smaller protein species in wild-type protoplasts. In vsr1, vsr3, and vsr4 mutant protoplasts, the amount of processed form was slightly reduced and a small proportion of phaseolin (6%–11%) was secreted into the incubation medium (Fig. 1C), indicating that the vsr1, vsr3, and vsr4 mutants have a minor defect in trafficking of phaseolin in leaf cells. These results are consistent with the notion that other members of the VSR family may participate in the trafficking of soluble cargo in vegetative tissues (Zouhar et al., 2010).
To confirm these results at the cellular level, we examined localization of these cargo proteins in vsr1 and vsr4 mutants. AALP:GFP or phaseolin were transformed into wild-type, vsr1, or vsr4 protoplasts, and localization of these cargo proteins was examined directly or by immunohistochemistry using antiphaseolin antibody. Similar to the wild-type protoplasts, the majority of vsr1 and vsr4 mutant protoplasts transformed with AALP:GFP produced vacuolar patterns (Fig. 1D). In vsr1 and vsr4 mutant protoplasts, phaseolin localized primarily to a structure with a diameter of 4 μm (Fig. 1E), as reported previously (Park et al., 2004). These results confirm that although a certain portion of these cargo proteins is secreted into the medium, vsr1, vsr3, and vsr4 mutant plants do not have any substantial effect on the localization pattern.
VSR1, VSR3, and VSR4 Exhibit Functional Redundancy in Their Role for the Vacuolar Trafficking of Soluble Proteins in Vegetative Cells
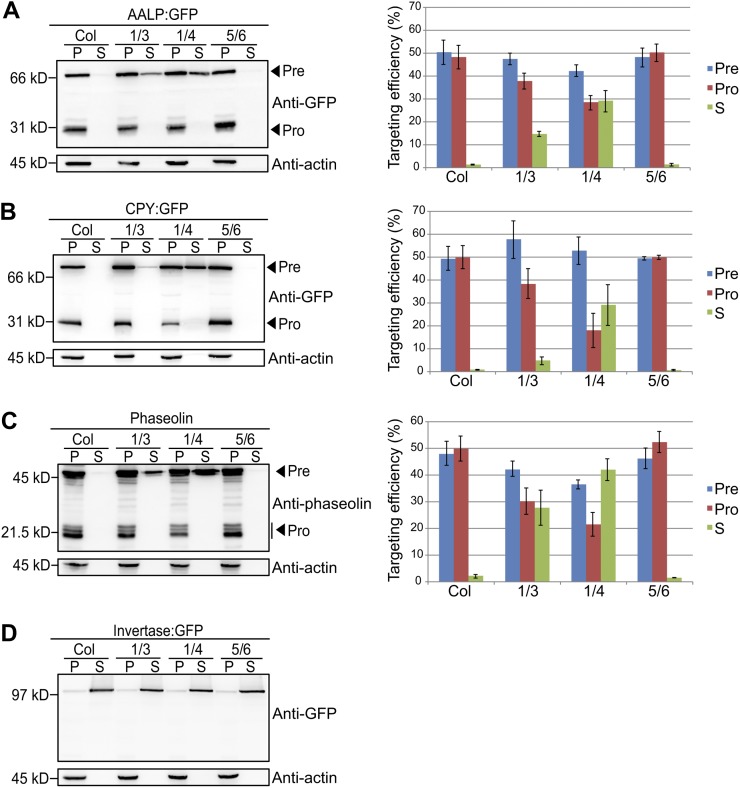
The results shown in Figure 1 prompted us to test whether VSRs have a redundant function in vegetative tissues. Therefore, vacuolar trafficking of AALP:GFP and CPY:GFP was examined in protoplasts from vsr1vsr3, vsr1vsr4, and vsr5vsr6 double mutants. AALP:GFP or CPY:GFP was introduced into wild-type or double mutant protoplasts, and the vacuolar trafficking of these proteins was examined by western blotting using anti-GFP antibody. The amount of AALP:GFP secreted into the medium in vsr1vsr3 and vsr1vsr4 protoplasts was 15% and 29% of total expressed proteins, respectively; concomitantly, the processed form of AALP:GFP decreased to 38% and 29% of total expressed proteins in vsr1vsr3 and vsr1vsr4 protoplasts, respectively (Fig. 2A). These results indicate that the double mutant had much more severe defects in protein trafficking to the LV. In contrast, in vsr5vsr6 mutant protoplasts AALP:GFP was not secreted into the medium and the amount of the processed form was comparable with that of wild-type protoplasts, indicating that vacuolar trafficking of AALP:GFP is not affected in vsr5vsr6 double mutants. CPY:GFP produced similar results to those of AALP:GFP. In vsr1vsr3 and vsr1vsr4 mutant protoplasts, the amount of CPY:GFP that was secreted was 5% and 29% of total expressed proteins, respectively, and the processed forms of CPY:GFP were reduced to 39% and 19% of total proteins, respectively, whereas the vsr5vsr6 mutant did not show any defect in vacuolar trafficking of CPY:GFP (Fig. 2B). Furthermore, a large proportion of AtCPY was secreted into the incubation medium when transiently expressed in vsr1vsr4 mutant protoplasts, but not in wild-type protoplasts (Supplemental Figure S2). These results indicate that VSR1, VSR3, and VSR4, but not VSR5 and VSR6, are involved in vacuolar trafficking of ssVSS-containing proteins in vegetative tissues, and they are functionally redundant in protein trafficking to the LVs.
Figure 2.
VSR1, VSR3, and VSR4 are involved in the vacuolar trafficking of soluble cargo proteins. A to C, Western-blot analysis of AALP:GFP, CPY:GFP, or phaseolin trafficking in protoplasts from vsr1vsr3, vsr1vsr4, and vsr5vsr6 double mutant plants. Protoplasts derived from wild-type and indicated vsr mutant plants were transformed AALP:GFP (A), CPY:GFP (B) or phaseolin (C). After 24 h of incubation, protein extracts were prepared from the protoplasts (P) and from the incubation medium (S). Twenty-five percent of protein extracts from the protoplasts and incubation medium were used for western-blot analysis. Targeting efficiency was expressed as a relative value of the amount of precursor (Pre), processed (Pro), and secreted protein (S) to the amount of total protein. Actin was detected as a control for protein leakage from protoplasts and as a loading control. Three independent transformation experiments were performed to obtain an average targeting efficiency. Error bars indicate sd (n = 3). 1/3, vsr1vsr3 mutant; 1/4, vsr1vsr4 mutant; 5/6, vsr5vsr6 mutant. D, Western-blot analysis of invertase:GFP in the wild type and vsr double mutants. Protoplasts were transformed with invertase:GFP and protein extracts were prepared from the protoplasts (P) and from the incubation medium (S). Twenty-five percent of protein extracts from the protoplasts and incubation medium were used for western-blot analysis. [See online article for color version of this figure.]
We also examined trafficking of phaseolin in these double mutants. Phaseolin was introduced into protoplasts from leaf tissues of these double-mutant plants, and its trafficking was examined by western-blot analysis using antiphaseolin antibody. Similar to the trafficking patterns observed with ssVSS-containing vacuolar proteins, trafficking of phaseolin was defective in both vsr1vsr3 and vsr1vsr4, but not vsr5vsr6, mutants. In vsr1vsr3 and vsr1vsr4 mutant protoplasts, 28% and 42% of phaseolin was secreted into the incubation medium, respectively, whereas in vsr5vsr6 mutant protoplasts, trafficking of phaseolin was as efficient as that observed in wild-type protoplasts (Fig. 2C). These results confirm that VSR1, VSR3, and VSR4, but not VSR5 and VSR6, are involved in the trafficking of phaseolin. Together, these results suggest that VSR1, VSR3, and VSR4 are redundantly involved in trafficking of both LV and PSV proteins in leaf cells. As a control, we examined whether these vsr mutants have any effect on the secretory pathway. Protoplasts from wild-type, vsr1vsr3, vsr1vsr4, and vsr5vsr6 mutant plants were transformed with invertase:GFP to express a chimeric protein of secretory invertase and GFP (Kim et al., 2005), and its trafficking was examined by western-blot analysis. Figure 2D shows that invertase:GFP was efficiently secreted in vsr double mutants including vsr5vsr6, confirming that these VSRs are specific to trafficking of proteins to the LV and PSV.
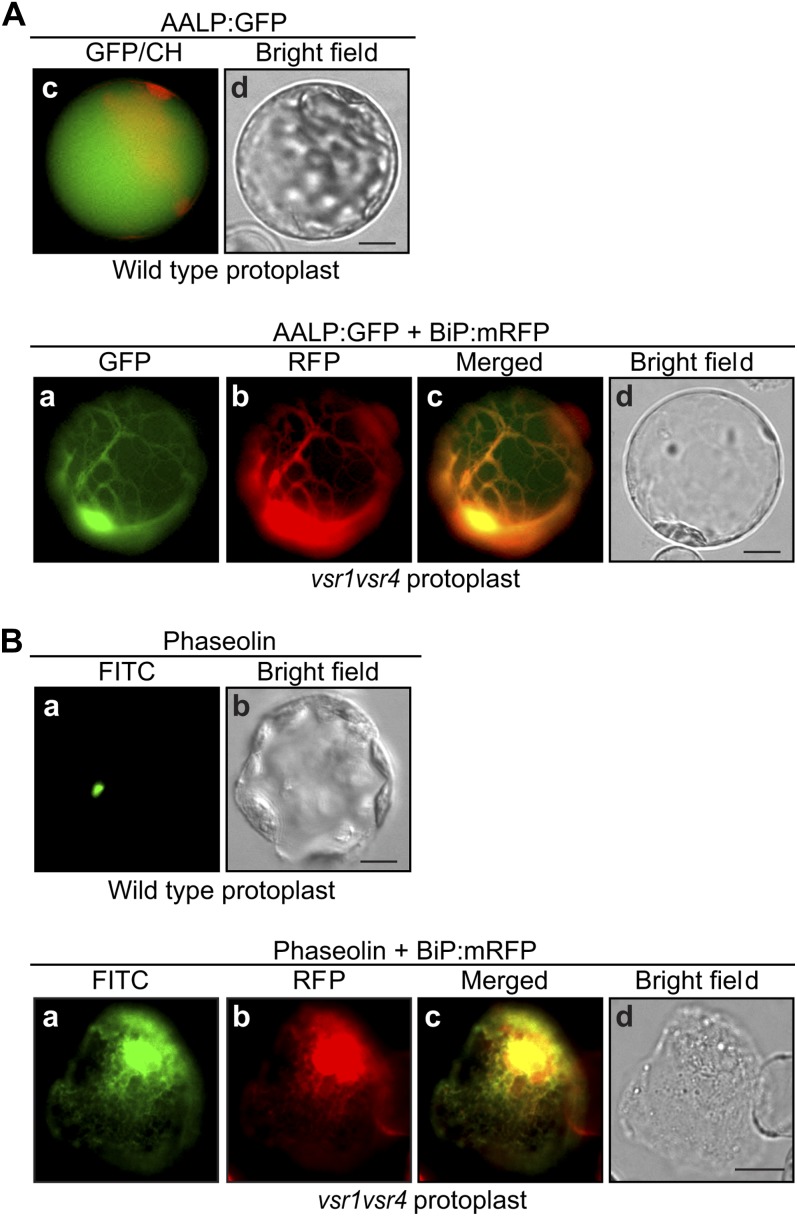
To obtain independent evidence for the role of VSR1 and VSR4, localization of AALP:GFP was examined in the vsr1vsr4 mutant. AALP:GFP and ER-localized chaperone binding protein:monomeric red fluorescent protein (BiP:mRFP) were introduced into vsr1vsr4 mutant protoplasts, and localization of these proteins was examined under a fluorescence microscope. In vsr1vsr4 mutant protoplasts, the majority of protoplasts produced the vacuolar pattern and also network pattern, which colocalized with an ER marker protein, BiP:mRFP (Fig. 3A), indicating that trafficking of AALP:GFP is inhibited and some proportion of AALP:GFP localizes to the ER. Next, we examined the localization of phaseolin in vsr1vsr4 double mutant plants. vsr1vsr4 double mutant protoplasts were cotransformed with phaseolin and BiP:mRFP, and the localization of phaseolin was examined by immunostaining with antiphaseolin antibody. The majority of transformed protoplasts displayed the network pattern instead of the speckle pattern. The network pattern of phaseolin overlapped with that of BiP:mRFP at the ER (Fig. 3B), indicating that phaseolin localizes primarily to the ER. These data confirm that VSR1 and VSR4 function redundantly in trafficking of both AALP:GFP and phaseolin to the LV and the subcellular structure that gives the speckle pattern, respectively.
Figure 3.
Vacuolar proteins accumulate in the ER in vsr1vsr4 double mutant plants. A, Subcellular localization of AALP:GFP in the vsr1vsr4 double mutant protoplasts. Wild-type protoplasts were transformed with AALP:GFP and vsr1vsr4 mutant protoplasts were cotransformed with AALP:GFP and BiP:mRFP. At 24 h after transformation, localization of these proteins was examined by fluorescence microscopy. CH, chloroplast autofluorescence. Bar = 20 µm. B, Subcellular localization of phaseolin in the vsr1vsr4 double mutant protoplasts. Wild-type protoplasts were transformed with phaseolin and vsr1vsr4 protoplasts were cotransformed with phaseolin and BiP:mRFP. Localization of phaseolin was determined by immunostaining with an antiphaseolin antibody followed by an FITC-labeled secondary antibody. BiP:mRFP was observed directly. Bar = 20 µm.
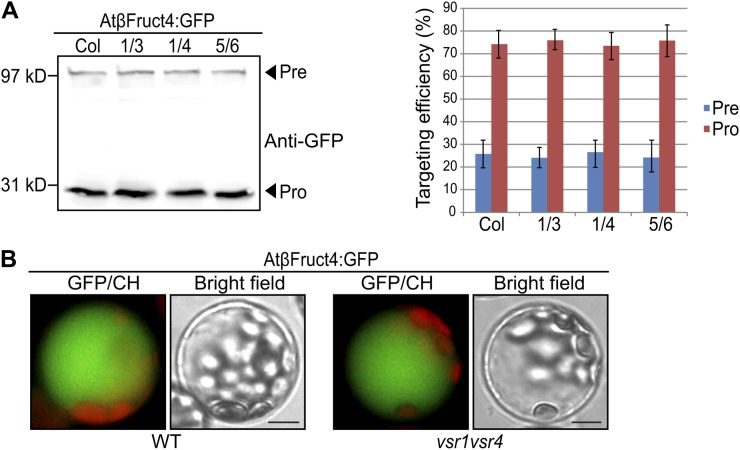
Next, we tested whether VSRs are also involved in trafficking of membrane proteins destined for the tonoplasts of the LV. We examined trafficking of AtβFruct4:GFP, a chimeric protein of AtβFructosidase4 and GFP in vsr double mutants (Jung et al., 2011). It has been proposed that AtβFruct4:GFP is delivered and incorporated into the tonoplast first, and then subsequently released into the lumen of the LV by proteolytic processing (Jung et al., 2011). As reported previously, in wild-type protoplasts AtβFruct4:GFP was efficiently targeted to the central vacuole when examined by western-blot analysis using anti-GFP antibody. In addition, in vsr double-mutant protoplasts AtβFruct4:GFP was targeted to the LV as efficiently as it was in wild-type protoplasts (Fig. 4A), indicating that VSR1 and VSR4 are not involved in trafficking of membrane proteins to the LV. To confirm this, we examined the localization of AtβFruct4:GFP in vsr1vsr4 mutant protoplasts. The majority of transformed vsr1vsr4 mutant protoplasts gave green fluorescent signals in the central vacuole (Fig. 4B), confirming that it is targeted to the LV. These results suggest that VSRs are not involved in the vacuolar trafficking of membrane proteins.
Figure 4.
Vacuolar trafficking of AtβFruct4:GFP is not inhibited in vsr mutant plants. A, Western-blot analysis of AtβFruc4t:GFP trafficking in vsr1vsr3, vsr1vsr4, and vsr5vsr6 double mutants. Protoplasts from wild-type and indicated vsr mutant plants were transformed with AtβFruct4:GFP. At 24 h after transformation, proteins extracted from protoplasts were analyzed by western blotting using anti-GFP antibody. Targeting efficiency was expressed as a relative value of the amount of precursor (Pre) and processed protein (Pro) to the amount of total proteins. Error bars indicate sd (n = 3). Col, Col-0 wild type; 1/3, vsr1vsr3 mutant; 1/4, vsr1vsr4 mutant; 5/6, vsr5vsr6 mutant. B, Subcellular localization of AtβFruct4:GFP in vsr1vsr4 mutant protoplasts. Protoplasts derived from the wild type and vsr1vsr4 were transformed with AtβFruct4:GFP. Localization of AtβFruct4:GFP was examined under a fluorescent microscope. WT, Col-0 wild-type. Bars = 20 μm.
Transient Expression of VSR1 and VSR4, But Not VSR5, Rescues the Defect in Trafficking of Both Lytic and PSV Proteins in Protoplasts of vsr1vsr4 Double Mutant Plants
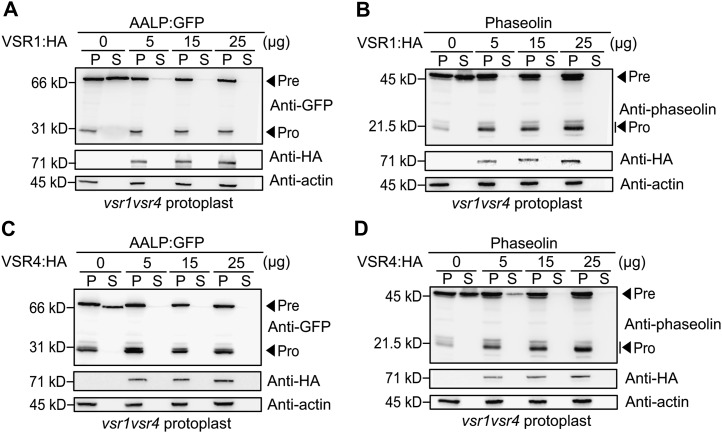
To further test the role of VSRs in vacuolar trafficking and their functional redundancy, we examined whether VSR1 can complement the defect in vacuolar trafficking of AALP:GFP in protoplasts from vsr1vsr4 double mutant plants. Varying amounts of the epitope hemagglutinin (HA)-tagged VSR1 (VSR1:HA) plasmid were introduced into vsr1vsr4 mutant protoplasts together with AALP:GFP, and the vacuolar trafficking of AALP:GFP was analyzed by western blotting using anti-GFP antibody. The C-terminal HA epitope-tagged VSR1 (VSR1:HA) colocalizes primarily with GFP:VSR1 at the PVC, indicating that the HA epitope tag at the C terminus does not interfere with its proper localization of VSRs in protoplasts (Kim et al., 2005). The amount of secreted AALP:GFP was gradually decreased with increasing amounts of VSR1:HA (Fig. 5A), indicating that transiently expressed VSR1:HA rescues the defect in the vacuolar trafficking of AALP:GFP in the vsr1vsr4 double mutant background. Similarly, transiently expressed VSR1:HA reduced the amounts of secreted CPY:GFP and phaseolin in a dose-dependent manner (Figure 5B; Supplemental Figure S3A).
Figure 5.
Expression of VSR1 or VSR4 rescues the vacuolar trafficking defect in vsr1vsr4 mutant protoplasts. A and B, Effect of VSR1:HA expression on the trafficking of AALP:GFP and phaseolin in vsr1vsr4 double mutants. Protoplasts derived from vsr1vsr4 mutant plants were cotransformed with the indicated amount of VSR1:HA together with either AALP:GFP (A) or phaseolin (B). At 24 h after transformation, protein extracts were prepared from transformed protoplasts (P) and incubation medium (S) and analyzed by western blotting using anti-GFP or antiphaseolin antibody. Expression of VSR1:HA was detected by anti-HA antibody. Actin was detected as a control for protein leakage from protoplasts and as a loading control. Pre, precursor form; Pro, processed form. C and D, Effect of VSR4:HA expression on the trafficking of AALP:GFP and phaseolin in the vsr1vsr4 double mutants. Protoplasts derived from vsr1vsr4 mutant plants were cotransformed with the indicated amount of VSR4:HA together with either AALP:GFP (C) or phaseolin (D), and protein extracts prepared from transformed protoplasts (P) and incubation medium (S) were analyzed by western blotting using anti-GFP or antiphaseolin antibody. Expression of VSR4:HA was detected by anti-HA antibody. Actin was detected as a control for protein leakage from protoplasts and as a loading control. Pre, precursor form; Pro, processed form.
In a reciprocal experiment, we examined whether VSR4 can complement the vacuolar trafficking defect of vsr1vsr4 double mutant plants. Varying amounts of the VSR4:HA plasmid were introduced into vsr1vsr4 mutant protoplasts together with AALP:GFP, CPY:GFP, or phaseolin, and their trafficking efficiency was determined by western-blot analysis using anti-GFP or antiphaseolin antibody. At 5 μg of VSR4:HA, AALP:GFP was no longer secreted into the medium (Fig. 5C). CPY:GFP and phaseolin showed similar recovery in their vacuolar trafficking from coexpression of VSR4:HA, confirming that VSR4 complements the defect in trafficking of vacuolar and PSV proteins in vsr1vsr4 double mutant plants (Figure 5D; Supplemental Figure S3B). These results strongly suggest that VSR1 and VSR4 are functionally redundant in trafficking of both ssVSS- and ctVSS-containing cargo proteins in vegetative tissues.
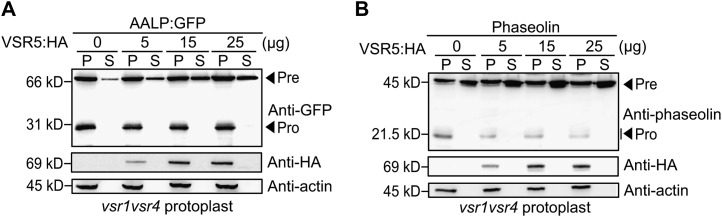
Among the double mutants, vsr5vsr6 protoplasts did not have any noticeable defect in the trafficking of vacuolar and PSV proteins. This raised the possibility that VSR5 and VSR6 are not involved in the trafficking of these proteins. To test this possibility, we examined whether VSR5 can complement the defect in the trafficking of vacuolar and PSV proteins in protoplasts from vsr1vsr4 double-mutant plants. AALP:GFP, CPY:GFP, or phaseolin were introduced into vsr1vsr4 protoplasts together with increasing amounts of VSR5:HA, and trafficking of these proteins was determined by western-blot analysis. VSR5:HA did not complement the defect in vsr1vsr4 mutants (Fig. 6, A and B; Supplemental Figure S4). In fact, overexpression of VSR5:HA aggravated the defect in the trafficking of these proteins and caused further secretion into the medium in a dose-dependent manner, indicating that VSR5 cannot complement the defect in trafficking of these proteins in the vsr1vsr4 mutant. However, it is intriguing that high levels of VSR5 aggravate the trafficking defects in the vsr1vsr4 mutant. One possible explanation is that VSR5 may compete with VSR1, VSR3, and VSR4 for the molecular machineries for receptor recycling. The sorting receptors recycle back to the TGN via the retromer complex (Seaman, 2005). In Arabidopsis, VSP35, a component of the retromer complex, interacts with VSR and lack of VPS35 inhibits the efficient trafficking of storage proteins to the PSV (Oliviusson et al., 2006; Yamazaki et al., 2008). Consistent with this notion, the amino acid sequence of the C-terminal domain of VSR5 and VSR6 is highly similar to that of VSR1, VSR3, and VSR4.
Figure 6.
Ectopic expression of VSR5:HA increases the secretion of cargo proteins in vsr1vsr4 double mutant protoplasts. A and B, Effect of VSR5:HA expression on the trafficking of AALP:GFP and phaseolin in the vsr1vsr4 double mutant. Protoplasts derived from vsr1vsr4 mutant plants were cotransformed with the indicated amount of VSR5:HA together with either AALP:GFP (A) or phaseolin (B). Protein extracts from transformed protoplasts (P) and incubation medium (S) were analyzed by western blotting using anti-GFP or antiphaseolin antibody. Expression of VSR5:HA was detected by anti-HA antibody. Actin was detected as a control for protein leakage from protoplasts and as a loading control. Pre, precursor form; Pro, processed form.
The Luminal Domain of VSRs Determines the Specificity of VSRs in Vacuolar Trafficking
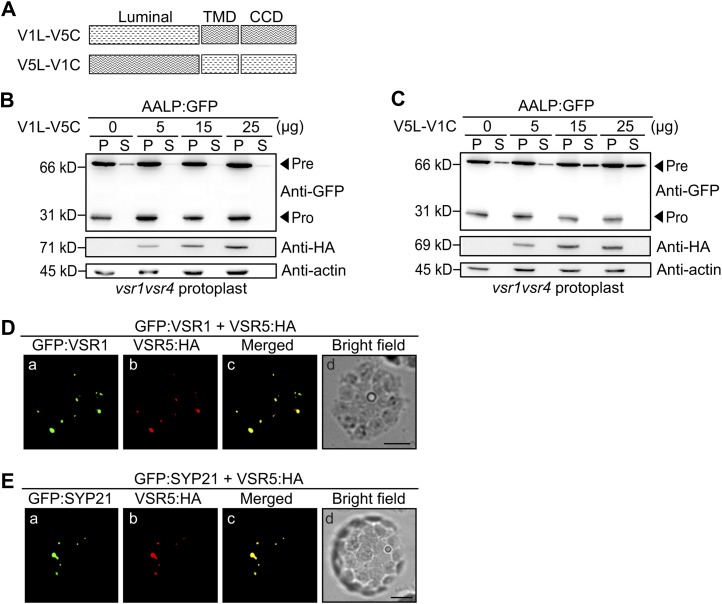
The luminal proteinase-associated (PA) domain contains a binding site for the vacuolar targeting signal (Cao et al., 2000). The seven AtVSRs are divided into two groups based on the sequence homology of the PA domains (Zouhar et al., 2010; De Marcos Lousa et al., 2012): one group with VSR1, VSR2, VSR3, and VSR4 and the other group with VSR5, VSR6, and VSR7. Therefore, it is possible that the specificity of VSRs in vacuolar trafficking may be determined by the luminal domain. To test this idea, we generated domain-swapping mutants between VSR1 and VSR5 by exchanging their luminal domains, denoted as V1N-V5C:HA and V5N-V1C:HA (Fig. 7A). These constructs were examined for complementation of the defect in protein trafficking in the vsr1vsr4 mutant. V1N-V5C:HA or V5NV1C:HA were cotransformed into protoplasts from vsr1vsr4 mutants with AALP:GFP, and vacuolar trafficking of AALP:GFP was examined by western-blot analysis using anti-GFP antibody. V1NV5C:HA, but not V5N-V1C:HA, efficiently complemented the defect in trafficking of AALP:GFP in the vsr1vsr4 double mutant protoplasts (Fig. 7, B and C). Similar results were obtained with the PSV protein phaseolin (Supplemental Figure S5, A and B). These results strongly suggest that the cargo specificity is determined by the luminal domain of VSRs, and the luminal domains of VSR1, VSR3, and VSR4 encode different cargo specificity from that of VSR5 and VSR6.
Figure 7.
The luminal domain of VSRs determines the cargo specificity for vacuolar trafficking. A, Illustration of chimeric constructs of V1N-V5C:HA and V5N-V1C:HA, in which the luminal domain was swapped between VSR1 and VSR5. V1N-V5C:HA, Fusion of VSR1 luminal domain (amino acid residues 1–563) to the transmembrane domain (TMD) and C-terminal cytosolic domain (CCD) of VSR5:HA; V5N-V5C, fusion of VSR5 luminal domain (amino acid residues 1–562) to the TMD and CCD of VSR1. B and C, Effect of V1N-V5C:HA and V5N-V1C:HA on the vacuolar trafficking of AALP:GFP in vsr1vsr4 mutant protoplasts. Protoplasts derived from vsr1vsr4 mutant plants were cotransformed with the indicated amount of V1N-V5C:HA (B) or V5N-V1C:HA (C) together with AALP:GFP. Protein extracts from transformed protoplasts (P) and incubation medium (S) were analyzed by western blotting using anti-GFP antibody. Expression of V1N-V5C:HA and V5N-V1C:HA was detected by anti-HA antibody. Actin was detected as a control for protein leakage from protoplasts and as a loading control. Pre, precursor form; Pro, processed form. D, Colocalization of GFP:VSR1 and VSR5:HA. Wild-type protoplasts were transformed with GFP:VSR1 and VSR5:HA, and the localization of VSR5:HA was examined by immunostaining with anti-HA antibody followed by TRITC-labeled anti-rabbit IgG antibody. GFP:VSR1 was observed directly. Bars = 20 μm. E, Colocalization of GFP:SYP21 and VSR5:HA. Wild-type protoplasts were transformed with GFP:SYP21 and VSR5:HA. Transformed protoplasts were immunostained with anti-HA antibody followed by TRITC-labeled anti-rabbit IgG antibody. Localization was examined under a fluorescent microscope. GFP:SYP21 was observed directly. Bars = 20 μm.
An alternative possibility is that these two groups of VSRs may localize to different organelles. However, previous studies show that all seven VSRs localize to the PVC (Li et al., 2002; Tse et al., 2004). To rule out this possibility, we examined the localization of VSR1 and VSR5 in protoplasts. VSR5:HA was cotransformed into wild-type protoplasts together with GFP:VSR1 or GFP:SYP21, and the localization of these proteins was examined by immunostaining using anti-HA antibody. VSR5:HA exhibited a punctate staining pattern that closely overlapped with that of both GFP:AtVSR1 and GFP:SYP21 (Fig. 7, D and E). This result further supports the idea that the failure to complement the defect of protein trafficking in the vsr1vsr4 double mutants by VSR5 likely results from the difference in cargo specificity in the PA domains between VSR5 and VSR1 or VSR4.
rmr Double Mutants Do Not Display a Defect in the Vacuolar Trafficking of Soluble Cargo Proteins
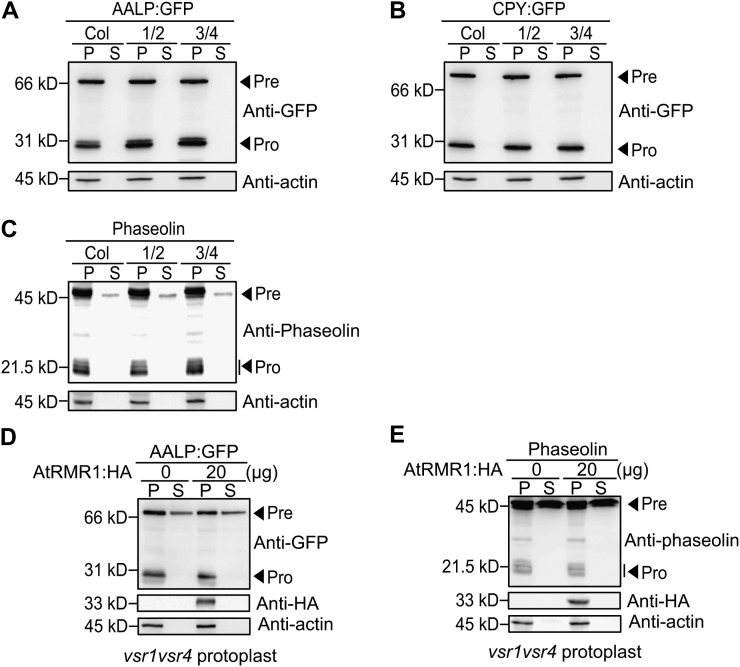
Previous studies suggested that RMR1 is a cargo receptor for storage proteins (Park et al., 2005; Shen et al., 2011). To gain insight into the role of RMRs in the vacuolar trafficking in vegetative tissues, we examined the trafficking of soluble cargoes in protoplasts from rmr1rmr2 and rmr3rmr4 double mutants. AALP:GFP, CPY:GFP, or phaseolin were introduced into wild-type or mutant protoplasts, and the vacuolar trafficking of these proteins was examined by western blotting using anti-GFP or antiphaseolin antibody. In rmr1rmr2 and rmr3rmr4 mutant protoplasts, trafficking of AALP:GFP or CPY:GFP was as efficient as that observed in wild-type protoplasts. These proteins were not secreted into the medium and the amount of the processed form was comparable with that of wild-type protoplasts (Fig. 8, A and B). Similar results were obtained with the ctVSS-containing cargo protein phaseolin. rmr1rmr2 and rmr3rmr4 mutant did not exhibit any defect in the trafficking of phaseolin (Fig. 8C). Thus, these results from the rmr mutants did not agree with those of previous studies where overexpression of a dominant negative form of AtRMR1 was used (Park et al., 2005). One possible explanation is that seven AtRMR isoforms are functionally redundant and mutations in two out of seven may not cause any significant defect in vacuolar trafficking in leaf protoplasts.
Figure 8.
rmr double mutants do not display a defect in the vacuolar trafficking of soluble cargo proteins. A to C, Western-blot analysis of AALP:GFP, CPY:GFP, or phaseolin trafficking in protoplasts from rmr1rmr2 and rmr3rmr4 mutant plants. Protoplasts from wild-type and rmr mutant plants were transformed AALP:GFP (A), CPY:GFP (B) or phaseolin (C). Protein extracts from protoplasts (P) and incubation medium (S) were analyzed by western blotting using anti-GFP or antiphaseolin antibody. Actin was detected using antiactin antibody as a control for protein leakage from protoplasts and as a loading control. Pre, precursor form; Pro, processed form. D and E, Failure to complement the vacuolar trafficking defect in the vsr1vsr4 double mutants by overexpression of AtRMR1:HA. Protoplasts from vsr1vsr4 mutant plants were cotransformed with the indicated amount of AtRMR1:HA together with either AALP:GFP (A) or phaseolin (B). Protein extracts from protoplasts (P) and incubation medium (S) were analyzed by western blotting using anti-GFP or antiphaseolin antibodies. Expression of AtRMR1:HA was detected by anti-HA antibody. Actin was detected using antiactin antibody as a control for protein leakage from protoplasts and as a loading control. Pre, precursor form; Pro, processed form.
It has been shown that both RMR1 and VSR4 interact with ctVSS-containing peptide (Suen et al., 2010; Shen et al., 2011). Thus, it is possible that RMR1 and VSRs may cooperate in the trafficking of phaseolin. To test this idea, we examined whether AtRMR1 can complement the vacuolar trafficking defect of the vsr1vsr4 double mutant. AtRMR1:HA was introduced into vsr1vsr4 mutant protoplasts with AALP:GFP or phaseolin, and the vacuolar trafficking of these proteins was examined by western blotting using anti-GFP or antiphaseolin antibody. As shown in Figure 8, D and E, expression of AtRMR1:HA did not rescue the defects in trafficking of AALP:GFP and phaseolin in the vsr1vsr4 mutant, indicating that AtRMR does not complement the vsr mutations in vacuolar trafficking. It is possible that AtRMRs and AtVSRs may play a role at different stages of vacuolar trafficking in leaf cells.
DISCUSSION
The seven Arabidopsis VSRs are divided into three classes based on sequence homology: class 1 contains VSR1 and VSR2; class 2 contains VSR3 and VSR4; and class 3 contains VSR5, VSR6, and VSR7 (De Marcos Lousa et al., 2012). Among the seven VSRs, the members of class 1 and 2 are more closely related to each other, whereas members of class 3 are more distantly related to members of class 1 and 2. This study provided evidence that three VSRs belonging to class 1 and 2 are involved in trafficking of soluble proteins to the LV in vegetative cells, including VSR1, VSR3, and VSR4. However, no evidence for involvement in trafficking to the LV was found for the class 3 members VSR5 and VSR6. When examined in vsr1vsr3 and vsr1vsr4 mutant protoplasts, two LV proteins, AALP:GFP and CPY:GFP, and a PSV protein, phaseolin, were secreted into the incubation medium. These proteins also accumulated to higher levels as unprocessed forms in vsr1vsr3 and vsr1vsr4 mutant protoplasts, and these trafficking defects were complemented by VSR1 or VSR4 but not VSR5. The unprocessed proteins did not accumulate to higher levels in the double mutant vsr5vsr6. The underlying mechanism by which VSR1, VSR3, and VSR4, but not VSR5 and VSR6, are involved in vacuolar trafficking was tested by investigating two different possibilities. One possibility was a difference in their subcellular localization. A previous study showed that VSRs are localized to the plasma membrane in growing pollen tubes (Wang et al., 2011b). However, in protoplasts from leaf tissues, both VSR1 and VSR5 localize primarily to the PVC, thus arguing against this possibility. For the second possibility, we tested whether these two different groups of VSRs differ in their recognition of cargoes by using luminal domain-swapping mutants. The results show that replacement of the luminal domain of VSR5 with that of VSR1 can complement vsr1vsr4 double mutants, whereas replacement of the luminal domain of VSR1 with that of VSR5 cannot complement the double mutant plants. These results support the second possibility and demonstrate that the luminal domains of VSR1, VSR3, and VSR4, but not VSR5 and VSR6, can recognize both LV and PSV cargoes. The luminal domains of BP80 of pea, a close homolog of VSR3 and VSR4, and Arabidopsis VSR1 contain binding sites for ssVSS and ctVSS (Kirsh et al., 1994; Cao et al., 2000; Shimada et al., 2003). Current data do not provide information about the functional role of VSR5 and VSR6 in plants.
Our study provides evidence that VSR1, VSR3, and VSR4 are involved in LV trafficking in vegetative cells. Previous studies showed that these three VSRs, VSR1, VSR3, and VSR4, play a role in trafficking of proteins to the PSV (Shimada et al., 2003; Zouhar et al., 2010). The vsr1 mutant plants exhibit secretion of PSV proteins to the apoplasts in seeds (Shimada et al., 2003). Although vsr3 and vsr4 single mutants did not show any secretion of PSV proteins, vsr1vsr3 and vsr1vsr4 double mutant plants exhibit defects in protein trafficking to the PSV in seeds (Zouhar et al., 2010). The secretion of PSV proteins into the apoplasts in seeds was not observed in vsr5 and vsr6 single mutants or vsr5vsr6 double mutants. Similarly, protoplasts from leaf tissues of vsr1vsr3 and vsr1vsr4, but not vsr5vsr6 double mutants, also exhibited defects in trafficking of the LV proteins AALP:GFP and CPY:GFP, and the PSV protein phaseolin. These proteins were secreted into the incubation medium, and the unprocessed full-length proteins increased to high levels. Moreover, the results from trafficking assays of both LV and PSV proteins in protoplasts of vsr single and double mutants support the earlier notion that VSR1 and VSR2 are involved in LV trafficking (daSilva et al., 2005; Foresti et al., 2010; Kim et al., 2010).
The results from the trafficking assay in protoplasts of mutant leaf tissues are both consistent and inconsistent with those from intact mutant plants. Endogenous AtAleurain (AALP) was secreted into the apoplasts in leaf tissues of vsr1vsr4 double mutants (Zouhar et al., 2010), which was consistent with our results in vsr mutant protoplasts. However, AtAleurain was not secreted into apoplasts in vsr1 or vsr4 single mutants, which was in contrast with the results showing that AALP:GFP was secreted into the incubation medium at higher levels in vsr protoplasts than in wild-type protoplasts. Furthermore, our results showed that CPY:GFP is secreted in protoplasts derived from the vsr single mutants and from the vsr1vsr3 and vsr1vsr4 double mutant plants. Thus, the secretion of CPY:GFP in these mutants is in contrast with the behavior of CPY in intact mutant plants (Zouhar et al., 2010). Endogenous CPY was not secreted in intact vsr1vsr4 double mutant plants, nor was it secreted in intact vsr1 and vsr4 single mutant plants. A simple explanation for this discrepancy in the behavior of CPY and AtAleurain (AALP) in intact plants versus protoplasts is that GFP-fused constructs with AtAleurain (AALP) and CPY behave differently from their untagged endogenous proteins. Another explanation is that the amount of proteins loaded into the vacuolar trafficking pathways is different in intact plants and protoplasts, or that the amount of proteins loaded into the protoplasts was higher than that in intact plants. Protein levels can easily be overexpressed in transformed protoplasts (Denecke et al., 2012). In intact plants, the capacity of vacuolar trafficking pathways in the vsr single and double mutants is still large enough to handle the amount of expressed CPY proteins, although the capacity is reduced in the mutants by the loss of VSR1, VSR4, or both. Similarly, the capacity of the vacuolar trafficking pathway in the vsr single mutants is still large enough to handle the amount of AtAleurain, whereas that in the vsr1vsr4 double mutant is further reduced to a level so that the amount of expressed AtAleurain could be greater than the capacity of the vacuolar trafficking pathways. This suggests that minor changes in the capacity of vacuolar trafficking pathways cannot be detected if the amount of proteins transported to the vacuole is still within the capacity of trafficking pathways. In contrast, a change in the capacity of the vacuolar trafficking pathways in protoplasts can be easily assessed by applying varying amounts of cargo proteins.
Among the seven VSRs that have been identified in Arabidopsis, the results show that VSR1, VSR3, and VSR4 are involved in both lytic and PSV trafficking pathways. This raises an intriguing question of how proteins are specifically transported to the two different vacuole types, the LV and the PSV, because other molecular machineries involved in vacuolar trafficking differentiate between these two types of vacuoles. For example, the VTI11 isoform of VTI (a v-SNARE localized involved in trafficking from the TGN to the PVC) is primarily involved in the LV pathway, whereas the VTI12 isoform appears to be primarily involved in the PSV pathway, although they are functionally redundant to a certain degree (Surpin et al., 2003; Sanmartín et al., 2007). Among three VPS35 isoforms, a certain degree of specificity can be observed between the lytic and PSV pathways; VPS35a is primarily involved in the LV pathway and VPS35c is primarily involved in the PSV pathway (Yamazaki et al., 2008; Hashiguchi et al., 2010). The epsin-like monomeric adaptors EpsinR1 and EpsinR2 show specificity toward the VTI isoforms; EpsinR1 interacts with VTI11, whereas EpsinR2 interacts with VTI12 (Song et al., 2006; Lee et al., 2007). Currently, it is not clearly understood how this occurs. One possibility is that the trafficking of proteins to these two vacuoles may not require specific sorting because the PSV is specific to seed cells, whereas the LV exists in vegetative tissue cells (Frigerio et al., 2008; Zouhar and Rojo, 2009; De Marcos Lousa et al., 2012). Therefore, the cell type-specific presence of these two vacuole types may be enough to confer specific targeting of proteins to these two organelles. However, there are also reports showing that two different types of vacuoles exist in single cells in vegetative tissues. The presence of a structure to which phaseolin is targeted in protoplasts from leaf tissues of Arabidopsis has been demonstrated, and localization of two different potassium channel isoforms in two different vacuoles has been reported in rice (Oryza sativa; Park et al., 2004, 2005; Isayenkov et al., 2011). Another possibility is that other factors are involved in specificity determination. However, current evidence in support of this possibility is lacking. Further studies are necessary to elucidate how the specificity is determined.
MATERIALS AND METHODS
Plant Material and Growth
vsr and rmr mutants have been described previously (Zouhar et al., 2010). The vsr1 mutant is in the Wassilewskija background and the other mutants are in the Col-0 background. Plants were grown on B5 plates in a culture room under a 16-h-light/8-h-dark cycle. Leaf tissues of 2- to 3-week-old plants were used to isolate protoplasts.
Construction of Plasmid DNAs
AtCPY (At3g10410) was isolated by PCR from an Arabidopsis (Arabidopsis thaliana) complementary DNA (cDNA) library using specific primers CPY-F and CPY-R. The PCR products was digested with XbaI/BamHI and ligated to a pUC-based expression vector. To generate the AtCPY:GFP fusion construct, the termination codon of AtCPY was deleted by PCR using CPY-F and CPY-G-R primers, and fused to the N terminus of the GFP-coding region. For the construction of AtVSR4:HA, full-length AtVSR4 cDNA obtained from the RIKEN Arabidopsis cDNA collection (Seki et al., 1998; 2002) was amplified by PCR using VSR4-F and VSR5-R primers. AtVSR5 (At2g34940) was isolated by PCR from an Arabidopsis cDNA library using specific primers VSR5-F and VSR5-R. The PCR products were digested with XbaI/BamHI and ligated to a pUC-based expression vector containing the 35S cauliflower mosaic virus promoter, HA epitope, and nopaline synthase terminator. VSR1 and VSR5 domain-swap mutants were generated using a two-step PCR strategy. The luminal domain of VSR1 was amplified using the VSR1-F and V15-R primers. Likewise, the C-terminal domain of VSR5 was amplified using primer HA-R and V15-F containing an extended region complementary to the 3′ end of the VSR1 fragment. These two fragments were the templates for a second round of PCR using VSR1-F and HA-R primers, resulting in V1N-V5C-containing nucleotides 1–1,689 of VSR1 and nucleotides 1,687–1,857 of VSR5. V5N-V1C was produced using the same approach. V5N-V1C consisted of nucleotides 1–1,686 of VSR5 and nucleotides 1,690–1,872 of VSR1. The PCR products were digested with XbaI/XhoI and ligated to a pUC-based expression vector. The primer sequences used to prepare the constructs are shown in Supplemental Table S1. The sequence of all constructs was confirmed by DNA sequencing.
Transient Expression and Microscopy
The plasmids used in protoplast transformation were purified using Qiagen columns according to the manufacturer’s protocol. Arabidopsis protoplasts were prepared from leaf tissues, and purified DNA plasmids were introduced into protoplasts by polyethylene glycol-mediated transformation (Jin et al., 2001). Expression of these constructs was monitored at various time points after transformation. Images were obtained using a fluorescence microscope (Axioplan 2; Carl Zeiss) equipped with a 40×/0.75 objective (Plan-NEOFLUAR) and a cooled charge-coupled device camera (Senicam; PCO Imaging). The filter sets used were XF116 (exciter, 474AF20; dichroic, 500DRLP; emitter, 510AF23) and XF33/E (exciter, 535DF35; dichroic, 570DRLP; emitter, 605DF50; Omega, Inc.) for GFP/fluorescein isothiocyanate (FITC) and RFP/tetramethyl rhodamine isothiocyanate (TRITC), respectively. For the laser-scanning confocal microscopy, transformed protoplasts were observed with a Zeiss LSM 510 META laser-scanning confocal microscope (Carl Zeiss). Excitation/emission wavelengths were 488/505 to 530 nm for GFP, and 543/560 to 615 nm for RFP. Captured images were processed using Adobe Photoshop CS5.
Immunohistochemistry
Transformed protoplasts were used for immunohistochemistry. Transformed protoplasts were allowed to adhere to poly-l-Lys-coated slides (Sigma) for 30 min, fixed in W6 buffer (154 mm NaCl, 125 mm CaCl2, 2.5 mm maltose, 5 mm KCl, 10 mm HEPES, pH 7.2) containing 4% paraformaldehyde for 60 min, and permeabilized in TSW buffer (10 mm Tris-HCl, pH 7.4, 0.9% NaCl, 0.25% gelatin, 0.02% SDS, 0.1% Triton X-100) for 10 min. Fixed protoplasts were subsequently incubated overnight at 4°C with anti-HA (Roche) or antiphaseolin (Frigerio et al., 1998) antibodies in the same buffer. After washing three times with TSW buffer, protoplasts were incubated for 1 h at room temperature with TRITC-conjugated goat anti-rat, anti-mouse IgG or FITC-conjugated goat anti-rabbit IgG secondary antibodies (Zymed Laboratories). After another three washes in TSW buffer, protoplasts were mounted in Mowiol (Hoechst), containing 2.5% 1,4-diazobicyclo-[2.2.2]-octane (Sigma).
Protein Preparation and Western-Blot Analysis
Transformed protoplasts were harvested at 24 h after transformation and resuspended in lysis buffer (25 mm HEPES-NaOH, pH 7.4, 150 mm NaCl, 3 mm EDTA, 2 mm EGTA, and complete protease inhibitor cocktail [Roche]). Protoplasts were lysed by brief sonication and subjected to centrifugation at 10,000g at 4°C for 10 min to remove debris. To prepare proteins present in the medium, cold TCA (100 μL) was added to the medium (1 mL) and protein aggregates were precipitated by centrifugation at 10,000g at 4°C for 15 min. After washing with acetone, protein pellet was dissolved in a solution of 0.1 n NaOH. Proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes using blotting apparatuses (Hoefer). For the western blotting, monoclonal anti-GFP (Clontech), antiphaseolin (Frigerio et al., 1998), monoclonal antiactin (MP Biomedicals), and anti-AtCPY antibody (Zouhar et al., 2010) were used. Protein blots were developed using western-blot detection solution (Supex) and visualized using the LAS3000 image capture system (FUJIFILM). The immunoblots were quantified by measuring the intensity of the protein bands with LAS3000 software.
Sequence data from this article can be found in the Genbank/EMBL data libraries under the following accession numbers: vsr4, N594467; vsr5, N544991; vsr6, N873290; rmr1, N100448; rmr2, N870412; rmr3, N552180; and rmr4, N24974.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression analysis of AtVSRs in Arabidopsis.
Supplemental Figure S2. Vacuolar trafficking of AtCPY is inhibited in the vsr1vsr4 double mutant.
Supplemental Figure S3. Ectopic expression of AtVSR1 or AtVSR4 rescues the vacuolar trafficking of AtCPY:GFP in vsr1vsr4 double mutant protoplasts.
Supplemental Figure S4. Expression of AtVSR5:HA increases the secretion of AtCPY:GFP in vsr1vsr4 double mutant protoplasts.
Supplemental Figure S5. Effect of AtV1N-V5C:HA and AtV5N-V1C:HA on the vacuolar trafficking of phaseolin.
Supplemental Table S1. Sequences of primers used in this study.
Glossary
- LV
lytic vacuole
- PSV
protein storage vacuole
- ER
endoplasmic reticulum
- ssVSS
sequence-specific vacuolar sorting signal
- ctVSS
C-terminal vacuolar sorting sequence
- PVC
prevacuolar compartment
- TGN
trans-Golgi network
- FITC
fluorescein isothiocyanate
- TRITC
tetramethyl rhodamine isothiocyanate
- Col-0
Columbia-0
- cDNA
complementary DNA
- PA
proteinase-associated
- HA
hemagglutinin
References
- Ahmed SU, Bar-Peled M, Raikhel NV. (1997) Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein-sorting receptors of eukaryotic cells. Plant Physiol 114: 325–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham DC, Raikhel NV. (2000) Unique features of the plant vacuolar sorting machinery. Curr Opin Cell Biol 12: 491–495 [DOI] [PubMed] [Google Scholar]
- Cao X, Rogers SW, Butler J, Beevers L, Rogers JC. (2000) Structural requirements for ligand binding by a probable plant vacuolar sorting receptor. Plant Cell 12: 493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli S, Vitale A. (2005) The phaseolin vacuolar sorting signal promotes transient, strong membrane association and aggregation of the bean storage protein in transgenic tobacco. J Exp Bot 56: 1379–1387 [DOI] [PubMed] [Google Scholar]
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J. (2005) Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17: 132–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marcos Lousa C, Gershlick DC, Denecke J. (2012) Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. Plant Cell 24: 1714–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke J, Aniento F, Frigerio L, Hawes C, Hwang I, Mathur J, Neuhaus JM, Robinson DG. (2012) Secretory pathway research: the more experimental systems the better. Plant Cell 24: 1316–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti O, Gershlick DC, Bottanelli F, Hummel E, Hawes C, Denecke J. (2010) A recycling-defective vacuolar sorting receptor reveals an intermediate compartment situated between prevacuoles and vacuoles in tobacco. Plant Cell 22: 3992–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A. (1998) Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Hinz G, Robinson DG. (2008) Multiple vacuoles in plant cells: rule or exception? Traffic 9: 1564–1570 [DOI] [PubMed] [Google Scholar]
- Hadlington JL, Denecke J. (2000) Sorting of soluble proteins in the secretory pathway of plants. Curr Opin Plant Biol 3: 461–468 [DOI] [PubMed] [Google Scholar]
- Hashiguchi Y, Niihama M, Takahashi T, Saito C, Nakano A, Tasaka M, Morita MT. (2010) Loss-of-function mutations of retromer large subunit genes suppress the phenotype of an Arabidopsis zig mutant that lacks Qb-SNARE VTI11. Plant Cell 22: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz G, Colanesi S, Hillmer S, Rogers JC, Robinson DG. (2007) Localization of vacuolar transport receptors and cargo proteins in the Golgi apparatus of developing Arabidopsis embryos. Traffic 8: 1452–1464 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinforma 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Robinson DG. (2009) Transport vesicle formation in plant cells. Curr Opin Plant Biol 12: 660–669 [DOI] [PubMed] [Google Scholar]
- Hwang I. (2008) Sorting and anterograde trafficking at the Golgi apparatus. Plant Physiol 148: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov S, Isner JC, Maathuis FJ. (2011) Rice two-pore K+ channels are expressed in different types of vacuoles. Plant Cell 23: 756–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong GW, Hwang I. (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13: 1511–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe NA, Craddock CP, Frigerio L. (2005) Pathways for protein transport to seed storage vacuoles. Biochem Soc Trans 33: 1016–1018 [DOI] [PubMed] [Google Scholar]
- Jung C, Lee GJ, Jang M, Lee M, Lee J, Kang H, Sohn EJ, Hwang I. (2011) Identification of sorting motifs of AtβFruct4 for trafficking from the ER to the vacuole through the Golgi and PVC. Traffic 12: 1774–1792 [DOI] [PubMed] [Google Scholar]
- Jurgens G. (2004) Membrane trafficking in plants. Annu Rev Cell Dev Biol 20: 481–504 [DOI] [PubMed] [Google Scholar]
- Kim H, Park M, Kim SJ, Hwang I. (2005) Actin filaments play a critical role in vacuolar trafficking at the Golgi complex in plant cells. Plant Cell 17: 888–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kang H, Jang M, Chang JH, Miao Y, Jiang L, Hwang I. (2010) Homomeric interaction of AtVSR1 is essential for its function as a vacuolar sorting receptor. Plant Physiol 154: 134–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC. (1994) Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc Natl Acad Sci USA 91: 3403–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval V, Chabannes M, Carrière M, Canut H, Barre A, Rougé P, Pont-Lezica R, Galaud JP. (1999) A family of Arabidopsis plasma membrane receptors presenting animal β-integrin domains. Biochim Biophys Acta 1435: 61–70 [DOI] [PubMed] [Google Scholar]
- Lee GJ, Kim H, Kang H, Jang M, Lee DW, Lee S, Hwang I. (2007) EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate. Implications for EpsinR2 function in protein trafficking in plant cells. Plant Physiol 143: 1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YB, Rogers SW, Tse YC, Lo SW, Sun SS, Jauh GY, Jiang L. (2002) BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol 43: 726–742 [DOI] [PubMed] [Google Scholar]
- Masclaux FG, Galaud JP, Pont-Lezica R. (2005) The riddle of the plant vacuolar sorting receptors. Protoplasma 226: 103–108 [DOI] [PubMed] [Google Scholar]
- Miao Y, Yan PK, Kim H, Hwang I, Jiang L. (2006) Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol 142: 945–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemes S, Labs M, Scheuring D, Krueger F, Langhans M, Jesenofsky B, Robinson DG, Pimpl P. (2010) Sorting of plant vacuolar proteins is initiated in the ER. Plant J 62: 601–614 [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG. (2006) Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18: 1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Kim SJ, Vitale A, Hwang I. (2004) Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 134: 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Lee D, Lee GJ, Hwang I. (2005) AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol 170: 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes FC, Buono R, Otegui MS. (2011) Plant endosomal trafficking pathways. Curr Opin Plant Biol 14: 666–673 [DOI] [PubMed] [Google Scholar]
- Robinson DG, Oliviusson P, Hinz G. (2005) Protein sorting to the storage vacuoles of plants: a critical appraisal. Traffic 6: 615–625 [DOI] [PubMed] [Google Scholar]
- Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV. (2003) A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 7389–7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín M, Ordóñez A, Sohn EJ, Robert S, Sánchez-Serrano JJ, Surpin MA, Raikhel NV, Rojo E. (2007) Divergent functions of VTI12 and VTI11 in trafficking to storage and lytic vacuoles in Arabidopsis. Proc Natl Acad Sci USA 104: 3645–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Nakano A. (2007) Mechanisms of COPII vesicle formation and protein sorting. FEBS Lett 581: 2076–2082 [DOI] [PubMed] [Google Scholar]
- Seaman MN. (2005) Recycle your receptors with retromer. Trends Cell Biol 15: 68–75 [DOI] [PubMed] [Google Scholar]
- Seki M, Carninci P, Nishiyama Y, Hayashizaki Y, Shinozaki K. (1998) High-efficiency cloning of Arabidopsis full-length cDNA by biotinylated CAP trapper. Plant J 15: 707–720 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Kamiya A, Ishida J, Satou M, Sakurai T, Nakajima M, Enju A, Akiyama K, Oono Y, et al. (2002) Functional annotation of a full-length Arabidopsis cDNA collection. Science 296: 141–145 [DOI] [PubMed] [Google Scholar]
- Shen Y, Wang J, Ding Y, Lo SW, Gouzerh G, Neuhaus JM, Jiang L. (2011) The rice RMR1 associates with a distinct prevacuolar compartment for the protein storage vacuole pathway. Mol Plant 4: 854–868 [DOI] [PubMed] [Google Scholar]
- Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. (2003) Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 16095–16100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EJ, Kim ES, Zhao M, Kim SJ, Kim H, Kim YW, Lee YJ, Hillmer S, Sohn U, Jiang L, et al. (2003) Rha1, an Arabidopsis Rab5 homolog, plays a critical role in the vacuolar trafficking of soluble cargo proteins. Plant Cell 15: 1057–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lee MH, Lee GJ, Yoo CM, Hwang I. (2006) Arabidopsis EPSIN1 plays an important role in vacuolar trafficking of soluble cargo proteins in plant cells via interactions with clathrin, AP-1, VTI11, and VSR1. Plant Cell 18: 2258–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen PK, Shen J, Sun SSM, Jiang L. (2010) Expression and characterization of two functional vacuolar sorting receptor (VSR) proteins, BP-80 and AtVSR4 from culture media of transgenic tobacco BY-2 cells. Plant Sci 179: 68–76 [Google Scholar]
- Surpin M, Zheng H, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, et al. (2003) The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 15: 2885–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, Jiang L. (2004) Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16: 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rogers JC, Jiang L. (2011a) Plant RMR proteins: unique vacuolar sorting receptors that couple ligand sorting with membrane internalization. FEBS J 278: 59–68 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhuang XH, Hillmer S, Robinson DG, Jiang LW. (2011b) Vacuolar sorting receptor (VSR) proteins reach the plasma membrane in germinating pollen tubes. Mol Plant 4: 845–853 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Shimada T, Takahashi H, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. (2008) Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol 49: 142–156 [DOI] [PubMed] [Google Scholar]
- Zouhar J, Muñoz A, Rojo E. (2010) Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4 sort vacuolar storage cargo in seeds and vegetative tissues. Plant J 64: 577–588 [DOI] [PubMed] [Google Scholar]
- Zouhar J, Rojo E. (2009) Plant vacuoles: where did they come from and where are they heading? Curr Opin Plant Biol 12: 677–684 [DOI] [PubMed] [Google Scholar]