Abstract
ABI3 (for ABSCISIC ACID INSENSITIVE3), a transcription factor of the abscisic acid signal transduction pathway, plays a major role during seed development, dormancy inception, and dormancy maintenance. This protein appears to also function in meristematic and vegetative plant tissues and under certain stress conditions. We have isolated the ABI3 gene ortholog (CnABI3) from yellow cedar (Callitropsis nootkatensis) and found that it was functionally similar to other ABI3 genes of angiosperms. Here, we report that using a yeast (Saccharomyces cerevisiae) two-hybrid approach, we have identified another protein of yellow cedar (CnAIP2; for CnABI3 INTERACTING PROTEIN2) that physically interacts with CnABI3. Functional analyses revealed that CnAIP2 plays important roles during key transitions in the plant life cycle: (1) CnAIP2 impaired seed development and reduced seed dormancy; (2) CnAIP2 promoted root development, particularly the initiation of lateral roots, and the CnAIP2 gene promoter was exquisitely auxin sensitive; and (3) CnAIP2 promoted the transition from vegetative growth to reproductive initiation (i.e. flowering). The nature of the effects of CnAIP2 on these processes and other evidence place CnAIP2 in the category of a “global” regulator, whose actions are antagonistic to those of ABI3.
Processes that occur during seed development are critical for the establishment of the next generation, including the viability and vigor of seedlings. At dispersal, the quiescent mature seed commences germination upon encountering favorable environmental conditions. Some seeds fail to complete germination under favorable conditions, even though they are viable. These dormant seeds must be exposed to environmental cues such as periods of warm, dry conditions (after ripening), moist chilling, or even smoke for dormancy to be terminated. ABI3 (for ABSCISIC ACID INSENSITIVE3), a component of the abscisic acid (ABA) signal transduction pathway, plays a major role during seed development and in dormancy inception (Nambara et al., 1995; Finkelstein et al., 2002, 2008; Kermode, 2003, 2005; Nambara and Marion-Poll, 2003; Rodríguez-Gacio et al., 2009). Although there are clearly other components of ABA signaling that are involved in seed development, and in dormancy maintenance of the mature seed, ABI3 has received considerable attention as a major regulator. Numerous recent reports have documented the diverse functions of ABI3 during different stages of the plant life cycle. ABI3 of the deeply dormant conifer species yellow cedar (Callitropsis nootkatensis, formerly known as Chamaecyparis nootkatensis) appears to play a role in the maintenance of seed dormancy (Zeng et al., 2003).
ABI3 and VIVIPAROUS1 (VP1; the ABI3 ortholog in maize [Zea mays]) were initially considered to be seed-specific transcription factors; later findings revealed that the role of ABI3/VP1 is not restricted to seeds, and the protein appears to function in meristematic and vegetative plant tissues and under certain stress conditions (Rohde et al., 2000). The broad range of functions of ABI3/VP1 proteins is further exemplified by the fact that they can act as activators or repressors of target genes. In this capacity, they do not work alone but in combination with other proteins such as DNA-binding proteins and transcription factors. For example, they can act through ABA-responsive elements (ABREs) of target gene promoters, leading to the transcription of ABA-responsive genes. In doing so, they do not bind to the ABREs directly but rather interact with them indirectly through their interactions with other proteins (Hobo et al., 1999a, 1999b). ABI3/VP1 may recruit additional DNA-binding proteins to the promoters of storage protein genes via its ability to alter chromatin structure (e.g. nucleosome positioning; Li et al., 2001). Regulation of the expression of an Arabidopsis (Arabidopsis thaliana) 2S storage protein gene (At2S3) appears to involve FUSCA3 (FUS3) and LEAFY COTYLEDON2 (LEC2), both of which bind directly to promoter elements consisting of alternating pyrimidines and purines (RY repeats 1 and 2), and ABI3, which acts in an indirect manner, probably via its interaction with bZIP proteins that bind to the G-box (Kroj et al., 2003).
It is likely that at different stages of the plant life cycle, ABI3 interacts with different sets of proteins to mediate a variety of functions. In angiosperms, several proteins that interact with ABI3 and VP1 have been identified by yeast (Saccharomyces cerevisiae) two-hybrid methods (Hobo et al., 1999b; Jones et al., 2000; Kurup et al., 2000; Nakamura et al., 2001). A bZIP factor, TRAB1, was isolated and showed bona fide interactions with both VP1 and with ABREs of target genes (Hobo et al., 1999b). Likewise, the Arabidopsis ABI3 protein physically interacts with ABI5, also a bZIP factor and a homolog of TRAB1 (55% amino acid similarity; Nakamura et al., 2001), with signaling functions in both seeds and vegetative tissues (Brocard et al., 2002; Lopez-Molina et al., 2002; Arroyo et al., 2003). Four other proteins show specific interactions in vivo and in vitro with ABI3 of Arabidopsis (Kurup et al., 2000) and may constitute a regulatory network along with ABI3 that controls embryo development, dormancy, and germination. Among the various proteins that show specific interactions with Arabidopsis ABI3 are the bZIP proteins, AtbZIP10 and AtbZIP25, which physically interact with ABI3 to regulate seed storage protein gene expression (Lara et al., 2003). AIP2 (for ABI3 INTERACTING PROTEIN2) of Arabidopsis is an E3 ligase of the ubiquitin 26S proteasome pathway that participates in down-regulating ABA signaling by targeting ABI3 for posttranslational destruction (Zhang et al., 2005). Three proteins have been identified that interact with wild oat (Avena fatua) VP1 (Jones et al., 2000). AfVIP2 encodes a ring-type zinc finger domain protein and is more highly expressed in dormant embryos; expression of the AfVIP3 gene, encoding a paralog of the human C1 transcription factor involved in cell cycle control, is greater in germinated seeds (Jones et al., 2000). In rice (Oryza sativa), GF14, a 14-3-3 protein, appears to act as a structural linkage by interacting with both VP1 and EmBP1 as part of the VP1 regulatory complex on the Em gene promoter (Schultz et al., 1998).
Yellow cedar is an important conifer species, with a habitat extending from the California-Oregon border in forested montane areas, through the coastal regions of British Columbia, and north to Prince William Sound in Alaska (Hennon et al., 2008). Seeds of yellow cedar exhibit deep dormancy at maturity; the dispersed seeds require several months of cool, moist conditions to break dormancy, an adaptive process that prevents germination during the cold winter months. Even under laboratory conditions, the seeds require a minimum 3-month dormancy-breaking treatment to elicit germination. We have isolated the ABI3 gene from yellow cedar CnABI3, and we further contributed to defining the role of ABI3 in conifer seeds (Zeng et al., 2003; Zeng and Kermode, 2004). Using a yeast two-hybrid approach, we have identified three yellow cedar proteins that interact with CnABI3. Here, we report on the characterization of one of them, CnAIP2, that plays important roles during several key transitions of the plant life cycle. When overexpressed in Arabidopsis, this protein impairs seed development and reduces seed dormancy and ABA sensitivity. It has a promotive effect on root development, particularly the initiation of lateral roots. Expression of CnAIP2pro in roots and seeds is greatly enhanced by auxin. Finally, CnAIP2 promotes early flowering. The widespread functions of CnAIP2 show that, like ABI3, this protein is a “global” regulator. The nature of its effects in different life cycle processes intimates that CnAIP2 negatively regulates ABI3/CnABI3 or functions in an antagonistic manner to ABI3/CnABI3 actions.
RESULTS
Identification of CnAIP2 as a CnABI3-Interacting Protein by a Yeast Two-Hybrid Approach
We used the Stratagene CytoTrap yeast two-hybrid system to identify yellow cedar proteins that interact with CnABI3. From about 3 million transformants screened, and after several rounds of strict verification, three proteins were eventually identified to interact with CnABI3. We named the interacting proteins CnAIP1, CnAIP2, and CnAIP3. The studies in this report are focused on CnAIP2.
The complementary DNA (cDNA) fragment of CnAIP2 derived from the pMyr plasmid lacked the 5′ end of the coding sequence. We amplified the missing coding region successfully using a 5′-RACE method (Supplemental Materials and Methods S1).
The full-length CnAIP2 cDNA is 2,016 bp and encodes a 672-amino acid protein with a molecular mass of 73.3 kD and a theoretical pI of 5.39. A nuclear localization of the protein is predicted at 94% (predicted by the online program PSORT at http://psort.hgc.jp/form.html). The amino acid sequence of CnAIP2 showed some degree of similarity (approximately 30% identical amino acids) to the FRIGIDA family proteins of Arabidopsis, whose function lies in imposing a vernalization requirement for flowering in winter accessions (Michaels et al., 2004).
CnABI3, like other ABI3/VP1 proteins, has four conserved domains: an acidic activation domain, A1, and three basic domains, B1, B2, and B3. In order to test which domains of CnABI3 participate in the interactions with CnAIP2, we generated deletion constructs of CnABI3 to include a number of combinations of the four domains in the bait vector (Fig. 1A; Supplemental Materials and Methods S1). As shown in Figure 1A, we used seven different constructs of pSos-CnABI3 (containing a full or partial CnABI3 coding region) to cotransform yeast cells with pMyr-CnAIP2 to examine protein-protein interactions. Each of the cotransformations was done twice independently, and the same results were obtained (Fig. 1A). The results indicate that the B1 and B2 domains of CnABI3 are required for the interaction with CnAIP2.
Figure 1.
Protein-protein interactions between CnAIP2 and CnABI3/AtABI3. A, Domains of CnABI3 that interact with CnAIP2. Four conserved domains of CnABI3 were differentially deleted in each construct, and the effect on interaction was determined. Results from a yeast two-hybrid test indicate that B1 and B2 domains of CnABI3 are required for the interaction. B, Protein-protein interactions between the conifer CnAIP2 protein and the Arabidopsis ABI3 protein examined by a yeast two-hybrid assay.
The CnAIP2 Protein Also Physically Interacts with Arabidopsis ABI3
A physical interaction occurs between yellow cedar CnABI3 and CnAIP2 proteins. Our previous research found that CnABI3 was similar to other ABI3 genes of angiosperms and has shown that CnABI3 functions nearly perfectly in the Arabidopsis abi3 null mutant (abi3-6), restoring almost all of the mutant’s severe phenotypes (Zeng and Kermode, 2004). Thus, we were interested to find out if Arabidopsis ABI3 also interacts with CnAIP2. We cloned the coding sequence of the ABI3 gene into the pSos vector and carried out yeast two-hybrid assays to analyze protein-protein interactions. The results showed that there was a strong physical interaction between ABI3 of Arabidopsis and CnAIP2 of yellow cedar (Fig. 1B).
Expression of CnAIP2 in Yellow Cedar and Transgenic Arabidopsis
CnAIP2 Expression in Yellow Cedar Seeds during Dormancy, Germination, and Early Seedling Growth
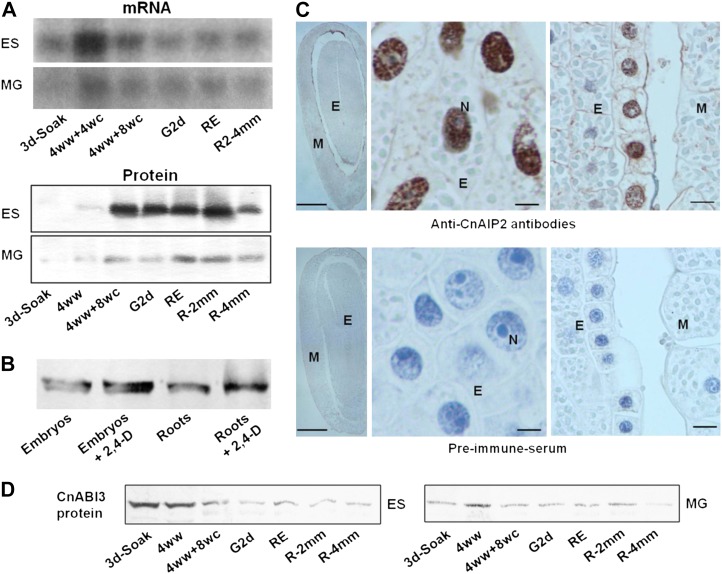
We first examined the steady-state levels of CnAIP2 mRNA and protein in yellow cedar seeds (embryos and megagametophytes) at different stages during dormancy breakage, germination, and postgerminative (early seedling) growth. The full dormancy-breaking treatment for yellow cedar seeds consists of a 3-d soak, 4 weeks of warm, moist conditions, and 8 weeks of moist chilling. CnAIP2 mRNA was initially low in embryos of mature seeds that had been subjected to only a 3-d water soak, but it increased markedly after seeds had received some moist chilling (particularly after 4 weeks of subsequent moist chilling; Fig. 2A, ES). A decline in transcripts occurred at the later stages, in embryos of seeds that had received the full dormancy-breaking treatment, and during and following seed germination (Fig. 2A, ES). The same general pattern of changes was evident in megagametophytes, albeit at a lower expression level (Fig. 2A, MG). The steady-state levels of CnAIP2 protein showed an interesting pattern of changes, particularly in view of the changes in transcripts during later stages. As was the case with the transcript abundance pattern, CnAIP2 protein abundance was initially low in 3-d soaked seeds but increased substantially after seeds had received some exposure to moist chilling, in this case 8 weeks (Fig. 2A, 4ww+8wc). The level of CnAIP2 protein remained high over the later stages (through germination and very early growth), unlike transcript levels, which indicates a relatively long half-life (little degradation) of CnAIP2 protein after its synthesis during these stages. The levels of CnAIP2 protein at different stages intimate that it is potentially required for dormancy breakage, for germination, and for very early seedling growth. Another interesting observation is that protein levels of CnAIP2 in megagametophytes were much lower than in embryos (Fig. 2A), which further indicates that CnAIP2 is specifically needed for germination (culminating in the elongation of the radicle) and likely not for reserve mobilization in megagametophytes, as this process occurs later during seedling growth (Xia et al., 2002). The amounts of CnABI3 protein during the different stages of yellow cedar were also investigated (Fig. 2D); interestingly, the abundance of CnAIP2 and CnABI3 proteins, particularly in the embryo at the various stages, appears to be negatively correlated. This reflects the negative regulation of CnAIP2 on ABI3-related functions (see below). Nonetheless the presence of both proteins at the various stages is further evidence of their physical interaction.
Figure 2.
Expression and localization of CnAIP2 in yellow cedar seeds. A, Steady-state levels of CnAIP2 mRNA and proteins in mature soaked dormant seeds, during a dormancy-breaking treatment, and during germination and early postgerminative growth. ES, Embryos and young seedlings; MG, megagametophytes; 3d-Soak, mature seeds subjected to a 3-d water soak (25°C); 4ww, mature seeds subjected to a 3-d soak and 4 weeks of warm, moist conditions (25°C); 4ww+4wc and 4ww+8wc, mature seeds subjected to a 3-d soak and 4 weeks of warm, moist conditions, followed by 4 weeks or 8 weeks of moist chilling (4°C), respectively; G2d, dormancy-terminated seeds placed in germination conditions for 2 d; RE, seeds at radicle emergence; R2-4mm, young seedlings with radicles of 2 to 4 mm. Ten micrograms of total RNA (for northern blots) and 100 µg of total proteins (for western blots) were loaded in each lane. B, CnAIP2 protein in embryos of mature seeds subjected to the full 3-month dormancy-breaking treatment and seedling roots (approximately 10 mm) after incubation overnight with 0 and 20 µm 2,4-D in MS medium. Fifty micrograms of total proteins was loaded on each lane. C, Immunohistochemistry to localize CnAIP2 in yellow cedar seeds. Sections of mature seeds previously subjected to the 3-month dormancy-breaking treatment (including embryo [E] and megagametophyte [M]) were incubated with either anti-CnAIP2 antibodies (top panel) or preimmune serum (taken from the same mouse) as a control (bottom panel). Brown color indicates positive labeling with anti-CnAIP2 antibodies. Sections were counterstained with hematoxylin; lithium carbonate was used to stain the nucleic acids blue and to show nuclei (N). Bars = 1,000, 10, and 25 µm for the left, middle, and right panels, respectively. D, CnABI3 protein in the same tissues and stages as shown in A for the CnAIP2 protein.
As presented below, a chimeric gene driven by the CnAIP2 promoter (CnAIP2pro-GUS) is strongly promoted by auxin in Arabidopsis. The synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D) effectively increased the abundance of the CnAIP2 protein in both embryos of yellow cedar seeds that had received a full dormancy-breaking treatment and in yellow cedar seedling roots (Fig. 2B).
CnAIP2 Is Localized in the Nucleus
As stated above, the CnAIP2 protein has a predicated nuclear subcellular localization. To verify this, immunohistochemistry was performed using yellow cedar seeds that had received a full dormancy-breaking treatment. The CnAIP2 protein was predominantly localized in the nuclei of the embryo (Fig. 2C), especially in the outer layer of embryonic cells adjacent to the megagametophyte (Fig. 2C).
Expression of CnAIP2pro-GUS in Arabidopsis
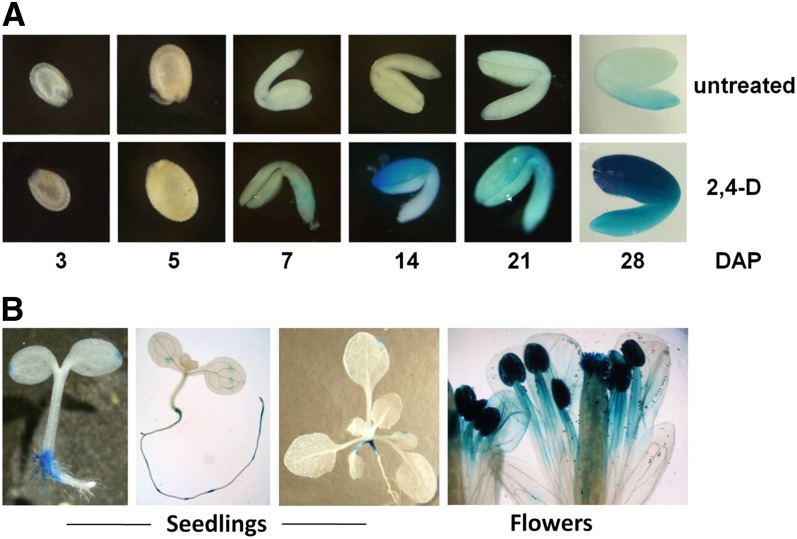
We isolated the promoter region of CnAIP2 by a genome-walking approach, and a 2.6-kb fragment was subsequently amplified. In order to examine the expression characteristics of CnAIP2pro, a chimeric construct was generated to fuse the CnAIP2 promoter fragment with the GUS gene coding sequence, and this construct was used to transform Arabidopsis. The chimeric gene driven by the CnAIP2 promoter was highly expressed in roots and in flowers, as determined by GUS histochemical staining (Fig. 3B). The expression level was much lower in seeds (being detected 7–28 d after pollination [DAP]; Fig. 3A) and was undetectable in most young leaves of seedlings (in some cases, the leaves showed slight expression at primary and secondary veins; the cotyledons of young seedlings showed expression at the tips that may correspond to auxin maxima; Fig. 3B). Analyses of ABI3pro::GUS (GFP) chimeric genes in Arabidopsis similarly show that ABI3 expression occurs in embryos of seeds (Parcy et al., 1994; Ng et al., 2004), in flowers (Ng et al., 2004), and in seedling roots treated with ABA (Ng et al., 2004) or auxin (Brady et al., 2003).
Figure 3.
Expression patterns of GUS driven by the CnAIP2 promoter at different stages during the Arabidopsis life cycle. A, Developing and mature seeds were stained with X-gluc directly or incubated in one-half-strength MS liquid medium with 20 µm 2,4-D overnight before X-gluc staining. Seed coats of seeds older than 7 DAP were removed before incubating in X-gluc or 2,4-D solution. B, Seedling and flower tissues were collected and directly stained with X-gluc without any treatment. Chlorophyll in green tissues was removed by incubating samples in 95% ethanol overnight. Photographs are cropped at different magnifications to show proper details.
Expression levels of CnAIP2pro were greatly enhanced by auxin in seeds (Fig. 3A) and roots (see below). As noted above, GUS expression of untreated developing (7–28 DAP) seeds was relatively low; however, expression was strongly enhanced after the seeds had been incubated in 2,4-D in Murashige and Skoog (MS) liquid medium overnight (Fig. 3A, bottom panel). At the later stages of development (7–28 DAP), the testa (seed coat) was first removed from seeds before auxin/5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-gluc) incubation because, otherwise, we found that it prevented the compounds from entering the seeds effectively. We did not remove seed coats of 3- and 5-DAP seeds because the embryos were too small. With or without the application of auxin, we noticed that during development, the expression was initially stronger in cotyledons of some seeds while stronger in the radicle of others; eventually, expression was evident throughout the whole seed (Fig. 3A).
After germination, GUS activity was evident in cotyledons and hypocotyls of 1-d-old seedlings; in 2-d-old and older seedlings, expression was mostly localized in roots and primarily at the sites of prospective lateral root formation (Fig. 3B; see also below). GUS activity was almost undetectable in expanded green cotyledons and leaves but later reached very high levels in flowers. Here, the highest expression occurred in the anthers and stigma and, to a lesser extent, in the style and upper regions of the filaments (Fig. 3B).
CnAIP2 Negatively Regulates Seed Development and Dormancy Maintenance
Seed Development Is Impaired in Arabidopsis Expressing CnAIP2
It is not technically feasible to characterize the phenotypic consequences of overexpressed or silenced CnAIP2 gene expression in transgenic yellow cedar plants and seeds as a means of elucidating some of the functions of the protein during the plant life cycle. Indeed, generating the transgenic materials would take several decades. Therefore, to explore any phenotypes associated with expression of the CnAIP2 protein, we made a construct using the cauliflower mosaic virus 35S promoter to drive the coding sequence of CnAIP2 and transformed Arabidopsis (ecotype Columbia [Col-0]) plants with the chimeric construct. We found that a great proportion of transgenic plants expressing CnAIP2 showed various degrees of disruption of seed development. Some lines were sterile, and some aborted seeds during their development (Fig. 4A). Only about 30% of the T1 transgenic plants produced viable mature seeds. Yeast two-hybrid assays showed that CnAIP2 exhibited physical protein-protein interactions with the Arabidopsis ABI3 protein (see above). Although it is unknown if CnAIP2 negatively regulates ABI3 through this physical interaction, the phenotypes associated with CnAIP2 expression in relation to impaired seed development clearly contrast with the known functions of ABI3/CnABI3 proteins, in which they play critical roles in promoting seed maturation processes. CnAIP2 protein levels in 2-week-old siliques (together with seeds if there were any in the siliques) from some of the T1 transgenic plants showed a general positive correlation to the degree of impaired seed development (Fig. 4B). Transgenic lines impaired in the production of viable seeds generally showed a higher amount of CnAIP2 protein, while those that produced normal seeds exhibited a lower level of CnAIP2 protein, such as lines 6 and 7 (line 11 was an exception; Fig. 4B). Interestingly, all of the T1 transgenic plants that produced viable normal seeds continued to produce 100% normal seeds in all succeeding generations. Only the normal seeds were used in subsequent experiments.
Figure 4.
Seed development of some CnAIP2-expressing lines was impaired, leading to abortion at early stages. A, An example of siliques expressing CnAIP2 with impaired seed development, in comparison with wild-type (WT) siliques as a control. All siliques were collected 2 weeks after pollination. B, CnAIP2 protein levels in 2-week-old siliques. Lines 1, 2, 4, 8, 9, and 10 were unable to produce seeds; lines 3 and 5 produced small siliques with a few seeds; lines 6, 7, and 11 produced normal seeds. The arrow indicates the CnAIP2 protein. [See online article for color version of this figure.]
CnAIP2 Seeds Exhibit Reduced Dormancy
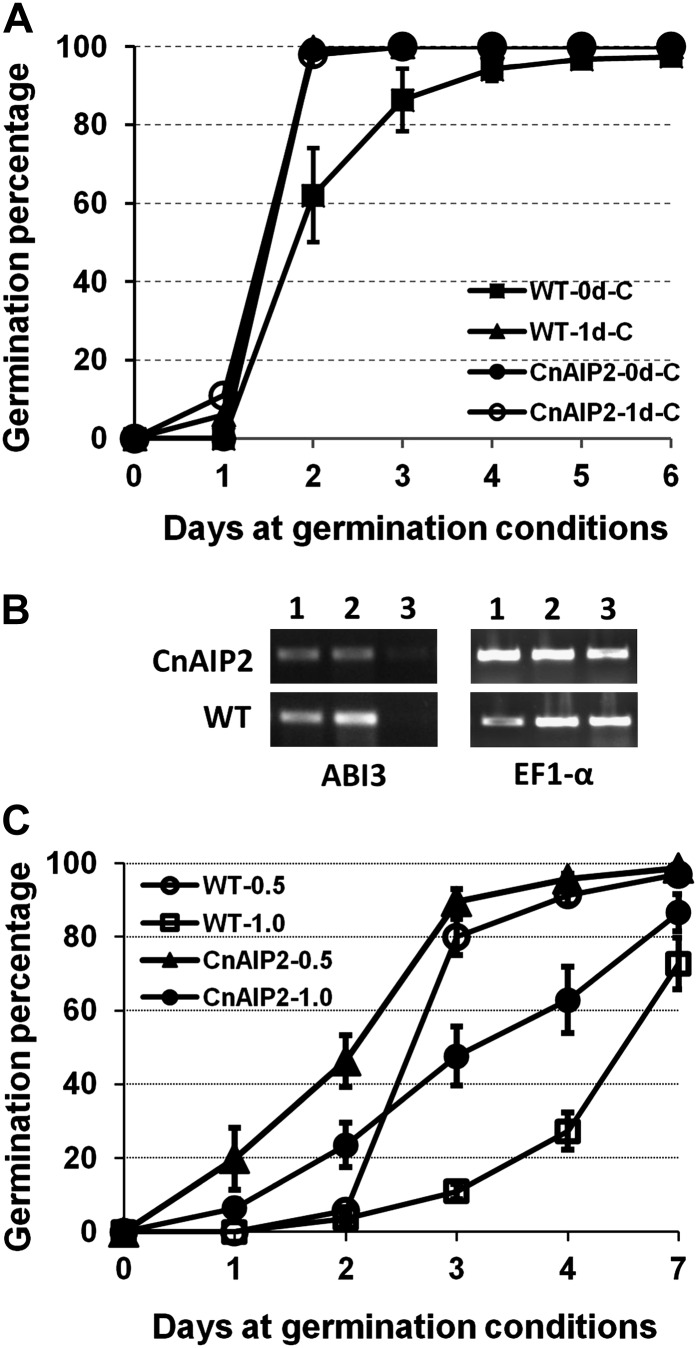
We observed that all of the CnAIP2 transgenic Arabidopsis lines that were able to produce viable seeds exhibited very similar and stable phenotypes in the following generations in terms of germination, growth performance, and time of flowering. Therefore, we randomly screened homozygous lines for further experiments. Freshly harvested mature dry seeds from wild-type (Col-0) and CnAIP2 plants were subjected to different durations of moist chilling prior to being placed in germination conditions. Seeds were scored as germinated if they exhibited radicle emergence. CnAIP2 seeds showed a lower degree of dormancy as compared with wild-type seeds; even in the complete absence of previous moist chilling, the population germinated on one-half-strength MS plates to the same degree as wild-type seeds that had first been subjected to 1 d of moist chilling (Fig. 5A). In the absence of previous moist chilling, the CnAIP2 seed population exhibited 100% germination on day 2 in germination conditions as compared with 60% germination for the wild-type seeds. One-day moist-chilled CnAIP2 seeds germinated faster than their wild-type counterparts; this was evident even after just 1 d in germination conditions (Fig. 5A). Both wild-type and CnAIP2 seeds that had undergone 2 to 4 d of moist chilling exhibited 100% germination after 1 d in germination conditions.
Figure 5.
Germination characteristics and ABI3 expression levels of wild-type (WT) and CnAIP2 transgenic seeds. A, Germination performance of seeds either without (0d-C) or with (1d-C) a 1-d moist-chilling treatment prior to transfer of seeds to germination conditions. B, RT-PCR transcript analysis to detect ABI3 expression levels in seeds under germination conditions without a prior moist-chilling treatment. The housekeeping gene EF1-α was used as the control. Numbers above the gels indicate days in germination conditions on one-half-strength MS plates. C, Germination performance of seeds in the presence of ABA. Seeds were subjected to a 4-d moist-chilling treatment (4°C) prior to transfer to germination conditions. Plates contained one-half-strength MS medium with either 0.5 or 1.0 µm (+)-ABA. On plates without ABA, all seeds germinated 1 d after their transfer to germination conditions. Data represent means ± se of duplicate tests, each with 50 to 100 seeds.
We then used reverse transcription (RT)-PCR transcript analysis to examine the expression level of ABI3 during germination in seeds that had not received any moist chilling. The housekeeping gene EF1-α (for elongation factor1-α) was used as a control. The steady-state level of ABI3 transcripts was generally lower in CnAIP2 seeds than in wild-type seeds (Fig. 5B). After 3 d in germination conditions, when most of the CnAIP2 and wild-type seeds had already germinated, the abundance of ABI3 transcripts decreased to undetectable levels (Fig. 5B).
Germination of CnAIP2 Seeds Is Less Sensitive to Inhibition by Exogenous ABA
To examine possible differences in ABA sensitivity, mature wild-type and CnAIP2 seeds were placed on one-half-strength MS medium containing 0.5 or 1 µm ABA and subjected to a 4-d moist-chilling treatment (4°C) to break dormancy. The plates were then transferred to germination conditions (21°C, 16-h photoperiod). The CnAIP2 seeds consistently exhibited a lower sensitivity to ABA as far as the inhibition of their germination was concerned (Fig. 5C). For example, after 2 d in germination conditions, CnAIP2 seeds in 0.5 µm ABA exhibited 46% germination, while equivalently treated wild-type seeds exhibited only 5% germination. A similar trend was evident in the presence of 1 µm ABA (Fig. 5C).
CnAIP2 Promotes Root Development
CnAIP2 Is Highly Expressed in Roots, Particularly at Lateral Root Initiation Sites
As part of our examination of the spatial and temporal patterns of CnAIP2pro-GUS expression in Arabidopsis, we undertook GUS histochemical staining of young seedlings (Fig. 6A). GUS was expressed during early postgerminative growth (as early as in 1-d-old seedlings), mostly in cotyledons and hypocotyls; some seedlings showed expression in the radicle as well. In 2- and 3-d-old seedlings, GUS expression in most seedlings changed and showed highest expression levels in the upper part of the root directly adjacent to the hypocotyl (Fig. 6A, a). Later, in seedlings older than 4 d, expression progressed to lower portions of the roots and was mostly localized to the sites of lateral root initiation (Fig. 6A, b–f). At these stages, we were unable to detect GUS expression in most expanded cotyledons and leaves (Fig. 6A, b, c, and g). The very distinct localization of GUS activity (areas of blue staining) was apparent in the early stages of lateral root development at the initiation sites even prior to the lateral roots themselves becoming morphologically visible (Fig. 6A, d). GUS expression stayed at high levels even after lateral roots were fully developed (Fig. 6A, f).
Figure 6.
Expression characteristics and auxin inducibility of CnAIP2pro-GUS in young seedlings and promotive effects of CnAIP2 on seedling root growth and lateral root initiation. A, Histochemical analyses conducted on young seedlings expressing CnAIP2pro-GUS. a to c, Seedlings at 2, 4, and 7 d after germination; d to f, GUS expression at the lateral root initiation sites; g, 4-d-old seedling treated with 1 µm 2,4-D overnight prior to X-gluc staining. Chlorophyll was removed by incubating seedlings in 95% ethanol overnight after X-gluc staining. Photographs are cropped at different magnifications to show proper details. B, Effect of auxins on CnAIP2-GUS expression of young seedlings. Activities of GUS were determined by the fluorometric assay conducted on 3-d-old seedlings incubated overnight (16 h) in one-half-strength MS liquid medium with various concentrations (µm) of auxins as indicated. Data represent means ± se of duplicate experiments. MU, Methylumbelliferone. C and D, Effects of CnAIP2 expression (CnAIP2-OE) on root length (C) and number of lateral roots (D) showing CnAIP2 transgenic seedlings versus wild-type (WT; control) seedlings after 1 week and 2 weeks at germination/growth conditions. Seeds were subjected to a 4-d moist-chilling treatment (4°C) on one-half-strength MS plates prior to transfer of plates to germination conditions. Data represent means ± sd of approximately 50 plants of wild-type or CnAIP2 transgenic plants. Asterisks indicate significant differences (**P < 0.001, *P < 0.05) from the wild type determined by Student’s t test.
CnAIP2pro Expression in Roots Is Greatly Enhanced by Auxin
Auxin, especially 2,4-D, substantially increased the GUS expression levels of young seedlings (Fig. 6A, g). GUS activity as determined by fluorometric assays (Jefferson et al., 1987) indicated a greater than 70-fold increase in 3-d-old seedlings in the presence of 20 µm 2,4-D as compared with the control without hormone (Fig. 6B). The natural auxin indole-3-acetic acid had a weaker effect than 2,4-D but still dramatically enhanced CnAIP2pro-GUS expression (Fig. 6B). Interestingly, ABI3 is expressed in seedling roots treated with auxin (Brady et al., 2003) or ABA (Ng et al., 2004), and auxin is involved in the ABI3/VP1 regulation of lateral root initiation (Suzuki et al., 2001; Brady et al., 2003).
CnAIP2 Protein Promotes Root Development in Arabidopsis
We used 35S-CnAIP2 and wild-type seeds that had been germinated on large plates to assess their root length and the number of lateral roots. The CnAIP2 protein strongly promoted root development, especially at the early stages of seedling growth. The average root length of CnAIP2 seedlings was 58% longer than that of wild-type seedlings at week 1 in germination/growth conditions and was 24% longer than that of wild-type seedlings after 2 weeks (Fig. 6C). The average numbers of lateral roots of CnAIP2 seedlings were 140% and 53% higher than those of the wild-type seedlings at the end of weeks 1 and 2, respectively (Fig. 6D).
CnAIP2 Promotes Flowering in Arabidopsis
The GUS gene driven by the CnAIP2 promoter was expressed very strongly in flower tissues, most notably in the anthers (pollen) and stigma (Fig. 3B).
Synthesis of CnAIP2 protein in transgenic Arabidopsis plants caused early flowering in all of the transgenic lines, and this phenotype was very stable in later generations. Flowering time was recorded as the day from germination completion (radicle emergence) to the day when the first flower bud emerged on a plant. The number of primary leaves formed at flowering was also recorded. As shown, the CnAIP2-expressing plants flowered much earlier than the wild-type (Col-0) plants (Fig. 7A). On average, the CnAIP2 plants took 17 d and the wild-type plants took 22.5 d to flower (Fig. 7B), and these plants at flowering possessed an average of seven and 12 leaves, respectively (Fig. 7B). The abi3 null mutant plants (abi3-6) also took 17 d to flower and possessed seven leaves at flowering (Fig. 7B). When we expressed the CnAIP2 gene in Arabidopsis abi3-6 mutants, we could not detect any further promotion of the early-flowering phenotype of abi3-6 plants (Fig. 7B). Moreover, no noticeable phenotypes occurred as a consequence of expressing the CnAIP2 gene in the abi3-6 mutant. Thus, expressing CnAIP2 in wild-type plants mimics the phenotype of the abi3 null mutant in terms of flowering time. These results intimate that CnAIP2 regulates flowering by negatively regulating ABI3 through their physical (protein-protein) interactions.
Figure 7.

CnAIP2 promotes flowering in Arabidopsis (Col) plants. A, Appearance of CnAIP2/wild-type (WT) and wild-type plants at day 29 after germination. B, Graphs show means of flowering time ± sd and average number of leaves ± sd at flowering of CnAIP2, wild-type, and abi3-6 plants (data of each genotype were determined for approximately 100 plants). Flowering time is recorded from the day of germination completion to the day when the first flower bud emerged on a plant. The asterisk indicates significant difference (P < 0.001) from the wild type determined by Student’s t test. [See online article for color version of this figure.]
Transgenic CnAIP2 and Wild-Type Arabidopsis Plants Exhibit Distinct Patterns of Gene Expression during Key Transitions
We selected some of the key stages that showed different phenotypes between CnAIP2 and wild-type plants (seed development, flowering, and root development) and used microarray analyses to examine differentially expressed genes at those stages. Some genes that play roles in each of the stages were selected for further RT-PCR transcript analyses, which additionally served to verify some of the microarray results showing substantial differential expression between the two lines.
Seeds of CnAIP2 and Wild-Type Arabidopsis Plants Exhibit Differential Gene Expression during Development
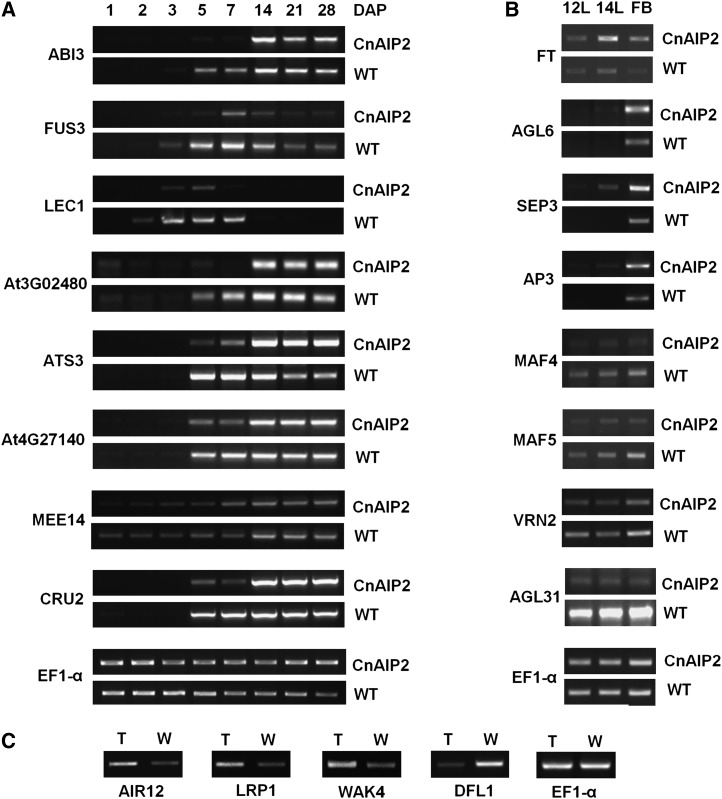
CnAIP2 impaired Arabidopsis seed development at the early stages (see above). The microarray analyses showed that a few dozen genes related to seed development had altered expression levels in the CnAIP2 transgenic seeds as compared with the wild type, mostly at the earlier sampling stage, week 1 or 7 DAP (Table I); many fewer genes showed altered expression at 28 DAP (Table I). Notably, ABI3, FUS3, and LEAFY COTYLEDON1 (LEC1), three critical (global) genes of seed development, showed decreased expression in the transgenic seeds, which was further verified by the RT-PCR analysis, especially during earlier developmental stages (Fig. 8A). Note that for a more thorough understanding, we collected developing seeds from eight time points during seed development, with a more detailed collection at the earlier stages for the RT-PCR analysis (i.e. at 1, 2, 3, 5, 7, 14, 21, and 28 DAP). Other seed development-related genes selected for verification by RT-PCR were seed-storage protein or embryo-development related and included CRUCIFERIN2 (CRU2), At4G27140 (2S SEED STORAGE PROTEIN1), MATERNAL EFFECT EMBRYO ARREST14 (MEE14), ARABIDOPSIS THALIANA SEED GENE3 (ATS3), and AT3G02480 (ABA-responsive protein related). Verifying the microarray studies, these genes showed distinct differences between the CnAIP2 transgenic and wild-type seeds during the early stages of development. Of the eight genes analyzed by RT-PCR, seven of them showed significant differences at stages before 7 DAP but exhibited similar expression during the later stages (14–28 DAP); an exception was the FUS3 gene, whose expression was consistently lower in the CnAIP2 transgenic seeds as compared with the wild type throughout seed development (Fig. 8A).
Table I. Fold changes in gene expression between CnAIP2 and the wild type at different life cycle stages as determined by microarray analyses.
Genes shown are examples of those that may play roles in each of the stages.
| Gene Name (Plays Role in) | Expression Fold Change |
|
|---|---|---|
| CnAIP2/Wild Type | Wild Type/CnAIP2 | |
| 7-DAP seeds | ||
| AT2G33830 (dormancy/auxin-associated protein) | 26.5 | |
| AT2G45180 (seed storage/LTP family protein) | 8.1 | |
| AT3G22490 (seed development, LEA protein) | 31.1 | |
| AT4G36600 (LEA domain-containing protein) | 5.9 | |
| AT5G23360 (ABA-responsive protein-related) | 4.0 | |
| ATEXPA2 (seed development) | 11.0 | |
| DAG1 (dormancy and germination) | 3.1 | |
| DREB1A (responses to ABA, dehydration in seed) | 7.8 | |
| DYL1 (dormancy-associated protein) | 16.3 | |
| GEA6 (seed development, late embryogenesis) | 41.7 | |
| M10 (seed development, LEA-like protein) | 8.6 | |
| MEE14 (embryo development) | 15.6 | |
| MEE38 (embryo development) | 6.3 | |
| RAB18 (response to ABA) | 5.8 | |
| UNE15 (embryo development) | 27.2 | |
| ABA1 (ABA biosynthesis) | 2.5 | |
| ABI3 (seed development) | 4.5 | |
| AHG1 (ABA responses, seed germination) | 4.7 | |
| AT1G02820 (LEA3 family protein) | 5.6 | |
| AT1G52690 (LEA protein, putative) | 8.9 | |
| AT2G03850 (LEA domain-containing protein) | 7.8 | |
| AT2G18370 (seed storage/lipid transfer protein) | 37.5 | |
| AT2S3 (lipid binding/nutrient reservoir) | 8.3 | |
| AT3G02480 (ABA-responsive protein-related) | 87.7 | |
| AT3G15670 (LEA protein, putative) | 5.1 | |
| AT3G22620 (seed storage/LTP family protein) | 12.1 | |
| AT3G54510 (early responsive to dehydration) | 22.7 | |
| AT3G58550 (seed storage/LTP family protein) | 46.5 | |
| AT4G21020 (LEA domain-containing protein) | 17.2 | |
| AT4G22120 (early responsive to dehydration) | 11.3 | |
| AT4G27140 (2S seed storage protein1) | 27.5 | |
| AT5G44310 (LEA domain-containing protein) | 9.3 | |
| ATPER1 (antioxidant/seed dormancy) | 23.4 | |
| ATS1 (embryo development) | 16.7 | |
| ATS3 (seed development) | 25.6 | |
| CRA1 (seed storage protein) | 22.1 | |
| CRU2 (seed storage protein) | 36.0 | |
| CRU3 (seed storage protein) | 22.7 | |
| DOG1 (dormancy and germination) | 5.5 | |
| EDA4 (embryo development) | 15.2 | |
| FUS3 (seed development) | 6.5 | |
| LEA (dehydrin LEA) | 12.3 | |
| LEC1 (seed development) | 1.4 | |
| MEE55 (embryo development) | 5.8 | |
| OLEO2 (seed lipid accumulation) | 8.9 | |
| TIP3;1 (water channel) | 20.7 | |
| 28-DAP seeds | ||
| AT2G10940 (seed storage/LTP family protein) | 3.3 | |
| AT5G49950 (embryogenesis) | 4.2 | |
| ATS3 (seed development) | 5.8 | |
| DOG1 (seed dormancy and germination) | 2.5 | |
| DREB1A (response to ABA, dehydration) | 3.5 | |
| ERD10 (response to ABA, seed germination) | 3.2 | |
| ERD14 (response to ABA, seed germination) | 3.1 | |
| GAMMA-TIP (water channel protein) | 4.3 | |
| AT2G03850 (LEA domain-containing protein) | 4.9 | |
| ABI2 (ABA signal transduction) | 2.8 | |
| AHG1 (ABA responses, seed development and germination) | 2.1 | |
| AMY1 (starch mobilization, responsive to ABA, GA) | 5.0 | |
| AT1G54070 (dormancy/auxin-associated protein) | 4.5 | |
| AT4G01600 (ABA-responsive protein-related) | 3.1 | |
| AT4G22120 (early responsive to dehydration) | 23.8 | |
| AT5G38195 (seed storage/lipid transfer protein) | 12.3 | |
| AT5G53270 (seed maturation family protein) | 7.9 | |
| EDA7 (embryo development) | 4.9 | |
| EMB1879 (embryo development) | 18.6 | |
| EMB2756 (embryo development) | 6.4 | |
| FUS3 (seed development) | 4.2 | |
| MEE38 (embryo development) | 17.6 | |
| MEE66 (embryo development) | 6.9 | |
| MEE69 (embryo development) | 4.2 | |
| Flower bud | ||
| AGL19 (positive regulator of flowering) | 8.2 | |
| AGL6 (flower development) | 2.8 | |
| AP3 (flower development) | 2.7 | |
| AT1G29140 (flower development) | 45.0 | |
| AT1G76530 (auxin efflux carrier family protein) | 8.7 | |
| AT2G21220 (auxin-responsive protein, putative) | 12.7 | |
| AT3G43120 (auxin-responsive protein-related) | 9.2 | |
| AT4G13790 (auxin-responsive protein, putative) | 20.6 | |
| AT5G13350 (auxin responsive) | 8.6 | |
| AT5G18010 (auxin-responsive protein, putative) | 6.0 | |
| AT5G45880 (flower development) | 35.3 | |
| ATMYB21 (flower development) | 75.3 | |
| COL1 (circadian rhythms, flower development) | 3.3 | |
| COL2 (circadian rhythms, flower development) | 3.0 | |
| ELF4 (flower development) | 2.4 | |
| FT (positive regulator of flower development) | 19.7 | |
| MAF3 (flowering regulation) | 3.2 | |
| SEP3 (flower development) | 2.2 | |
| VIN3 (flowering regulation) | 6.2 | |
| AGL31 (negative regulator of flowering) | 45.2 | |
| MAF4 (negative regulator of flowering) | 15.6 | |
| MAF5 (negative regulator of flowering) | 5.0 | |
| VRN2 (negative regulator of flowering) | 33.6 | |
| 9-d roots | ||
| AIR1 (lateral root morphogenesis) | 3.4 | |
| AIR12 (lateral root morphogenesis) | 6.0 | |
| ARR12 (root development) | 2.9 | |
| AT1G17345 (auxin-responsive protein) | 3.4 | |
| AT5G13380 (auxin-responsive protein) | 7.7 | |
| AT5G20810 (auxin-responsive protein) | 3.6 | |
| ATNRT2:1 (lateral root development) | 7.1 | |
| HB53 (root development) | 3.4 | |
| IAA14 (response to auxin, lateral root development) | 4.4 | |
| LRP1 (lateral root development) | 3.4 | |
| WAK4 (lateral root development) | 3.8 | |
| XLG3 (root development) | 4.1 | |
| AT1G48690 (auxin-responsive protein) | 5.1 | |
| AT1G56150 (auxin-responsive protein) | 3.1 | |
| AT5G03310 (auxin-responsive family protein) | 9.0 | |
| DFL1 (negatively regulates lateral root formation) | 2.1 | |
| IAA13 (response to auxin) | 2.2 | |
| IAA6 (response to auxin) | 2.3 | |
| NPH4 (lateral root primordium development) | 2.8 | |
| PIN5 (PIN-FORMED5, auxin polar transport) | 3.4 | |
| PUCHI (lateral root morphogenesis) | 3.7 | |
Figure 8.
RT-PCR transcript analysis to detect gene expression in developing seeds (A) prior to and during flowering (B) and in roots from 9-d-old seedlings (C). The housekeeping gene EF1-α was used as the control for mRNA amounts between the samples. A, Developing seeds at different DAP, as indicated. WT, Wild type. B, 12L and 14L indicate leaves (and shoot meristem tissues) from 12-d-old and 14-d-old seedlings, respectively, and FB indicates flower bud. C, T indicates transgenic CnAIP2 plants and W indicates wild-type (Col-0) plants.
CnAIP2 and Wild-Type Arabidopsis Plants Exhibit Differential Gene Expression during Flowering
The CnAIP2 transgenic plants consistently showed an early-flowering phenotype. Microarray analyses (Table I) showed that more than 20 genes related to flowering regulation had altered expression levels in the transgenic plants. Most notable was the expression level of the positive regulator of flowering, FLOWER LOCUS T (FT), which was 20-fold higher than that of the wild-type plants (Table I). This was also verified by RT-PCR, along with analyses of flower development genes such as AGAMOUS-LIKE6 (AGL6), APETALA3 (AP3), and SEPALLATA3 (SEP3; Fig. 8B). Note that for RT-PCR amplification, we also included samples of 12-d and 14-d rosette leaves and shoot meristem tissues before flower buds had emerged (the earliest flower bud of CnAIP2 plants appeared on day 15). It was obvious that FT, SEP3, and AP3 accumulated to higher amounts in the CnAIP2 vegetative tissues before flowering (Fig. 8B). Genes whose products negatively regulate flowering, such as MADS AFFECTING FLOWERING4 (MAF4), MAF5, REDUCED VERNALIZATION RESPONSE2 (VRN2), and AGAMOUS LIKE MADS BOX PROTEIN31 (AGL31), were expressed at significantly higher levels in the wild-type plants as compared with the CnAIP2 plants (Table I; Fig. 8B).
CnAIP2 and Wild-Type Arabidopsis Seedlings Exhibit Differential Gene Expression during Root Development
CnAIP2 promoted root development and lateral root initiation, especially at the early stages (see above). We used 9-d roots (roots of seedlings 9 d after radicle emergence) to compare gene expression profiles between the transgenic and wild-type seedlings. More than one dozen genes encoding proteins that positively regulate root and lateral root development had higher expression levels in the CnAIP2 transgenic seedlings (Table I; Fig. 8C). These included AUXIN-INDUCED IN ROOT CULTURES12 (AIR12), LATERAL ROOT PRIMORDIUM1 (LRP1), EXTRA-LARGE GTP-BINDING PROTEIN3 (XLG3), ARABIDOPSIS RESPONSE REGULATOR12 (ARR12), and WALL-ASSOCIATED KINASE4 (WAK4; Table I; Fig. 8C). On the other hand, negative regulators such as DWARF IN LIGHT1 (DFL1) showed a decreased level of expression in roots of the CnAIP2 transgenic seedlings as compared with those of wild-type seedlings (Table I; Fig. 8C).
DISCUSSION
A General Note Regarding the Experimental Systems of This Research
We are interested in understanding the mechanisms that underlie the deep dormancy of seeds of yellow cedar, a high-elevation conifer species having significant importance to the forest industry. To study proteins that interact with CnABI3, we isolated CnAIP2 from yellow cedar, a protein uncovered through a yeast two-hybrid approach. Due to the extremely long life cycle of yellow cedar and to difficulties associated with genetic transformation of this species, we conducted most of our functional analyses in Arabidopsis. We are fully aware of the evolutionary distance between yellow cedar and Arabidopsis. However, a considerable number of genes have conserved functions between gymnosperms and angiosperms. Indeed, particularly relevant to this study, our previous research showed that yellow cedar CnABI3 functions nearly perfectly in the Arabidopsis ABI3 null mutant abi3-6, restoring almost all of the mutant’s severe phenotypes (Zeng and Kermode, 2004). Furthermore, ectopic expression of the conifer CnABI3 gene in transgenic tobacco (Nicotiana tabacum) activates the promoters of seed storage protein genes, similar to that of the Arabidopsis ABI3 gene (Zeng et al., 2003; Kermode et al., 2007). Thus, due to the severe limitations of conventional approaches for the functional characterization of conifer genes/proteins (i.e. conducted in the homologous plant host), the approach of Arabidopsis expression is useful, allowing us to investigate the biological functions of CnABI3 and its interacting proteins in a technically feasible and timely manner.
CnAIP2 Plays Important Functions in Life Cycle Transitions with Opposite Roles to ABI3
Seed Development and Germination
Both in yellow cedar and Arabidopsis, our findings indicated that the abundance and role of CnAIP2 were generally opposite to those of ABI3/CnABI3. In yellow cedar seeds, CnAIP2 protein abundance of 3-d soaked mature dormant seeds was very low as compared with that during dormancy breakage, especially after seeds had been exposed to moist chilling and during germination and early seedling growth. This is in contrast to changes in the abundance of the CnABI3 protein, which is much higher in mature imbibed seeds whose dormancy is maintained (Zeng et al., 2003). Expression of both CnABI3 and CnAIP2 appears to involve some control at the posttranscriptional/posttranslational level. CnAIP2 may be potentially required for dormancy breakage, germination, and early seedling growth, since the protein increased dramatically after the full cold, moist treatment and remained at high levels during germination and early growth. At the same time, the abundance of the CnAIP2 transcripts and CnAIP2 protein in megagametophytes was much lower than in embryos (and young seedlings), which again intimates that CnAIP2 is needed for germination, although unequivocal evidence cannot rest on these findings alone. Patterns of accumulation in the megagametophyte indicate that the protein is not required for reserve mobilization in this tissue.
One of the distinctive effects of the overproduction of the CnAIP2 protein in Arabidopsis (effected by expression of the chimeric 35Spro-CnAIP2 gene) was an impairment of seed development, especially early development. Only 30% of the T1 plants produced viable seeds. Therefore, the abundance of the protein during seed development is likely subject to strict regulation. The mature yellow cedar (3-d-soaked) seeds contained low amounts of CnAIP2 protein as compared with the other stages that were characterized. Expression of the CnAIP2pro-GUS chimeric gene in Arabidopsis (by assessment of GUS activities) was generally low (almost undetectable) in the majority of developing seeds; yet, expression appeared to be temporally regulated in seeds, being higher at the later stages of development. In Arabidopsis seeds transgenically expressing the 35Spro-CnAIP2 gene, there was a general correlation between the amount of CnAIP2 protein and the degree of disruption of seed development; yet, even in some of the transgenic seeds, the level of CnAIP2 protein synthesis was sufficiently low to allow seeds to survive. All such lines produced 100% viable seeds in the following generations. Indeed, other phenotypes such as early flowering were also stable in all subsequent generations. Perhaps the most revealing analyses were the microarray and RT-PCR studies that showed that important genes, those considered to be global regulators of seed developmental processes, such as ABI3, FUS3, and LEC1, were down-regulated in the CnAIP2 seeds, as were genes associated with, or specific to, embryo development. Expression of the LEC1 gene, for example, which was apparent in wild-type seeds at 3 to 7 DAP, was virtually undetectable in the CnAIP2 transgenic seeds. In most cases, down-regulated expression of developmental genes was exhibited in the early stages (i.e. before 7 DAP); starting from 14 DAP onward, the expression levels of all of the “developmental” genes characterized (with the exception of FUS3) returned to wild-type levels. The normal expression evident at the later stages may have enabled seed survival. (It is important to note that the transgenic plants that we used for the microarray analyses were T5 plants, and these plants produced 100% viable seeds, in sharp contrast to T1 plants, which only produced approximately 30% viable seeds. Therefore, there was no inherent bias in sampling.) The microarray analyses, in particular, strikingly showed that the numbers of seed- and embryo development-related genes with altered expression levels in the transgenic seeds (either up- or down-regulated relative to those of wild-type seeds) were much greater at the early developmental stage (7 DAP) than at the later stage (28 DAP).
Also striking was the effect of the CnAIP2 protein on reducing seed dormancy (lowering the requirement for seed exposure to moist chilling), promoting germination and lowering seed sensitivity to ABA as far as germination is concerned. The actions of CnAIP2 in seed development and germination were again clearly opposite those of ABI3/CnABI3 proteins. There could well be a direct negative regulation between these two proteins through their protein-protein interactions.
We previously reported that CnABI3 functions nearly perfectly in the Arabidopsis ABI3 null mutant abi3-6, producing desiccation-tolerant normal seeds (Zeng and Kermode, 2004). Interestingly, expression of CnAIP2 protein in the CnABI3-Arabidopsis abi3-6 plants led to an inability of seeds to survive after desiccation. We tried this dual transformation twice. The first round yielded a small number of double transgenic plants, but all of the T2 seeds were unable to germinate. The second transformation failed entirely, and no double transgenic plants could be generated. This may not be entirely unexpected, since single transformation with the CnAIP2 gene in wild-type Arabidopsis resulted in 70% of the transgenic plants not being able to produce viable seeds; when both CnAIP2 and CnABI3 proteins were expressed in plants without a functional Arabidopsis ABI3 gene, the effect may have been to further sequester CnABI3 (through the physical interaction), thus magnifying the negative effect on seed viability.
Root Growth and Lateral Root Initiation
Lateral roots are important in the uptake of water and the acquisition of nutrients from soil as well as in the establishment (anchoring) of the seedling. ABI3 participates in the inhibition of lateral root initiation (see below). Following seed germination, we could clearly see the dynamics of CnAIP2pro expression (monitored by GUS activities) in roots. Temporally, the initial site of expression was at the top part of the root directly underneath the hypocotyl; subsequently, expression extended to lower parts of the root, mostly localized at the sites of lateral root formation. This could be detected at the early stages of lateral root development (stages I–III, as described by Péret et al. [2009]) even before the lateral roots were visible; expression stayed strong after the lateral root was fully developed. The performance of root growth in seedlings expressing CnAIP2 protein indicated that CnAIP2 played a role in promoting root development, particularly in promoting lateral root initiation. The microarray results also showed that expression of CnAIP2 in Arabidopsis up-regulated some genes such as WAK4, ARR12, and LRP1 that encode positive regulators of root and lateral root development (Lally et al., 2001; Yokoyama et al., 2007; Krichevsky et al., 2009). At the same time, genes such as DFL1 that negatively specify regulators of root development had lower expression in CnAIP2 roots. DFL1 is involved in auxin signal transduction and inhibits lateral root cell differentiation in light (Nakazawa et al., 2001). We used 9-d roots for the microarray analysis because we found that the action of CnAIP2 in promoting root development was most strongly exhibited in the early stages of seedling growth (i.e. before seedlings were 2 weeks old). In Arabidopsis and other species, auxin plays a vital role in lateral root development (Casimiro et al., 2001; Marchant et al., 2002; Dubrovsky et al., 2008; Fukaki and Tasaka, 2009; Chen et al., 2011; Moriwaki et al., 2011; Zhu et al., 2012). It is important to note that CnAIP2 expression was strongly promoted by auxin. This was verified in seeds and roots of both yellow cedar and Arabidopsis. These findings imply that CnAIP2 is involved in ABA (through its interaction with ABI3) and auxin signal transduction pathways and possibly other hormone signaling/stress pathways. Indeed, it may be a positive and perhaps global component of auxin-mediated lateral root formation. The microarray analyses showed that a number of the auxin-responsive genes were altered in their expression levels between the roots of the CnAIP2 transgenic and wild-type plants. Besides auxin, other hormones participate and interact with each other to regulate lateral root formation, in which ABA generally has a negative role. Accordingly, expression of ABI3 was found to increase ABA inhibition of root growth (Parcy et al., 1994; Parcy and Giraudat, 1997), and maize VP1 is also implicated in the inhibition of root/lateral root development when expressed in Arabidopsis to complement the abi3-6 mutation (Suzuki et al., 2001). Therefore, CnAIP2 appears to act in a manner opposite to that of VP1/ABI3 in yet another process. In another report, ABI3 was found to play some role in the auxin regulation of lateral root development (Brady et al., 2003). The fact that ABI3 itself is auxin inducible in lateral root primordia (Brady et al., 2003), and the obvious auxin inducibility of CnAIP2-GUS expression in seedlings, provide indirect evidence of their interaction in root development.
The role of CnAIP2 in promoting root development was mostly exhibited in the early stages of seedling development, which again contrasts with the inhibitory actions of ABI3 in delaying or “safeguarding” against a resumption of development until the conditions are optimal.
The Transition from Vegetative Growth to Reproductive Initiation: Flowering
Another significant feature of CnAIP2 was its ability to promote early flowering. In Arabidopsis CnAIP2-expressing plants, the average flowering time was equivalent to the ABI3 null mutant abi3-6 plants. The early-flowering phenotype was very stable and consistent in all subsequent generations tested. Again, CnAIP2 might achieve this by negatively regulating ABI3. This is indicated by the fact that CnAIP2 did not alter the flowering time in the abi3-6 mutant, which lacks a functional ABI3 gene. Our microarray data showed that several genes that positively regulate flowering (i.e. promote the transition to flowering or promote the floral development process per se) had higher expression levels in the CnAIP2 plants. Most strikingly, expression of FT was almost 20 times higher in the transgenic plants (wild-type and CnAIP2 tissues were sampled during the same hour of the day). This probably was one of the major reasons why CnAIP2 plants flowered so early, since FT is one of the most important floral integrator genes, a promoter of flowering that has been referred to as “florigen” (Zeevaart, 2006; Jaeger and Wigge, 2007), the mobile protein that travels through the phloem to the apex where it interacts with FLOWERING D to up-regulate floral identity genes (Turck et al., 2008). The expression of genes encoding MADS domain transcription factors that represent some of the collaborative flowering regulators and/or floral organ identity regulators (e.g. AP3 of class B and SEP3 of class E) was also higher in the CnAIP2 transgenic plants. Likewise, the expression of other regulatory genes such as AGL6 and AGL19 was higher in the CnAIP2 transgenic plants; both of these genes encode products that are potent activators of flowering (Schönrock et al., 2006; Koo et al., 2010). AGL6, for example, is a floral promoter with a dual role: that of inhibiting the transcription of the FLC/MAF genes (see below) while promoting the expression of FT (Yoo et al., 2011). Coincidentally, the expression of genes encoding negative regulators of flowering, such as AGL31, MAF4, and MAF5, was down-regulated in the CnAIP2 plants, consistent with the early-flowering phenotype. It is worth noting that while the amino acid sequence of CnAIP2 showed similarity to that of FRIGIDA proteins, the role of CnAIP2 was opposite the reported role of FRIGIDA proteins, namely to delay flowering and impose a vernalization requirement (Johanson et al., 2000; Michaels et al., 2004). We are conducting detailed analyses to compare the role of the ABI3-FRI and CnABI3-CnAIP2 protein interactions in the regulation of flowering time.
Increasing evidence indicates that flowering and seed dormancy/germination are key life cycle processes that are closely related to each other, and some common proteins may participate as gatekeepers of these transitions. CnAIP2 is likely one of the key regulatory proteins. Another important regulator is FLC, a protein that regulates flowering and also plays a role during germination; its functions are assisted by other flowering pathway proteins (FT, SOC1, and AP1; Chiang et al., 2009). Some common mechanisms are suggested to regulate flowering and bud/seed dormancy involving circadian regulators and genes responsive to temperature as well as genes of hormonal pathways that act in both processes (Horvath, 2009).
Overall Significance
Yellow cedar is a high-elevation conifer species that requires 2 years to complete seed development. Dormancy breakage is elicited by exposure of the dispersed mature seeds to a prolonged period of moist and cold conditions (6–18 months in natural stands). This prevents germination of shed seeds in the fall and during the cold winter months. Thus, we can safely state that in yellow cedar, a strict control over its life cycle transitions is of the utmost importance; indeed, the survival of this species (particularly in view of its specific habitat) depends on this. There is a striking similarity between the prolonged moist chilling required by seeds of some species (particularly conifers) to acquire a competence for germination and the acquisition of flowering competence in response to vernalization. In both cases, an environmental signal leads to the ability of the seed or plant to perceive and react to a subsequent signal or set of conditions that is required for the actual “switch” or life cycle transition from vegetative to reproductive development or from dormancy to germination. For the transition to take place, an additional signal(s) is necessary: for example, optimal temperature and perhaps light of a certain wavelength to initiate germination as well as a certain daylength (photoperiod) for flowering induction after vernalization of winter annuals (Amasino, 2004). The CnABI3/ABI3 interacting protein, CnAIP2, plays important roles in several key transitions of the plant life cycle, most notably during seed development and flowering. The phenotypes reveal that the functions of CnAIP2 appear to be opposite those of ABI3/CnABI3 and further intimate that CnAIP2 may regulate ABI3 in a negative manner through the protein-protein interactions between the two. ABI3 generally functions as a gatekeeper (or safeguard) during transitions of the plant life cycle (i.e. in dormancy maintenance), as a germination “checkpoint,” and in maintaining vegetative growth and delaying flowering. To these functions of ABI3, however, plants and seeds need some factors as counterregulators. As global regulators, these antagonistic factors contribute to maintaining appropriate spatial and developmental regulation of gene expression, achieving a delicate balance during plant and seed development and ensuring successful development and growth. CnAIP2 is a suitable candidate as an ABI3 counterregulator. Intriguingly, this counterregulation appears to involve auxin, a hormone connected to anther maturation processes (Cecchetti et al., 2008), early seed and embryo development (especially the morphogenesis phase; Harada et al., 2010), lateral root formation (Guyomarc’h et al., 2010), and even flowering time regulation (Mai et al., 2011) and temperature sensing (Rietveld et al., 2000). Figure 9 presents a summary of CnAIP2-CnABI3 interactions in which both proteins may function as global regulators of life cycle transitions but in an antagonistic manner.
Figure 9.
Summary diagram showing life cycle transitions potentially regulated by ABI3-CnAIP2 interactions and some of the target genes that are regulated in an antagonistic manner by CnAIP2 and ABI3. Arrow-headed lines indicate positive roles, and T-headed lines indicate negative roles. Representative genes in dotted squares are affected in CnAIP2-expressing plants.
MATERIALS AND METHODS
Construction of Bait Vector and Target cDNA Library
Stratagene’s CytoTrap XR library construction kit was used for the yeast (Saccharomyces cerevisiae) two-hybrid assay to detect protein-protein interactions between CnABI3 and target proteins. The coding region of CnABI3 cDNA was cloned into pSos and engineered in frame and downstream of the hSOS protein between restriction sites NcoI and SalI. Total RNA of yellow cedar (Callitropsis nootkatensis) seeds (year-1 developing seeds and mature seeds at different stages of a 3-month stratification treatment) was extracted using a method optimized for conifers (Wang et al., 2000), and mRNA was purified with the PolyATtract mRNA isolation system II (Promega). A cDNA library was constructed using 7 μg of mRNA, and the resultant cDNA library was inserted into the pMyr vector between restriction sites EcoRI and XhoI following the instruction manual of the kit.
Yeast Two-Hybrid Analyses to Identify Proteins That Interact with CnABI3
Yeast cdc25H competent cells were first transformed with pSos-CnABI3. Positive transformants were verified to be suitable in the yeast two-hybrid assay. A yeast cell culture from a single colony containing pSos-CnABI3 was used in the preparation of competent cells. The pMyr-cDNA library was transformed to the competent cells, and possible protein-protein interactions were identified and verified according to methods in the instruction manual of the CytoTrap kit. Procedures for the amplification of the 5′-vend cDNA of CnAIP2 and the CnABI3 deletion constructs used to analyze the CnABI3 domains required for a physical interaction with CnAIP2 are noted in Supplemental Materials and Methods S1.
Expression Analyses of CnAIP2 in Yellow Cedar Seeds
Yellow cedar seeds of seed lot 30156 were subjected to a dormancy-breaking treatment (1 month of warm, moist conditions at 25°C and 2 months of moist chilling at 4°C) and then placed in germination conditions (30°C days, 20°C nights with an 8-h photoperiod). Megagametophytes and embryos were excised from seeds at different stages and stored at –80°C before RNA and protein extraction. Northern- and western-blot analyses were carried out to examine steady-state levels of CnAIP2 mRNA and protein. RNA and protein extraction, and northern- and western-blot analyses, were performed using methods described by Zeng et al. (2003). For northern blots, the cDNA of CnAIP2 derived from the pMyr vector was used to prepare 32P-labeled probes. For western blots, a 1:500 dilution of antibodies was used. Antibodies of CnAIP2 were produced by genetic immunization at GENEART. Western-blot analyses to detect the CnABI3 protein were performed as described by Zeng et al. (2003).
Immunohistochemistry of Yellow Cedar Seeds
Seeds were first subjected to the 3-month full dormancy-breaking treatment. Seed coats were removed, and embryos and megagametophytes were fixed in 50% ethyl alcohol, 5% glacial acetic acid, and 10% formaldehyde for 1 week and then embedded in paraffin for sectioning. Immunohistochemistry procedures were performed by Wax-it Histology Service. To detect CnAIP2 proteins, sections of seeds were incubated with either anti-CnAIP2 antibodies or preimmune serum from the same mouse as the control. Sections were counterstained with hematoxylin and then with lithium carbonate to stain nucleic acids in the nuclei, which stain blue. A positive reaction to CnAIP2 proteins is indicated by the brown color, while nuclear counterstaining is in blue.
Generation of 35S-CnAIP2 Transgenic Plants
The full-length cDNA of CnAIP2 was amplified from pBluescript SK+-CnAIP2 using PCR with forward primer 5′-GGGATGTATAATAGTTATTTGTTTGGGATGG-3′ and reverse primer 5′-GGGCTACTCTAAATATGAAGAGGGAAG-3′. The PCR fragment was cloned into pGEM-T-Easy vector (Promega), resulting in pGEM-T-Easy-CnAIP2. The NotI (filled in with Klenow) cDNA fragment of CnAIP2 from pGEM-CnAIP2 was introduced into the XbaI/SacI (end polished with T4 DNA polymerase) sites of the binary vector pBI121. This generated the 35S-CnAIP2 plasmid that was used to transform Arabidopsis (Arabidopsis thaliana) Col-0 plants using the floral dip method (Clough and Bent, 1998).
Seed Dormancy/Germination Performance, ABA Sensitivity of Germination-Competent Seeds, and Root Development Analyses
To analyze the degree of seed dormancy, freshly harvested mature seeds of different lines were surface sterilized in 40% commercial bleach (2.4% hypochlorite) for 10 min and washed three times with sterilized water (5 min each). Seeds were then sown on one-half-strength MS solid medium in petri dishes and immediately incubated in a growth chamber (Conviron model E15) in germination conditions (21°C, 16-h light period) without moist-chilling treatments. In parallel experiments, seeds underwent 1 to 4 d of cold treatment at 4°C before transferring to germination conditions. Germination was defined as radicle emergence, and germination percentage was recorded daily. All tests were carried out in duplicate, each with 50 to 100 seeds.
For testing of ABA sensitivity as far as a subsequent inhibition of seed germination was concerned, seeds were surface sterilized as above and sown on one-half-strength MS medium containing 0.5 or 1.0 μm (+)-ABA and moist chilled at 4°C for 4 d. The plates were then transferred to germination conditions. Germination performance was recorded daily.
To measure root development, seeds were arranged in a line on each large plate (150 mm diameter) and subjected to a 4-d moist-chilling treatment before transfer of the plates to growth conditions. Plates were placed vertically in the growth chamber so that roots grew downward in a “straight line” manner for easier measurement. Root length and the number of lateral roots were recorded after 1 and 2 weeks of incubation in germination conditions. A lateral root was counted when it visibly emerged from the main root.
Flowering Time Measurement
Seeds of different lines were placed on one-half-strength MS plates and subjected to a 4-d moist-chilling treatment (at 4°C) before transfer to a growth chamber for germination. Seedlings at similar stages after germination (approximately 4–5 d) were transferred to soil and grown in a growth chamber at 21°C with a 16-h photoperiod and an average light intensity (photosynthetic photo flux) of 96 μmol m−2 s−1 (Quantum Meter; APOGEE Instruments). Plants of all the tested lines were grown side by side in the same growth chamber. Growth performance was monitored daily. Flowering time was scored as the number of days from germination to the day when the first flower bud emerged. The number of primary leaves at flowering was also recorded.
Microarray Analysis and RT-PCR Verification
CnAIP2 transgenic and wild-type plants were grown side by side under the same conditions. Analyses were conducted on developing seeds (7 and 28 DAP), on flower buds, and on roots of seedlings 9 d after germination on MS plates; these materials were collected in triplicate for RNA extraction (NucleoSpin RNA Plant; Macherey-Nagel). Wild-type and CnAIP2 flower buds (before the appearance of open white flowers) were sampled during the same hour of the day but on different days because CnAIP2 plants flowered earlier. Microarray tests, data processing, and statistical analyses were performed by The Laboratory for Advanced Genome Analysis at the Vancouver Prostate Centre and as detailed in Supplemental Materials and Methods S1. A number of genes related to the phenotypes that showed differential expression levels (at least a 2-fold difference) in the microarray analysis were selected for RT-PCR transcript analysis for verification of the microarray data. Primers were designed to span at least one intron, so that any PCR product from genomic DNA could be distinguished in case residual genomic DNA was not eliminated. Primer sequences of each gene are listed in Supplemental Table S1. RT-PCR transcript analysis procedures are noted in Supplemental Materials and Methods S1.
Sequence data from this article can be found in the GenBank data library under accession numbers AY268951 (CnAIP2 cDNA) and FJ501963 (CnAIP2 promoter).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Primers for RT-PCR.
Acknowledgments
We thank Peter McCourt (University of Toronto) for supplying the Arabidopsis abi3-6 mutant and Jerome Giraudat (Centre National de la Recherche Scientifique) for the Arabidopsis ABI3 gene. We are also grateful to Anne Haegert of the Laboratory for Advanced Genome Analysis at the Vancouver Prostate Centre for her help with the microarray analyses.
Glossary
- ABA
abscisic acid
- ABREs
ABA-responsive elements
- 2,4-D
2,4-dichlorophenoxyacetic acid
- DAP
days after pollination
- MS
Murashige and Skoog
- X-gluc
5-bromo-4-chloro-3-indolyl-β-glucuronic acid
- Col-0
ecotype Columbia
- RT
reverse transcription
- cDNA
complementary DNA
References
- Amasino R. (2004) Take a cold flower. Nat Genet 36: 111–112 [DOI] [PubMed] [Google Scholar]
- Arroyo A, Bossi F, Finkelstein RR, León P. (2003) Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol 133: 231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P. (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34: 67–75 [DOI] [PubMed] [Google Scholar]
- Brocard IM, Lynch TJ, Finkelstein RR. (2002) Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol 129: 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti V, Altamura MM, Falasca G, Costantino P, Cardarelli M. (2008) Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 20: 1760–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Bao ML, Sun YZ, Yang YJ, Xu XH, Wang JH, Han N, Bian HW, Zhu MY. (2011) Regulation of auxin response by miR393-targeted transport inhibitor response protein 1 is involved in normal development in Arabidopsis. Plant Mol Biol 77: 619–629 [DOI] [PubMed] [Google Scholar]
- Chiang GC, Barua D, Kramer EM, Amasino RM, Donohue K. (2009) Major flowering time gene, flowering locus C, regulates seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA 106: 11661–11666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E. (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105: 8790–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59: 387–415 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69: 437–449 [DOI] [PubMed] [Google Scholar]
- Guyomarc’h S, Lucas M, Laplaze L. (2010) Lateral/secondary roots. In Encyclopedia of Life Sciences. John Wiley & Sons, Chichester, UK. http://onlinelibrary.wiley.com/doi/10.1002/9780470015902.a0002060.pub2/full (December 18, 2011) [Google Scholar]
- Harada JJ, Belamonte MF, Kwong RW. (2010) Plant embryogenesis (zygotic and somatic). In Encyclopedia of Life Sciences. John Wiley & Sons, Chichester, UK. http://onlinelibrary.wiley.com/doi/10.1002/9780470015902.a0002042.pub2/full (December 18, 2011) [Google Scholar]
- Hennon PE, D’Amore DV, Wittwer DT, Caouette JP. (2008) Yellow-cedar decline: conserving a climate-sensitive tree species as Alaska warms. In Integrated Restoration of Forested Ecosystems to Achieve Multiresource Benefits. Proceedings of the 2007 National Silviculture Workshop, general technical report PNW-Gtr-733. US Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR, pp 233–245.
- Hobo T, Asada M, Kowyama Y, Hattori T. (1999a) ACGT-containing abscisic acid response element (ABRE) and coupling element 3 (CE3) are functionally equivalent. Plant J 19: 679–689 [DOI] [PubMed] [Google Scholar]
- Hobo T, Kowyama Y, Hattori T. (1999b) A bZIP factor, TRAB1, interacts with VP1 and mediates abscisic acid-induced transcription. Proc Natl Acad Sci USA 96: 15348–15353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath D. (2009) Common mechanisms regulate flowering and dormancy. Plant Sci 177: 523–531 [Google Scholar]
- Jaeger KE, Wigge PA. (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C. (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347 [DOI] [PubMed] [Google Scholar]
- Jones HD, Kurup S, Peters NC, Holdsworth MJ. (2000) Identification and analysis of proteins that interact with the Avena fatua homologue of the maize transcription factor VIVIPAROUS 1. Plant J 21: 133–142 [DOI] [PubMed] [Google Scholar]
- Kermode AR. (2003) Seed development: physiology of maturation. In B Thomas, D Murphy, B Murray, eds, Encyclopedia of Applied Plant Sciences. Academic Press, Waltham, MA, pp 1261–1279. [Google Scholar]
- Kermode AR. (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24: 319–344 [Google Scholar]
- Kermode AR, Zeng Y, Hu X, Lauson S, Abrams SR, He X. (2007) Ectopic expression of a conifer Abscisic Acid Insensitive3 transcription factor induces high-level synthesis of recombinant human alpha-L-iduronidase in transgenic tobacco leaves. Plant Mol Biol 63: 763–776 [DOI] [PubMed] [Google Scholar]
- Koo SC, Bracko O, Park MS, Schwab R, Chun HJ, Park KM, Seo JS, Grbic V, Balasubramanian S, Schmid M, et al. (2010) Control of lateral organ development and flowering time by the Arabidopsis thaliana MADS-box gene AGAMOUS-LIKE6. Plant J 62: 807–816 [DOI] [PubMed] [Google Scholar]
- Krichevsky A, Zaltsman A, Kozlovsky SV, Tian GW, Citovsky V. (2009) Regulation of root elongation by histone acetylation in Arabidopsis. J Mol Biol 385: 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroj T, Savino G, Valon C, Giraudat J, Parcy F. (2003) Regulation of storage protein gene expression in Arabidopsis. Development 130: 6065–6073 [DOI] [PubMed] [Google Scholar]
- Kurup S, Jones HD, Holdsworth MJ. (2000) Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J 21: 143–155 [DOI] [PubMed] [Google Scholar]
- Lally D, Ingmire P, Tong HY, He ZH. (2001) Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 13: 1317–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara P, Oñate-Sánchez L, Abraham Z, Ferrándiz C, Díaz I, Carbonero P, Vicente-Carbajosa J. (2003) Synergistic activation of seed storage protein gene expression in Arabidopsis by ABI3 and two bZIPs related to OPAQUE2. J Biol Chem 278: 21003–21011 [DOI] [PubMed] [Google Scholar]
- Li G, Chandrasekharan MB, Wolffe AP, Hall TC. (2001) Chromatin structure and phaseolin gene regulation. Plant Mol Biol 46: 121–129 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32: 317–328 [DOI] [PubMed] [Google Scholar]
- Mai YX, Wang L, Yang HQ. (2011) A gain-of-function mutation in IAA7/AXR2 confers late flowering under short-day light in Arabidopsis. J Integr Plant Biol 53: 480–492 [DOI] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Bezerra IC, Amasino RM. (2004) FRIGIDA-related genes are required for the winter-annual habit in Arabidopsis. Proc Natl Acad Sci USA 101: 3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki T, Miyazawa Y, Kobayashi A, Uchida M, Watanabe C, Fujii N, Takahashi H. (2011) Hormonal regulation of lateral root development in Arabidopsis modulated by MIZ1 and requirement of GNOM activity for MIZ1 function. Plant Physiol 157: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Lynch TJ, Finkelstein RR. (2001) Physical interactions between ABA response loci of Arabidopsis. Plant J 26: 627–635 [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M. (2001) DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J 25: 213–221 [DOI] [PubMed] [Google Scholar]
- Nambara E, Keith K, McCourt P, Naito S. (1995) A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 121: 629–636 [Google Scholar]
- Nambara E, Marion-Poll A. (2003) ABA action and interactions in seeds. Trends Plant Sci 8: 213–217 [DOI] [PubMed] [Google Scholar]
- Ng DW, Chandrasekharan MB, Hall TC. (2004) The 5′ UTR negatively regulates quantitative and spatial expression from the ABI3 promoter. Plant Mol Biol 54: 25–38 [DOI] [PubMed] [Google Scholar]
- Parcy F, Giraudat J. (1997) Interactions between the ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J 11: 693–702 [DOI] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J. (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Rietveld PL, Wilkinson C, Franssen HM, Balk PA, van der Plas LH, Weisbeek PJ, Douwe de Boer A. (2000) Low temperature sensing in tulip (Tulipa gesneriana L.) is mediated through an increased response to auxin. J Exp Bot 51: 587–594 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Gacio MdelC, Matilla-Vázquez MA, Matilla AJ. (2009) Seed dormancy and ABA signaling: the breakthrough goes on. Plant Signal Behav 4: 1035–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Kurup S, Holdswoth M. (2000) ABI3 emerges from the seed. Trends Plant Sci 5: 418–419 [DOI] [PubMed] [Google Scholar]
- Schönrock N, Bouveret R, Leroy O, Borghi L, Köhler C, Gruissem W, Hennig L. (2006) Polycomb-group proteins repress the floral activator AGL19 in the FLC-independent vernalization pathway. Genes Dev 20: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz TF, Medina J, Hill A, Quatrano RS. (1998) 14-3-3 proteins are part of an abscisic acid-VIVIPAROUS1 (VP1) response complex in the Em promoter and interact with VP1 and EmBP1. Plant Cell 10: 837–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kao CY, Cocciolone S, McCarty DR. (2001) Maize VP1 complements Arabidopsis abi3 and confers a novel ABA/auxin interaction in roots. Plant J 28: 409–418 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Wang SX, Hunter W, Plant A. (2000) Isolation and purification of functional total RNA from woody branches and needles of Sitka and white spruce. Biotechniques 28: 292–296 [DOI] [PubMed] [Google Scholar]
- Xia JH, Stewart D, Kermode AR. (2002) Modified moist chilling treatments that promote germination and post-germinative reserve mobilization of different seed lots of yellow-cedar. Seed Sci Technol 30: 263–277 [Google Scholar]
- Yokoyama A, Yamashino T, Amano Y, Tajima Y, Imamura A, Sakakibara H, Mizuno T. (2007) Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48: 84–96 [DOI] [PubMed] [Google Scholar]
- Yoo SK, Wu X, Lee JS, Ahn JH. (2011) AGAMOUS-LIKE 6 is a floral promoter that negatively regulates the FLC/MAF clade genes and positively regulates FT in Arabidopsis. Plant J 65: 62–76 [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. (2006) Florigen coming of age after 70 years. Plant Cell 18: 1783–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Kermode AR. (2004) A gymnosperm ABI3 gene functions in a severe abscisic acid-insensitive mutant of Arabidopsis (abi3-6) to restore the wild-type phenotype and demonstrates a strong synergistic effect with sugar in the inhibition of post-germinative growth. Plant Mol Biol 56: 731–746 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Raimondi N, Kermode AR. (2003) Role of an ABI3 homologue in dormancy maintenance of yellow-cedar seeds and in the activation of storage protein and Em gene promoters. Plant Mol Biol 51: 39–49 [DOI] [PubMed] [Google Scholar]
- Zhang X, Garreton V, Chua NH. (2005) The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev 19: 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZX, Liu Y, Liu SJ, Mao CZ, Wu YR, Wu P. (2012) A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol Plant 5: 154–161 [DOI] [PubMed] [Google Scholar]