Abstract
Riboflavin (vitamin B2) is the precursor of the flavin coenzymes flavin mononucleotide and flavin adenine dinucleotide. In Escherichia coli and other bacteria, sequential deamination and reduction steps in riboflavin biosynthesis are catalyzed by RibD, a bifunctional protein with distinct pyrimidine deaminase and reductase domains. Plants have two diverged RibD homologs, PyrD and PyrR; PyrR proteins have an extra carboxyl-terminal domain (COG3236) of unknown function. Arabidopsis (Arabidopsis thaliana) PyrD (encoded by At4g20960) is known to be a monofunctional pyrimidine deaminase, but no pyrimidine reductase has been identified. Bioinformatic analyses indicated that plant PyrR proteins have a catalytically competent reductase domain but lack essential zinc-binding residues in the deaminase domain, and that the Arabidopsis PyrR gene (At3g47390) is coexpressed with riboflavin synthesis genes. These observations imply that PyrR is a pyrimidine reductase without deaminase activity. Consistent with this inference, Arabidopsis or maize (Zea mays) PyrR (At3g47390 or GRMZM2G090068) restored riboflavin prototrophy to an E. coli ribD deletant strain when coexpressed with the corresponding PyrD protein (At4g20960 or GRMZM2G320099) but not when expressed alone; the COG3236 domain was unnecessary for complementing activity. Furthermore, recombinant maize PyrR mediated NAD(P)H-dependent pyrimidine reduction in vitro. Import assays with pea (Pisum sativum) chloroplasts showed that PyrR and PyrD are taken up and proteolytically processed. Ablation of the maize PyrR gene caused early seed lethality. These data argue that PyrR is the missing plant pyrimidine reductase, that it is plastid localized, and that it is essential. The role of the COG3236 domain remains mysterious; no evidence was obtained for the possibility that it catalyzes the dephosphorylation that follows pyrimidine reduction.
Riboflavin is the substrate for biosynthesis of the essential flavocoenzymes FMN and FAD, which occur in all kingdoms of life and have roles in diverse redox reactions as well as in other processes such as DNA repair, light sensing, and bioluminescence (Fischer and Bacher, 2005). Plants and many microorganisms can synthesize riboflavin, but humans and other animals cannot, so they must obtain it from the diet (Powers, 2003). Plant foods are important sources of riboflavin for humans, and the riboflavin pathway is a target for engineering biofortified crops (Fitzpatrick et al., 2012).
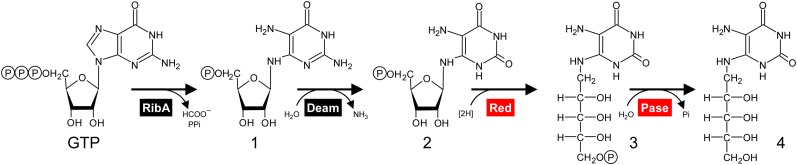
Riboflavin biosynthesis proceeds via the same pathway in bacteria and plants (Fischer and Bacher, 2005; Roje, 2007). This pathway starts from GTP, which is converted by GTP cyclohydrolase II (named RibA in Escherichia coli) to the pyrimidine derivative 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone 5′-P. Deamination of the pyrimidine ring then yields 5-amino-6-ribosylamino-2,4(1H,3H)-pyrimidinedione 5′-P, and subsequent reduction of the ribosyl moiety gives 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P. After dephosphorylation, this product is condensed with 3,4-dihydroxy-2-butanone 4-P to give 6,7-dimethyl-8-ribityllumazine, whose dismutation yields riboflavin. Figure 1 shows the first four steps of this pathway.
Figure 1.
The first four steps of the riboflavin biosynthesis pathway in bacteria and plants. The enzymes involved are GTP cyclohydrolase II (RibA), pyrimidine deaminase (Deam), pyrimidine reductase (Red), and a specific phosphatase (Pase). Enzymes for which the plant genes are not known are colored red. Intermediates are as follows: 1, 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone 5′-P; 2, 5-amino-6-ribosylamino-2,4(1H,3H)-pyrimidinedione 5′-P; 3, 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P; 4, 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione.
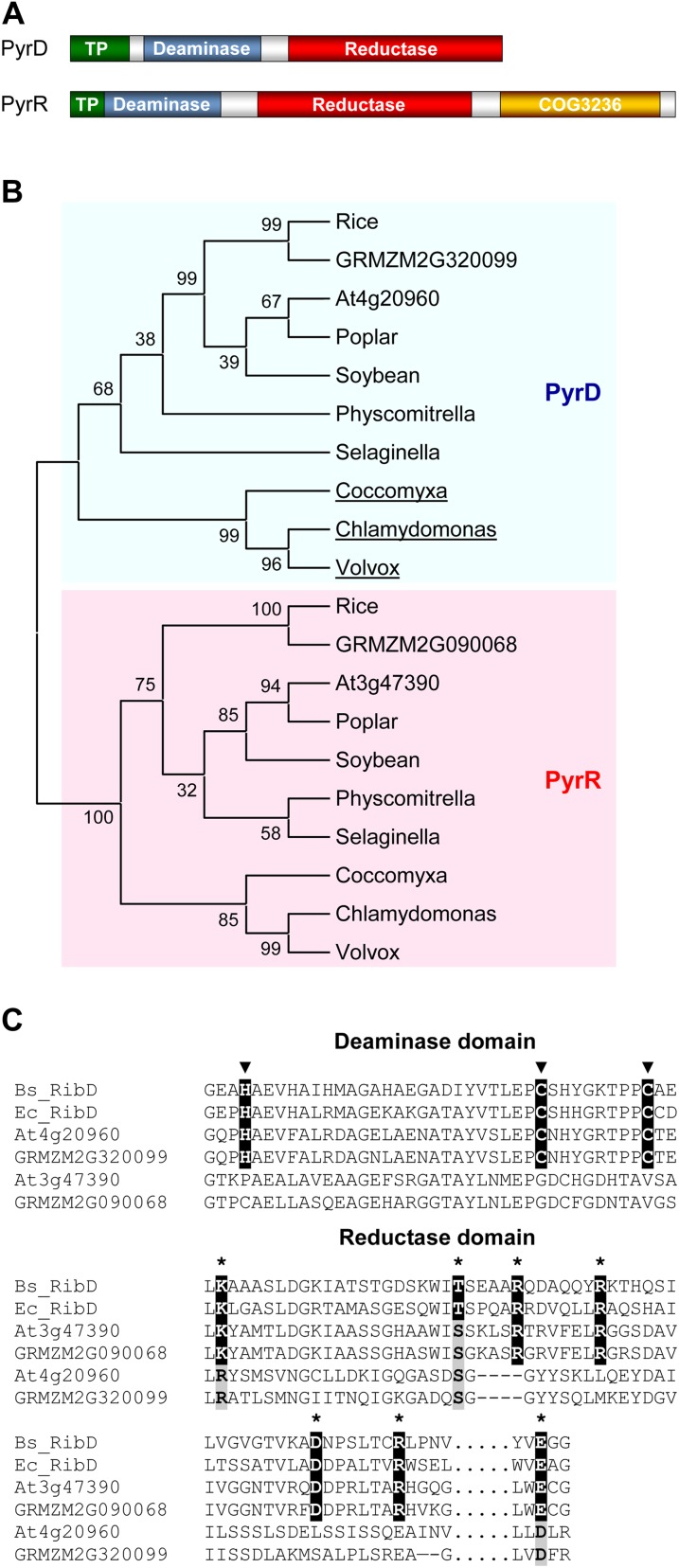
In E. coli, the deamination and reduction steps are catalyzed by a single bifunctional enzyme, RibD, which has N-terminal deaminase and C-terminal reductase domains that retain their respective activities when expressed separately (Richter et al., 1997; Magalhães et al., 2008). The situation in plants seems superficially similar but is in fact more complex (Gerdes et al., 2012). The bidomain bacterial RibD protein has two types of homologs in plants (Fischer et al., 2004; Chatwell et al., 2006; Chen et al., 2006), here called PyrD and PyrR, both with apparent deaminase and reductase domains (Fig. 2A). Only PyrD, represented by At4g20960, has been studied biochemically; it was found to have pyrimidine deaminase but not reductase activity (Fischer et al., 2004). The function of PyrR, represented by At3g47390, is unknown, although it has been inferred to have reductase activity (Chatwell et al., 2006; Chen et al., 2006; Ouyang et al., 2010) and perhaps to lack deaminase activity (Ouyang et al., 2010). Another mystery surrounding PyrR proteins is the presence of an extra C-terminal domain of unknown function (COG3236 in the Clusters of Orthologous Groups database; Fig. 2A); this domain occurs as a stand-alone protein in many bacteria. One possibility is that it catalyzes the dephosphorylation that follows the pyrimidine reduction step in the pathway (Fig. 1). The phosphatase involved is most likely substrate specific, but it has not been identified in plants or any other organism (Roje, 2007; Gerdes et al., 2012), and genes for enzymes in the same pathway, especially for successive steps, are quite commonly fused (Suhre, 2007). A mutation (phs1) that deleted the COG3236 domain from Arabidopsis (Arabidopsis thaliana) PyrR resulted in a photosensitive phenotype that could be rescued by supplied FAD (Ouyang et al., 2010).
Figure 2.
Structure and phylogeny of plant PyrD and PyrR proteins. A, Domain architectures. The examples shown are Arabidopsis At4g20960 and At3g47390; the predicted plastid targeting peptide (TP) varies in length between species. B, Phylogenetic tree of PyrD and PyrR proteins. Sequences were aligned with ClustalW; the tree was built by the neighbor-joining method with MEGA5. Bootstrap values (%) for 1,000 replicates are next to the nodes. Only the tree topology is shown. Note that the PyrD proteins of green algae (underlined) lack a reductase domain. C, Alignments showing the conservation of the zinc-binding residues (arrowheads) in the deaminase domain of PyrD but not PyrR proteins and the conservation of the predicted substrate-binding residues (asterisks) in the reductase domain of PyrR but not PyrD proteins. The deaminase sequences correspond to residues 45 to 85 of B. subtilis RibD (synonym RibG); the reductase sequences correspond to residues 150 to 210 and (separated by dots) 288 to 292 of B. subtilis RibD. Identical zinc- or substrate-binding residues are black, and conservative replacements are gray. Dashes indicate gaps that maximize the alignment.
The plant riboflavin synthesis pathway is considered to be plastidial (Roje, 2007), but this location is based almost solely on bioinformatics and high-throughput proteome analyses (Gerdes et al., 2012). In only one case is there more definitive experimental support: in vitro chloroplast import data for the pathway’s penultimate enzyme, 6,7-dimethyl-8-ribityllumazine synthase (Jordan et al., 1999). Similarly, clear genetic support for the function of most plant riboflavin synthesis enzymes is lacking (Gerdes et al., 2012), the exception being an Arabidopsis RibA homolog (Hedtke and Grimm, 2009).
The work reported here established, using maize (Zea mays) and Arabidopsis, that PyrR is indeed the missing pyrimidine reductase, that it lacks deaminase activity, and that its COG3236 domain is not essential for pyrimidine reductase activity and most likely lacks phosphatase activity. We also demonstrated the import of PyrR and PyrD into chloroplasts in vitro and confirmed that the gene for PyrR is essential.
RESULTS
Bioinformatic Analysis of E. coli RibD Homologs PyrD and PyrR
The overall sequence identity between PyrD and PyrR proteins is quite low: 25% for those from Arabidopsis (At4g20960 and At3g47390, respectively) and 27% for their maize orthologs (GRMZM2G320099 and GRMZM2G090068, respectively). Phylogenetic analysis of PyrD and PyrR proteins from diverse plants indicates that they are paralogs that diverged at the base of the plant lineage (Fig. 2B). The extra C-terminal COG3236 domain of PyrR proteins appears to be equally ancient, being present in PyrR proteins from green algae to angiosperms (Fig. 2A). The sequence divergence between PyrD and PyrR, their ancient paralogy, and their conservation throughout the plant kingdom strongly imply a divergence in function. That the diverged function of PyrR remains one in riboflavin synthesis is supported by coexpression analyses of Arabidopsis microarray data at the ATTED database (Obayashi et al., 2011). Such analyses place the gene for PyrR near the center of a coexpression network containing the genes for PyrD and three other riboflavin pathway enzymes (Supplemental Fig. S1).
Sequence alignments with the well-characterized Bacillus subtilis RibD (Chen et al., 2006; also called RibG) and E. coli RibD (Stenmark et al., 2007) enzymes show that, unlike PyrD proteins, PyrR proteins have a deaminase domain that lacks the catalytically essential zinc-binding residues and a reductase domain in which the predicted substrate-binding residues are all present (Fig. 2C). This reciprocity in missing residues predicts that PyrR proteins are monofunctional pyrimidine reductases and that PyrD proteins are monofunctional pyrimidine deaminases. The latter prediction has been experimentally confirmed for Arabidopsis PyrD, as noted above (Fischer et al., 2004). That PyrD proteins are monofunctional deaminases is also supported by the observation that the PyrD proteins of green algae, unlike those of other plants, completely lack a reductase domain (Fig. 2B).
The PyrR and PyrD proteins of Arabidopsis, maize, and other plants have N-terminal extensions that are generally predicted to be chloroplast-targeting peptides by TargetP (Emanuelsson et al., 2007), Predotar (Small et al., 2004), and Wolf PSORT (Horton et al., 2007) algorithms. This is in accordance with the predictions and limited experimental data for other riboflavin pathway enzymes (Gerdes et al., 2012). The putative targeting peptide sequences were removed from the PyrR and PyrD constructs used for the complementation and biochemical studies described in the next two sections.
Plant PyrR Genes Complement the Reductase But Not the Deaminase Function of E. coli RibD
For complementation assays of the function of Arabidopsis and maize PyrR, we constructed an E. coli strain (CmpX13ΔribD) containing a full deletion of the ribD gene and a chromosomal copy of the ribM riboflavin transporter gene from Corynebacterium glutamicum. Expression of RibM enables E. coli riboflavin auxotrophs to grow when provided with relatively low (50 μm) concentrations of riboflavin, which they cannot otherwise do (Mathes et al., 2009).
As expected, the riboflavin prototrophy of CmpX13ΔribD was restored by plasmid-driven expression of the native E. coli ribD gene but not by expression of the Arabidopsis PyrD gene alone (Fischer et al., 2004; Fig. 3A). Expression of the Arabidopsis PyrR gene alone likewise failed to restore riboflavin prototrophy, but coexpression of both PyrR and PyrD did so (Fig. 3A). Similarly, neither maize PyrD nor PyrR complemented the ribD phenotype when expressed singly, but they did so when expressed together (Fig. 3B). These results indicate that Arabidopsis and maize PyrR proteins have pyrimidine reductase activity and lack pyrimidine deaminase activity. To attempt to define the region of PyrR that bears the reductase activity, two truncated versions of the Arabidopsis protein were tested for complementing activity: one lacking the C-terminal COG3236 domain, the other lacking both COG3236 and deaminase domains. The protein remained active without the COG3236 domain but not without the deaminase domain (Fig. 3B). The latter result contrasts with the situation for Arabidopsis PyrD, whose deaminase domain is active when expressed on its own (Fischer et al., 2004).
Figure 3.
Functional complementation of E. coli ribD deletant strain CmpX13ΔribD by genes encoding Arabidopsis and maize PyrR and PyrD. A, Strain CmpX13ΔribD was transformed with pETDuet-1 vector alone (V) or harboring E. coli ribD or Arabidopsis (At) PyrR and PyrD singly or together. For each construct, three independent isolates were cultured on LB medium containing 50 µg mL−1 kanamycin, 50 µg mL−1 ampicillin, and 1 mm IPTG, without or with 50 µm riboflavin, for 72 h at 22°C. B, As in A except that the vector harbored maize (Zm) PyrR and PyrD singly or together or Arabidopsis PyrD plus versions of Arabidopsis PyrR lacking either the COG3236 domain (AtPyrRΔ) or the COG3236 and deaminase domains (AtPyrR*). A control with Arabidopsis PyrD plus full-length Arabidopsis PyrR was included.
Recombinant PyrR Has Pyrimidine Reductase Activity in Vitro
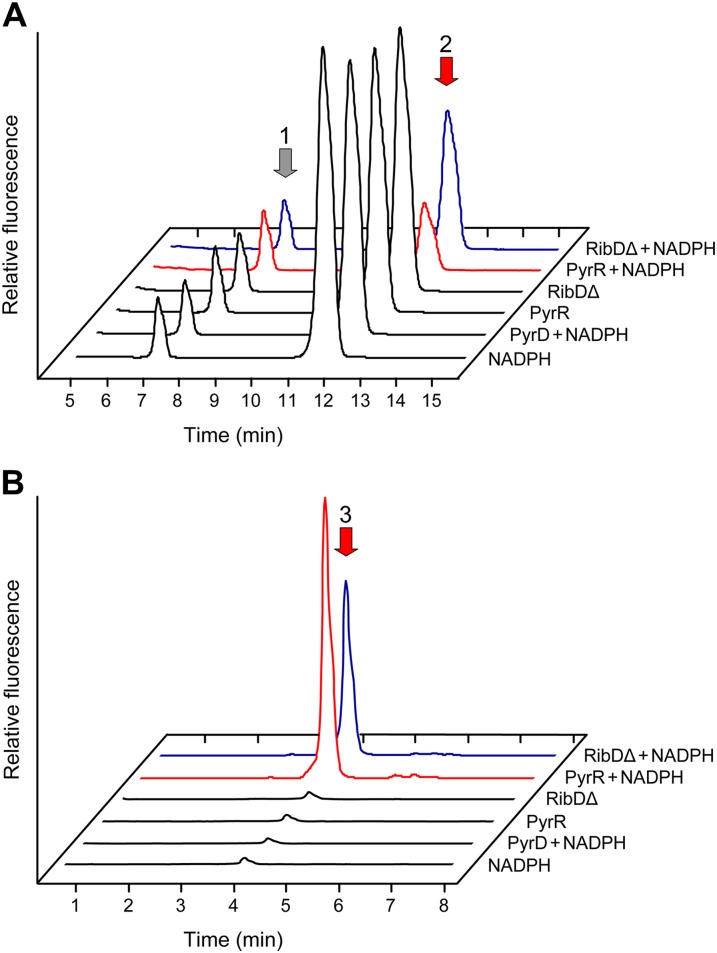
The reductase substrate, 5-amino-6-ribosylamino-2,4(1H,3H)-pyrimidinedione 5′-P (Fig. 1), was synthesized from GTP enzymatically using E. coli RibA and RibD in the absence of NAD(P)H. The reaction mixture, which contained some unconverted 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone 5′-P (Fig. 4A), was deproteinized by ultrafiltration before use in reductase assays. Reactants and products were analyzed as their diacetyl derivatives and detected by fluorometric HPLC (Fig. 4; Richter et al., 1997).
Figure 4.
Verification of the pyrimidine reductase activity of recombinant maize PyrR. E. coli RibA was used to convert GTP to 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone 5′-P (peak 1), which was converted to the reductase substrate, 5-amino-6-ribosylamino-2,4(1H,3H)-pyrimidinedione 5′-P (peak 2), by E. coli RibD in the absence of NADPH. After ultrafiltration to remove RibA and RibD, the reaction products were incubated for 1 h with PyrR plus and minus 200 μm NADPH or, as controls, the reductase domain of E. coli (RibDΔ) plus and minus NADPH, recombinant maize PyrD plus NADPH, or NADPH alone, and the formation of the reductase product, 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P (peak 3), was monitored. Substrates and products were analyzed by fluorometric HPLC after diacetyl derivatization. A, Disappearance of the reductase substrate after incubation with PyrR plus NADPH (red trace) or the E. coli RibD reductase domain plus NADPH (blue trace; positive control). As calculated from their areas, peak 2 fell by 39% ± 2% and 62% ± 6% (means of triplicate samples ± se) in the RibDΔ plus NADPH and PyrR plus NADPH treatments, respectively, relative to the corresponding controls without NADPH. B, Appearance of the reductase product after incubation with PyrR plus NADPH (red trace) or the E. coli RibD reductase domain plus NADPH (blue trace; positive control). The small product peaks in the negative controls may be attributable to traces of NAD(P)H complexed with the RibD protein used to synthesize the substrate and with the RibDΔ protein (Chen et al., 2006).
Assays were made on Ni2+ affinity-purified recombinant maize PyrR (GRMZM2G090068) and PyrD (GRMZM2G320099), with the purified reductase domain of E. coli RibD (Magalhães et al., 2008) as a positive control. In the presence of NADPH, maize PyrR mediated the conversion of 5-amino-6-ribosylamino-2,4(1H,3H)-pyrimidinedione 5′-P to 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P, as evidenced by the appearance of the latter compound (Fig. 4B) and the disappearance of the former (Fig. 4A). The RibD reductase-positive control did likewise but PyrD did not, as expected (Fischer et al., 2004; Magalhães et al., 2008). Maize PyrR also mediated the reduction reaction when NADH (200 μm) replaced NADPH, although the amount of product formed was only 39% ± 9% (mean ± se) of that with NADPH (data not shown). The identity of the PyrR product was validated by HPLC-mass spectrometry (MS)/MS/MS (MS3) analysis of the HPLC-purified diacetyl derivative peak. The retention time and fragmentation pattern were almost identical to those of an authentic standard prepared using E. coli RibD (Supplemental Fig. S2). Ions at mass-to-charge ratio (m/z) 309 and 321 represent the loss of phosphate (neutral loss of 80 and 98, respectively), confirming the presence of a phosphate ester, and the ion at m/z 193 is characteristic of the expected pyrimidine moiety (Supplemental Fig. S2). Together, these results confirm that maize PyrR has NAD(P)H-linked pyrimidine reductase activity and, because the assay contained no proteins besides PyrR, show that this activity does not require the presence of PyrD.
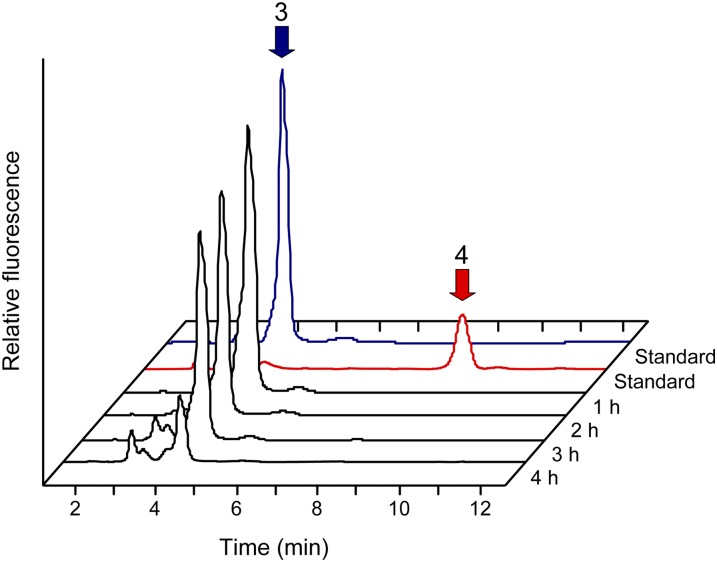
To test whether the COG3236 domain catalyzes dephosphorylation of the PyrR product (5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P), authentic 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P was prepared using E. coli RibD, treated with alkaline phosphatase, and derivatized with diacetyl. The dephosphorylated derivative then served as a chromatographic standard, enabling the determination of whether a similar chromatographic peak appeared in profiles of maize PyrR reductase assay products. No peak comigrating with the dephosphorylated standard was detected in analyses of the PyrR assay products, even when the assay time was prolonged for up to 4 h (Fig. 5). Nor was such a peak observed when divalent metal cations (0.5 mm) that are cofactors for various phosphatases (Mn2+, Co2+, Zn2+, Ni2+, or Ca2+) were individually added to the reaction mixture and the Mg2+ concentration was lowered to 1 mm to approximate the free Mg2+ concentration in the chloroplast stroma (Ishijima et al., 2003; data not shown). The detection limit for the dephosphorylated product was equivalent to 0.1% of the amount of phosphorylated product. These results provide no evidence for the PyrR protein having phosphatase activity.
Figure 5.
Evidence against PyrR having pyrimidine phosphatase activity. Pyrimidine reductase assays were run as in Figure 4 with maize PyrR plus 200 μm NADPH for 1, 2, 3, or 4 h, and the reaction mixtures were analyzed by fluorometric HPLC after diacetyl derivatization. Note that no conversion of 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P (peak 3) to its dephosphorylated form (peak 4) was detectable even after 4 h. Note also the progressive decomposition of 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione 5′-P, which is known to be unstable (Magalhães et al., 2008). The top-most (blue and red) traces show the running positions of authentic standards, prepared using E. coli RibD (peak 3) or RibD plus alkaline phosphatase (peak 4).
PyrR and PyrD Are Imported into Chloroplasts
As noted above, PyrR and PyrD proteins are predicted to be plastid localized, and there is some support for this from high-throughput proteomic data for Arabidopsis and maize (Zybailov et al., 2008, 2009; Sun et al., 2009; Ferro et al., 2010). To substantiate plastid localization, we carried out in vitro protein import assays in which isolated pea chloroplasts were incubated with radiolabeled full-length PyrR or PyrD proteins from Arabidopsis and maize. The chloroplastic Arabidopsis YgfZ protein At1g60990 (Waller et al., 2012) was used as a positive control. After incubation with each of the PyrR and PyrD proteins, chloroplasts contained a labeled product that resisted thermolysin digestion and was of lower molecular mass than the supplied precursor, as expected for an imported protein (Fig. 6).
Figure 6.
Protein import into isolated pea chloroplasts. The Arabidopsis (At) and maize (Zm) PyrR and PyrD sequences, together with a control for chloroplast import (At1g60990; AtYgfZ), were translated in vitro in the presence of [3H]Leu. The translation products were incubated in the light with chloroplasts (C), which were then repurified on a 10% Percoll gradient without (−) or with (+) prior thermolysin (TH) treatment to remove adsorbed proteins. Proteins were separated by SDS-PAGE and visualized by fluorography; exposure times were adjusted to give comparable band intensities. Samples were loaded next to an aliquot of the translation product (TP). Molecular masses (kD) are indicated. Note that the banding pattern is similar before and after thermolysin treatment for AtPyrD, AtPyrR, and ZmPyrR, because these proteins were very efficiently taken up and processed, as was the control protein YgfZ.
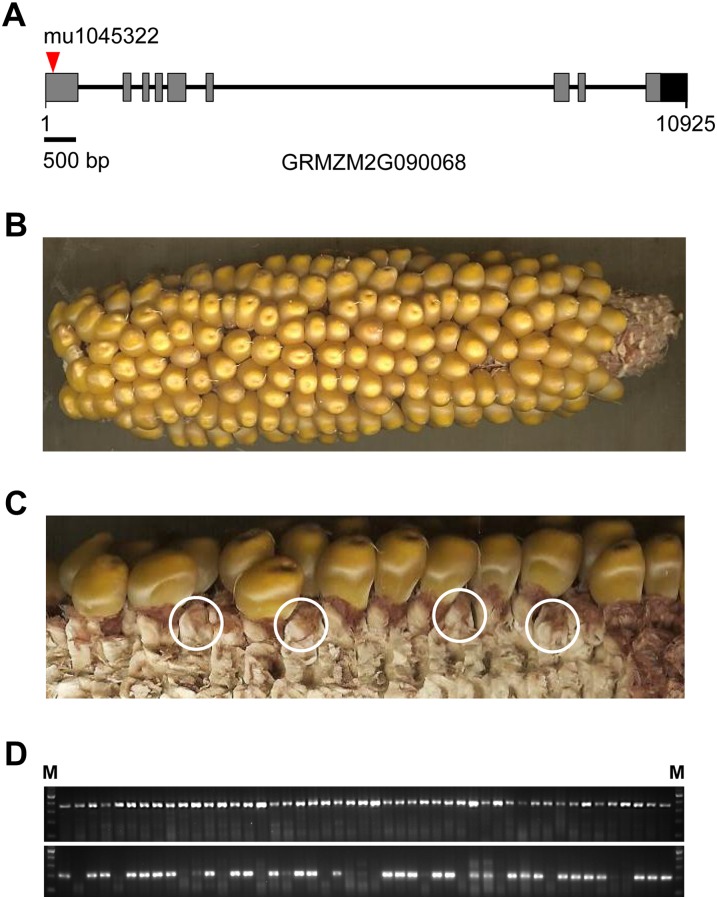
Ablation of Maize PyrR Causes Early Seed Lethality
Searches of the maize genome detected a single gene encoding PyrR (GRMZM2G090068). This gene consists of nine exons. If current understanding of riboflavin synthesis in plants (Gerdes et al., 2012) is correct, ablating PyrR should block riboflavin synthesis and hence be lethal. To test this prediction, we searched the maize UniformMu database (McCarty et al., 2005) for lines carrying Mutator (Mu) element insertions likely to abolish the expression of an active GRMZM2G090068 protein. One such line (UFMu-05381) with an insertion (mu1045322) located in the first exon was found (Fig. 7A). Genotype analysis of 21 seedlings from this line detected only heterozygous mutant and nonsegregating homozygous wild-type individuals. Inspection of ears from self-pollinated heterozygotes revealed that, in addition to morphologically normal kernels (Fig. 7B), ears contained florets with unexpanded ovaries. Of 92 total florets examined by dissection (Fig. 7C), 68 contained kernels and 24 contained unexpanded ovaries, consistent with a 3:1 (χ2 = 0.81) segregation of an early seed-lethal phenotype. Molecular genotyping (Fig. 7D) of seedlings grown from the normal seeds confirmed the absence of viable homozygous mutant progeny. Within the normal seed class, heterozygous and homozygous wild-type individuals occurred in a 2:1 ratio (33 heterozygotes, 15 homozygous wild types, and zero homozygous mutants; χ2 = 0.76). The evidence for 3:1 segregation for an early seed-lethal phenotype and for 2:1 segregation of heterozygotes in the normal seed class indicates that while both male and female gametophytes carrying the mutant allele are able to effect fertilization, homozygous mutant seed abort shortly after fertilization. Hence, PyrR is evidently required for both endosperm and embryo formation in the seed.
Figure 7.
Ablation of maize PyrR. A, Gene structure of maize PyrR. The location of the Mu element insertion (mu1045322) in the UFMu-05381 line is indicated by the arrowhead. The gray boxes represent exons, and the black box indicates the putative 3′ untranslated region. B, The self-pollinated ear of a heterozygote. C, Segregation of aborted seed. To expose florets that failed to produce seed (examples are shown in circles), kernels and glumes were removed from all florets in a section of the ear shown in B. D, Genotype analysis of progeny from a self-pollinated heterozygote. The top panel shows the PCR products (368 bp) obtained using a gene-specific primer pair (mu1045322-F1/R1), and the bottom panel shows the PCR products (approximately 220 bp) amplified by the gene-specific primer (mu1045322-F1) and a Mu-specific primer (TIR6). The PCR products were visualized by ethidium bromide staining. M, DNA ladder (100 bp).
DISCUSSION
Our data provide bioinformatic, genetic, and biochemical evidence that the Arabidopsis and maize PyrR proteins have pyrimidine reductase but not deaminase activity and show that PyrR is the only enzyme in maize with pyrimidine reductase activity. Therefore, it is reasonable to conclude that PyrR is the missing pyrimidine reductase in plant riboflavin synthesis. The strong sequence conservation among PyrR proteins, and their clear distinction from PyrD proteins, mean that the functional annotation of PyrR proteins as pyrimidine reductase can be confidently propagated by homology from Arabidopsis and maize to the rest of the plant kingdom. The results also show that the PyrR enzyme parallels bacterial pyrimidine reductases in accepting either NADPH or NADH but differs in preferring NADPH (Richter et al., 1997) and that, like other plant riboflavin synthesis enzymes (Gerdes et al., 2012), both PyrR and PyrD are targeted to plastids.
As nearly all bacteria have a bifunctional, two-domain RibD enzyme (Osterman and Overbeek, 2003), it is surprising that plants should instead have a monofunctional reductase and a monofunctional deaminase, each having an enzymatically inactive domain. The surprise comes on two counts: first, that catalytic subfunctionalization occurred in the plant lineage, and second, that the catalytically incompetent domain has been retained. Structural and biochemical data on the bifunctional bacterial enzymes offer some perspective on both counts. Thus, although the RibD substrates and products (Fig. 1, compounds 1–3) are chemically unstable (Magalhães et al., 2008) and so might be expected to be channeled between deaminase and reductase domains, RibD structures show no such channel and in fact show the deaminase and reductase active sites facing in opposite directions (Chen et al., 2006; Stenmark et al., 2007). Consistent with this structural evidence for the independence of the two active sites, the RibD deaminase and reductase domains are catalytically competent when expressed separately (Richter et al., 1997; Magalhães et al., 2008), and single-domain deaminase proteins occur naturally in green algae (Fig. 2B). These observations imply that subfunctionalization is inherently quite possible because the domains are not functionally interlocked. Structural data also suggest a possible rationale for the retention of catalytically inactive domains in PyrR and PyrD: that they are required for oligomerization. E. coli RibD is a dimer whose monomers interact via the reductase domain (Stenmark et al., 2007), and B. subtilis RibD is a tetramer whose monomers interact via both domains (Chen et al., 2006).
In addition, our data confirm that the C-terminal COG3236 domain characteristic of PyrR proteins is not needed for reductase activity. This finding fits with the absence of this domain from the bifunctional deaminase-reductase RibD proteins of bacteria and with the observation that the Arabidopsis phs1 mutation, which deletes the COG3236 domain without affecting protein expression, is not lethal (Ouyang et al., 2010). Although negative data are necessarily not definitive, failure to detect phosphatase activity in PyrR, with or without the addition of various potential metal cofactors, argues that the COG3236 domain is most probably not the missing phosphatase in the riboflavin pathway. This inference is strengthened by comparative genomic evidence, inasmuch as COG3236 belongs to the NADAR protein family, whose members with known activities are N-glycosidases, not phosphatases (de Souza and Aravind, 2012).
Finally, if the role of the COG3236 domain is not dephosphorylation, what is it? Possible clues come from comparative genomics and from the phenotype of the Arabidopsis phs1 mutant. Thus, besides being fused to PyrR in plants, COG3236 is also fused in certain γ-proteobacteria (e.g. Vibrio spp., Photobacterium profundum) to a homolog of RibA, the first enzyme of riboflavin synthesis (de Souza and Aravind, 2012), and the phs1 mutant is flavin deficient (Ouyang et al., 2010). These strands of evidence both reinforce a connection with the riboflavin pathway. Given the reactive, labile nature of the intermediates at the head of this pathway (Magalhães et al., 2008), it is reasonable to invoke an ancillary role for COG3236 in preempting or repairing damage to, or cellular damage from, these intermediates (Gerdes et al., 2012; Seaver et al., 2012).
MATERIALS AND METHODS
Bioinformatics
Protein sequences were taken from GenBank, MaizeSeqence.org, and the Joint Genome Institute Genome Portal and, when necessary, verified using ESTs from the GenBank dbEST database. Alignments were made with Multalin (Corpet, 1988) or ClustalW (Chenna et al., 2003), and phylogenetic trees were drawn by the neighbor-joining method using MEGA5 (Tamura et al., 2011). Arabidopsis (Arabidopsis thaliana) gene coexpression was analyzed using the ATTED-II Network Drawer tool (Obayashi et al., 2011).
Bacterial Strains and cDNAs
An Escherichia coli ribD deletant strain containing a chromosomal copy of the Corynebacterium glutamicum ribM riboflavin transporter gene (CmpX13ΔribD) was constructed from strain CmpX13 (Mathes et al., 2009), which harbors ribM, by using homologous recombination (Yu et al., 2000) to replace ribD with a kanamycin resistance cassette. Correct deletion of the ribD gene was confirmed by PCR. CmpX13ΔribD was maintained on Luria-Bertani (LB) medium containing 50 μm riboflavin and 50 μg mL−1 kanamycin.
The latest Arabidopsis genome annotation release (The Arabidopsis Information Resource 10) calls two versions of the product of the gene encoding PyrR (At3g47390; GenBank accession nos. NP_001190026 and NP_190323). The NP_001190026 version is supported by a full-length cDNA (GenBank accession no. AX546684), closely resembles PyrR sequences from other eudicots, and is the one we used. The NP_190323 version appears to have an intron-splicing error that corrupts the protein sequence between residues 437 and 468. Arabidopsis ecotype Columbia-0 PyrR (At3g47390) and PyrD (At4g20960) cDNAs were amplified from leaf RNA using Phusion DNA polymerase (Finnzymes), cloned into pETDuet-1, and sequenced. A 5-bp insertion starting at nucleotide 358 of the open reading frame was removed from the At3g47390 sequence by SOEing (Heckman and Pease, 2007). A full-length maize (Zea mays) PyrD cDNA (ZM_BFc0036A01, GenBank accession no. BT033973) and a 5′-truncated PyrR cDNA (ZM_BFb0063N12.r, GenBank accession no. DR822967) were obtained from the Arizona Genomics Institute (University of Arizona) and sequenced. The PyrR 5′ region encoding the N-terminal 249 residues (corresponding to the GRMZM2G090068 predicted gene product) was chemically synthesized (GenScript) and spliced to the truncated cDNA sequence by SOEing as above. The resulting full-length cDNA sequence was deposited in GenBank (accession no. JX838796). The primers used in this and the subcloning work described in the following sections are listed in Supplemental Table S1.
Arabidopsis and Maize PyrR and PyrD Constructs
For functional complementation experiments, Arabidopsis PyrR and PyrD or maize PyrR and PyrD, minus their predicted N-terminal targeting peptides, were cloned separately or together into the pETDuet-1 expression vector (Novagen); for use as a positive control, E. coli ribD was cloned into the same vector. Modified versions of Arabidopsis PyrR lacking the COG3236 domain, or lacking both the COG3236 and the deaminase domains, were also cloned into pETDuet-1 together with Arabidopsis PyrD. The positions at which the various truncations were made are shown in Supplemental Figure S3. For protein expression, all four proteins, minus the targeting regions as above, were cloned into pET28b (Novagen), adding the N-terminal hexa-His tag encoded by the vector. All constructs were verified by sequencing.
Functional Complementation Experiments
The pETDuet-1 vector alone, or containing E. coli ribD, Arabidopsis or maize PyrR and/or PyrD, or Arabidopsis PyrR shorn of the COG3236 domain or of both the COG3236 and deaminase domains, were introduced into strain CmpX13ΔribD. Transformants were plated on LB medium containing 50 µg mL−1 kanamycin, 50 µg mL−1 ampicillin, and 1 mm isopropyl-β-thiogalactopyranoside (IPTG) without or with 50 µm riboflavin. Incubation was at 22°C for 72 h, after which images of the plates were captured.
Protein Overexpression and Isolation
The pET28b constructs containing Arabidopsis or maize PyrR or PyrD were introduced into E. coli strain BL21-CodonPlus (DE3)-RIPL. Cultures (1 L) were grown at 37°C in LB medium containing 50 μg mL−1 kanamycin. When A600 reached 0.6, IPTG was added (final concentration, 0.5 mm) and incubation was continued for 5 h at 25°C. Subsequent steps were carried out at 4°C. Cells were harvested by centrifugation, resuspended in 50 mm NaH2PO4, 300 mm NaCl, and 10 mm imidazole, pH 8.0, and sonicated. To the cleared supernatant, 1 mL of Ni2+-nitrilotriacetic acid agarose 50% slurry (Qiagen) was added, followed by rotary shaking for 1 h at 4°C. The mixture was then poured into a column and allowed to drain by gravity. After washing with 16 mL of 50 mm NaH2PO4, 300 mm NaCl, and 20 mm imidazole, pH 8.0, proteins were eluted with 2 mL of this buffer containing 250 mm imidazole, desalted on PD-10 columns (GE Healthcare) equilibrated in 20 mm triethanolamine-HCl, pH 7.5, 100 mm NaCl, and 2 mm β-mercaptoethanol, 10% (v/v) glycerol, and concentrated to approximately 1.2 mg mL−1 in an Amicon Ultra-0.5 mL 10K unit (Millipore). Purified proteins were frozen in liquid N2 and stored at −80°C. Protein was estimated by dye binding (Bradford, 1976) with bovine serum albumin as the standard. As pilot tests showed that very little Arabidopsis PyrR could be obtained in soluble form, maize PyrR and PyrD were used for characterization work. Recombinant E. coli RibA, RibD, and RibD with an inactivated deaminase domain (RibDΔ) were prepared and purified by Ni2+ affinity chromatography essentially as described (Magalhães et al., 2008).
Pyrimidine Reductase Assay
The substrate for pyrimidine reductase, 5-amino-6-ribosylamino-2,4(1H,3H)-pyrimidinedione 5′-P, was synthesized in a 50-μL reaction mixture containing 10 mm Tris-HCl, pH 8.0, 8 mm MgCl2, 30 mm dithiothreitol, 5 mm GTP, 100 μg of E. coli RibA, and 50 μg of E. coli RibD. The MgCl2 concentration was lowered to 2 mm, and the amount of RibA was doubled when the substrate was to be used in tests for phosphatase activity. After incubation for 30 min at 37°C, proteins were removed by ultrafiltration in an Amicon unit as above; the ultrafiltrate was used as substrate for the pyrimidine reductase assay at once or after freezing in liquid N2 and storage at −80°C. Standard pyrimidine reductase assays (100 μL) contained 10 mm HEPES-NaOH, pH 7.5, 50 μL of ultrafiltrate, 200 μm NADPH or NADH, and 16 μg of enzyme and were incubated for 1 h at 37°C. A solution (50 μL) containing 1% (v/v) diacetyl and 15% (w/v) TCA was added, and the mixture was incubated for 1 h at 37°C as described (Richter et al., 1997). Samples (5 μL) of the derivatization mixture were diluted 10-fold and analyzed by fluorometric HPLC for the appearance of the reduced product using a Discovery C18 column (250 × 4.6 mm, 5 µm particle size) eluted with 7% (v/v) methanol and 30 mm formic acid at 1.5 mL min−1 and for disappearance of the substrate using the same column eluted with 10% (v/v) methanol and 0.1% trifluoroacetic acid at 1.5 mL min−1. Excitation and emission wavelengths were, respectively, 408 and 485 nm for the product derivative and 330 and 435 nm for the substrate derivative. To prepare dephosphorylated product derivative as a standard, 50-μL reaction mixtures containing 50 mm HEPES-NaOH, pH 8.0, 5 mm MgCl2, 5 mm GTP, 100 μg of E. coli RibA and 50 μg of E. coli RibD, 200 μm NADPH, 10 mm Glc-6-P, and 1 unit of Glc-6-P dehydrogenase were incubated for 30 min, after which 1 unit of calf alkaline phosphatase was added and incubation was continued for 30 min at 37°C. Samples were then derivatized as above. The derivative of the product formed by maize PyrR was prepared as described for pyrimidine reductase assays, scaled up 10-fold.
Liquid Chromatography-Mass Spectrometry Analysis of RibD and PyrR Products
RibD and PyrR products were analyzed using a Thermo LTQ Velos with an Accela 600 UPLC apparatus and Accela open autosampler (Thermo Fisher). The mass spectrometer was operated in positive heated-electrospray mode under the following conditions: 3,000 V for the spray needle, 125°C for the source temperature, flow rates 40 arbitrary units sheath gas, 5 arbitrary units auxiliary gas, and 300°C capillary temperature. Gradient elution was employed on a hypersil GOLD column (100 × 2.1 mm, 3-μm particle size; Thermo Fisher) at a flow rate of 140 μL min−1 with 0.1% formic acid in liquid chromatography-mass spectrometry water (Sigma; A) and 0.1% formic acid in liquid chromatography-mass spectrometry methanol (Sigma; B) using these conditions: 99% A for 2 min, decreased linearly to 50% A in 1 min, held isocratic at 50% A for 8 min, then increased to 99% A for reequilibration (4 min). Total run time was 15 min per sample (10-μL injections). Full scan spectra (mass spectrometry) were collected from m/z 100 to 1,000; tandem mass spectrometry data were collected for the RibD product, while only full-scan spectra and MS3 data were collected for the PyrR product. Tandem mass spectrometry was performed (helium as collision gas) on m/z 407.1 with collision energy of 25%, a graph axis q of 0.25, isolation time of 10 ms, and isolation width of 1.0 atomic mass unit collecting the mass range 110 to 500. MS3 was performed on m/z 389.2 (the major product ion from the dissociation of m/z 407.1) with collision energy of 25%, q of 0.25, isolation time of 10 ms, and isolation width of 1.0 atomic mass unit collecting the mass range 105 to 500.
Chloroplast Import Experiments
Chloroplast import was assayed essentially as described previously (Pribat et al., 2010). Briefly, cDNAs encoding full-length Arabidopsis and maize PyrR and PyrD proteins were PCR amplified using forward primers containing a Kozak sequence and cloned into pGEM-4Z (Promega). Coupled in vitro transcription-translation, organelle preparation, import conditions, thermolysin treatment, and repurification of organelles were as described (Pribat et al., 2010). At1g60990 (Waller et al., 2012) was used as a positive control for chloroplast import. Import times used in the reactions were 5 min for At1g60990 and 20 min for Arabidopsis PyR, Arabidopsis PyrD, maize PyrR, and maize PyrD.
Identification of a Maize GRMZM2G090068 Mutant
The maize GRMZM2G090068::Mu insertion line was identified in the inbred UniformMu population (McCarty et al., 2005). Plants were grown in a greenhouse and at the University of Florida research farm in Citra. For genotyping, seedlings from seeds of self-pollinated ears were grown in plastic pots at 24°C in a walk-in growth chamber under a 16/8-h light/dark cycle and sampled for DNA at 14 d after planting. For genotype analysis, a segment (1–2 cm) of seedling leaf was harvested and stored in a 2-mL microcentrifuge tube. For genomic DNA extraction, leaf samples were pulverized, suspended in 0.8 mL of DNA extraction buffer (0.31 m NaCl, 50 mm Tris-HCl, pH 8.0, 20 mm EDTA, and 1% sarkosyl), and then extracted with 0.8 mL of TE8 (10 mm Tris-HCl, 1 mm EDTA, pH 8.0)-saturated phenol:chloroform (1:1). After centrifugation (12,000g, 10 min), the aqueous phase (0.7 mL) was transferred to a fresh microcentrifuge tube. To the supernatant, 70 μL of 3 m sodium acetate (pH 5.2) was added and mixed gently. Then, 0.65 mL of isopropanol was added and mixed gently by inversion. After centrifugation (12,000g, 5 min), the isopropanol solution was drained and 0.5 mL of 70% ethanol was added to wash the DNA pellet. After recentrifugation, the ethanol was drained and the DNA pellet was air dried for a few minutes and dissolved in 0.4 mL of TE8 buffer (pH 8.0). DNA was quantified using a NanoDrop 1000 instrument (Thermo Fisher), and 100 ng of DNA from each sample was used for PCR analysis. The following primer pairs were used: 5′-CGTCTCATCCGCTCCAGCATCCAGC-3′ and 5′-GACCACCCAGGAATCAGCGCTGTC-3′ (mu1045322-F1/R1) for amplification of the wild-type allele and 5′-CGTCTCATCCGCTCCAGCATCCAGC-3′ and 5′-AGAGAAGCCAACGCCAWCGCCTCYATTTCGTC-3′ (mu1045322-F1/TIR6) for detection of the Mu insertion allele.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number JX838796.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ATTED coexpressed gene network connecting the expression of Arabidopsis PyrR (At3g47390) with that of PyrD (At4g20960) and genes encoding three other riboflavin synthesis enzymes.
Supplemental Figure S2. Liquid chromatography-mass spectrometry analysis of the diacetyl derivative of the compound formed by E. coli RibD or maize PyrR in the presence of 200 μm NADPH.
Supplemental Figure S3. Amino acid sequences of Arabidopsis and maize PyrR and PyrD proteins showing the points at which truncations were made to remove various regions.
Supplemental Table S1. Oligonucleotide primers used to clone and subclone Arabidopsis and maize PyrR and PyrD cDNAs.
Acknowledgments
We thank T. Mathes for E. coli strain CmpX13, J.S. Blanchard for the His-tagged RibD and modified RibD constructs, and S. Berthet for assistance with cloning Arabidopsis cDNAs.
Glossary
- m/z
mass-to-charge ratio
- LB
Luria-Bertani
- IPTG
isopropyl-β-thiogalactopyranoside
References
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chatwell L, Krojer T, Fidler A, Römisch W, Eisenreich W, Bacher A, Huber R, Fischer M. (2006) Biosynthesis of riboflavin: structure and properties of 2,5-diamino-6-ribosylamino-4(3H)-pyrimidinone 5′-phosphate reductase of Methanocaldococcus jannaschii. J Mol Biol 359: 1334–1351 [DOI] [PubMed] [Google Scholar]
- Chen SC, Chang YC, Lin CH, Lin CH, Liaw SH. (2006) Crystal structure of a bifunctional deaminase and reductase from Bacillus subtilis involved in riboflavin biosynthesis. J Biol Chem 281: 7605–7613 [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16: 10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza RF, Aravind L. (2012) Identification of novel components of NAD-utilizing metabolic pathways and prediction of their biochemical functions. Mol Biosyst 8: 1661–1677 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2: 953–971 [DOI] [PubMed] [Google Scholar]
- Ferro M, Brugière S, Salvi D, Seigneurin-Berny D, Court M, Moyet L, Ramus C, Miras S, Mellal M, Le Gall S, et al. (2010) AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteomics 9: 1063–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Bacher A. (2005) Biosynthesis of flavocoenzymes. Nat Prod Rep 22: 324–350 [DOI] [PubMed] [Google Scholar]
- Fischer M, Römisch W, Saller S, Illarionov B, Richter G, Rohdich F, Eisenreich W, Bacher A. (2004) Evolution of vitamin B2 biosynthesis: structural and functional similarity between pyrimidine deaminases of eubacterial and plant origin. J Biol Chem 279: 36299–36308 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TB, Basset GJ, Borel P, Carrari F, DellaPenna D, Fraser PD, Hellmann H, Osorio S, Rothan C, Valpuesta V, et al. (2012) Vitamin deficiencies in humans: can plant science help? Plant Cell 24: 395–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes S, Lerma-Ortiz C, Frelin O, Seaver SMD, Henry CS, de Crécy-Lagard V, Hanson AD. (2012) Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J Exp Bot 63: 5379–5395 [DOI] [PubMed] [Google Scholar]
- Heckman KL, Pease LR. (2007) Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc 2: 924–932 [DOI] [PubMed] [Google Scholar]
- Hedtke B, Grimm B. (2009) Silencing of a plant gene by transcriptional interference. Nucleic Acids Res 37: 3739–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35: W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima S, Uchibori A, Takagi H, Maki R, Ohnishi M. (2003) Light-induced increase in free Mg2+ concentration in spinach chloroplasts: measurement of free Mg2+ by using a fluorescent probe and necessity of stromal alkalinization. Arch Biochem Biophys 412: 126–132 [DOI] [PubMed] [Google Scholar]
- Jordan DB, Bacot KO, Carlson TJ, Kessel M, Viitanen PV. (1999) Plant riboflavin biosynthesis: cloning, chloroplast localization, expression, purification, and partial characterization of spinach lumazine synthase. J Biol Chem 274: 22114–22121 [DOI] [PubMed] [Google Scholar]
- Magalhães ML, Argyrou A, Cahill SM, Blanchard JS. (2008) Kinetic and mechanistic analysis of the Escherichia coli ribD-encoded bifunctional deaminase-reductase involved in riboflavin biosynthesis. Biochemistry 47: 6499–6507 [DOI] [PubMed] [Google Scholar]
- Mathes T, Vogl C, Stolz J, Hegemann P. (2009) In vivo generation of flavoproteins with modified cofactors. J Mol Biol 385: 1511–1518 [DOI] [PubMed] [Google Scholar]
- McCarty DR, Settles AM, Suzuki M, Tan BC, Latshaw S, Porch T, Robin K, Baier J, Avigne W, Lai J, et al. (2005) Steady-state transposon mutagenesis in inbred maize. Plant J 44: 52–61 [DOI] [PubMed] [Google Scholar]
- Obayashi T, Nishida K, Kasahara K, Kinoshita K. (2011) ATTED-II updates: condition-specific gene coexpression to extend coexpression analyses and applications to a broad range of flowering plants. Plant Cell Physiol 52: 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman A, Overbeek R. (2003) Missing genes in metabolic pathways: a comparative genomics approach. Curr Opin Chem Biol 7: 238–251 [DOI] [PubMed] [Google Scholar]
- Ouyang M, Ma J, Zou M, Guo J, Wang L, Lu C, Zhang L. (2010) The photosensitive phs1 mutant is impaired in the riboflavin biogenesis pathway. J Plant Physiol 167: 1466–1476 [DOI] [PubMed] [Google Scholar]
- Powers HJ. (2003) Riboflavin (vitamin B-2) and health. Am J Clin Nutr 77: 1352–1360 [DOI] [PubMed] [Google Scholar]
- Pribat A, Noiriel A, Morse AM, Davis JM, Fouquet R, Loizeau K, Ravanel S, Frank W, Haas R, Reski R, et al. (2010) Nonflowering plants possess a unique folate-dependent phenylalanine hydroxylase that is localized in chloroplasts. Plant Cell 22: 3410–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter G, Fischer M, Krieger C, Eberhardt S, Lüttgen H, Gerstenschläger I, Bacher A. (1997) Biosynthesis of riboflavin: characterization of the bifunctional deaminase-reductase of Escherichia coli and Bacillus subtilis. J Bacteriol 179: 2022–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roje S. (2007) Vitamin B biosynthesis in plants. Phytochemistry 68: 1904–1921 [DOI] [PubMed] [Google Scholar]
- Seaver SM, Henry CS, Hanson AD. (2012) Frontiers in metabolic reconstruction and modeling of plant genomes. J Exp Bot 63: 2247–2258 [DOI] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C. (2004) Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4: 1581–1590 [DOI] [PubMed] [Google Scholar]
- Stenmark P, Moche M, Gurmu D, Nordlund P. (2007) The crystal structure of the bifunctional deaminase/reductase RibD of the riboflavin biosynthetic pathway in Escherichia coli: implications for the reductive mechanism. J Mol Biol 373: 48–64 [DOI] [PubMed] [Google Scholar]
- Suhre K. (2007) Inference of gene function based on gene fusion events: the Rosetta-stone method. Methods Mol Biol 396: 31–41 [DOI] [PubMed] [Google Scholar]
- Sun Q, Zybailov B, Majeran W, Friso G, Olinares PD, van Wijk KJ. (2009) PPDB, the Plant Proteomics Database at Cornell. Nucleic Acids Res 37: D969–D974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller JC, Ellens KW, Alvarez S, Loizeau K, Ravanel S, Hanson AD. (2012) Mitochondrial and plastidial COG0354 proteins have folate-dependent functions in iron-sulphur cluster metabolism. J Exp Bot 63: 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. (2000) An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA 97: 5978–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B, Friso G, Kim J, Rudella A, Rodríguez VR, Asakura Y, Sun Q, van Wijk KJ. (2009) Large scale comparative proteomics of a chloroplast Clp protease mutant reveals folding stress, altered protein homeostasis, and feedback regulation of metabolism. Mol Cell Proteomics 8: 1789–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ. (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS ONE 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]