Reduction/oxidation (redox) metabolism and associated signaling are key components of cross tolerance to biotic and abiotic stresses in plants. Climate change factors such as predicted increases in temperature and the availability of atmospheric carbon dioxide (CO2) and ozone (O3) will have a profound effect on oxidative signaling in plants, particularly in relation to photosynthetic metabolism and environmental stress responses. Redox signaling is responsive to myriad environmental signals through influences on metabolism and the triggered activation of the suite of oxidative burst-generating enzymes, whose function is to enhance the oxidation state of the apoplast/cell wall environment. Like reactive oxygen species (ROS), the abundance of low molecular antioxidants such as ascorbate, glutathione, tocopherols and carotenoids, and antioxidant enzymes is modified by environmental triggers. Oxidants and antioxidants do not operate in isolated linear redox signaling pathways. Rather, they are part of a much larger stress signaling network that integrates information from many pathways including hormones and sugars to regulate plant growth and defense responses. Here, we explore the likely responses of oxidants and antioxidants to global change factors and discuss how they might modify gene expression, influencing overall plant fitness through altered stress responses.
OXIDANTS AND ANTIOXIDANTS
Of the many oxidants in cells, ROS have received the greatest attention because of their inherent reactivity and their functions as cellular signaling molecules (D’Autréaux and Toledano, 2007). The signaling function of ROS has been extensively documented (Kovtun et al., 2000; Apel and Hirt, 2004; Miller et al., 2008), but the mechanisms involved remain controversial, particularly with regard to the specificity that is required for signaling. Much uncertainty remains concerning whether superoxide, hydrogen peroxide, and singlet oxygen are themselves signaling molecules or whether their generation and accumulation simply create an oxidative environment that facilitates signaling by other pathways such as calcium mobilization, thiol-disulfide exchange, protein-protein interactions, and transcription factor-binding properties. Hydrogen peroxide is able to operate efficiently in signaling through chemical reactions with specific atoms of target proteins that lead to covalent protein modifications. The primary targets of ROS signals are amino acids such as Cys. Low-Mr thiols such as glutathione and protein Cys residues are particularly well suited for reactions with oxidants such as hydrogen peroxide (D’Autréaux and Toledano, 2007). ROS accumulation is also intrinsically linked to the production of another major plant signaling molecule, nitric oxide (Neill et al., 2002).
The lifetime of ROS is determined largely by the complex antioxidant network, which is composed of antioxidant enzymes such as catalase, ascorbate peroxidase (APX), and the other enzymes of the ascorbate/glutathione cycle (Fig. 1) and low-Mr antioxidants such as ascorbate, glutathione, carotenoids, and tocopherols. Ascorbate and glutathione are the major low-Mr hydrophilic antioxidants of plants. Together with peroxiredoxins, these metabolites play a major role in ROS detoxification (Foyer and Shigeoka, 2011). The roles of individual enzymes and metabolites of the antioxidant system have been elucidated largely from studies on mutants that are deficient in different components of the system. For example, Arabidopsis (Arabidopsis thaliana) mutants that are deficient in CATALASE2 (cat2), which is the enzyme responsible for the removal of hydrogen peroxide produced during photorespiration, show a marked accumulation of glutathione together with a decrease in the ratio of reduced glutathione (GSH) to glutathione disulfide when grown in air. Moreover, cat2 mutants are characterized by a constitutive up-regulation of pathogen response proteins, particularly those that are commonly induced by salicylic acid (SA)-dependent pathways (Queval et al., 2007; Chaouch et al., 2010). Knockout mutants that are deficient in the cytosolic form (gr1) of glutathione reductase have a similar phenotype to the wild-type plants. However, cat2gr1 double mutants display a strong stress-response phenotype, suggesting that glutathione-dependent antioxidative pathways are increasingly solicited when peroxisomal catalase activity is compromised (Mhamdi et al., 2010).
Figure 1.
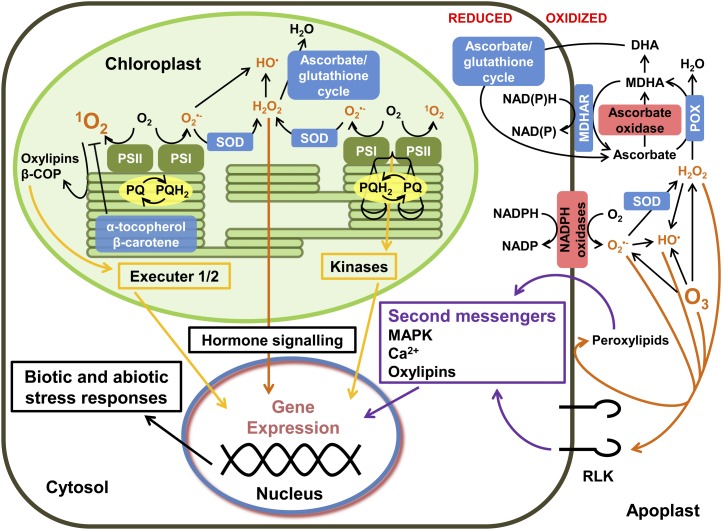
Schematic representation of subcellular hydrogen peroxide (H2O2) metabolism and its generation by the pathway of photorespiration and subsequent signaling through the glutathione pool linked to the ascorbate/glutathione cycle, as well as the production of ascorbate in the mitochondria and its subsequent transport to the apoplast, where it is oxidized by apoplastic enzymes related to extracellular redox signaling pathways. AGC, Ascorbate-glutathione cycle; AO, ascorbate peroxidase; CAT, catalase; DH, dehydrogenase; DHA, dehydroascorbate; DHAR, DHA reductase; GO, glycolate oxidase; GR, glutathione reductase; MDHA, monodehydroascorbate; MDHAR, monodehydroascorbate reductase; OX, oxidase; PET, photosynthetic electron transfer chain; Pi, inorganic phosphate; POX, peroxidase; RET, respiratory electron transfer chain.
While the ability of the ascorbate and glutathione pools to act as redox buffers is undoubtedly one of their most important attributes, current evidence suggests that they have precise and distinct roles in redox signaling. Glutathione makes a key contribution to hydrogen peroxide-dependent signaling pathways within the intracellular environment (Han et al., 2012). It is a major arbiter of the intracellular redox potential (Foyer and Noctor, 2005). In contrast to glutathione, ascorbate appears to exert its greatest influence by setting thresholds for apoplastic and cytoplasmic signaling. Hence, oxidants and antioxidants fulfill signaling roles within the antioxidant signaling network, using kinase-dependent and -independent pathways that are initiated through redox-sensitive receptors, most probably modulated by thiol status. While the antioxidant system is highly efficient, its regulation is finely tuned to allow fluctuations in the extent of ROS accumulation in order to facilitate appropriate signaling functions.
Within the context of redox signaling, the plant cell must be considered as a set of discrete compartments, as illustrated in Figure 1, each of which has different antioxidants and antioxidant-buffering capacities that are determined by differences in synthesis, transport, and/or degradation of key components. In each discrete cellular location, therefore, redox signaling can be controlled and buffered independently. This permits redox-sensitive signal transduction to occur in locations such as the apoplast and the thylakoid lumen, whereas other highly buffered spaces have a much higher threshold for ROS signals. Through interactions with the hormone signaling network, oxidants and antioxidants participate in numerous signaling pathways, including those involved in chloroplast-to-nucleus and mitochondria-to-nucleus retrograde communication, that serve to coordinate gene expression in the nuclear and cytoplasmic genomes (Fey et al., 2005; Bräutigam et al., 2007; Cela et al., 2011; Ramel et al., 2012).
ASCORBATE-BASED REDOX SHUTTLE SYSTEMS ACROSS THE PLASMA MEMBRANE
Ascorbate is synthesized in the mitochondria, the final step of the pathway, which is catalyzed by l-galactono-1,4-lactone dehydrogenase (GLDH), residing in the respiratory electron transport chain, where it directly donates electrons to cytochrome c (Bartoli et al., 2000; Millar et al., 2003). GLDH is a plant-specific ancillary subunit of complex I, and its presence is required for the stability of the complex (Millar et al., 2003; Pineau et al., 2008). GLDH activity is regulated by respiration and by redox controls such as glutathionylation (Leferink et al., 2009). Ascorbate produced in the mitochondria is transported throughout the cell and across the plasma membrane into the apoplast, where it is oxidized by ascorbate oxidase (Fig. 1). Ascorbate oxidase initiates the apoplastic pathway for ascorbate degradation, which produces metabolites such as oxalic acid and threonic acid (Green and Fry, 2005).
Apoplastic ascorbate plays an important role in plant resistance to O3. Moreover, there is a general correlation between O3 sensitivity and tissue ascorbate contents (Sandermann, 2008). Arabidopsis mutants that are deficient in ascorbate (vtc) are characterized by high sensitivity to O3 (Conklin et al., 2000) as well as constitutive up-regulation of SA-dependent defense responses and an enhanced basal resistance to biotrophic pathogens (Pastori et al., 2003; Pavet et al., 2005; Mukherjee et al., 2010). Plants respond to O3 and other apoplastic oxidants by rapidly exporting ascorbate into the apoplast (Parsons and Fry, 2010). The products of ascorbate breakdown such as oxalate are considered to contribute to a network of signals in the apoplast that transfer information concerning the redox state of the extracellular environment to the nucleus. In addition, many of the apoplast/cell wall functions depend on its relatively low antioxidant status. Despite the presence of many potential antioxidants, such as flavonoids and polyamines, the redox-buffering capacity of the apoplast is much weaker than inside the cell (Pignocchi et al., 2003). Unlike the cytoplasm, the apoplast is deficient in NAD(P)H, and the apoplastic ascorbate pool is markedly more oxidized than cytoplasmic ascorbate (Pignocchi et al., 2003; Pignocchi and Foyer, 2003).
Like the tonoplast membrane, the plasma membrane contains an ascorbate-dependent cytochrome b561, which is reduced on one face by ascorbate and oxidized on the other by monodehydroascorbate or phenolics that can act as substrates for monodehydroascorbate reductase (Preger et al., 2005). The ultimate electron acceptor in the apoplast is either oxygen or 3,4-dihydroxyphenolic compounds such as chlorogenic acid and catechin that influence cell wall composition. While redox shuttling between the chloroplast and cytosol is well characterized, with well-described roles in the integration of stromal and cytosolic metabolism (Taniguchi and Miyake, 2012), little is known about the redox shuttling between the cytosol and apoplast and how it may participate in the coordination of intracellular processes and cell wall metabolism. In addition to driving plasma membrane electron transport chains, ascorbate-based redox shuttle systems ensure adequate provision of reductant to the apoplast while exporting excess reducing equivalents from the cytosol and thus helping to maintain a proper metabolic balance between energy and reducing equivalents.
The oxidized form of ascorbate, dehydroascorbate, can only be regenerated in the cytosol after transport across the plasma membrane (Pignocchi and Foyer, 2003). Crucially, the plasma membrane ascorbate and dehydroascorbate transport systems fail to maintain a highly reduced apoplastic ascorbate pool, probably because of the action of apoplastic ascorbate oxidase. This results in a steep ascorbate gradient across the plasma membrane, which contributes to a futile cycle of ascorbate synthesis, oxidation, and recycling that spans the intracellular and extracellular environments. Such a futile cycle might function to limit and monitor the availability of reduced ascorbate within the cellular environment and hence mitochondrial function, but it may also serve to decrease the overall levels of cellular reductant and generate the oxidized forms (monodehydroascorbate and dehydroascorbate) in the apoplastic environment. The operation of futile cycles involving cellular reductants like ascorbate can also be used to control ROS-mediated signal transmission that occurs in response to atmospheric pollutants, pathogens, and hormones (Pignocchi et al., 2003).
THE INFLUENCE OF GLOBAL CHANGE FACTORS ON REDOX SIGNALING PATHWAYS
The climate and atmosphere of the earth have varied greatly over geologic time. For example, the step-wise increase in atmospheric oxygen that occurred in the late Precambrian is considered to be pivotal in the rapid evolution of macroscopic multicellular life. In recent times, the CO2 and O3 levels in the troposphere have increased significantly because of industrialization and emissions arising from the burning of fossil fuels (Prentice et al., 2001; Ainsworth et al., 2008). Increases in atmospheric CO2 concentrations favor enhanced carbon gain because of the stimulation of photosynthesis, and they are likely to ameliorate O3 damage due to reduced O3 uptake and increased carbon assimilation. However, the beneficial effects of elevated CO2 on photosynthesis and hence productivity, particularly in C3 plants, may be offset by increases in global temperatures. By the end of this century, the Earth’s atmosphere may have CO2:oxygen ratios as high as 0.0047, but average global temperatures are likely to increase by up to 3.9°C (Lopes and Foyer, 2011). While no negative effects of elevated atmospheric CO2 have been observed in C4 crops, which will benefit from higher temperatures, atmospheric CO2 enrichment coupled to declining oxygen levels is likely to favor C3 plants, particularly in environments where NH4+ is the most abundant nitrogen source (Long et al., 2005).
Elevated atmospheric CO2 levels have a stimulating effect on photosynthesis because of decreased photorespiration, particularly in C3 plants. Photorespiration is a major source of hydrogen peroxide, which is widely accepted to be one of the most important oxidative signaling molecules leading to the activation of abiotic and biotic stress responses (Neill et al., 2002; Miller et al., 2008). Future atmospheric CO2 enrichment will favor a decline in photorespiratory hydrogen peroxide production relative to oxidative signals produced by other processes, particularly photosynthetic and respiratory electron transport. Moreover, the predicted increases in tropospheric O3 will favor enhanced oxidative signaling from other compartments of the cell, particularly the apoplast. The responses of antioxidant metabolism in the leaves of plants grown with atmospheric CO2 enrichment are variable, but the consensus appears to be that growth at elevated CO2 reduces oxidative stress (Booker and Fiscus, 2005). However, it is interesting that a similar increase in protein carbonylation was observed in the leaves of Arabidopsis and soybean (Glycine max) plants that were grown either with CO2 enrichment or exposed to O3 (Qiu et al., 2008). While the majority of the proteins that were responsive to both treatments such as ferredoxin-thioredoxin reductase were decreased in abundance, others such as APX1 and the β-subunit of ATP synthase were more abundant in plants grown under high CO2 or treated with O3. Because the protein carbonylation patterns were similar under both conditions, the authors concluded that plants grown with CO2 enrichment suffer enhanced oxidative stress (Qiu et al., 2008). However, a possible alternative explanation might be that CO2 enrichment and exposure to O3 increase the redox gradient across the plasma membrane. In this explanation, CO2 enrichment would decrease oxidative signaling processes in the intracellular environment (by decreasing photorespiration) relative to the apoplastic environment, while exposure to O3 would enhance oxidative signaling in the extracellular apoplastic environment relative to the intracellular compartment. Hence, both treatments would increase the redox gradient across the plasma membrane, leading to similar changes in signaling pathways regulating gene expression, including genes encoding specific proteases that target proteins with carbonyl groups for degradation.
Exposure to even very low O3 concentrations can trigger large and sustained increases in plant defenses to biotic threats. For example, when tomato (Solanum lycopersicum) fruit are treated with low levels of O3, they are thereafter better able resist fungal growth (Tzortzakis et al., 2007). Moreover, pretreatment with O3 prior to fungal infection produces fruit that remain resistant to subsequent fungal spoilage even when stored in an O3-free atmosphere (Tzortzakis et al., 2007). Low-O3 treatments induce plant innate immune responses (Tzortzakis et al., 2011) and pathogen-associated molecular patterns, which result in enhanced tolerance to microbes, often including genetically programmed cell suicide pathways (Sandermann et al., 1998), a process that involves the activation of an anion channel in the plasma membrane (Kadono et al., 2010).
Leaves respond to O3 fumigation by increasing secondary metabolism in a seemingly unspecific manner, with increases in compounds such as phenylpropanoids and polyamines, as well as increasing the production of the stress hormone ethylene and modifications in the patterns of biogenic volatile organic compounds (Iriti and Faoro, 2009). O3 rapidly breaks down in plants because it reacts with water and other cellular components to form other ROS, particularly hydrogen peroxide, which trigger downstream responses including hormone cascades (Overmyer et al., 2003) and increased antioxidant metabolism (Gillespie et al., 2012), features that underpin cross tolerance to biotic and abiotic stresses. The leaf response to O3 not only clearly illustrates the overlap between plant defense responses against pathogens and environmental pollutants but demonstrates the fundamental role of oxidation as a key trigger in both processes. It is tempting to speculate that O3-triggered cellular oxidation is central to the creation of short- and long-term molecular stress “memories” by facilitating the activation of a general suite of preemptive plant defenses.
The phenomenon of cross tolerance to different stresses that is triggered by an exposure to a single stress is widespread in plants. It involves the synergistic coactivation of nonspecific stress-responsive pathways that cross biotic-abiotic stress boundaries. The synergistic coactivation of plant stress responses confers a preemptive advantage by enabling a general increase in stress resistance following exposure to a single stress condition (Collins et al., 1995; Ryu et al., 1995). While the precise molecular and physiological mechanisms remain poorly defined, the acquisition of cross tolerance is linked to enhanced production of ROS and oxidative signaling pathways that operate at the interface between redox and hormone signaling networks (Bartoli et al., 2012). Plant stress hormones such as ethylene, SA, abscisic acid (ABA), and jasmonates (JAs), which induce tolerance to a wide spectrum of stresses, also promote ROS production through the activation of NADPH oxidases (Bartoli et al., 2012).
ANTIOXIDANT DEFENSES AND PROTECTION OF PHOTOSYNTHESIS
Photosynthesis is a major source of ROS, which are generated through processes associated with energy transfer and electron transport (Fig. 2) and also indirectly through the initiation of the photorespiratory pathway (Foyer and Noctor, 2009). The intracellular environment is protected from uncontrolled oxidation by a robust antioxidant defense network. All the enzymes of the ascorbate/glutathione cycle are dual targeted to chloroplasts and mitochondria, while other forms of the pathway enzymes are found in the cytosol and peroxisomes. In chloroplasts, ascorbate also fulfills crucial roles in photosynthesis. It helps to prevent oxidation of stromal proteins and protects PSII by participating in the violaxanthin deepoxidase reaction, which generates zeaxanthin, an essential component of the thermal energy dissipation mechanisms that protect PSII from the potentially harmful effects of high light. If the oxygen-evolving complex is inactivated by high light, then lumen ascorbate can act as a temporary electron donor to PSII (Tóth et al., 2009). Ascorbate is also required in the thylakoid membranes for the regeneration of the reduced form of α-tocopherol, which is the major tocochromanol (vitamin E) compound accumulating in chloroplasts exposed to high-light stress. As well as physically quenching and chemically scavenging singlet oxygen, tocopherols are also major scavengers of lipid peroxyl radicals in the thylakoid membranes (Liebler, 1993; Maeda et al., 2005). The lipophilic antioxidants, such as carotenoids, tocopherols, and plastoquinol, fulfill essential roles in policing oxidants such as singlet oxygen that are generated within the thylakoid membranes. Singlet oxygen generated by PSII is responsible for most (over 90%) of the observed nonenzymatic peroxidation of thylakoid membrane lipids (Triantaphylidès et al., 2008). α-Tocopherol and β-carotene operate synergistically to eliminate singlet oxygen from PSII (Trebst et al., 2002; Munné-Bosch, 2005). Plastoquinol can also act as an efficient singlet oxygen scavenger (Nowicka and Kruk, 2012). However, the reactivity of singlet oxygen means that the most effective means of defense is to minimize singlet oxygen production by preventing the overreduction of PSII that allows back reactions leading to triplet chlorophyll.
Figure 2.
Schematic representation of the intracellular and extracellular redox signaling networks involving hydrogen peroxide (H2O2) and singlet oxygen (1O2) generated by chloroplasts and extracellular apoplastic processes and O3. β-COP, β-Carotene oxidation products; DHA, dehydroascorbate; HO•, hydroxyl radical; MAPK, mitogen-activated protein kinase; MDHA, monodehydroascorbate; MDHAR, monodehydroascorbate reductase; O2•−, superoxide; POX, peroxidase; PQ, oxidized plastoquinone; PQH2, reduced plastoquinone; SOD, superoxide dismutase.
Light-induced damage to PSII resulting in photoinhibition is an inherent problem in the design of the photosynthetic machinery. It is generally considered that primary photodamage to PSII is caused by the absorption of light by the manganese cluster of the PSII reaction center. Moreover, singlet oxygen inhibits the PSII repair cycle (Nishiyama et al., 2006; Takahashi and Badger, 2011). This vulnerability to light means that each PSII reaction center is rebuilt about once every 20 to 30 min, even under optimal irradiances. However, the light-induced turnover of D1 protein might be viewed as a system that fine-tunes electron transport and metabolic processes to prevailing environmental conditions (Foyer and Shigeoka, 2011). The acidification of the thylakoid lumen limits the capacity for electron transport from the plastoquinone pool to the cytochrome b6/f complex. Hence, under high light, the transport of electrons from PSII may be limited at the level of plastoquinol oxidation, and this would favor an increase in the lifetime of the reduced PSII acceptor. In such circumstances, the likelihood of a back reaction in PSII is increased, as is, as a consequence, the probability of singlet oxygen formation.
Flux control exerted at the level of the abundance of plastocyanin may serve to adjust the electron transport rate under conditions of low carbon assimilation (Schottler et al., 2004). Decreases in the abundance of plastocyanin may protect PSI from overreduction and hence favor decreased superoxide and hydrogen peroxide production. The PROTON GRADIENT REGULATION5 protein protects PSI against light-induced damage, particularly in plants exposed to fluctuating light (Suorsa et al., 2012). Leaves employ a range of other protection mechanisms that minimize light-induced damage to PSII and PSI, including light avoidance strategies such as leaf and chloroplast movements, light scattering by anthocyanins or other phenolic compounds, and reduction of pigment antenna size that contributes to the overall protection of PSII under high light (Krieger-Liszkay et al., 2008). A range of photoprotection mechanisms exist within the light-harvesting and electron transport system, including thermal energy dissipation, cyclic electron flow around PSI, pseudocyclic electron flow coupled to the ascorbate/glutathione cycle, the malate valve, and photorespiration (Demmig-Adams and Adams, 2006). Global change factors that enhance these photoprotection processes would diminish the excitation energy pressure on PSII and limit high-light-induced damage.
CHLOROPLASTS AS SENSORS OF ENVIRONMENTAL CHANGE
In natural environments, plants grow under fluctuating environmental conditions, requiring frequent acclimation of photosynthesis and related metabolism to drive plant growth and development. The sensor function of chloroplasts is intrinsically linked to photosynthesis and its regulation. The photosynthetic electron transport chain uses redox signals as direct and dynamic means of regulating metabolism and gene expression (Foyer et al., 2012). Chloroplast-to-nucleus signaling pathways allow the optimization of photosynthetic electron transport efficiency in response to environmental change. Within this context, the redox state of the plastoquinone pool is an important regulator of chloroplast-to-nucleus signaling regulating nuclear gene expression through pathways involving plastid-localized protein kinases such as STATE TRANSITION7 (STN7) or STN8 (Pfannschmidt et al., 2001; Sullivan and Gray, 2002). The production of superoxide and hydrogen peroxide by PSI and the generation of singlet oxygen at PSII also contribute to the network of signals that provide information on the redox state of the electron transport system (Wagner et al., 2004; Lee et al., 2007). Moreover, carotenoids, ascorbate, and glutathione not only limit the lifetime of oxidant signals but also participate in signal transduction pathways (Foyer and Noctor, 2005; Ramel et al., 2012). As discussed above, signals downstream of hydrogen peroxide produced by the electron transport system are likely to be mediated by changes in the redox state of the glutathione pool as well as by direct interactions with redox-sensitive proteins (D’Autréaux and Toledano, 2007). The hydrogen peroxide-dependent activation of SA-dependent pathways is well characterized (Desikan et al., 2001; Vandenabeele et al., 2004; Davletova et al., 2005; Vanderauwera et al., 2005). However, recent evidence suggests that hydrogen peroxide-dependent changes in the glutathione pool can act independently of NONEXPRESSER OF PATHOGENESIS-RELATED GENE1, which is the best-characterized thiol-dependent protein involved in pathogenesis responses, to activate SA-dependent defense responses (Han et al., 2012).
The carotenoid-based pathways of ABA and strigolactone synthesis begin in chloroplasts, as do pathways of oxylipin synthesis. The oxidation of free α-linolenic acid by electron-carrying lipoxygenases is a “wound” signal that results in downstream JA synthesis (Gfeller et al., 2010). Thereafter, a chloroplastic 13-lipoxygenase converts 18:3 and 16:3 fatty acids into their hydroperoxide forms such as 13-hydroperoxylinolenic acid, from which a range of different metabolites are produced, including cis+-12-oxophytodienoic acid. This metabolite can either be retained in the chloroplasts, where it is used as a precursor for the synthesis of other oxylipins, or it can be transported to the peroxisomes, where JA is produced (Gfeller et al., 2010). ABA and brassinosteroids trigger NADPH oxidase-dependent bursts of hydrogen peroxide that are integral to the regulation of stomatal closure and hence leaf transpiration rates and water use efficiency (Karpinski et al., 1999; Fryer et al., 2003). The ABA-dependent signaling pathway involves ABA receptor proteins, GLUTATHIONE PEROXIDASE3, protein kinases such as OPEN STOMATA1, and protein phosphatases such as ABA INSENSITIVE1 (ABI1) and ABI2 (Bartoli et al., 2012).
Genetic evidence has demonstrated unequivocally that singlet oxygen is a signaling molecule in plants. Given the reactivity of singlet oxygen, it is unlikely that it escapes the chloroplast, so signal transduction is considered to begin close to the site of singlet oxygen generation. Two plastid proteins, EXECUTER1 and EXECUTER2, participate in the translocation of singlet oxygen-derived signals from the plastid to the nucleus (Wagner et al., 2004; Lee et al., 2007). Moreover, the oxidation of β-carotene also leads to the generation of signals that reprogram gene expression or trigger cell suicide pathways (Ramel et al., 2012). The singlet oxygen-dependent oxidation of β-carotene produces a range of volatile derivatives such as β-cyclocitral, which induces changes in the expression of a large set of singlet oxygen-responsive genes (Ramel et al., 2012). However, unlike EXECUTER1 and EXEXUTER2 (Wagner et al., 2004; Lee et al., 2007), the volatile derivative pathway appears to exert little effect on hydrogen peroxide-responsive genes (Ramel et al., 2012).
An analysis of the transcriptome profiles of a range of Arabidopsis genotypes that are deficient in either hydrogen peroxide processing enzymes or in low-Mr antioxidants revealed a surprisingly small degree of overlap in the transcriptome patterns of leaves lacking different components of the antioxidant system (Queval and Foyer, 2012). Relatively few transcripts encoding chloroplast proteins were changed in abundance in response to antioxidant depletion. However, a large number of transcripts encoding core and accessory components of the thylakoid NADPH dehydrogenase complex were decreased in abundance when the activity of the thylakoid ascorbate peroxidase (tAPX) was decreased or in mutants with low levels of glutathione. Similarly, the levels of mRNAs encoding auxiliary proteins involved in the PSII repair cycle were specifically decreased in mutants deficient in glutathione (Queval and Foyer, 2012). These data, taken together with the finding that mRNAs encoding components of the thylakoid NADPH dehydrogenase complex were enhanced in abundance in Arabidopsis leaves following the transfer from high-CO2 growth conditions to air (Foyer et al., 2012), suggest that the expression of genes encoding proteins involved in this complex, which is involved in the regulation of cyclic electron flow around PSI, is highly sensitive to redox- and/or energy-related controls.
LEAF TRANSCRIPTOME PROFILES RESULTING FROM PERTURBATIONS IN THE BALANCE BETWEEN OXIDANTS AND ANTIOXIDANTS
To provide greater insights into how global change factors might influence cross-tolerance phenomena associated with alterations in the balance between oxidants and antioxidants, we have compared the leaf transcriptome profiles of Arabidopsis genotypes that are deficient in photorespiratory hydrogen peroxide processing (cat2; Queval et al., 2012), chloroplastic hydrogen peroxide processing (tapx; Maruta et al., 2012), ascorbate synthesis (vtc1 and vtc2; Kerchev et al., 2011), GSH synthesis (rootmeristemless1 [rml1]; Vernoux et al., 2000), or wild-type plants that had been exposed to 500 nL L−1 O3 for 6 h (Short et al., 2012). For simplicity, we have focused here on two components as examples of common responses of plants to environmental stress: the activation of the anthocyanin synthesis pathway (Fig. 3) and increases in mRNAs encoding receptor-like kinases (Figs. 4 and 5). The data used in Figures 3 to 5 were either generated in our own laboratories (cat2, vtc1, vtc2, and rml1) or taken from the literature (tapx [Maruta et al., 2012] and wild-type plants subjected to O3 fumigation [Short et al., 2012]). All the microarray data used in this analysis are available at the Web sites listed in Table I. For our analysis, transcriptomic data from the mutants were processed as described previously (Queval and Foyer, 2012). Data from the O3 fumigation have been subjected to Student’s t test and false discovery rate calculation using the Limma algorithm, and genes showing false discovery rate-corrected P values of less than 0.05 and relative change in expression of at least 2 compared with nonfumigated Arabidopsis were retained for the comparison.
Figure 3.
Redox regulation of the phenylpropanoid pathway leading to anthocyanin synthesis in Arabidopsis genotypes that are deficient in chloroplastic hydrogen peroxide processing (tapx), ascorbate synthesis (vtc1 and vtc2), GSH synthesis (rml1), or wild-type plants that had been exposed to 500 nL L−1 O3 for 6 h. A, Genes encoding components of the anthocyanin biosynthesis pathway or associated regulatory genes. B, Accessory or putative genes involved in anthocyanin biosynthesis. Blue bars, rml1; dark green bars, vtc2; light green bars, vtc1; black bars, tapx; red bars, O3. C, Simplified representation of the anthocyanin biosynthesis pathway to indicate the components encoded by the genes identified in A and B. 4CL, 4-COUMARATE:COA LIGASE; DFR, DIHYDROFLAVONOL 4-REDUCTASE; LDOX, LEUCOANTHOCYANIDIN DIOXYGENASE; PIF3, PHYTOCHROME INTERACTING FACTOR3; TT, TRANSPARENT TESTA.
Figure 4.
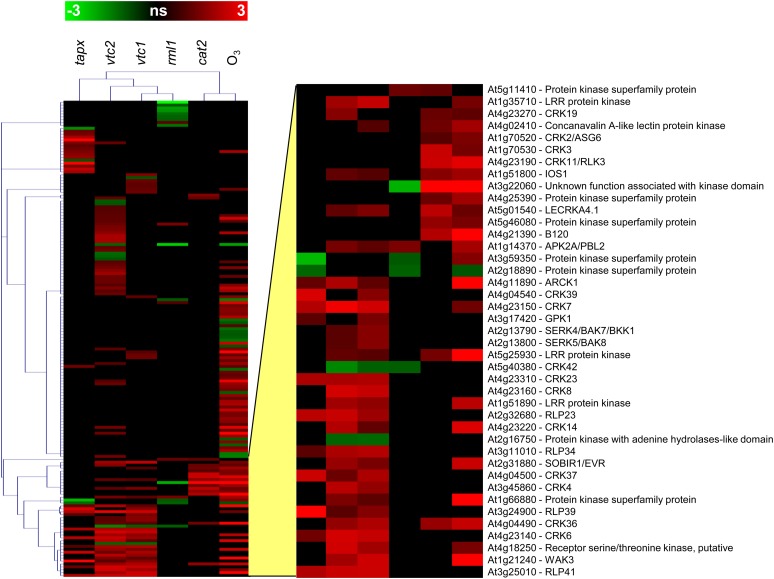
Hierarchical clustering (Pearson correlation) of RLK transcripts that respond to changes in cellular redox signaling in Arabidopsis genotypes that are deficient in photorespiratory hydrogen peroxide processing (cat2), chloroplastic hydrogen peroxide processing (tapx), ascorbate synthesis (vtc1 and vtc2), GSH synthesis (rml1), or wild-type plants that had been exposed to 500 nL L−1 O3 for 6 h. The color scale indicates relative log2 changes in expression; ns, nonsignificant.
Figure 5.
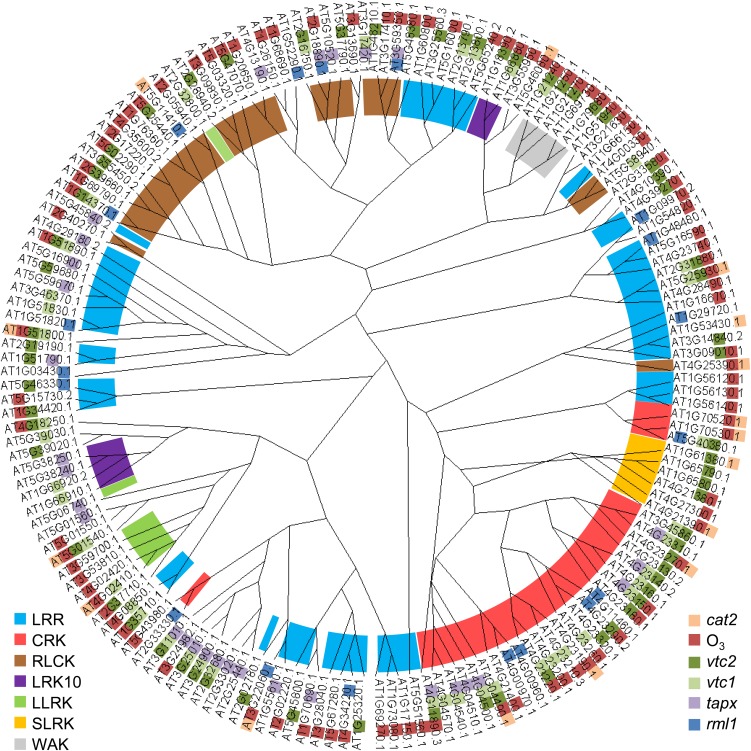
Radial cladogram showing the classification of the RLKs identified in Figure 4 based on their protein sequences. Protein sequences were aligned and homologies calculated using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2). The cladogram was drawn using Dendroscope3 (Huson and Scornavacca, 2012). The color classification inside the circle indicates RLK families at bottom left. The color classification of the Arabidopsis Genome Initiative codes indicates the oxidant condition (mutant or treatment) affecting RLK expression at bottom right. LLRK, Legume-lectin RLK; LRK10, wheat LRK10-like RLK; RLCK, receptor-like cytoplasmic kinase; SLRK, S-locus glycoprotein-like RLK.
Table I. Background information related to transcriptome data for Arabidopsis mutants with deficiencies in antioxidant capacity and for wild-type plants subjected to 500 nL L−1 O3 fumigation for 6 h.
| Mutant or Treatment | Reference | Data Web Site |
|---|---|---|
| vtc1 | Kerchev et al. (2011) | http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23331 |
| vtc2 | Kerchev et al. (2011) | http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE23331 |
| rml1 | D. Schnaubelt, Y. Dong, G. Queval, P. Diaz-Vivancos, M. Makgopa, G. Howell, M. Hannah, and C. Foyer, unpublished data | http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE36893 |
| cat2 | Queval et al. (2012) | http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE27985 |
| tapx | Maruta et al. (2012) | http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE35526 |
| O3 | Short et al. (2012) | http://www.ebi.ac.uk/arrayexpress/experiments/E-MEXP-342 |
DIFFERENTIAL REGULATION OF ANTHOCYANIN GENE EXPRESSION BY LOW ASCORBATE AND LOW GSH
Anthocyanins are often used as a visible phenotypic marker of stress. The synthesis of anthocyanins is coordinately controlled by a complex network of inducible transcription factors such as PRODUCTION OF ANTHOCYANIN PIGMENT1 (PAP1) and PAP2 that are regulated by hormones, nutrients, and photoreceptors such as cryptochromes and phytochromes, as illustrated in Figure 3 (Shin et al., 2007; Rubin et al., 2009). Anthocyanin synthesis is also highly responsive to redox regulation (Vanderauwera et al., 2005; Bashandy et al., 2009). It was recently shown that ascorbate, which exerts a strong influence on plant growth and defense pathways through the activation of SA- and ABA-dependent pathways and a repression of JA signaling (Pastori et al., 2003; Kerchev et al., 2011), is an important endogenous regulator of anthocyanin synthesis (Page et al., 2012). The analysis shown in Figure 3 confirms that ascorbate regulates the accumulation of mRNAs involved in flavonol and anthocyanin precursor synthesis (Page et al., 2012), such as PHENYLALANINE AMMONIA-LYASE1 (PAL1), 4-COUMARATE:COENZYME A LIGASE3, CHALCONE SYNTHASE (CHS), CHALCONE FLAVANONE ISOMERASE1 (CFI), FLAVANONE 3-HYDROXYLASE (F3H), and FLAVONOL SYNTHASE1 (FLS1), as well as the MYB transcription factor PAP1 and an ELONGATED HYPOCOTYL5 (HY5) homolog HYH (Fig. 3). Moreover, ascorbate modulates the blue light- and chloroplast-derived signal transduction systems that regulate anthocyanin accumulation. However, the abundance of mRNAs encoding components of flavonol glycoside biosynthesis, which branches from the anthocyanin pathway, is unchanged by low ascorbate. The induction of the phenylpropanoid biosynthesis pathway, particularly the expression of PAL and CHS by O3, has been reported for several plant systems (Iriti and Faoro, 2009). The results of the analysis shown in Figure 3 confirm this finding in Arabidopsis, where mRNAs encoding two PAL isoforms were increased in abundance after exposure to O3.
In marked contrast to the ascorbate-dependent repression of the expression of CHS, CFI, and F3H, low GSH enhanced the abundance of these mRNAs (Fig. 3A). Moreover, low GSH increased transcripts such as DIHYDROFLAVONOL 4-REDUCTASE, LEUCOANTHOCYANIDIN DIOXYGENASE, and an anthocyanin acyltransferase (AAT) that encode later steps of the anthocyanin biosynthetic pathway and also several related transporters, UGTs, and glutathione S-transferases (Fig. 3, A and B). Three members of the Myb/bHLH/WD-repeat complex family (PAP1, PAP2, and TRANSPARENT TESTA8), which activate anthocyanin biosynthesis (González et al., 2008), as well as the phytochrome interactor PIF3 were induced by GSH deficiency, as illustrated in Figure 3. Moreover, transcripts of LATERAL ORGAN BOUNDARY DOMAIN39 (LBD39), which is a repressor of anthocyanin biosynthesis (Rubin et al., 2009), were decreased by low GSH (Fig. 3, A and C).
While the control of anthocyanin synthesis by a set of closely related MYB transcription factors, such as PAP1/MYB75 and PAP2/MYB90 (Fig. 3), that activate the transcription of general phenylpropanoid and anthocyanin-specific genes has been reported, little information is currently available concerning the regulation of phenylpropanoid biosynthesis, especially with respect to the homeostatic mechanisms that coordinate and maintain metabolite levels within the required range. The data presented here provide evidence of the specific roles of different antioxidants in this regulation. Moreover, the inverse effects of O3 and low ascorbate would argue against simple models in which phenylpropanoid biosynthesis is triggered by enhanced oxidation. The data in Figure 3 indicate that the relative abundance of different antioxidants in the cell could exert much more specific effects on the regulation of the expression of genes encoding specific components of the phenylpropanoid pathway.
OXIDANT AND ANTIOXIDANT REGULATION OF RECEPTOR-LIKE KINASES
Accumulating evidence suggests that existing models greatly oversimplify redox signaling and that it is important to clearly distinguish between different signaling cascades and signaling tasks initiated by fine-tuning of the balance between oxidants and antioxidants in different cellular compartments. The following consideration of the regulation of the expression patterns of receptor-like/Pelle kinases (RLKs) shows that low ascorbate induces a unique profile of RLK regulation, which has some overlap with the transcript profiles induced by O3 but not those induced by deficiencies in other hydrogen peroxide-scavenging systems.
RLKs are Ser/Thr protein kinases that regulate a wide range of physiological processes, including development, hormone perception, and abiotic and biotic stress responses in plants (Gish and Clark, 2011). They typically contain a signal peptide, a variable extracellular domain, a transmembrane region, and a conserved intracellular protein kinase domain. The extracellular ligand-binding domain, which perceives signals and is important in cell-to-environment and cell-to-cell communication, is commonly used to classify RLKs into distinct subgroups (Shiu and Bleecker, 2003). There are more than 600 genes encoding RLKs in Arabidopsis, and they represent about 2% of the genome, but only relatively few of them, mostly Leu-rich repeat-RLKs, have been functionally characterized (Gish and Clark, 2011).
The Cys-rich RLKs (CRKs), previously called Domain of Unknown Function26 RLKs, form a large subgroup with more than 40 members, many of which are involved in the regulation of programmed cell death and plant defense responses. Transcripts encoding several CRKs were increased in abundance following exposure to O3 in a manner that was very similar to the response to pathogen attack (Wrzaczek et al., 2010). Moreover, a comprehensive phenotypic characterization of a knockout collection for the entire CRK family has confirmed their role in oxidative signaling (J. Kangasjärvi, personal communication). The expression of CRKs is regulated by high light, and several CRKs are differentially expressed in mutants that are defective in hormone signaling, particularly SA, ethylene, and JA-dependent pathways (Wrzaczek et al., 2010). The analysis illustrated in Figures 4 and 5 shows that many more transcripts encoding RLKs were increased in leaves exposed to O3 than in leaves that were deficient in catalase, tAPX, or glutathione. Surprisingly, although a set of RLKs were affected in a similar way in both vtc1 and vtc2, another group of RLK mRNAs were differentially abundant in vtc2 but not in vtc1 (Fig. 4).
Growth at high levels of O3 resulted in coordinated transcriptional reprogramming of antioxidant metabolism, chlorophyll biosynthesis, and respiratory pathways (Gillespie et al., 2012). However, the transcriptional response of antioxidant metabolism to elevated O3 was less marked compared with ambient atmospheric CO2 availability (Gillespie et al., 2012). In contrast to plants exposed to 500 nL L−1 O3 for 6 h, which resulted in changes in the abundance of a large number (95) of RLK mRNAs, very few (16) RLK mRNAs were responsive to photorespiratory hydrogen peroxide, as indicated by the mutants deficient in the major photorespiratory form of catalase (cat2; Supplemental Table S1). While 62 RLKs were induced by low ascorbate and seven RLK mRNAs were repressed (Supplemental Table S1), only five were induced by low GSH and 14 RLKs were repressed. Moreover, only four RLKs were similarly regulated by the two antioxidants (Supplemental Table S1), suggesting a high degree of specificity in ascorbate-dependent and GSH-dependent regulation of RLK expression. Furthermore, four RLKs were repressed and 26 were induced in response to low ascorbate peroxidase activity in the thylakoid membranes (tapx). Less than one-half (10) of the induced RLKs in tapx showed the same pattern in response to ascorbate deficiency. Of the low ascorbate-induced RLK genes, 18 belong to the CRK family. Despite the specific responses of the individual RLK genes to the different redox signals described here, the pattern of expression did not reveal an exclusive RLK family response to a given signal (Fig. 5). The literature information available on the RLKs responding to specific oxidative signals suggests that they are involved in a diverse range of physiological processes, as discussed below. However, the scarcity of literature information on RLK functions has not allowed the production of a more precise map than that shown in Figure 5 linking the different classes of RLK to specific redox signals.
All of the 16 RLK mRNAs that were responsive to increased photorespiratory hydrogen peroxide availability in the cat2 mutant were increased in abundance, and of these, 13 were also induced by O3 (Fig. 4). Hence, this subset of RLKs might form part of a general response to enhanced oxidation, whether this is perceived first in the apoplast or in the peroxisomes. Of the 13 genes that are induced by O3 and by higher photorespiratory hydrogen peroxide availability, four were also induced by low ascorbate. This small group that is generally responsive to enhanced oxidation includes Leu-rich repeat kinase, which contributes to Hyaloperonospora arabidopsidis susceptibility, IMPAIRED OOMYCETE SUSCEPTIBILITY1 (IOS1; Hok et al., 2011), and the negative regulators of ABA responses LECTIN RECEPTOR KINASE A4.1 (LecRKA4.1) and CRK36 (Xin et al., 2009; Tanaka et al., 2012). The other RLK mRNAs that show a similar response to enhanced oxidation in the cat2 mutant and following O3 fumigation of wild-type plants are CRK3 and CRK11. CRK11 is induced by pathogens, SA, and oxidative stress (Czernic et al., 1999), while CRK3, like CRK2, might participate in ABA and GA signaling (Bassel et al., 2011). It has also been suggested that CRK3 interacts with an isoform of Gln synthase (GLN1;1) to regulate nitrogen remobilization during leaf senescence (Li et al., 2006). Transcripts encoding another RLK (PROLINE-RICH EXTENSIN-LIKE RECEPTOR KINASE10 [PERK10]), which is thought to be involved in the regulation of primary metabolism, were decreased in abundance following exposure to O3. Depletion of PERK10 in loss-of-function mutants leads to enhanced starch accumulation (Mentzen et al., 2008).
Transcripts encoding a range of other RLKs that were increased following fumigation with O3 are involved in development, hormone perception, and abiotic and biotic stress responses in Arabidopsis (Supplemental Table S1). For example, the expression of PEPTIDE SIGNALING MOLECULE RECEPTOR1 (PEPR1) and PEPR2 is induced by pathogens, wounding, and methyl jasmonate (Yamaguchi et al., 2010). The PEPR1 gene product shows guanyl cyclase activity and is involved in calcium-dependent defense gene expression (Qi et al., 2010). Exposure to O3 has been found to induce a large cluster of genes involved in calcium signaling, including several calcium/calmodulin-dependent protein kinases (Short et al., 2012). Like PEPR1, the PHYTOSULFOKINE RECEPTOR1 shows both kinase and guanylate cyclase activity (Kwezi et al., 2011). CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) is required for the elicitation of defense signaling in response to the perception of chitin (Miya et al., 2007). The expression of BOTRYTIS-INDUCED KINASE1 and RPM1-INDUCED PROTEIN KINASE is modulated by the Xanthomonas campestris AvirulenceAC effector, suggesting that these function in signaling pathways that operate downstream of FLAGELLIN-SENSITIVE2 (FLS2) and CERK1 (Feng et al., 2012).
Exposure to O3 also results in the altered abundance of transcripts encoding RLKs that have functions in the regulation of intracellular and intercellular transport. For example, NUCLEAR SHUTTLE PROTEIN INTERACTING KINASE3 mRNAs, which were less abundant following O3 treatment (Supplemental Table S1), are considered to be involved in plant responses to viruses (Fontes et al., 2004). PLASMODESMATA-LOCATED PROTEIN1 (PDLP1) mRNAs were more abundant following O3 treatment. The PDLP family of proteins is considered to promote virus movement through the plasmodesmata by regulating tubule formation in response to viral movement proteins (Amari et al., 2010). For example, PDLP5 transcripts accumulate in response to infestation by the biotrophic pathogen Pseudomonas syringae; they are involved in the induction of SA-dependent innate immunity responses as well as the repression of plasmodesmata trafficking (Lee et al., 2011b).
The plant response to O3 also involves RLKs that are involved in the regulation of developmental processes. For example, HAE induces floral organ abscission (Jinn et al., 2000), while CAST AWAY acts together with EVERSHED (EVR) and HAE to inhibit cell separation (Burr et al., 2011). ROOT HAIR SPECIFIC16 overexpression causes branched root hair morphology (Won et al., 2009). RECEPTOR-LIKE PROTEIN KINASE1 is required for embryo patterning (Nodine et al., 2007) but it also has roles in ABA signaling, both in responses to drought and in the regulation of senescence (Osakabe et al., 2010; Lee et al., 2011a). Similarly, LecRK1 is induced in stomatal guard cells after bacterial infection and leads to the inhibition of ABA-mediated stomatal immunity (Desclos-Theveniau et al., 2012). This mechanism may have evolved to avoid the deleterious effects of a prolonged inhibition of photosynthesis that would be caused by decreased CO2 availability following prolonged stomata closure (Desclos-Theveniau et al., 2012).
Given the roles of ascorbate in the apoplast and cell wall compartments of the cell (Pignocchi and Foyer, 2003), it is interesting that transcripts encoding four WALL-ASSOCIATED KINASEs (WAKs) were enhanced in abundance in the vtc2 mutant that is deficient in this redox metabolite. The WAK proteins are considered to have functions in pectin metabolism in the apoplast (Kohorn et al., 2006). The wak2 mutants show decreased vacuolar invertase activity, consistent with the sugar-mediated regulation of turgor maintenance and cell growth (Kohorn et al., 2006). WAKL10 is a guanylyl cyclase that is expressed in response to pathogens (Meier et al., 2010).
Transcripts encoding SOMATIC EMBRYOGENESIS RECEPTOR KINASE4 (SERK4) and SERK5, which were much more abundant in the leaves of the vtc1 and vtc2 mutants than in the wild type (Fig. 4), are considered to fulfill roles in the control of cell death, particularly in response to pathogens (Albrecht et al., 2008). Moreover, SERK4 is able to restore the shoot phenotype of brassinosteroid-insensitive mutants (Albrecht et al., 2008). The SERK4 gene product interacts with FLS2, which is involved in the bacterial elicitor flagellin perception and innate immunity response (Gómez-Gómez and Boller, 2000). FLS2 was induced in response to both low GSH and low ascorbate (Supplemental Table S1).
Transcripts encoding the pathogen-responsive IOS1 (Hok et al., 2011) were more abundant in the leaves of the vtc1 and vtc2 mutants that have low ascorbate than in the wild type (Fig. 4), as was SUPPRESSOR OF BIR1 (SOBIR1). Other RLK genes, such as CRK5, CRK6, CRK10, and BAK1-INTERACTING RECEPTOR-LIKE KINASE1 (BIR1), were induced in vtc2 but not in vtc1. CRK5, CRK6, and CRK10 are involved in plant defense processes and have WRKY-binding sites in their promoter regions (Du and Chen, 2000; Chen et al., 2003). They are responsive to both pathogen- and SA-induced WRKY transcription factors (Du and Chen, 2000; Chen et al., 2003). Moreover, CRK5 can induce cell death, even in the absence of SA (Chen et al., 2003). The bir1 mutants exhibit spontaneous cell death (Gao et al., 2009). BIR1 is a repressor of the defense response that includes the repression of the SOBIR1 defense pathway (Gao et al., 2009). BIR1 (EVR) is also a repressor of abscission (Leslie et al., 2010).
mRNAs encoding several RLKs such as CRK13, which modulate SA signaling, were altered in abundance in vtc1 (Supplemental Table S1), consistent with the global changes in other SA-related transcripts that have previously been reported in the vtc1 and vtc2 mutants (Pastori et al., 2003; Kerchev et al., 2011). The overexpression of CRK13 resulted in the activation of SA signaling pathways, leading to the accumulation of pathogenesis-related proteins and enhanced resistance to P. syringae (Acharya et al., 2007). Therefore, the enhanced expression of CRK13 in vtc1 is consistent with the enhanced resistance to P. syringae observed in the low-ascorbate mutants (Pavet et al., 2005). Moreover, transcripts encoding other RLKs with functions in ABA signaling were also modified in the low-ascorbate genotypes, consistent with the elevated ABA content and altered ABA-mediated expression patterns observed in these mutants (Kerchev et al., 2011). For example, LecRKA4.1 was induced in response to low ascorbate and LecRKA4.3 was induced in tapx (Fig. 4; Supplemental Table S1). These RLKs are negative regulators of ABA responses in seed germination (Xin et al., 2009). ABA- AND OSMOTIC STRESS-INDUCIBLE RECEPTOR-LIKE CYTOSOLIC KINASE1 (ARCK1) and CRK36 transcripts were increased in abundance in both the vtc1 and vtc2 mutants (Fig. 4). The CRK36 and ARCK1 proteins form a complex that negatively controls ABA and osmotic stress signal transduction (Tanaka et al., 2012). Among the 14 RLKs uniquely affected by tAPX deficiency were BRASSINOSTEROID INSENSITIVE1 (BRI1)-LIKE, which regulates vascular differentiation and is a functional homolog of the brassinosteroid receptor BRI1 (Caño-Delgado et al., 2004) and ROP-BINDING PROTEIN KINASE1 (RBK1). RBK1 expression is associated with vasculature, induced by pathogens, and interacts with Rop GTPases, which are a plant-specific class of small GTPases (Molendijk et al., 2008).
A smaller number of RLK genes were regulated by low GSH compared with those modified by low ascorbate. Of these, ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN5 (AHP5) and RLK7 were induced by low GSH (Supplemental Table S1). The AHP family proteins play a role in the phosphorelay involved in cytokinin signaling (Hutchison et al., 2006), and RLK7 controls germination speed and tolerance to oxidative stress (Pitorre et al., 2010). Taken together, these data suggest that, in contrast to the GSH pool, which exerts only a minor influence on the abundance of RLK mRNAs, the abundance of ascorbate might be an important regulator of the expression of RLK genes.
CONCLUSION AND PERSPECTIVE
It is generally accepted that exposure to environmental stresses disrupts the metabolic balance of plant cells, leading to increases in oxidant production, and accumulation, leading to oxidative signaling (Miller et al., 2008). However, the evidence discussed here demonstrates that while oxidants and antioxidants are essentially opposing elements in redox signaling cascades, each component (oxidant and antioxidant) within a given cellular compartment or niche is likely to fulfill an individual signaling task. The intracellular environment, apart from certain compartments such as the endoplasmic reticulum, tends to be highly reducing, while the extracellular environment of the cell wall/apoplast tends to be highly oxidizing, with a net flow of reductant in the form of ascorbate from the interior of the cell to the external surface, where ascorbate oxidase is used to maintain a strong redox gradient across the plasma membrane. The strong induction in plasma membrane-associated RLKs in plants that have low leaf ascorbate levels, where ascorbate levels in the apoplast are undetectable (Veljovic-Jovanovic et al., 2001), would support the view that the redox gradient across the plasma membrane is a crucial redox sensor and regulator of gene expression. The extent of this gradient is greatly influenced by changes in metabolism during development and by stress, which often favors a steepening of the gradient, because of the regulated generation of stress-induced oxidative burst.
Differential oxidant and antioxidant concentrations between compartments permit redox-driven vectorial signaling, through processes such as ascorbate-driven electron transport or futile cycles. With respect to global change parameters, attention must be focused on the apoplast as a major site where oxidants are produced in response to stress and are perceived. Fluctuations in the redox state of the apoplast will facilitate interactions between receptor proteins containing oxidizable thiols that are sited in or near the membrane surface. The extent of the redox gradient across the plasma membrane triggers or elaborates membrane signaling, for example, through receptor kinases and channel activity, modifying calcium release and aquaporin or peroxiporin function.
We suggest that the redox gradient across the plasma membrane is a key sensor of global change and a crucial regulator of redox signaling. The predicted increases in the abundance of CO2 and O3 in the atmosphere by 2050 will have a profound influence on the balance between intracellular and extracellular oxidation, favoring a steepening of the redox gradient across the plasma membrane. Changes in the redox gradient across the plasma membrane will inevitably modify the stress signaling pathways that contribute to cross-tolerance phenomena and perhaps also the molecular patterns by which plants create a “memory” of stress by reprogramming gene expression and epigenetic pathways. While the presented analysis is based on results obtained from a somewhat limited number of mutants/transgenic plants, the future analysis of signal transduction effects, especially data based on transient redox shifts and induced RNA silencing, will undoubtedly lead to the identification of redox signaling components, processes, and mechanisms that can be used to improve stress tolerance traits while enabling crops to exploit the future high atmospheric CO2 environment for yield enhancement. This will be a critical step toward adapting crops to future growing environments.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Expression change values (in log2) of RLKs that are differentially expressed in response to changes in cellular redox signaling pathways in various Arabidopsis genotypes.
Glossary
- CO2
carbon dioxide
- O3
ozone
- ROS
reactive oxygen species
- GSH
reduced glutathione
- SA
salicylic acid
- GLDH
l-galactono-1,4-lactone dehydrogenase
- ABA
abscisic acid
- JA
jasmonate
- tAPX
thylakoid ascorbate peroxidase
- RLK
receptor-like/Pelle kinase
- CRK
Cys-rich RLK
References
- Acharya BR, Raina S, Maqbool SB, Jagadeeswaran G, Mosher SL, Appel HM, Schultz JC, Klessig DF, Raina R. (2007) Overexpression of CRK13, an Arabidopsis cysteine-rich receptor-like kinase, results in enhanced resistance to Pseudomonas syringae. Plant J 50: 488–499 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Leakey ADB. (2008) Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol 147: 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC. (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amari K, Boutant E, Hofmann C, Schmitt-Keichinger C, Fernández-Calvino L, Didier P, Lerich A, Mutterer J, Thomas CL, Heinlein M, et al. (2010) A family of plasmodesmal proteins with receptor-like properties for plant viral movement proteins. PLoS Pathog 6: e1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Bartoli C, Pastori G, Foyer CH. (2000) Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol 123: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Casalongue C, Simontacchi M, Márquez-García B, Foyer CH. (2012) Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ Exp Bot (in press) [Google Scholar]
- Bashandy T, Taconnat L, Renou JP, Meyer Y, Reichheld JP. (2009) Accumulation of flavonoids in an ntra ntrb mutant leads to tolerance to UV-C. Mol Plant 2: 249–258 [DOI] [PubMed] [Google Scholar]
- Bassel GW, Glaab E, Márquez J, Holdsworth MJ, Bacardit J. (2011) Functional network construction in Arabidopsis using rule-based machine learning on large-scale data sets. Plant Cell 23: 3101–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker FL, Fiscus EL. (2005) The role of ozone flux and antioxidants in the suppression of ozone injury by elevated CO2 in soybean. J Exp Bot 418: 2139–2151 [DOI] [PubMed] [Google Scholar]
- Bräutigam K, Dietzel L, Pfannschmidt T. (2007) Plastid-nucleus communication: anterograde and retrograde signalling in development and function of plastids. In R Bock, ed, Cell and Molecular Biology of Plastids, Vol 19: Topics in Current Genetics. Springer, Berlin, pp 409–455 [Google Scholar]
- Burr CA, Leslie ME, Orlowski SK, Chen I, Wright CE, Daniels MJ, Liljegren SJ. (2011) CAST AWAY, a membrane-associated receptor-like kinase, inhibits organ abscission in Arabidopsis. Plant Physiol 156: 1837–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caño-Delgado A, Yin Y, Yu C, Vafeados D, Mora-García S, Cheng JC, Nam KH, Li J, Chory J. (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131: 5341–5351 [DOI] [PubMed] [Google Scholar]
- Cela J, Chang C, Munné-Bosch S. (2011) Accumulation of γ- rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol 52: 1389–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois-Meurinne M, Van Breusegem F, Saindrenan P, Noctor G. (2010) Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol 153: 1692–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Du L, Chen Z. (2003) Sensitization of defense responses and activation of programmed cell death by a pathogen-induced receptor-like protein kinase in Arabidopsis. Plant Mol Biol 53: 61–74 [DOI] [PubMed] [Google Scholar]
- Collins GG, Nie XL, Saltveit ME. (1995) Heat shock proteins and chilling sensitivity of mung bean hypocotyls. J Exp Bot 46: 795–802 [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. (2000) Identification of ascorbic acid deficient Arabidopsis thaliana mutants. Genetics 154: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernic P, Visser B, Sun W, Savoure A, Deslandes L, Marco Y, Van Montagu M, Verbruggen N. (1999) Characterization of an Arabidopsis thaliana receptor-like protein kinase gene activated by oxidative stress and pathogen attack. Plant J 18: 321–327 [DOI] [PubMed] [Google Scholar]
- D’Autréaux B, Toledano MB. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824 [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW. (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172: 11–21 [DOI] [PubMed] [Google Scholar]
- Desclos-Theveniau M, Arnaud D, Huang TY, Lin GJ, Chen WY, Lin YC, Zimmerli L. (2012) The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Mackerness SAH, Hancock JT, Neill SJ. (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Chen Z. (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24: 837–847 [DOI] [PubMed] [Google Scholar]
- Feng F, Yang F, Rong W, Wu X, Zhang J, Chen S, He C, Zhou JM. (2012) A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485: 114–118 [DOI] [PubMed] [Google Scholar]
- Fey V, Wagner R, Brautigam K, Wirtz M, Hell R, Dietzmann A, Leister D, Oelmuller R, Pfannschmidt T. (2005) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280: 5318–5328 [DOI] [PubMed] [Google Scholar]
- Fontes EP, Santos AA, Luz DF, Waclawovsky AJ, Chory J. (2004) The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev 18: 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63: 1637–1661 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28: 1056–1071 [Google Scholar]
- Foyer CH, Noctor G. (2009) Redox regulation in photosynthetic organisms: signaling, acclimation and practical implications. Antioxid Redox Signal 11: 862–905 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Shigeoka S. (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155: 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Ball L, Oxborough K, Karpinski S, Mullineaux PM, Baker NR. (2003) Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J 33: 691–705 [DOI] [PubMed] [Google Scholar]
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, et al. (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44 [DOI] [PubMed] [Google Scholar]
- Gfeller A, Dubugnon L, Liechti R, Farmer EE. (2010) Jasmonate biochemical pathway. Sci Signal 3: cm3. [DOI] [PubMed] [Google Scholar]
- Gillespie KM, Xu F, Richter KT, Mcgrath JM, Markelz RJC, Ort DR, Leakey ADB, Ainsworth EA. (2012) Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell Environ 35: 169–184 [DOI] [PubMed] [Google Scholar]
- Gish LA, Clark SE. (2011) The RLK/Pelle family of kinases. Plant J 66: 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- González A, Zhao M, Leavitt JM, Lloyd AM. (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53: 814–827 [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC. (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature 433: 83–87 [DOI] [PubMed] [Google Scholar]
- Han Y, Mhamdi A, Chaouch S, Queval G, Zechmann B, Noctor G. (2012) Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok S, Danchin EG, Allasia V, Panabieres F, Attard A, Keller H. (2011) An Arabidopsis (malectin-like) leucine-rich repeat receptor-like kinase contributes to downy mildew disease. Plant Cell Environ 34: 1944–1957 [DOI] [PubMed] [Google Scholar]
- Huson DH, Scornavacca C. (2012) Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol 61: 1061–1067 [DOI] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, González M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, et al. (2006) The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18: 3073–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriti M, Faoro R. (2009) Chemical diversity and defense metabolism: how plants cope with pathogens and ozone pollution. Int J Mol Sci 10: 3371–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn TL, Stone JM, Walker JC. (2000) HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev 14: 108–117 [PMC free article] [PubMed] [Google Scholar]
- Kadono T, Tran D, Errakhi R, Hiramatsu T, Meimoun P, Briand J, Iwaya-Inoue M, Kawano T, Bouteau F. (2010) Increased anion channel activity is an unavoidable event in ozone-induced programmed cell death. PLoS ONE 5: e13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. (1999) Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 284: 654–657 [DOI] [PubMed] [Google Scholar]
- Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier PJ, Hancock RD, Foyer CH. (2011) The transcription factor ABI-4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23: 3319–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kobayashi M, Johansen S, Riese J, Huang LF, Koch K, Fu S, Dotson A, Byers N. (2006) An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J 46: 307–316 [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Fufezan C, Trebst A. (2008) Singlet oxygen production in PS II and related protection mechanism. Photosynth Res 98: 551–564 [DOI] [PubMed] [Google Scholar]
- Kwezi L, Ruzvidzo O, Wheeler JI, Govender K, Iacuone S, Thompson PE, Gehring C, Irving HR. (2011) The phytosulfokine (PSK) receptor is capable of guanylate cyclase activity and enabling cyclic GMP-dependent signaling in plants. J Biol Chem 286: 22580–22588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, Hong SW, Whang SS, Lim PO, Nam HG, Koo JC. (2011a) Age-dependent action of an ABA-inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol 52: 651–662 [DOI] [PubMed] [Google Scholar]
- Lee JY, Wang X, Cui W, Sager R, Modla S, Czymmek K, Zybaliov B, van Wijk K, Zhang C, Lu H, et al. (2011b) A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 23: 3353–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Kim C, Landgraf F, Apel K. (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 10270–10275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leferink NGH, van Duijn E, Barendregt A, Heck AJR, van Berkel WJH. (2009) Galactonolactone dehydrogenase requires a redox-sensitive thiol for optimal production of vitamin C. Plant Physiol 150: 596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie ME, Lewis MW, Youn JY, Daniels MJ, Liljegren SJ. (2010) The EVERSHED receptor-like kinase modulates floral organ shedding in Arabidopsis. Development 137: 467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RJ, Hua W, Lu YT. (2006) Arabidopsis cytosolic glutamine synthetase AtGLN1;1 is a potential substrate of AtCRK3 involved in leaf senescence. Biochem Biophys Res Commun 342: 119–126 [DOI] [PubMed] [Google Scholar]
- Liebler DC. (1993) The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol 23: 147–169 [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Morgan PB. (2005) Global food insecurity: treatment of major food crops with elevated carbon dioxide or ozone under large-scale fully open-air conditions suggests recent models may have overestimated future yields. Philos Trans R Soc Lond B Biol Sci 360: 2011–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MS, Foyer CH. (2011) The impact of high CO2 on plant abiotic stress tolerance. In JL Araus, GA Slafer, eds, Crop Stress Management and Global Climate Change. CABI, London, pp 85–104 [Google Scholar]
- Maeda H, Sakuragi Y, Bryant DA, DellaPenna D. (2005) Tocopherols protect Synechocystis sp. strain PCC 6803 from lipid peroxidation. Plant Physiol 138: 1422–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Noshi M, Tanouchi M, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. (2012) H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J Biol Chem 287: 11717–11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Ruzvidzo O, Morse M, Donaldson L, Kwezi L, Gehring C. (2010) The Arabidopsis wall associated kinase-like 10 gene encodes a functional guanylyl cyclase and is co-expressed with pathogen defense related genes. PLoS ONE 5: e8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzen WI, Peng J, Ransom N, Nikolau BJ, Wurtele ES. (2008) Articulation of three core metabolic processes in Arabidopsis: fatty acid biosynthesis, leucine catabolism and starch metabolism. BMC Plant Biol 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou JP, et al. (2010) Arabidopsis GLUTATHIONE REDUCTASE 1 plays a crucial role in leaf responses to intracellular H2O2 and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol 153: 1144–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH. (2003) Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol 133: 443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R. (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133: 481–489 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk AJ, Ruperti B, Singh MK, Dovzhenko A, Ditengou FA, Milia M, Westphal L, Rosahl S, Soellick TR, Uhrig J, et al. (2008) A cysteine-rich receptor-like kinase NCRK and a pathogen-induced protein kinase RBK1 are Rop GTPase interactors. Plant J 53: 909–923 [DOI] [PubMed] [Google Scholar]
- Mukherjee M, Larrimore KE, Ahmed NJ, Bedick TS, Barghouthi NT, Traw MB, Barth C. (2010) Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Mol Plant Microbe Interact 23: 340–351 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S. (2005) The role of α-tocopherol in plant stress tolerance. J Plant Physiol 162: 743–748 [DOI] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J. (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5: 388–395 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N. (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757: 742–749 [DOI] [PubMed] [Google Scholar]
- Nodine MD, Yadegari R, Tax FE. (2007) RPK1 and TOAD2 are two receptor-like kinases redundantly required for Arabidopsis embryonic pattern formation. Dev Cell 12: 943–956 [DOI] [PubMed] [Google Scholar]
- Nowicka B, Kruk J. (2012) Plastoquinol is more active than α-tocopherol in singlet oxygen scavenging during high light stress of Chlamydomonas reinhardtii. Biochim Biophys Acta 1817: 389–394 [DOI] [PubMed] [Google Scholar]
- Osakabe Y, Mizuno S, Tanaka H, Maruyama K, Osakabe K, Todaka D, Fujita Y, Kobayashi M, Shinozaki K, Yamaguchi-Shinozaki K. (2010) Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis. J Biol Chem 285: 9190–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Broshe M, Kangasjärvi J. (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Page M, Sultana N, Paszkiewicz K, Florance H, Smirnoff N. (2012) The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant Cell Environ 35: 388–404 [DOI] [PubMed] [Google Scholar]
- Parsons HT, Fry SC. (2010) Reactive oxygen species-induced release of intracellular ascorbate in plant cell-suspension cultures and evidence for pulsing of net release rate. New Phytol 187: 332–342 [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH. (2005) Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis thaliana. Plant Physiol 139: 1291–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Schutze K, Brost M, Oelmuller R. (2001) A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J Biol Chem 276: 36125–36130 [DOI] [PubMed] [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH. (2003) The function of ascorbate oxidase in tobacco. Plant Physiol 132: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Foyer CH. (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Curr Opin Plant Biol 6: 379–389 [DOI] [PubMed] [Google Scholar]
- Pineau B, Layoune O, Danon A, De Paepe R. (2008) L-Galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. J Biol Chem 283: 32500–32505 [DOI] [PubMed] [Google Scholar]
- Pitorre D, Llauro C, Jobet E, Guilleminot J, Brizard JP, Delseny M, Lasserre E. (2010) RLK7, a leucine-rich repeat receptor-like kinase, is required for proper germination speed and tolerance to oxidative stress in Arabidopsis thaliana. Planta 232: 1339–1353 [DOI] [PubMed] [Google Scholar]
- Preger V, Scagliarini S, Pupillo P, Trost P. (2005) Identification of an ascorbate-dependent cytochrome b of the tonoplast membrane sharing biochemical features with members of the cytochrome b561 family. Planta 220: 365–375 [DOI] [PubMed] [Google Scholar]
- Prentice IC, Farquhar GD, Fasham MJR, Goulden ML, Heimann M, Jaramillo VJ, Kheshgi HS, Le Quere C, Scholes RJ, Wallace DWR. (2001) The carbon cycle and atmospheric carbon dioxide. In JT Houghton, Y Ding, DJ Griggs, M Noguer, PJ van der Linden, D Xiaosu, eds, Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York, pp 183–239 [Google Scholar]
- Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA. (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA 107: 21193–21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q-S, Huber JL, Booker FL, Jain V, Leakey ADB, Fiscus EL, Yau PM, Ort DR, Huber SC. (2008) Increased protein carbonylation in leaves of Arabidopsis and soybean in response to elevated CO2. Photosynth Res 97: 155–166 [DOI] [PubMed] [Google Scholar]
- Queval G, Foyer CH. (2012) Redox regulation of photosynthetic gene expression. Philos Trans R Soc Lond B 367: 3475–3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G. (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52: 640–657 [DOI] [PubMed] [Google Scholar]
- Queval G, Neukermans J, Vanderauwera S, Van Breusegem F, Noctor G. (2012) Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ 35: 374–387 [DOI] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M. (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA 109: 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR. (2009) Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21: 3567–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SB, Costa A, Xin Z, Li PH. (1995) Induction of cold hardiness by salt stress involves synthesis of cold and abscisic acid-responsive proteins in potato (Solanum commersonii Dun). Plant Cell Physiol 36: 1245–1251 [Google Scholar]
- Sandermann H. (2008) Ecotoxicology of ozone: bioactivation of extracellular ascorbate. Biochem Biophys Res Commun 366: 271–274 [DOI] [PubMed] [Google Scholar]
- Sandermann H, Ernst D, Heller W, Langebartels C. (1998) Ozone: an abiotic elicitor of plant defense reactions. Trends Plant Sci 3: 47–50 [Google Scholar]
- Schottler MA, Kirchhoff H, Weis E. (2004) The role of plastocyanin in the adjustment of the photosynthetic electron transport to the carbon metabolism in tobacco. Plant Physiol 136: 4265–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Park E, Choi G. (2007) PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. (2003) Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132: 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short EF, North KA, Roberts MR, Hetherington AM, Shirras AD, McAinsh MR. (2012) A stress-specific calcium signature regulating an ozone-responsive gene expression network in Arabidopsis. Plant J 71: 948–961 [DOI] [PubMed] [Google Scholar]
- Sullivan JA, Gray JC. (2002) Multiple plastid signals regulate the expression of the pea plastocyanin gene in pea and transgenic tobacco plants. Plant J 32: 763–774 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Badger MR. (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16: 53–60 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Osakabe Y, Katsura S, Mizuno S, Maruyama K, Kusakabe K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. (2012) Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis. Plant J 70: 599–613 [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Miyake H. (2012) Redox-shuttling between chloroplast and cytosol: integration of intra-chloroplast and extra-chloroplast metabolism. Curr Opin Plant Biol 15: 252–260 [DOI] [PubMed] [Google Scholar]
- Tóth SZ, Puthur JT, Nagy V, Garab G. (2009) Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol 149: 1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A, Depka B, Hollander-Czytko H. (2002) A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett 516: 156–160 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzortzakis NG, Singleton I, Barnes JD. (2007) Deployment of low-level ozone enrichment for the preservation of chilled fresh produce. Postharvest Biol Technol 43: 261–270 [Google Scholar]
- Tzortzakis NG, Taybi T, Roberts R, Singleton I, Borland A, Barnes JD. (2011) Low-level atmospheric ozone exposure induces protection against Botrytis cinerea with down-regulation of ethylene-, jasmonate- and pathogenesis-related genes in tomato fruit. Postharvest Biol Technol 61: 152–159 [Google Scholar]
- Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F. (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39: 45–58 [DOI] [PubMed] [Google Scholar]
- Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inzé D, Van Breusegem F. (2005) Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol 139: 806–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S, Pignocchi C, Noctor G, Foyer CH. (2001) Low vitamin C in the vtc-1 mutant of Arabidopsis thaliana is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426–435 [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al. (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Przybyla D, Camp ROD, Kim C, Landgraf F, Lee KP, Wursch M, Laloi C, Nater M, Hideg E, et al. (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306: 1183–1185 [DOI] [PubMed] [Google Scholar]
- Won SK, Lee YJ, Lee HY, Heo YK, Cho M, Cho HT. (2009) Cis-element- and transcriptome-based screening of root hair-specific genes and their functional characterization in Arabidopsis. Plant Physiol 150: 1459–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzaczek M, Brosche M, Salojarvi J, Kangasjarvi S, Idanheimo N, Mersmann S, Robatzek S, Karpinski S, Karpinska B, Kangasjarvi J. (2010) Transcriptional regulation of the CRK/DUF26 group of receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biol 10: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Wang A, Yang G, Gao P, Zheng ZL. (2009) The Arabidopsis A4 subfamily of lectin receptor kinases negatively regulates abscisic acid response in seed germination. Plant Physiol 149: 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. (2010) PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22: 508–522 [DOI] [PMC free article] [PubMed] [Google Scholar]