Abstract
Purpose
The International Peripheral T-Cell Lymphoma Project was undertaken to better understand the subtypes of T-cell and natural killer (NK) –cell lymphomas.
Patients and Methods
Angioimmunoblastic T-cell lymphoma (AITL) was diagnosed according to the 2001 WHO criteria by a central review process consisting of panels of expert hematopathologists. Clinical, pathologic, immunophenotyping, treatment, and survival data were correlated.
Results
Of 1,314 patients, 243 (18.5%) were diagnosed with AITL. At presentation, generalized lymphadenopathy was noted in 76% of patients, and 89% had stages III to IV disease. Skin rash was observed in 21% of patients. Hemolytic anemia and hypergammoglobulinemia occurred in 13% and 30% of patients, respectively. Five-year overall and failure-free survivals were 33% and 18%, respectively. At presentation, prognostic models were evaluated, including the standard International Prognostic Index, which comprised the following factors: age ≥ 60 years, stages III to IV disease, lactic dehydrogenase (LDH) > normal, extranodal sites (ENSs) > one, and performance status (PS) ≥ 2; the Prognostic Index for Peripheral T-Cell Lymphoma, comprising: age ≥ 60 years, PS ≥ 2, LDH > normal, and bone marrow involvement; and the alternative Prognostic Index for AITL (PIAI), comprising: age > 60 years, PS ≥ 2, ENSs > one, B symptoms, and platelet count < 150 × 109/L. The simplified PIAI had a low-risk group (zero to one factors), with 5-year survival of 44%, and a high-risk group (two to five factors), with 5-year survival of 24% (P = .0065).
Conclusion
AITL is a rare clinicopathologic entity characterized by an aggressive course and dismal outcome with current therapies.
INTRODUCTION
Angioimmunoblastic T-cell lymphoma (AITL) is a rare subtype of peripheral T-cell lymphoma (PTCL), first described as a distinct clinicopathologic entity in the 1970s.1,2 AITL accounts for approximately 1% to 2% of non-Hodgkin's lymphoma and 15% to 20% of PTCL.3 The incidence of AITL is low, with only 0.05 new patient cases per 100,000 people in the United States, without sex predilection.3,4
Typically, the architecture of the lymph node is effaced, with only a few benign follicles retained. A characteristic feature is extension of the infiltrate beyond the lymph node capsule, with a preserved and somewhat dilated subcapsular sinus. Between the follicles, there is a proliferation of high endothelial venules. The neoplastic T cells are positive for CD2, CD3, CD4, CD10, CXCL-13, PD1, and often BCL-6.5–7 Scattered large immunoblastic cells with more basophilic cytoplasm frequently are CD20 positive, polytypic, and often Epstein Barr Virus (EBV) –encoded RNA (EBER) positive. Stains for follicular dendritic cells such as CD21 and CD23 typically show meshworks of follicular dendritic cells outside of the follicles and often around vessels.8 T-cell receptor gene rearrangements are negative in up to 30% of patients with AITL. Conversely, immunoglobulin gene rearrangement can be found in as many as 10% of patients with AITL, most likely representing expanded EBV-positive B-cell clones. The disease is frequently associated with autoimmune phenomena, such as the presence of circulating immune complexes, cold agglutinins, hemolytic anemia, and rheumatoid factor and antismooth-muscle antibodies. Hypergammaglobulinemia is present in approximately 50% of patients, typically polyclonal.1
Various treatment modalities have been employed, including steroids, cytotoxic chemotherapy, and immunomodulators.9–11 The clinical course of AITL is aggressive, with a median survival of fewer than 3 years, regardless of type of treatment.12,13 The aim of the present study was to better characterize the diagnostic and clinical aspects of AITL in a large and representative series of patient cases confirmed by central expert pathology review. Herein, we present the findings for 243 patient cases of AITL from the International Peripheral T-Cell Lymphoma Project.14
PATIENTS AND METHODS
Twenty-two institutions in North America, Europe, and the Far East participated in the study (Appendix Table A1, online only). Approval for the study was obtained from the institutional review board at the coordinating center (University of Nebraska Medical Center, Omaha, NE) and at each participating center as per the institutional standard. Those selected for the study were previously untreated patients age ≥ 19 years, with de novo PTCL or natural killer/T-cell lymphoma, excluding mycosis fungoides/Sézary syndrome, who first presented between January 1, 1990, and December 31, 2002. At each institution, the local pathologist reviewed the diagnostic pathology slides and reports for each patient case and recorded the results of local immunophenotypic, cytogenetic, and molecular studies. Clinical characteristics of the patients, including treatment data and follow-up information, were also required.
From each institution, phenotype datasheets, diagnostic slides, and tissue blocks were sent to one of five regional centers for review by an expert hematopathologist. These centers included Omaha, Nebraska (D.D.W.); Leeds, United Kingdom (K.A. MacLennan); Würzburg, Germany (T.R.); Bologna, Italy (S.A.P.); and Nagoya, Japan (S. Nakamura). A standard panel of immunostains was performed on each patient case, including CD20, CD2, CD3, CD4, CD5, CD8, CD30, CD56, TCR-β, TIA-1, Ki67, and in situ hybridization stains for EBERs. Other immunostains, polymerase chain reaction analyses, and fluorescence in situ hybridization cytogenetic studies were performed as needed, and all patients were diagnosed according to 2001 WHO classification criteria.15 The percentages of transformed tumor cells (blasts) and tumor cells expressing CD30 or Ki67 were estimated in each patient case, as were the percentages of tumor cells and background cells expressing either CD4 or CD8. The number of cells staining for EBERs was evaluated semiquantitatively by counting positive cells in the 10 most positive fields using a 10× ocular lens and 20× objective and calculating the average number per field (f): 0/f = 0; < 1/f = 1+; 1 to 9/f = 2+; 10 to 50/f = 3+; and > 50/f = 4+.
At each center, the diagnostic slides for each patient case were classified independently by each expert according to WHO classification criteria. Initial diagnosis was based on examination of the hematoxylin and eosin– and/or Giemsa-stained slides, immunostains, and phenotype datasheets, but with only limited clinical information from the time of initial diagnosis, including biopsy site and site of the largest tumor mass (ie, diagnosis one). After recording this diagnosis, the expert was presented with the complete clinical datasheet, and a second diagnosis was rendered (ie, diagnosis two). The previous diagnosis could not be changed based on the clinical information subsequently revealed.
In addition to the independent diagnosis rendered by each of the four expert hematopathologists, a consensus diagnosis was also reached in each patient case. A consensus was considered to have been reached if at least three of the four experts on the panel agreed on the second diagnosis (diagnosis two). All patient cases without a consensus diagnosis and all unclassifiable cases were jointly reviewed on a multiheaded microscope and discussed by the four experts in a daily consensus conference, and an attempt was made to reach a consensus diagnosis. If additional sections, immunostains, molecular or cytogeneitc studies, or other information were required, a diagnostic algorithm was developed by the panel, and the additional materials were obtained.
The clinical information included coded patient and site identifiers, sex, ethnicity, date of birth, date and site of diagnostic biopsy, other sites of disease, and Ann Arbor stage. Additional data recorded included symptoms at diagnosis, site and diameter of the largest tumor mass, performance status (PS), and history of immunosuppressive therapy or immune system disorder. Laboratory data recorded included hemoglobin, platelet count, WBC count, presence of circulating lymphoma cells, and serologies for HIV and human T-cell leukemia virus 1. The serum lactate dehydrogenase (LDH), β2-microglobulin, and C-reactive protein levels, and the presence of hypercalcemia, hypogammaglobulinemia, hypergammaglobulinemia, monoclonal serum immunoglobulin, hemolytic anemia, and hemophagocytic syndrome, were recorded. Initial therapy and response; details of remission, progression, or relapse; and subsequent therapies, along with survival status and cause of death, were recorded.
Treatment outcome was determined by overall survival (OS) and failure-free survival (FFS). OS was defined as the time from diagnosis to death resulting from any cause, with surviving patient follow-up censored at the last contact date. FFS was defined as the time from diagnosis to first occurrence of progression, relapse after response, or death resulting from any cause. Follow-up of patients not experiencing any of these events was censored at the date of last contact. Estimates of OS and FFS distributions were calculated using the Kaplan-Meier method,16 and time-to-event distributions were compared using the log-rank test.17 For survival analysis, the International Prognostic Index (IPI)18 and Prognostic Index for PTCL, Unspecified (PIT),19 were evaluated. In addition, comparisons of clinical and prognostic factors were performed using the χ2 or Fisher's exact test. Multivariate analysis was performed with a Cox hazards regression model using stepwise selection. All clinical and pathologic data were centrally analyzed at University of Nebraska Medical Center and presented and discussed at a consensus conference attended by all the investigators.
RESULTS
There were a total of 1,314 patient cases of PTCL, of which 243 (18.5%) were diagnosed as AITL. AITL was more frequent in Europe (28.7% of all PTCL), followed by Asia (17.9%) and North America (16.0%). As shown in Table 1, the median age was 65 years (range, 20 to 86 years), with a male-to-female ratio of 1:3. At presentation, generalized lymphadenopathy was noted in 76% of patients, and splenomegaly and hepatomegaly were present in 35% and 26% of patients, respectively. Overall, 89% of patients had advanced-stage disease by Ann Arbor classification. Extranodal disease was present in 27% of patients. Skin rash was observed in 21% of patients. Hemolytic anemia was seen in 13% of patients, and dysproteinemia represented by hypergammaglobulinemia occurred in 30%; among these, monoclonal serum immunoglobulin was observed in 10% of patients. Overall, 6% of patients were treated with immunosuppressive drugs for treatment of symptoms, mostly steroids, at the time of diagnosis. The IPI and PIT could be calculated in 222 (91%) and 233 (96%) patient cases, respectively.
Table 1.
Clinical Characteristics of Patients With AITL (N = 243)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 65 | |
| Range | 20-86 | |
| Male sex | 137 | 56 |
| ECOG PS ≥ 2 | 89 of 239 | 37 |
| Stages III to IV | 214 of 241 | 89 |
| Extranodal sites ≥ two | 66 | 27 |
| BM involvement | 67 of 228 | 28 |
| Systemic symptoms | 168 | 69 |
| Hemolytic anemia | 32 of 205 | 13 |
| Anemia | 81 of 205 | 33 |
| Platelet count < 150,000/cmm | 60 | 25 |
| Elevated LDH | 146 of 223 | 60 |
| Elevated β2-microglobulin | 53 of 92 | 22 |
| Elevated CRP | 84 of 127 | 35 |
| Hypercalcemia | 3 of 184 | 1 |
| Hypergammaglobulinemia | 74 of 166 | 30 |
| HTLV-1 positive | 6 of 89 | 2 |
| HIV positive | 2 of 151 | 1 |
| Immunosuppressive drugs | 13 of 211 | 5 |
| Immune system disorder | 3 of 210 | 1 |
| IPI ≥ 2 | 192 of 222 | 79 |
| PIT ≥ 2 | 154 of 233 | 63 |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; BM, bone marrow; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; HTLV-1, human T-cell lymphotropic virus type I; IPI, International Prognostic Index; LDH, lactic dehydrogenase; PIT, Prognostic Index for Peripheral T-Cell Lymphoma, Unspecified.
Diagnostic Accuracy
Two diagnoses were made by each of the four expert hematopathologists in each patient case based mainly on histology, immunophenotype, and molecular genetic data (diagnosis one) and then with the additional complete clinical data (diagnosis two). The agreement of the experts on diagnosis two with consensus diagnosis of AITL was 81%. Change of a diagnosis of AITL (diagnosis one) to the correct (consensus) diagnosis after consideration of the clinical data occurred in only nine patient cases, whereas change of a diagnosis of other types of PTCL (diagnosis one) to the correct (consensus) diagnosis of AITL occurred in 17 patient cases.
Treatment and Outcome
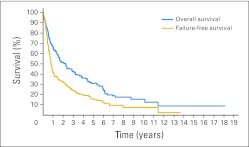
Five-year OS for the entire group was 32%, and 5-year FFS was 18% (Fig 1). A majority of the patients (82%) received combination chemotherapy containing an anthracycline, whereas the rest received combination chemotherapy without an anthracycline (7%), single-agent therapy (5%) or no chemotherapy (6%). The decision of no initial treatment was mostly the result of advanced disease and poor PS. Autologous stem-cell transplantation (ASCT) was administered to 31 patients (17%) and was part of initial treatment in 15 (8%). The complete remission rate for those receiving an anthracycline-containing regimen was 61%. However, there was no survival advantage for patients receiving combination chemotherapy with an anthracycline when compared with those receiving combination chemotherapy without an anthracycline. At the close of the study, 66% of patients had died, and only 9% were in remission at the time of death.
Fig 1.
Overall and failure-free survival for 243 patients with angioimmunoblastic T-cell lymphoma.
Clinical Prognostic Factors
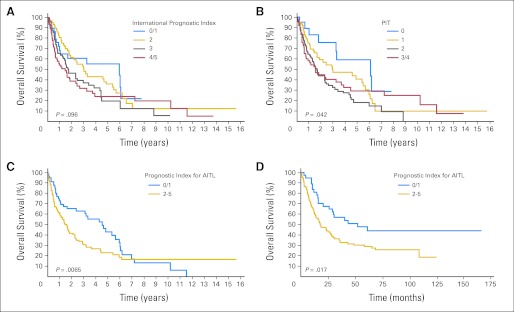
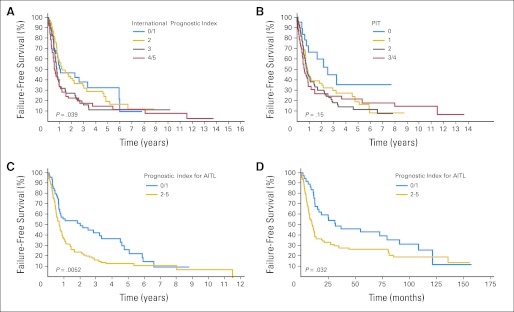
Of the prognostic factors in the IPI, only age > 60 years (P = .04), PS > 2 (P = .002), and extranodal sites (ENSs) > one (P = .012) were predictive of OS (Fig 2A). For FFS, only PS > 2 (P < .001) was predictive (Fig 3A). Neither stage (P = .60 and P = .68) nor elevated serum LDH (P = .56 and P = .55) were predictive of OS or FFS, respectively. Therefore, the IPI was not a particularly good prognostic model for AITL. OS and FFS as predicted by PIT score (age ≥ 60 years, PS ≥ 2, LDH > normal, and bone marrow involvement) are shown in Figures 2B and 3B.
Fig 2.
Overall survival (OS) for patients with angioimmunoblastic T-cell lymphoma (AITL) using the (A) International Prognostic Index, (B) Prognostic Index for Peripheral T-Cell Lymphoma, Unspecified (PIT), and (C) Prognostic Index for AITL (PIAI); (D) OS for GELA (Groupe d'Etude des Lymphomes de l'Adulte) validation cohort using the PIAI.
Fig 3.
Failure-free survival (FFS) for patients with angioimmunoblastic T-cell lymphoma (AITL) using the (A) International Prognostic Index, (B) Prognostic Index for Peripheral T-Cell Lymphoma, Unspecified (PIT), and (C) Prognostic Index for AITL (PIAI); (D) FFS for GELA (Groupe d'Etude des Lymphomes de l'Adulte) validation cohort using the PIAI.
We also evaluated other potential prognostic factors, and the following were adverse predictors of OS and FFS, respectively: B symptoms (P = .005 and P = .01) and platelet count < 150 × 109/L (P = .017 and P = .28). Therefore, we constructed a new prognostic model similar to the IPI for AITL using the five significant prognostic factors: age > 60 years, PS > 2, ENSs > one, B symptoms, and platelet count < 150 × 109/L (Figs 2C and 3C). A comparison of the three prognostic models (Table 2) revealed that the new prognostic index for AITL (PIAI) was more predictive of low- and high-risk subsets of patients with AITL.
Table 2.
Survival of Patients With AITL by Prognostic Model
| No. of Risk Factors | Patients (%) | 5-Year OS (%) | 5-Year FFS (%) |
|---|---|---|---|
| IPI | |||
| 0/1 | 14 | 56 | 34 |
| 2 | 28 | 38 | 21 |
| 3 | 30 | 20 | 12 |
| 4/5 | 28 | 25 | 16 |
| PIT | |||
| 0/1 | 36 | 46 | 22 |
| 2 | 37 | 19 | 12 |
| 3/4 | 27 | 30 | 22 |
| PIAI | |||
| 0/1 | 30 | 44 | 28 |
| 2-5 | 70 | 24 | 15 |
Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; FFS, failure-free survival; IPI, International Prognostic Index; PIAI, Prognostic Index for AITL; PIT, Prognostic Index for Peripheral T-Cell Lymphoma, Unspecified.
The PIAI was also tested in an independent set of patients with AITL for validation. The GELA (Groupe d'Etude des Lymphomes de l'Adulte) group analyzed 157 patients from its previously published report on patients with AITL20 using the PIAI prognostic model. In this independent validation cohort, low- versus high-risk PIAI maintained significance for OS and FFS (Figs 2D and 3D).
Pathologic Prognostic Factors
We also evaluated a variety of pathologic features as possible prognostic factors by univariate analysis, and the following were adverse predictors of OS and FFS, respectively: Ki67 proliferation > 30% (P = .018 and P = .015) and transformed tumor cells > 20% (P = .025 and P = .094). Pathologic features that were not predictive of survival were significant EBV infection (EBERs, three to ≥ 4), CD30 expression, and background CD8-positive T cells > 10%. However, by stepwise multivariate analysis, when controlling for the IPI, neither Ki67 proliferation nor transformed tumor cells was an independent predictor of survival. When controlling for the PIAI, however, Ki67 proliferation > 30% was an independent predictor of survival, with a hazard ratio of 1.6 for OS (P = .045) and 1.6 for FFS (P = .046).
DISCUSSION
In our study, AITL mainly affected older individuals, with a median age of 65 years (range, 20 to 86 years), and most patients had advanced stage (89%, stages III to IV), systemic symptoms (69%), extranodal involvement (27%), high IPI scores (79%, ≥ 2), elevated LDH (60%), and hypergammaglobulinemia (30%).
The centralized review of the diagnostic material by panels of expert hematopathologists, coupled with appropriate immunostains, permitted a consistent diagnosis and thereby successful validation of the 2001 WHO classification of this rare but distinct type of T-cell lymphoma. The agreement of the experts with the consensus diagnosis of AITL was 81%. Possible additional diagnostic markers such as CXCL13 or CD279 (PD1) may help to improve the accuracy and reproducibility of this diagnosis. The 28% of patients with large numbers of EBER-positive (three to ≥ four) accompanying B cells in our study sample is comparable to the rate (19%) recently reported in patients with AITL treated within GELA trials.20
So far, limited data are available on prognostic factors in AITL, yielding controversial results. Pangalis et al21 found that lymphocytopenia was more evident in patients who died as a result of AITL than in those who remained alive (P = .089). Aozasa et al22 suggested that the presence of clear and convoluted cells was predictive of poor prognosis. Moreover, achievement of complete remission is an important prognostic factor.23,24 The Kiel Lymphoma Study Group analyzed the prognostic impact of clinical observations and laboratory findings at presentation and found that survival was significantly related to age (P = .032), stage (P = .037), systemic symptoms (P = .007), skin rash/pruritus (P = .038), edema (P = .030), ascites (P = .013), high LDH (P = .007), and decreased hemoglobin (P = .020).9 Archimbaud et al24 identified prognostic factors associated with shorter survival, including rash (P < .001), lymph node eosinophilia (P = .03), elevated LDH (P = .03), and drug exposure in relation to the onset of the disease (P = .02); parameters associated with longer survival were localized lymphadenopathy (P = .01) and achievement of remission (P < .001). In our study, an alternative prognostic model for AITL was constructed using the five clinical prognostic factors: age > 60 years, PS ≥ 2, ENSs > one, B symptoms, and platelet count < 150 × 109/L.
We found an OS rate of only 33% at 5 years, which confirms the dismal outcome of AITL, despite the fact that most patients were treated with anthracycline-containing combination chemotherapy. Some patients (17%) also received high-dose chemotherapy (HDT) followed by ASCT. Our results are in agreement with the 5-year OS rate of 33% recently reported by GELA, which treated 157 patients in different clinical trials with intensive chemotherapy regimens, including some patients who also received consolidation with HDT plus ASCT.20 The treatment regimens currently used in most patients with AITL do not result in long-lasting remissions. Thus, novel treatment approaches are warranted. Two recent studies regarding the role of HDT with ASCT are promising. In a study by Schetelig et al25 of 29 patients, 76% experienced an adequate response, and the OS rate at 5 years was 44%. In a more recent large study of 146 patients treated by the European Group for Blood and Marrow Transplantation, an OS of 59% at 4 years was reported, with significantly better survival in those patients who underwent ASCT during first complete remission.26 Additional studies to confirm the results of HDT with ASCT should be considered.27
In addition, new agents with activity against PTCL are currently undergoing further evaluation either alone or in combination. There are a number of histone deacetylase inhibitors with activity in T-cell lymphoma, including vorinostat, romidepsin, panbinostat, and belinostat. Vorinostat and romidepsin are US Food and Drug Administration (FDA) approved for cutaneous T-cell lymphoma, with response rates in the range of 25% to 30%.28,29 A phase II trial of romidepsin for relapsed PTCL demonstrated a 29% overall response rate, which led to FDA approval.30 Belinostat has also been studied in patients with PTCL, with an overall response rate of 32%, including two complete responses.31 Histone deacetylase inhibitors may have additive and/or synergistic activity with other agents, including topoisomerase inhibitors, bortezomib, and others.
Pralatrexate, a potent folate antagonist, is also currently FDA approved for relapsed PTCL. This agent is administered intravenously and also has an overall response rate of approximately 30%.32 An immunoconjugate, brentuximab vetodin (SGN-35), has demonstrated an 86% overall response rate and 53% complete response rate for patients with relapsed CD30-positive ALCL.33 Further evaluation of agents directed at the immunosuppressive microenvironment in AITL is also an area of active research. Cyclosporine has been used in a small number of patients with AITL, with a 60% response rate.34 This is now being tested in a larger clinical trial. Other immune modulation and antiangiogenic agents, including rituximab and lenalidomide, are also being explored as single or combination agents for AITL treatment.35,36 However, few patients with AITL have been enrolled onto any of these clinical trials, resulting in limited data.
In conclusion, given the rarity and poor outcome of AITL, more prospective clinical trials are required to understand the pathogenesis of this disease so that specific biologic targeted therapies can be developed. Our more specific prognostic model for patients with AITL may also improve our ability to identify patients for alternative therapies. Significant progress in the treatment of AITL can be expected in the future from the use of novel, sophisticated, and powerful technologies of genomics and proteomics.37–39
Acknowledgment
We thank Christian Gisselbrecht, MD, and Nicholas Mounier, MD, and GELA (Groupe d'Etude des Lymphomes de l'Adulte) for providing the information for the validation cohort of patients for the prognostic index evaluation.
Appendix
Table A1.
Participating Sites and Physicians During the Study
| Participants |
|---|
| British Columbia Cancer Agency, Vancouver, British Columbia, Canada |
| Kerry Savage, MD |
| Joseph Connors, MD |
| Randy Gascoyne, MD |
| Mukesh Chhanabhai, MD |
| National Cancer Institute, Bethesda, MD |
| Wyndham Wilson, MD |
| Elaine S. Jaffe, MD |
| University of Nebraska Medical Center, Omaha, NE |
| James O. Armitage, MD |
| Julie M. Vose, MD |
| Dennis D. Weisenburger, MD |
| James Anderson, PhD |
| Fred Ullrich, MS |
| Martin Bast, BS |
| Massachusetts General Hospital, Boston, MA |
| Ephraim Hochberg, MD |
| Nancy Harris, MD |
| Agota Smogorzewska, MD |
| Los Angeles County Hospital–University of Southern California, Los Angeles, CA |
| Alexandra Levine, MD |
| Bharat N. Nathwani, MD |
| Arizona Cancer Center, Tucson, AZ |
| Thomas Miller, MD |
| Lisa Rimsza, MD |
| University of Barcelona Hospital, Barcelona, Spain |
| Emili Montserrat, MD |
| Armando Lopez-Guillermo, MD |
| Elias Campo, MD |
| Spanish National Cancer Center, Madrid, Spain |
| Marta Cuadros, MD |
| Javier Alvarez Ferreira, MD |
| Beatriz Martinez Delgado, MD |
| Norwegian Radium Hospital, Oslo, Norway |
| Harold Holte, MD |
| Jan Delabie, MD |
| University of Würzburg Hospital, Würzburg, Germany |
| Thomas Rüdiger, MD |
| Institute of Pathology, University of Wurzburg, Wurzburg, Germany |
| Konrad Müller-Hermelink, MD |
| Peter Reimer, MD |
| Krankenhaus Munchen-Schwabing, Munchen, Germany |
| Patrick Adam, MD |
| Martin Wilhelm, MD |
| Norbert Schmitz, MD |
| Christoph Nerl, MD |
| Saint Bartholomew's Hospital, London, United Kingdom |
| Andrew Lister, MD |
| Andrew Norton, MD |
| St James Hospital, Leeds, United Kingdom |
| Kenneth A. MacLennan, MD |
| University of Bologna Hospital, Bologna, Italy |
| Pier Luigi Zinzani, MD |
| Stefano A. Pileri, MD |
| Intergruppo Italiano Linfomi, Bologna, Italy |
| Massimo Federico, MD |
| Monica Bellei, PhD |
| Stefano Luminari, MD |
| Centre Hospitalier Lyon-Sud, Lyon, France |
| Bertrand Coiffier, MD |
| Francoise Berger, MD |
| King Chulalongkorn Hospital, Bangkok, Thailand |
| Intragumtornchai Tanin, MD |
| Pongsak Wannakrairot, MD |
| Queen Mary Hospital, Hong Kong, China |
| Wing Y. Au, MD |
| Raymond Liang, MD |
| Florence Loong, MD |
| Singapore General Hospital, Singapore |
| Sandeep Rajan, MD |
| Ivy Sng, MD |
| National Cancer Center Hospital of Japan, Tokyo, Japan |
| Kensei Tobinai, MD |
| Yoshihiro Matsuno, MD |
| Aichi Cancer Center, Nagoya, Japan |
| Yasuo Morishima, MD |
| Shigeo Nakamura, MD |
| Masao Seto, MD, PhD |
| Okayama University Hospital, Okayama, Japan |
| Mitsune Tanimoto, MD |
| Tadashi Yoshino, MD |
| Fukuoka University Hospital, Fukuoka, Japan |
| Junji Suzumiya, MD |
| Koichi Ohshima, MD |
| Samsung Medical Center, Seoul, Korea |
| Won-Seog Kim, MD |
| Young-Hyeh Ko, MD |
Footnotes
Written on behalf of the International Peripheral T-Cell Lymphoma Project.
Supported by grants from the University of Nebraska Foundation, Omaha, NE; Fondazione Cassa di Risparmio di Modena, Modena, Italy; and Associazione “Angela Serra” per la Ricerca sul Cancro, Modena, Italy.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: James O. Armitage, Allos Therapeutics (C), Genentech (C), Roche (C), Seattle Genetics (C), ZIOPHARM Oncology (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Massimo Federico, Nancy L. Harris, Julie M. Vose
Administrative support: Julie M. Vose
Provision of study materials or patients: Massimo Federico, Thomas Rudiger, Bharat N. Nathwani, Stefano Luminari, Bertrand Coiffier, Nancy L. Harris, Elaine S. Jaffe, Stefano A. Pileri, Kerry J. Savage, Dennis D. Weisenburger, James O. Armitage, Nicholas Mounier, Julie M. Vose
Collection and assembly of data: Thomas Rudiger, Monica Bellei, Stefano Luminari, Elaine S. Jaffe, Julie M. Vose
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Frizzera G, Moran EM, Rappaport H. Angio-immunoblastic lymphadenopathy with dysproteinaemia. Lancet. 1974;1:1070–1073. doi: 10.1016/s0140-6736(74)90553-4. [DOI] [PubMed] [Google Scholar]
- 2.Nathwani BN, Rappaport H, Moran EM, et al. Malignant lymphoma arising in angioimmunoblastic lymphadenopathy. Cancer. 1978;41:578–606. doi: 10.1002/1097-0142(197802)41:2<578::aid-cncr2820410226>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Rüdiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): Results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- 4.Morton L, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attygalle AD, Diss TC, Munson P, et al. CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2004;28:54–61. doi: 10.1097/00000478-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): A new diagnostic marker providing evidence that AITL derives from follicular helper cells. Am J Surg Pathol. 2006;30:490–494. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Dorfman DM, Brown JA, Shahsafaei A, et al. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung CY, Ho FC, Srivastabe G, et al. Usefulness of follicular dendritic cell pattern in classification of peripheral T-cell lymphomas. Histopathlogy. 1993;23:433–437. doi: 10.1111/j.1365-2559.1993.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 9.Siegert W, Agthe A, Griesser H, et al. Treatment of angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma using prednisone with or without the COPBLAM/IMVP-16 regimen: A multicenter study Kiel Lymphoma Study Group. Ann Intern Med. 1992;117:364–370. doi: 10.7326/0003-4819-117-5-364. [DOI] [PubMed] [Google Scholar]
- 10.Siegert W, Nerl C, Meuthen I, et al. Recombinant human interferon-alpha in the treatment of angioimmunoblastic lymphadenopathy: Results in 12 patients. Leukemia. 1991;5:892–895. [PubMed] [Google Scholar]
- 11.Advani R, Warnke R, Sikic BI, et al. Treatment of angioimmunoblastic T-cell lymphoma with cyclosporine. Ann Oncol. 1997;8:601–603. doi: 10.1023/a:1008275208592. [DOI] [PubMed] [Google Scholar]
- 12.Pautier P, Devidas A, Delmer A, et al. Angioimmunoblastic-like T-cell non Hodgkin's lymphoma: Outcome after chemotherapy in 33 patients and review of the literature. Leuk Lymphoma. 1999;32:545–552. doi: 10.3109/10428199909058412. [DOI] [PubMed] [Google Scholar]
- 13.Siegert W, Nerl C, Agthe A, et al. Angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma: Prognostic impact of clinical observations and laboratory findings at presentation—The Kiel Lymphoma Study Group. Ann Oncol. 1995;6:659–664. doi: 10.1093/oxfordjournals.annonc.a059281. [DOI] [PubMed] [Google Scholar]
- 14.Vose JM, Armitage JO, Weisenburger DD. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;25:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 18.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 19.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): A new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 20.Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood. 2008;111:4463–4470. doi: 10.1182/blood-2007-08-105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pangalis GA, Moran EM, Nathwani BN, et al. Angioimmunoblastic lymphadenopathy: Long-term follow-up study. Cancer. 1983;52:318–321. doi: 10.1002/1097-0142(19830715)52:2<318::aid-cncr2820520221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Aozasa K, Ohsawa M, Fujita MQ, et al. Angioimmunoblastic lymphadenopathy: Review of 44 patients with emphasis on prognostic behavior. Cancer. 1989;63:1625–1629. doi: 10.1002/1097-0142(19890415)63:8<1625::aid-cncr2820630832>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Ch'ang HJ, Su IJ, Chen CL, et al. Angioimmunoblastic lymphadenopathy with dysproteinemia: Lack of a prognostic value of clear cell morphology. Oncology. 1997;54:193–198. doi: 10.1159/000227687. [DOI] [PubMed] [Google Scholar]
- 24.Archimbaud E, Coiffier B, Bryon PA, et al. Prognostic factors in angioimmunoblastic lymphadenopathy. Cancer. 1987;59:208–212. doi: 10.1002/1097-0142(19870115)59:2<208::aid-cncr2820590205>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Schetelig J, Fetscher S, Reichle A, et al. Long-term disease-free survival in patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. Haematologica. 2003;88:1272–1278. [PubMed] [Google Scholar]
- 26.Kyriakou C, Canals C, Goldstone A, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: Complete remission at transplantation is the major determinant of outcome—Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:218–224. doi: 10.1200/JCO.2008.12.6219. [DOI] [PubMed] [Google Scholar]
- 27.Weidmann E, Gramatzki M, Wilhelm M, et al. Diagnosis and actual therapy strategies in peripheral T-cell lymphomas: Summary of an international meeting. Ann Oncol. 2004;15:369–374. doi: 10.1093/annonc/mdh085. [DOI] [PubMed] [Google Scholar]
- 28.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb muticenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 29.Bates S, Piekarz R, Wright J, et al. Final clinical results of a phase 2 NCI multicenter study of romidepsin in recurrent cutaneous T-cell lymphoma (molecular analyses included) Blood. 2008;112 abst 1568. [Google Scholar]
- 30.Coiffier B, Pro B, Prince M, et al. Final results from a pivotal, multicenter, international, open-label, phase 2 study of Romidepsin in progressive or relapsed peripheral T-cell lymphoma (PTCL) following prior systemic therapy. Blood. 2010;116 abstr 114. [Google Scholar]
- 31.Pohlman B, Advani R, Duvic M, et al. Final results of a phase II trial of belinostat (PXD101) in patients with recurrent or refractory peripheral or cutaneous T-cell lymphoma. Blood. 2009;114 doi: 10.1111/bjh.13222. abstr 920. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor OA, Horwitz S, Hamlin P, et al. Phase I/II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol. 2009;27:4357–4364. doi: 10.1200/JCO.2008.20.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shustov AR, Advani R, Brice P, et al. Complete remissions with Brentuximab Vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2010;116 abstr 961. [Google Scholar]
- 34.Advani R, Horwitz S, Zelenetz A, et al. Angioimmunoblastic T cell lymphoma: Treatment experience with cyclosporine. Leuk Lymphoma. 2007;48:521–525. doi: 10.1080/10428190601137658. [DOI] [PubMed] [Google Scholar]
- 35.Joly B, Frenkel V, Gaulard P, et al. Rituximab in combination with CHOP regimen in angioimmunoblastic T-cell lymphoma (AITL): Preliminary results in 9 patients treated in a single institution. Blood. 2005;106 abstr 2686. [Google Scholar]
- 36.Dueck GS, Chua N, Prasad A, et al. Activity of lenalidamide in a phase II trial for T-cell lymphoma: Report on the first 24 cases. J Clin Oncol. 2009;27(suppl):439s. abstr 8524. [Google Scholar]
- 37.Alizadeh AA, Advani RH. Evaluation and management of angioimmunoblastic T-cell lymphoma: A review of current approaches and future strategies. Clin Adv Hematol Oncol. 2008;6:899–909. [PubMed] [Google Scholar]
- 38.Dunleavy K, Wilson W, Jaffe ES. Angioimmunoblastic T-cell lymphoma: Pathobiological insights and clinical implications. Curr Opin Hematol. 2007;14:348–353. doi: 10.1097/MOH.0b013e328186ffbf. [DOI] [PubMed] [Google Scholar]
- 39.Iqbal J, Weisenburger DD, Greiner TC, et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication n angioimmunoblastic T-cell lymphoma. Blood. 2010;115:1026–1036. doi: 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]