Abstract
Purpose
Little is known about change in quality of life (QOL) among long-term cancer survivors. We examined change over time in QOL among long-term survivors of non-Hodgkin lymphoma and identified demographic, clinical, and psychosocial risk factors for poor outcomes.
Methods
Surveys were mailed to 682 lymphoma survivors who participated in a study 5 years earlier, when on average they were 10.4 years postdiagnosis. Standardized measures of QOL, perceptions of the impact of cancer, symptoms, medical history, and demographic variables were reported at both time points and examined using linear regression modeling to identify predictors of QOL over time.
Results
A total of 566 individuals participated (83% response rate) who were a mean of 15.3 years postdiagnosis; 52% were women, and 87% were white. One third of participants (32%) reported persistently high or improved QOL, yet a notable proportion (42%) reported persistently low or worsening QOL since the earlier survey. Participants who received only biologic systemic therapy reported improvement in physical health despite the passage of time. Older age, more comorbidity, and more or increasing negative and decreasing positive perceptions of cancer's impact were independent predictors of poor QOL. Lymphoma symptom burden, less social support, and having received a transplantation were related to negative perceptions of cancer's impact.
Conclusion
Moderate to severe symptom burden, limited social support, or having received a transplantation should alert the clinician to potential need for supportive services. Perceptions of cancer's impact are associated with QOL cross-sectionally and longitudinally; modifying these perceptions may thus provide a strategy for improving QOL.
INTRODUCTION
With an estimated 70,130 new patients in 2012, non-Hodgkin lymphoma (NHL) is one of the most common types of cancer.1 Advances in NHL treatment have resulted in a two-fold improvement in the 5-year survival rate, from 31% among whites in 1960 to 1963 to 67% for all races in 1999 to 2006. There are approximately 502,940 individuals in the United States living with a history of NHL.2
Given its high incidence and survival rates, NHL can be viewed as a prevalent chronic illness characterized by alternating symptom-free and symptom exacerbation phases that may require treatment. To the clinician, NHL represents a broad distribution of illness trajectories encompassing everything from the slow but persistent patterns of indolent lymphomas to the fast-growing aggressive lymphomas.3 To the patient, NHL is typically experienced as a chronic disease that powerfully affects life.4–6
Throughout the illness, attending to survivorship concerns is an important part of cancer care. Ideally, good survivorship care optimizes the patient's quality of life (QOL) within the context of his or her disease.7 To improve QOL, health services for NHL must target disease- and treatment-associated sequelae that impact patients throughout survivorship, potentially diminishing their QOL; an understanding of these sequelae is needed to guide health services planning.8 However, NHL survivors are an understudied group. Little is known about the longitudinal effects of recurrence and/or systemic treatment on QOL-related outcomes such as health and functioning.
To begin to address this gap, we surveyed a large NHL cohort in 2005 and 2010, focusing on symptoms of post-traumatic stress disorder (PTSD),9 QOL,10 and perceptions of the cancer experience as assessed by the Impact of Cancer (IOC) scale.11,12 The initial cohort was comprised of NHL survivors diagnosed at least 2 years before the 2005 survey, among whom 39% reported clinically significant PTSD symptoms and mental health status scores generally below age-stratified population norms. Longitudinal analyses of PTSD symptoms between 2005 and 2010 demonstrated that symptoms persisted or worsened for 37% of the sample.13 This finding prompted interest in describing change in QOL over time for NHL survivors.
The current article presents the longitudinal change in QOL-related outcomes among the respondents to the second survey in 2010, who were all at least 7 years postdiagnosis. A principal interest was to describe change in QOL experienced by these patients and identify individuals at risk for worsening QOL, with the goal of providing practical information to guide the development of survivorship care interventions. We focused on physical and mental health status as key QOL-related outcomes and hypothesized that these would remain poor and/or worsen for a subset of the NHL survivors who had select demographic (eg, less education) or clinical (eg, more comorbidity) characteristics or less social support, as informed by prior research.12 After determining that negative patient experience predicted QOL, we expanded our analyses to identify the risk factors for poor IOC scores. In addition, we included an examination of systemic treatment status to better inform clinical care.
METHODS
Study Design and Participants
This follow-up survey built on a prior study that examined QOL-related outcomes among NHL survivors identified from two academic medical center cancer registries (Duke University and University of North Carolina). In the initial cross-sectional study conducted in 2005, 886 participants completed a mail-in self-report survey that assessed PTSD symptoms, physical and mental health status, positive and negative IOC, lymphoma symptoms, types of treatment, disease status, and other outcomes. Details about the methods were previously published.9
The consent form from the original study included a statement of willingness to be recontacted within the next 5 years, which could be endorsed by the study participant. Considering that eligible patients (in 2005) had to have been diagnosed with NHL at least 2 years previously and be at least 18 years old, at recontact in 2010, they were at least 7 years postdiagnosis and ≥ 23 years old. Institutional review boards at Duke University and the University of North Carolina approved the study. Detailed follow-up survey procedures were previously published.13
Instruments and Measures
To assess QOL, the Medical Outcomes Study Short Form-36 (SF-36; version 2.0) was used; this general health measure contains 36 items that are grouped into eight subscales and two summary scores, the Physical Component Score (PCS; Physical Functioning, Role-Physical, Bodily Pain, and General Health) and Mental Component Score (MCS; Vitality, Social Functioning, Role-Emotional, and Mental Health). The QualityMetric Health Outcomes scoring software (QualityMetric, Lincoln, RI) was used, where 50 (standard deviation [SD], 10) represents the average (normed) score for each subscale and summary scale.14
Psychosocial status was assessed with two measures. The 20-item Medical Outcomes Study Social Support survey has a standardized score ranging from 20 to 100; higher scores represent better social support.15 The 37-item IOC (version 2) enables assessment of the patient's perceptions of positive life changes resulting from and negative impacts attributed to the cancer experience.11 The IOC contains four subscales quantifying positive perceptions (Altruism/Empathy, Health Awareness, Meaning of Cancer, and Positive Self-Evaluation) and four subscales quantifying negative perceptions (Appearance Concerns, Body Change Concerns, Life Interferences, and Worry); the mean of the subscales yield a Positive Impact Summary score and Negative Impact Summary score, respectively (range, 1 to 5 on each). Higher scores on the Positive Impact Summary indicate greater positive perceptions of the cancer experience; higher scores on the Negative Impact Summary indicate greater negative perceptions.
Demographics and clinical characteristics (eg, income, lymphoma recurrence, disease and treatment status) were collected via self-report. To assess the presence/extent of nonlymphoma clinical conditions, the 15-item Self-Administered Comorbidity Questionnaire, a self-report version of the Charlson comorbidity index, was used.16 Up to three points can be scored for each medical condition (1 point each for the problem, treatment, and functional limitation). Lymphoma symptoms such as fevers and night sweats were captured using the 15-item Functional Assessment of Cancer Therapy– Lymphoma module.17
Statistical Analysis
To compare follow-up study participants and nonparticipants with respect to initial demographic, clinical, and psychosocial characteristics and QOL, we tested for differences between responders and both nonresponders and decedents using t tests for continuous measures and χ2 tests for categorical measures. Computation of PCS and MCS scores consists of multiplying each SF-36 z score by its respective physical and mental factor score coefficient and summing the eight subscales, respectively, and then transforming each component score to the norm-based scoring based on the 1998 general US population mean. To permit comparison to age-related norms, the expected change in PCS score for each study participant was calculated and then summed and averaged to generate the expected PCS score for each treatment group.14 To depict change over time in QOL scores, a participant was assigned to an initial and follow-up category based on distance from the national age-based PCS and MCS norms. PCS and MCS scores less than, within, and greater than 0.5 SD of the norm indicated low, medium, and high categories, respectively.18
To assess the association between the demographic, clinical, and psychosocial characteristics and the follow-up SF-36 or IOC, we used a series of linear regression models, controlling for initial SF-36 or IOC. We first tested each characteristic separately (ie, only the candidate measure and initial SF-36 or IOC score in the model). Then, characteristics that were at least marginally significantly associated with follow-up SF-36 or IOC in these models (P < .10) were included in a multiple linear regression to estimate the independent associations of the initial survey predictors and follow-up survey correlates with follow-up SF-36 and IOC. For the psychosocial measures, change scores (follow-up score minus initial score) were included in this model rather than follow-up scores, to enable evaluation of the effect of changes in these measures independent of initial status. t tests were used to assess for differences between participants who reported no recurrence of disease and participants who reported a recurrence or were never in remission. The least squares means were obtained from analysis of variance models containing study age and remission status (in addition to treatment status). Data management and statistical analyses were conducted with SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
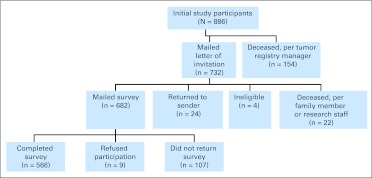
A total of 566 patients (83% response rate) participated in this follow-up survey (Fig 1). Among individuals who participated in the 2005 survey and who were assumed not to be dead in 2010, participants in the 2010 follow-up survey were compared with nonparticipants; follow-up survey participants were more likely to be white, have an income of more than $30,000, be married, be older, have received both chemotherapy and biologic therapy, have received systemic treatment, not have active disease, and have fewer lymphoma-related symptoms. Respondents also reported better QOL at the initial survey (Table 1). In 2005, the mean time since diagnosis for all follow-up survey participants was 10.4 years (SD, 7.1 years), and the most common lymphoma-related symptoms reported were tiring easily, trouble sleeping, worrying about new symptoms, and experiencing significant pain. The average interval between the initial and follow-up surveys was 4.8 years (SD, 0.17 years; range, 4.3 to 5.4 years). The mean age and time since diagnosis in 2010 were 67.4 years (SD, 12.4 years) and 15.2 years (SD, 7.2 years), respectively. The majority of participants (n = 329; 58.1%) were currently receiving care from an oncology or survivorship clinic at follow-up. Comparison of decedents to the 2005 sample demonstrates they were older, less educated, and had worse disease at baseline.
Fig 1.
CONSORT diagram.
Table 1.
Demographics and Clinical Characteristics, Psychosocial Status, and Quality of Life at the Time of the Initial Survey (2005): Comparison of Patients Who Did and Did Not Participate in the Follow-Up Survey (2010)
| Baseline Demographic or Clinical Characteristic | Participants(n = 566) |
Nonparticipants, Assumed Living (n = 144)a |
Pb | Deceased at Follow-Up(n = 176) |
Pc | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Demographics | ||||||||
| Female sex | 294 | 51.9 | 78 | 54.2 | .633 | 79 | 44.9 | .102 |
| White race | 494 | 87.3 | 108 | 75.0 | < .001 | 155 | 88.1 | .783 |
| Income < $30,000 | 113 | 22.2 | 53 | 41.4 | < .001 | 59 | 37.8 | < .001 |
| College or postgraduate degree | 242 | 43.6 | 50 | 35.7 | .091 | 45 | 26.6 | < .001 |
| Married or living with a partner | 452 | 80.4 | 96 | 68.1 | .001 | 124 | 71.3 | .011 |
| Age, years | < .001 | < .001 | ||||||
| Mean | 62.4 | 56.7 | 69.5 | |||||
| SD | 12.4 | 15.6 | 11.7 | |||||
| Clinical characteristicsd | ||||||||
| Had an indolent type of lymphoma | 270 | 50.3 | 76 | 55.9 | .243 | 99 | 60.7 | .019 |
| Was diagnosed at stage > I | 339 | 68.1 | 76 | 62.3 | .224 | 104 | 71.2 | .467 |
| Systemic treatment statuse | ||||||||
| Received chemotherapy only | 257 | 45.4 | 72 | 50.0 | .324 | 74 | 42.1 | .433 |
| Received biologic therapy only | 29 | 5.1 | 7 | 4.9 | .898 | 5 | 2.8 | .206 |
| Received chemotherapy and biologic therapy | 108 | 19.1 | 16 | 11.1 | .025 | 39 | 22.2 | .371 |
| Received a transplantation | 90 | 15.9 | 15 | 10.4 | .098 | 30 | 17.1 | .719 |
| Did not receive systemic treatment | 82 | 14.5 | 34 | 23.6 | .008 | 28 | 15.9 | .643 |
| Was currently receiving treatment | 58 | 10.4 | 22 | 15.6 | .083 | 37 | 21.8 | < .001 |
| Had active diseasef | 47 | 9.1 | 20 | 15.9 | .027 | 42 | 28.2 | < .001 |
| Had a recurrence of disease | 184 | 33.2 | 39 | 28.7 | .317 | 70 | 42.7 | .001 |
| Time since diagnosis, years | .156 | .747 | ||||||
| Mean | 10.4 | 9.5 | 10.2 | |||||
| SD | 7.1 | 6.5 | 7.3 | |||||
| Comorbidity scoreg | .166 | < .001 | ||||||
| Mean | 5.2 | 5.8 | 6.7 | |||||
| SD | 4.5 | 5.5 | 5.2 | |||||
| Had a second primary cancer | 71 | 12.7 | 16 | 11.4 | .673 | 33 | 19.4 | .027 |
| Lymphoma symptom scoreh | .001 | < .001 | ||||||
| Mean | 49.3 | 46.3 | 44.4 | |||||
| SD | 8.8 | 11.5 | 11.0 | |||||
| Psychosocial status scores | ||||||||
| Social Supporti | .079 | .514 | ||||||
| Mean | 83.4 | 80.7 | 84.3 | |||||
| SD | 16.0 | 17.1 | 17.3 | |||||
| IOC Negative Impactj | .080 | .003 | ||||||
| Mean | 2.2 | 2.3 | 2.4 | |||||
| SD | 0.7 | 0.9 | 0.8 | |||||
| IOC Positive Impactk | .978 | .181 | ||||||
| Mean | 3.5 | 3.5 | 3.4 | |||||
| SD | 0.8 | 0.8 | 0.8 | |||||
| Quality-of-life scores | ||||||||
| SF-36 PCSl | .033 | < .001 | ||||||
| Mean | 47.2 | 45.0 | 39.1 | |||||
| SD | 10.2 | 11.3 | 11.5 | |||||
| SF-36 MCSm | < .001 | < .001 | ||||||
| Mean | 50.4 | 45.7 | 45.7 | |||||
| SD | 10.7 | 11.3 | 11.7 | |||||
Abbreviations: IOC, Impact of Cancer; MCS, Mental Component Score; PCS, Physical Component Score; SD, standard deviation; SF-36, Medical Outcomes Study Short Form-36.
Assumed living; calculated as total initial sample (N = 866) minus participants (n = 566) minus deceased (n = 176).
P value for comparison of participants and nonparticipants, based on χ2 for percentages and t test for means.
P value for comparison of participants and decedents, based on χ2 for percentages and t test for means.
Does not include changes between baseline and follow-up surveys.
Systemic treatment at initial survey includes chemotherapy, biologic therapy, and bone marrow or stem-cell transplantation.
Was not in remission or cured of non-Hodgkin lymphoma.
Self-Administered Comorbidity Questionnaire; possible score range, 0 to 42.
Functional Assessment of Cancer Therapy–Lymphoma module; 15 items, possible score range, 0 to 60. Lower scores indicate greater symptoms.
Medical Outcomes Study Social Support total score; possible score range, 0 to 100; higher scores indicate higher support.
IOC Negative Summary score; possible score range, 1 to 5; higher scores indicate greater negative impacts.
IOC Positive Summary score; possible score range, 1 to 5; higher scores indicate greater positive impacts.
The median population score is 50; a higher score indicates better functioning.
The median population score is 50; a higher score indicates better functioning.
Change in QOL-Related Outcomes
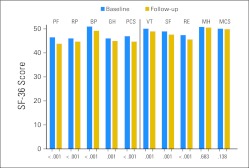
Figure 2 shows SF-36 scores at initial and follow-up surveys. All scores declined significantly (all P < .01), except for the MCS and Mental Health subscale. PCS and MCS mean scores were 45.0 (SD, 11.0) and 50.0 (SD, 10.9) at follow-up, respectively.
Fig 2.
Initial and follow-up norm-based survey scores on the Medical Outcomes Study Short Form-36 (SF-36) scales (n = 534). BP, Bodily Pain; GH, General Health; MCS, Mental Component Score; MH, Mental Health; PCS, Physical Component Score; PF, Physical Functioning; RE, Role-Emotional; RP, Role-Physical; SF, Social Functioning; VT, Vitality.
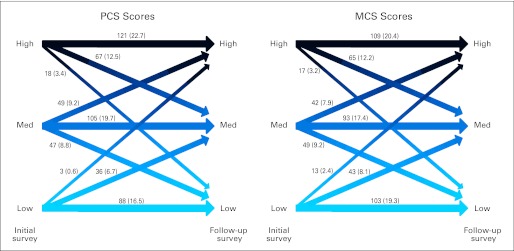
Figure 3 illustrates the change in QOL scores from the initial to follow-up surveys. Among the 534 participants who completed the PCS and MCS at both times, 88 (16.5%) and 98 (18.3%) reported improvement; 314 (58.8%) and 305 (57.1%) reported stability; and 132 (24.7%) and 131 (24.5%) reported worsening in scores, respectively. In total, 42% of patients reported either low physical or mental QOL, and 32% reported either high physical or mental QOL at a median of 12.9 years after their diagnosis.
Fig 3.
Change in quality of life: depiction of Medical Outcomes Study Short Form-36 scores [No. (%)] over 5 years (n = 534). High represents at least 0.5 standard deviation (SD) greater than the age-based norm; medium (Med) represents within 0.5 SD of the age-based norm; and low represents at least 0.5 SD less than the age-based norm. MCS, Mental Component Score; PCS, Physical Component Score.
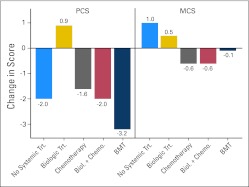
Figure 4 depicts the change in mean QOL scores among several systemic treatment regimens and compares each systemic treatment ever received to no systemic treatment. Only the biologic group reported improvement in mean PCS scores over time. The transplantation group reported a decline in PCS scores that exceeded the change for other groups. Smaller differences in the change in mean MCS scores were found between the no systemic treatment and systemic treatment groups.
Fig 4.
Change in Medical Outcomes Study Short Form-36 scores over 5 years by type of systemic treatment ever received (n = 534). Biol., biologic; BMT, bone marrow transplantation; Chemo., chemotherapy; MCS, Mental Component Score; PCS, Physical Component Score; Trt., treatment.
Predictors of QOL and IOC
Table 2 lists the results of linear regression models that controlled for initial QOL and initial QOL plus other demographic, clinical, and psychosocial variables. Significant predictors of lower PCS at follow-up in the final model were older age (P < .001), greater comorbidity (eg, back pain, high blood pressure, heart disease; P = .006), a more negative perception of the cancer experience as measured by IOC at initial survey (P < .001), and increases in negative (P < .001) and decreases in positive (P = .012) IOC scores. Both negative IOC at initial survey and increase in negative IOC scores over time were predictive of lower MCS (both P < .001) in the final model.
Table 2.
Multiple Linear Regression to Identify Predictors of Quality of Life at Follow-Up
| Variable | PCS |
MCS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for Initial PCS |
Adjusted for Initial PCS and Other Predictors (n = 519) |
Adjusted for Initial MCS |
Adjusted for Initial MCS and Other Predictors (n = 519) |
|||||||||
| Coefficient, β | SE | P | Coefficient, β | SE | P | Coefficient, β | SE | P | Coefficient, β | SE | P | |
| Initial PCS or MCS | 0.74 | 0.03 | < .001 | 0.52 | 0.04 | < .001 | 0.65 | 0.03 | < .001 | 0.47 | 0.04 | < .001 |
| Demographicsa | ||||||||||||
| Female sex | 0.17 | 0.69 | .807 | −1.39 | 0.73 | .055 | −1.03 | 0.69 | .134 | |||
| Nonwhite race | −0.63 | 1.04 | .544 | −1.42 | 1.10 | .201 | ||||||
| Income < $30,000 | −1.93 | 0.90 | .032 | −1.21 | 0.84 | .150 | −1.31 | 0.96 | .173 | |||
| Less than college degree | −0.36 | 0.72 | .616 | −1.00 | 0.74 | .177 | ||||||
| Not married | −0.25 | 0.89 | .782 | −0.09 | 0.94 | .925 | ||||||
| Age at study enrollment | −0.16 | 0.03 | < .001 | −0.21 | 0.03 | < .001 | −0.01 | 0.03 | .631 | |||
| Clinical characteristicsb | ||||||||||||
| Years since diagnosis | −0.06 | 0.05 | .237 | 0.004 | 0.05 | .943 | ||||||
| Recurrence in last 5 yearsc | 0.51 | 0.73 | .485 | 0.84 | 0.79 | .288 | ||||||
| Systemic treatment statusd | ||||||||||||
| Received chemotherapy | −0.47 | 1.03 | .651 | −2.16 | 1.09 | .049 | −1.36 | 1.05 | .195 | |||
| Received biologic treatment | 1.47 | 1.76 | .404 | 0.07 | 1.87 | .972 | 1.65 | 1.77 | .353 | |||
| Chemotherapy-biologic therapy interaction | 0.14 | 1.18 | .903 | −2.21 | 1.25 | .078 | −1.03 | 1.20 | .394 | |||
| Ever had transplantation | −0.34 | 1.24 | .783 | −1.26 | 1.32 | .339 | 0.49 | 1.30 | .703 | |||
| No systemic treatment ever | ||||||||||||
| Comorbidity scoree | −0.30 | 0.10 | .002 | −0.25 | 0.09 | .006 | −0.23 | 0.09 | .009 | −0.11 | 0.09 | .220 |
| Lymphoma symptom scoref | 0.68 | 0.47 | .143 | 2.13 | 0.51 | < .001 | 0.58 | 0.59 | .324 | |||
| Psychosocial statusg | ||||||||||||
| Social Supporth | 0.02 | 0.02 | .356 | 0.06 | 0.02 | .008 | 0.04 | 0.02 | .096 | |||
| IOC Negative Impacti | −0.80 | 0.54 | .136 | −3.05 | 0.53 | < .001 | −2.66 | 0.65 | < .001 | −3.70 | 0.78 | < .001 |
| IOC Positive Impactj | 0.003 | 0.45 | .994 | 0.0001 | 0.44 | .999 | −0.65 | 0.48 | .175 | |||
| Δ Social support | 0.02 | 0.02 | .340 | 0.03 | 0.02 | .165 | ||||||
| Δ IOC Negative Impact | −3.71 | 0.61 | < .001 | −5.21 | 0.60 | < .001 | −3.91 | 0.65 | < .001 | −4.88 | 0.67 | < .001 |
| Δ IOC Positive Impact | 1.18 | 0.61 | .052 | 1.49 | 0.59 | .012 | 1.05 | 0.65 | .104 | |||
| Model-adjusted R2 P | .48 | .59 | .41 | .50 | ||||||||
Abbreviations: IOC, Impact of Cancer; MCS, Mental Component Score; PCS, Physical Component Score.
Demographic variables are from the 2005 survey.
Clinical variables are from the 2005 survey (years since diagnosis, comorbidity, lymphoma symptom score, and systemic treatment status) and the follow-up 2010 survey (systemic treatment status and recurrence in last 5 years).
Participants who reported having a recurrence of their lymphoma within the last 5 years or who have never been in remission.
Mutually exclusive categories; participants reported receiving systemic treatment (ie, chemotherapy, biologic therapy such as rituximab, transplantation) at initial and/or follow-up survey.
Self-Administered Comorbidity Questionnaire; possible score range, 0 to 42.
Functional Assessment of Cancer Therapy–Lymphoma module; 15 items, possible score range, 0 to 60. Lower scores indicate greater symptoms.
Psychosocial variables are from the 2005 survey except for change variables.
Medical Outcomes Study Social Support total score; possible score range, 0 to 100; higher scores indicate higher support.
IOC Negative Summary score; possible score range, 1 to 5; higher scores indicate greater negative impacts.
IOC Positive Summary score; possible score range, 1 to 5; higher scores indicate greater positive impacts.
Table 3 lists the results of two linear regression models for the IOC Negative and Positive Impact Summaries, which adjusted for initial IOC and initial IOC plus other predictors. Having ever received a transplantation, more NHL-related symptoms, and less social support were predictive of greater perceptions of cancer having negatively impacted one's life at follow-up. Female sex, younger age, and increases in social support were predictive of greater positive perceptions.
Table 3.
Multiple Linear Regression to Identify Predictors of IOC at Follow-Up
| Variable | Negative Impact Summary |
Positive Impact Summary |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for Initial IOC Negative Impact |
Adjusted for Initial IOC Negative Impact and Other Predictors (n = 549) |
Adjusted for Initial IOC Positive Impact |
Adjusted for Initial IOC Positive Impact and Other Predictors (n = 546) |
|||||||||
| Coefficient, β | SE | P | Coefficient, β | SE | P | Coefficient, β | SE | P | Coefficient, β | SE | P | |
| Initial IOC Negative or Positive Impact | 0.78 | 0.03 | < .001 | 0.62 | 0.04 | < .001 | 0.73 | 0.03 | < .001 | 0.72 | 0.03 | < .001 |
| Demographicsa | ||||||||||||
| Female sex | −0.02 | 0.04 | .729 | 0.10 | 0.05 | .037 | 0.12 | 0.05 | .010 | |||
| Nonwhite race | −0.06 | 0.07 | .344 | 0.006 | 0.07 | .929 | ||||||
| Income < $30,000 | 0.10 | 0.06 | .072 | 0.07 | 0.06 | .197 | −0.01 | 0.06 | .850 | |||
| Less than college degree | −0.02 | 0.05 | .726 | −0.01 | 0.05 | .762 | ||||||
| Not married | −0.02 | 0.06 | .755 | 0.09 | 0.06 | .118 | ||||||
| Age at study enrollment | −0.003 | 0.002 | .056 | −0.003 | 0.002 | .074 | −0.004 | 0.002 | .041 | −0.004 | 0.002 | .036 |
| Clinical characteristicsb | ||||||||||||
| Years since diagnosis | 0.001 | 0.003 | .792 | −0.002 | 0.003 | .595 | ||||||
| Recurrence in last 5 yearsc | 0.04 | 0.05 | .423 | 0.05 | 0.05 | .340 | ||||||
| Systemic treatment statusd | ||||||||||||
| Received chemotherapy | 0.11 | 0.07 | .094 | 0.13 | 0.07 | .056 | 0.06 | 0.07 | .412 | 0.05 | 0.07 | .499 |
| Received biologic treatment | 0.12 | 0.12 | .310 | 0.18 | 0.12 | .126 | 0.11 | 0.12 | .361 | 0.12 | 0.12 | .304 |
| Chemotherapy-biologic therapy interaction | 0.14 | 0.08 | .073 | 0.15 | 0.08 | .058 | 0.14 | 0.08 | .083 | 0.13 | 0.08 | .109 |
| Ever had transplantation | 0.26 | 0.08 | .002 | 0.26 | 0.08 | .001 | 0.16 | 0.09 | .061 | 0.12 | 0.09 | .180 |
| No systemic treatment ever | − | |||||||||||
| Comorbidity scoree | 0.003 | 0.005 | .511 | 0.003 | 0.005 | .606 | ||||||
| Lymphoma symptom scoref | −0.15 | 0.04 | < .001 | −0.14 | 0.04 | < .001 | −0.03 | 0.03 | .341 | |||
| Psychosocial statusg | ||||||||||||
| Social Supporth | −0.003 | 0.001 | .020 | −0.003 | 0.001 | .024 | −0.0005 | 0.001 | .734 | 0.001 | 0.002 | .451 |
| Δ Social Support | −0.001 | 0.002 | .652 | 0.004 | 0.002 | .008 | 0.004 | 0.002 | .008 | |||
| Model-adjusted R2 P | .50 | .54 | .52 | .55 | ||||||||
Abbreviation: IOC, Impact of Cancer.
Demographic variables are from the 2005 survey.
Clinical variables are from the 2005 survey (years since diagnosis, comorbidity, lymphoma symptom score, and systemic treatment status) and the follow-up 2010 survey (systemic treatment status and recurrence in last 5 years).
Participants who reported having a recurrence of their lymphoma within the last 5 years or who have never been in remission.
Mutually exclusive categories; participants reported receiving systemic treatment (ie, chemotherapy, biologic therapy such as rituximab, transplantation) at initial and/or follow-up survey.
Self-Administered Comorbidity Questionnaire; possible score range, 0 to 42.
Functional Assessment of Cancer Therapy–Lymphoma module; 15 items, possible score range, 0 to 60. Lower scores indicate greater symptoms.
Psychosocial variables are from the 2005 survey except for change variables.
Medical Outcomes Study Social Support total score; possible score range, 0 to 100; higher scores indicate higher support.
DISCUSSION
This article describes change in QOL over 5 years among survivors of NHL and the relationship between QOL and many demographic, clinical, and psychosocial variables. These results have important clinical implications. First, regarding physical and mental health status, notable proportions of the sample reported persistently low status (16.5% and 19.3%, respectively) or worsening status (24.7% and 24.5%, respectively) over the 5-year study period. These trends are especially concerning when considering patterns of typical follow-up care. Although patients with persistently low QOL may continue being seen by a medical provider across the survivorship trajectory, patients whose QOL is not recognized as problematic early in survivorship but whose QOL steadily deteriorates over time may have graduated from acute care before their QOL becomes problematic. This point is supported by the finding that 38% of our follow-up sample who reported worsening SF-36 scores reported that they no longer received health care from an oncology or survivorship clinic. Consequently, they may lack much-needed support at later stages of survivorship when they have become invisible to the system of care.8,19
Second, because comorbidity and lymphoma symptom scores were predictive of QOL and negative IOC scores, respectively, intensive symptom management may help improve outcomes in NHL survivors. Our results suggest that the presence of moderate or severe symptom burden should alert the clinician to a potential need for psychosocial support. Additionally, symptom control may have a beneficial effect on QOL-related outcomes. Implementation of systems to make symptom screening and systematic documentation at point of care routine can likely support better symptom control.20–23
Third, PCS scores deteriorated to a lesser extent over the 5-year period than age-related norms might suggest (Fig 4). However, this may be partly attributed to selection bias, because the more affluent and able continued to participate (Table 1). Improved PCS scores reported by patients who received only biologic systemic therapy are encouraging. Consequently, the treatment (and presumably experiences during these treatment periods) may have implications for intervention. Clinicians might consider proactive comprehensive survivorship assessment in transplantation populations for appropriate services (eg, physical therapy, pain management, social work).
Fourth, although the mean decline in MCS was nonsignificant, the directional change was of improvement in emotional well-being among similar age-related norms over time. For example, the MCS general population norm for the 65 to 74 year age group is 1.6 points higher than that of the younger 55 to 64 year age group.14 In addition, studies conducted with breast cancer survivors24,25 and community-dwelling adults26 reported small improvements in MCS over 5 years. Because our sample included individuals who reported a recurrence or active disease, a future study might explore the role of expectancy or worry in mediating the decrease in emotional health among those with unremitting disease; if either plays a mediating role, then cognitive-behavioral approaches such as the Managing Uncertainty Day-to-Day intervention27 might ameliorate their decline. Although these indolent lymphomas may be viewed as simple cancers given that treatment is unneeded in some patients, for the patient, they represent a real cancer and, based on our findings, may justify early/continuous psychosocial intervention(s).
Arguably the most important and clinically meaningful finding of this study is the identified relationship between the impact of cancer and QOL. Negative perceptions of the impact of cancer at initial survey and worsening of negative perceptions from initial survey to follow-up predicted lower PCS and MCS scores. In separate analyses (not shown), our data suggest that negative IOC scores mediate the effects of two clinical measures (chemotherapy and lymphoma symptoms) and social support on MCS. The potential implications of these findings are profound: If we can determine which interventions alter perceptions of cancer's impact, then we may have a powerful approach to sustaining and enhancing QOL among NHL survivors, a population among which 25% are at risk for longitudinal deterioration of QOL.
First steps toward modifying the impact of cancer to improve QOL are to better understand impact (IOC) as a construct and its relationship to other potentially modifiable factors. Although positive and negative IOC scores might seem to represent two ends of a continuum, prior results related to their positive association with PTSD suggest that the IOC Positive and Negative Impact Summary scores measure distinct constructs.13 This may reflect many patients' ability to turn lemons into lemonade despite heightened awareness of abundant lemons.
This study had several limitations. First, there is potential for selection bias. Our sample included predominantly married and white individuals; however, this racial profile closely mirrors that of the national population of NHL survivors, thereby strengthening the generalizability of our findings. Second, individuals who participated in the survey had fewer symptoms and less active disease than those who did not participate or were deceased; thus, if results were skewed, the direction would likely be toward overestimation rather than underestimation of QOL during survivorship. Third, in an effort to minimize respondent burden, the 28-page survey lacked measures assessing other psychological attributes that might have elucidated protective mechanisms (eg, optimism, resilience). Fourth, the use of self-reported clinical status introduces potential error in that participants may not have thorough understanding of their clinical condition; this concern was minimized by using standardized instruments. Fifth, statistical tests were not subject to multiple comparison corrections in this article. Given the number of tests conducted, a more conservative significance level could be considered. Thus, some results may be interpreted cautiously. However, given that we used an a priori plan informed by theory and empirical data, we feel confident about the findings, particularly those related to the IOC in our discussion.
In summary, our results suggest that the presence of comorbidity or symptom burden in NHL survivors should alert the clinician to a potential need for supportive services. The IOC measure is associated with QOL-related outcomes cross-sectionally and longitudinally; if this relationship is causal, modifying IOC may provide a strategy for improving QOL. And, perhaps most importantly, our results show that QOL and the cancer experience, and their changes over time, are complex. Although we can signal specific subpopulations at risk, there isn't a one-size-fits-all approach; it is difficult to predict the long-term outcome for any particular person. The factors influencing QOL and their interplay must be monitored, integrating the most up-to-date analyses from the literature and individual patient experiences, to personalize psychosocial care. This requires integration of patient-reported monitoring as a standard of survivorship care using practical scales that can be efficiently embedded in routine practice and mapped back to more rigorous research instruments as needed.28
Acknowledgment
We thank the many survivors of non-Hodgkin lymphoma who participated in our study. We also thank the research staff of the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, including Kimberly Ward, Julia Thorp, and Madeline Mitchell, for assisting with the fielding of this survey.
Footnotes
Supported by the National Center for Research Resources (Grant No. UL1RR025747), the National Cancer Institute (Grant No. CA101492 and Cancer Care Quality Grant No. CA116339), the American Cancer Society (Grant No. DSW-0321301-SW), and the University of North Carolina Research Council.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Amy P. Abernethy, Novartis (C), Pfizer (C) Stock Ownership: None Honoraria: None Research Funding: Amy P. Abernethy, BioVex, DARA BioSciences, Helsinn Therapeutics, MICO, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Sophia K. Smith, Deborah K. Mayer, Sheryl Zimmerman, Patricia A. Ganz
Collection and assembly of data: Sophia K. Smith
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.National Cancer Institute: Non-Hodgkin lymphoma. http://www.cancer.gov/cancertopics/types/non-hodgkin.
- 2.Leukemia and Lymphoma Society: Non-Hodgkin lymphoma. http://www.lls.org/#/diseaseinformation/getinformationsupport/factsstatistics/nonhodgkinlymphoma/
- 3.American Cancer Society: What is non-Hodgkin lymphoma? Topics. http://www.cancer.org/Cancer/Non-HodgkinLymphoma/DetailedGuide/non-hodgkin-lymphoma-types-of-non-hodgkin-lymphoma.
- 4.Mols F, Aaronson NK, Vingerhoets AJ, et al. Quality of life among long-term non-Hodgkin lymphoma survivors: A population-based study. Cancer. 2007;109:1659–1667. doi: 10.1002/cncr.22581. [DOI] [PubMed] [Google Scholar]
- 5.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettengell R, Donatti C, Hoskin P, et al. The impact of follicular lymphoma on health-related quality of life. Ann Oncol. 2008;19:570–576. doi: 10.1093/annonc/mdm543. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention and Lance Armstrong Foundation: A national action plan for cancer survivorship: Advancing public health strategies. http://www.cdc.gov/cancer/survivorship/pdf/plan.pdf.
- 8.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 9.Smith SK, Zimmerman S, Williams CS, et al. Post-traumatic stress outcomes in non-Hodgkin's lymphoma survivors. J Clin Oncol. 2008;26:934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SK, Zimmerman S, Williams CS, et al. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115:3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespi CM, Smith SK, Petersen L, et al. Measuring the impact of cancer: A comparison of non-Hodgkin lymphoma and breast cancer survivors. J Cancer Surviv. 2010;4:45–58. doi: 10.1007/s11764-009-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SK, Crespi CM, Petersen L, et al. The impact of cancer and quality of life for post-treatment non-Hodgkin lymphoma survivors. Psychooncology. 2010;19:1259–1267. doi: 10.1002/pon.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SK, Zimmerman S, Williams CS, et al. Post-traumatic stress symptoms in long-term non-Hodgkin lymphoma survivors: Does time heal? J Clin Oncol. 2011;29:4526–4533. doi: 10.1200/JCO.2011.37.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware JE, Jr, Kosinski MA. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1 (ed 2) Lincoln, RI: QualityMetric; 2004. [Google Scholar]
- 15.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 16.Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 17.Webster K, Cashy J, Cella D, et al. Measuring quality of life (QOL) in patients with non-Hodgkin's lymphoma (NHL): The Functional Assessment of Cancer Therapy-Lymphoma (FACT-Lym) Qual Life Res. 2005;14:1650. abstr. [Google Scholar]
- 18.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt M, Simone JV. Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 20.Abernethy AP, Ahmad A, Zafar SY, et al. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. 2010;48(suppl):S32–S38. doi: 10.1097/MLR.0b013e3181db53a4. [DOI] [PubMed] [Google Scholar]
- 21.Abernethy AP, Wheeler JL, Zafar SY. Management of gastrointestinal symptoms in advanced cancer patients: The rapid learning cancer clinic model. Curr Opin Support Palliat Care. 2010;4:36–45. doi: 10.1097/SPC.0b013e32833575fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basch E, Abernethy AP. Commentary: Encouraging clinicians to incorporate longitudinal patient-reported symptoms in routine clinical practice. J Oncol Pract. 2011;7:23–25. doi: 10.1200/JOP.2010.000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basch E, Abernethy AP. Supporting clinical practice decisions with real-time patient-reported outcomes. J Clin Oncol. 2011;29:954–956. doi: 10.1200/JCO.2010.33.2668. [DOI] [PubMed] [Google Scholar]
- 24.Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early-stage breast cancer: A comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–855. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 26.Der-Martirosian C, Kritz-Silverstein D, Barrett-Connor E. Five-year stability in associations of health-related quality of life measures in community-dwelling older adults: The Rancho Bernardo Study. Qual Life Res. 2010;19:1333–1341. doi: 10.1007/s11136-010-9700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishel MH, Germino BB, Gil KM, et al. Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psychooncology. 2005;14:962–978. doi: 10.1002/pon.909. [DOI] [PubMed] [Google Scholar]
- 28.Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;28:4268–4274. doi: 10.1200/JCO.2010.28.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]