Introduction
Despite significant treatment advances and survival improvement in a variety of pediatric CNS tumors, there remains a large number of children who succumb to their disease. A variety of novel biologic and targeted therapies have been evaluated in an effort to improve survival. One class of therapeutics that is being widely explored is antiangiogenic agents.
Angiogenesis is the development of new vasculature and is critically important for tumor growth, invasion, and metastases.1 Vascular endothelial growth factor (VEGF) and its isoforms play a role in tumor angiogenesis, are overexpressed in a variety of human cancers, and may be associated with tumor progression.2 Among the many antiangiogenic agents, bevacizumab (BVZ) has been widely studied in pediatric and adult patients since its development in 1997.3–8 BVZ is a humanized monoclonal immunoglobulin G1 antibody against all human VEGF isoforms and their proteolytic fragments.9,10 BVZ has been shown to decrease vascular permeability and increase cellular apoptosis in a variety of tumor xenografts, including CNS tumors.11 As with most new agents, the understanding of its adverse effect profile is evolving as new patterns of adverse events are reported.
Numerous reports have documented osteonecrosis of the jaw associated with BVZ alone and/or in combination with other chemotherapies, steroids, and bisphosphonates. Maxillary osteonecrosis has previously been well described in association with the use of bisphosphonates and steroids.12–14 In the case of bisphosphonates, this phenomenon usually occurs after trauma. However, spontaneous osteonecrosis has developed in a subset of patients.15 It is hypothesized that bisphosphonates inhibit osteoclast function, thereby causing a defect in remodeling and wound healing of the jaw.15,16 There appears to be an increased risk of jaw osteonecrosis in adult patients treated with either single-agent BVZ or concomitantly with bisphosphonates.17–21 Although most case reports have described osteonecrosis of the jaw in adults, there are a few case reports that have described antiangiogenic-associated osteonecrosis in the appendicular skeleton (Table 1).22–24
Table 1.
Antiangiogenic-Associated Osteonecrosis Outside of the Jaw
| Reference | Sex | Age (years) | Primary Tumor | Antiangiogenic Agent | Duration of Therapy (months) | Osteonecrosis Location |
|---|---|---|---|---|---|---|
| Mir et al22 | M | 62 | Colon adenocarcinoma | Bevacizumab | 5 | Left femoral head |
| Mir et al22 | M | Unknown | Renal cell carcinoma | Sunitinib | 4.3 | Bilateral femoral heads |
| Mir et al22 | M | 64 | Rectal adenocarcinoma | Bevacizumab | 4.5 | Left femoral head |
| Koczywas and Cristea23 | F | 43 | Lung adenocarcinoma | Bevacizumab | 12 | Left humeral head |
| Guillet et al24 | M | 53 | Hepatocellular carcinoma | Sorafenib | 10 | Bilateral femoral heads |
| Current | F | 13 | Pilocytic astrocytoma | Bevacizumab | 17 | Lunate bone |
| Current | M | 17 | Ependymoma | Bevacizumab | 11 | Bilateral femurs |
| Current | F | 10 | Pilocytic astrocytoma | Bevacizumab | 6 | Bilateral distal femurs and proximal tibias |
We report osteonecrosis in the lunate bone of the wrist and the knee in three children with recurrent CNS tumors treated with BVZ in combination with irinotecan (CPT-11) on a phase II study that enrolled children with recurrent brain tumors. In this trial, patients received BVZ (10 mg/kg per day) and CPT-11 (125 to 250 mg/m2 per day) intravenously every 2 weeks (one course = 4 weeks of treatment) until disease progression, unacceptable toxicity, or a maximum of 24 months of therapy. As a result of possible deleterious effects of epiphyseal growth resulting from VEGF inhibition, patients were required to have a baseline x-ray of the left knee and periodically thereafter during therapy and if they had symptoms of skeletal pain.25 To our knowledge, this is the first case series to describe BVZ-associated osteonecrosis in children.
Case Report 1
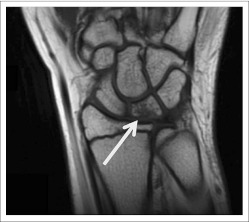
Patient 1 was a 13-year-old girl who began therapy after being enrolled on a study for a multiply recurrent hypothalamic/chiasmatic pilocytic astrocytoma with ventricular metastases. She had never received steroids or bisphosphonates. After 16 courses of treatment, she began complaining of wrist pain. On examination, the patient had tenderness to palpation of the left dorsal wrist with a decreased range of motion. A radiograph of the wrist did not reveal any bony abnormalities. However, magnetic resonance imaging (MRI) of the affected wrist revealed diffuse bone marrow edema with mild fragmentation and collapse of the lunate bone consistent with Kienbock's malacia (osteonecrosis of the lunate bone; Fig 1, arrow). The patient was taken off study because these findings were possibly attributable to BVZ. She was placed in a wrist brace, and her pain subsequently improved over a 6-week period.
Fig 1.
Case Report 2
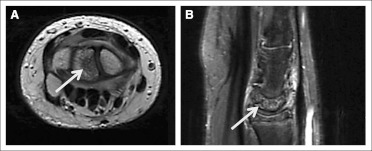
Patient 2 was a 17-year-old boy who began protocol therapy for a multiply recurrent supratentorial grade 2 ependymoma. The patient was on a low dose of steroids at the initiation of treatment, but the steroids were subsequently discontinued. After approximately 11 courses of therapy, a surveillance knee radiograph revealed increased sclerosis within the left lateral femoral condyle. The patient was asymptomatic without complaints of pain, discomfort, swelling, or a decreased range of motion. An MRI of both knees revealed bilateral medullary bone infarcts consistent with osteonecrosis of the diametaphyses (Fig 2, arrows). The patient was taken off study. Follow-up knee radiographs showed stable areas of sclerosis, and the patient remains without symptoms.
Fig 2.
Case Report 3
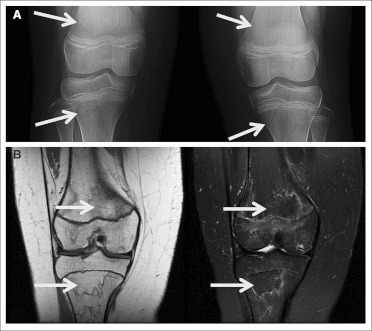
Patient 3 was a 10-year-old girl who began protocol therapy for a recurrent cervicomedullary pilocytic astrocytoma. The patient had been on a stable dose of steroids, which was successfully discontinued after the initiation of protocol therapy. She began complaining of bilateral knee pain after five courses of therapy. The pain was initially thought to be musculoskeletal in nature. Her pain continued, and radiographs of both knees revealed poorly defined areas of increased density (Fig 3A, arrows), and an MRI performed at an outside hospital revealed changes of the distal femurs that were suggestive of early osteonecrosis. Because of the increasing pain and radiographic findings, the patient was taken off study, and her pain diminished within a few months. The patient subsequently received involved field radiation therapy for her recurrent CNS tumor; however, she developed severe, symptomatic radiation-related swelling and was treated with both dexamethasone and BVZ (every 3 weeks). Shortly thereafter, she again complained of bilateral knee pain. Although repeat knee radiographs were normal, an MRI scan revealed avascular necrosis of both distal femurs and proximal tibias, with the left worse than the right, with noted involvement of the distal femoral epiphysis (Fig 3B, arrows). The BVZ was subsequently discontinued, and dexamethasone was replaced with hydrocortisone. The patient continued to have chronic hip and knee pain approximately 6 months since the end of BVZ and dexamethasone treatment.
Fig 3.
Discussion
BVZ is increasingly used in pediatric and adult patients with newly diagnosed and recurrent malignant diseases, including CNS tumors. Common adverse effects of BVZ include hypertension, proteinuria, hemorrhage, poor wound healing, and thromboembolic events.26 The understanding of rarer toxicities, however, is still evolving. Osteonecrosis has been previously well described in patients treated with either steroids or bisphosphonates alone and, more recently, in combination with BVZ-containing regimens. The published reports that have described BVZ-associated osteonecrosis have mostly been isolated to the jaw in adult patients with cancer.17,27–29 There is also a case report that described the development of lytic lesions within metaphyses of the radial and ulnar bones in an infant treated with BVZ for cutaneovisceral angiomatosis with thrombocytopenia syndrome.30 In this article, we described three unique pediatric cases of osteonecrosis after BVZ and CPT-11 treatment.
In addition to its known role in angiogenesis, VEGF plays a crucial role in osteogenic differentiation and bone formation, which may explain this toxicity.31 Preclinical data has documented the importance of impaired angiogenesis in the development of osteonecrosis.32 In case reports of BVZ-related osteonecrosis of the jaw, one hypothesis has been that BVZ impairs maxillary bone cell differentiation through a direct VEGF blockade.17,27–29 This blockade may result in compromised bone that is more susceptible to physiological trauma. It has also been hypothesized that the antiangiogenic properties of BVZ may impact the integrity of the microvasculature within the jaw and lead to a compromised osteon. This, too, may be exacerbated by minor trauma such as tooth brushing, which may make the jaw bones more susceptible to osteonecrosis.31
In our three cases, each patient had osteonecrosis outside of the jaw, which has been rarely described. Our patients developed osteonecrosis in locations (wrist and knee) of high impact, daily trauma, and/or repetitive use, which could easily have added to an already compromised microvasculature or defective bone differentiation process in the setting of BVZ use. It is unclear whether other risk factors also contributed to the formation of osteonecrosis in our patients. Patients 2 and 3 had a history of steroid use, which certainly could have induced or exacerbated the development of osteonecrosis. None of the patients described in the current report were taking concomitant bisphosphonates or had experienced any significant localized trauma.
These cases highlight the fact that osteonecrosis is a rare but real toxicity associated with BVZ use that can occur in pediatric patients and in locations remote from the jaw. They also suggest that routine imaging in patients with localized bony pain, swelling, or decreased range of motion is warranted to make this diagnosis early enough that BVZ can be discontinued before symptoms become chronic and/or irreversible.
Footnotes
Clinical trial information: NCT00381797.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 3.Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263–276. doi: 10.1517/14712598.3.2.263. [DOI] [PubMed] [Google Scholar]
- 4.Hinoda Y, Sasaki S, Ishida T, et al. Monoclonal antibodies as effective therapeutic agents for solid tumors. Cancer Sci. 2004;95:621–625. doi: 10.1111/j.1349-7006.2004.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zondor SD, Medina PJ. Bevacizumab: An angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother. 2004;38:1258–1264. doi: 10.1345/aph.1D470. [DOI] [PubMed] [Google Scholar]
- 6.Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol. 2008;26:399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 7.André N, Verschuur A, Rossler J, et al. Anti-angiogenic therapies for children with cancer. Curr Cancer Drug Targets. 2010;10:879–889. doi: 10.2174/156800910793357899. [DOI] [PubMed] [Google Scholar]
- 8.Taylor M, Rössler J, Geoerger B, et al. New anti-angiogenic strategies in pediatric solid malignancies: Agents and biomarkers of a near future. Expert Opin Investig Drugs. 2010;19:859–874. doi: 10.1517/13543784.2010.487654. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 10.Lin YS, Nguyen C, Mendoza JL, et al. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther. 1999;288:371–378. [PubMed] [Google Scholar]
- 11.Yuan F, Chen Y, Dellian M, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salort-Llorca C, Mínguez-Serra MP, Silvestre-Donat FJ. Maxillary osteonecrosis associated to antiangiogenic drugs. Med Oral Patol Oral Cir Bucal. 2011;16:e137–8. doi: 10.4317/medoral.16.e137. [DOI] [PubMed] [Google Scholar]
- 13.Bagan J, Scully C, Sabater V, et al. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): A concise update. Oral Oncol. 2009;45:551–554. doi: 10.1016/j.oraloncology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Mirzai R, Chang C, Greenspan A, et al. The pathogenesis of osteonecrosis and the relationships to corticosteroids. J Asthma. 1999;36:77–95. doi: 10.3109/02770909909065152. [DOI] [PubMed] [Google Scholar]
- 15.Han JW. Bisphosphonate related osteonecrosis of the jaws: Report of two cases. Imaging Sci Dent. 2011;41:129–134. doi: 10.5624/isd.2011.41.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kos M, Kuebler JF, Luczak K, et al. Bisphosphonate-related osteonecrosis of the jaws: A review of 34 cases and evaluation of risk. J Craniomaxillofac Surg. 2010;38:255–259. doi: 10.1016/j.jcms.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Francini F, Pascucci A, Francini E, et al. Osteonecrosis of the jaw in patients with cancer who received zoledronic acid and bevacizumab. J Am Dent Assoc. 2011;142:506–513. doi: 10.14219/jada.archive.2011.0220. [DOI] [PubMed] [Google Scholar]
- 18.Ngamphaiboon N, Frustino JL, Kossoff EB, et al. Osteonecrosis of the jaw: Dental outcomes in metastatic breast cancer patients treated with bisphosphonates with/without bevacizumab. Clin Breast Cancer. 2011;11:252–257. doi: 10.1016/j.clbc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Dişel U, Beşen AA, Özyılkan Ö, et al. A case report of bevacizumab-related osteonecrosis of the jaw: Old problem, new culprit. Oral Oncol. 2012;48:e2–e3. doi: 10.1016/j.oraloncology.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Hopp RN, Pucci J, Santos-Silva AR, et al. Osteonecrosis after administration of intravitreous bevacizumab. J Oral Maxillofac Surg. 2012;70:632–635. doi: 10.1016/j.joms.2011.02.104. [DOI] [PubMed] [Google Scholar]
- 21.Christodoulou C, Pervena A, Klouvas G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 22.Mir O, Coriat R, Gregory T, et al. Avascular necrosis of the femoral head: A rare class-effect of anti-VEGF agents. Invest New Drugs. 2011;29:716–718. doi: 10.1007/s10637-010-9406-6. [DOI] [PubMed] [Google Scholar]
- 23.Koczywas M, Cristea MC. Osteonecrosis of the humeral head in a patient with non-small cell lung cancer receiving bevacizumab. J Thorac Oncol. 2011;6:1960–1961. doi: 10.1097/JTO.0b013e31822e726f. [DOI] [PubMed] [Google Scholar]
- 24.Guillet M, Walter T, Scoazec JY, et al. Sorafenib-induced bilateral osteonecrosis of femoral heads. J Clin Oncol. 2010;28:e14. doi: 10.1200/JCO.2009.23.4252. [DOI] [PubMed] [Google Scholar]
- 25.Ryan AM, Eppler DB, Hagler KE, et al. Preclinical safety evaluation of rhuMAbVEGF, an antiangiogenic humanized monoclonal antibody. Toxicol Pathol. 1999;27:78–86. doi: 10.1177/019262339902700115. [DOI] [PubMed] [Google Scholar]
- 26.Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Katsenos S, Christophylakis C, Psathakis K. Osteonecrosis of the jaw in a patient with advanced non-small-cell lung cancer receiving bevacizumab. Arch Bronconeumol. 2012;48:218–219. doi: 10.1016/j.arbres.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Wynn RL. Bevacizumab (Avastin): An anti-angiogenic drug associated with osteonecrosis of the jaw. Gen Dent. 2011;59:410–413. [PubMed] [Google Scholar]
- 29.Van Poznak C. Osteonecrosis of the jaw and bevacizumab therapy. Breast Cancer Res Treat. 2010;122:189–191. doi: 10.1007/s10549-010-0933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AR, Hennessy JM, Kurth MA, et al. Reversible skeletal changes after treatment with bevacizumab in a child with cutaneovisceral angiomatosis with thrombocytopenia syndrome. Pediatr Blood Cancer. 2008;51:418–420. doi: 10.1002/pbc.21597. [DOI] [PubMed] [Google Scholar]
- 31.Estilo CL, Fornier M, Farooki A, et al. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26:4037–4038. doi: 10.1200/JCO.2007.15.5424. [DOI] [PubMed] [Google Scholar]
- 32.Kabata T, Matsumoto T, Yagishita S, et al. Vascular endothelial growth factor in rabbits during development of corticosteroid-induced osteonecrosis: A controlled experiment. J Rheumatol. 2008;35:2383–2390. doi: 10.3899/jrheum.070838. [DOI] [PubMed] [Google Scholar]