Abstract
Purpose
Tumor associated macrophages (TAMs) are considered with the capacity to have both negative and positive effects on tumor growth. The prognostic value of TAM for survival in patients with solid tumor remains controversial.
Experimental Design
We conducted a meta-analysis of 55 studies (n = 8,692 patients) that evaluated the correlation between TAM (detected by immunohistochemistry) and clinical staging, overall survival (OS) and disease free survival (DFS). The impact of M1 and M2 type TAM (n = 5) on survival was also examined.
Results
High density of TAM was significantly associated with late clinical staging in patients with breast cancer [risk ratio (RR) = 1.20 (95% confidence interval (CI), 1.14–1.28)] and bladder cancer [RR = 3.30 (95%CI, 1.56–6.96)] and with early clinical staging in patients with ovarian cancer [RR = 0.52 (95%CI, 0.35–0.77)]. Negative effects of TAM on OS was shown in patients with gastric cancer [RR = 1.64 (95%CI, 1.24–2.16)], breast cancer [RR = 8.62 (95%CI, 3.10–23.95)], bladder cancer [RR = 5.00 (95%CI, 1.98–12.63)], ovarian cancer [RR = 2.55 (95%CI, 1.60–4.06)], oral cancer [RR = 2.03 (95%CI, 1.47–2.80)] and thyroid cancer [RR = 2.72 (95%CI, 1.26–5.86)],and positive effects was displayed in patients with colorectal cancer [RR = 0.64 (95%CI, 0.43–0.96)]. No significant effect was showed between TAM and DFS. There was also no significant effect of two phenotypes of TAM on survival.
Conclusions
Although some modest bias cannot be excluded, high density of TAM seems to be associated with worse OS in patients with gastric cancer, urogenital cancer and head and neck cancer, with better OS in patients with colorectal cancer.
Introduction
Macrophages are a population of innate myeloid cells that are released from bone marrow as immature monocytic precursors and, after circulating in the blood stream, migrate into different tissues to undergo specific differentiation depending on local cues in the tissue [1], [2]. In response to different environment stimuli, macrophages can appear a range of different phenotypes [3]. The extremes of this range are recognized; the classically activated type M1 phenotype and the alternative activated M2 phenotype. The M1 macrophages are thought to be induced by interferon-γ, with or without lipopolysaccharide, tumor necrosis factor (TNF)-α, and activate cells of the adaptive immune system [4]. Differentiation of the M2 macrophages is induced by IL-4 or IL-13 and associated with parasite clearance, wound healing and dampen immune responses [5].
In 1863, it was fist found that a major leukocyte population was present in tumor, the so-called tumor-associated macrophages (TAM), which reflect the onset of cancer at site of previous chronic inflammation [1], [6]. These macrophages can induce neoplastic cell (cytotoxicity, apoptosis) and/or elicit tumor destructive reactions with the capacity to display both negative and positive effects on tumor growth depending on environmental stimuli of the tumor tissue [7], [8].
For long a large number of studies have been focused on identifying the prognostic value of TAM in solid tumors and most studies suggest that TAM is beneficial for tumor growth and, therefore, associated with poor prognosis [1]. However, there are some exceptions with high density of macrophages correlating with increased survival in different tumors [9]–[18] and even this contradiction has come up in the one type of tumor [11], [19]. This meta-analysis focused on the identifying diverse roles and functions of TAM and subpopulations of TAM for clinical outcome in patients with solid tumors.
Materials and Methods
Identification and Eligibility of Relevant Studies
We performed our meta-analysis according to a predetermined written protocol. To be eligible for our meta-analysis, studies had to deal with solid tumor at inclusion, to evaluate the correlation between TAM and survival, and to be published in English or Chinese languages. A computer-aided literature search of Pubmed (MEDLINE) 1950-present and EMBASE was conduced by combing search terms “cancer”, ”tumor”, “neoplasm”, “carcinoma”, “tumor-associated macrophage”, “tumor-infiltrating macrophage” and “intratumoral macrophage.” The deadline of the included articles was April 20th, 2012. Reference list from primary identified studies were also searched to prevent missing any studies by the electronic search strategies.
Inclusion criteria for primary studies were as follows: (1) proven diagnosis of solid tumor in humans, (2) using immunochemistry method to evaluate TAM by anti-CD68, M1-type TAM by anti-HLA-DR and M2-type TAM by anti-CD163, and (3) correlation of TAM with TNM staging, OS or DFS. Two independent reviewers processed primary assessment by identifying the eligibility of abstracts from database. Full articles were retrieved for further assessment if the eligibility was unclear from the abstracts. Any disagreements were resolved by serious discussion. We carefully examined the names of all authors and the medical centers involved in each publication to avoid duplication data. Whenever studies pertained to overlapping patients, we retained the studies with highest number of patients.
Definitions and Standardizations
We used preconcerted rules to standardize as much as possible the definition of TAM positivity. As 20% was the used as a cutoff value in majority of the included studies [18], [20]–[26], we defined TAM positivity as positive cell stain in at least 20% of tumor cells. When different definitions were used, we contacted the primary author of each articles to retrieve the cutoff value they used. When cutoff value was not possible to retrieve, we accepted the cutoff was closet to the 20% cutoff level. When cutoff value was closed to 20%, which ranged from 16.3% to 25%, we also accepted the cutoff as 20%.
Data Extraction
Data were carefully extracted from all of the included studies in duplicate by two of us, using a standard information collection form, with the following items, first author, year of publication, study design, median follow-up time, country of origin, number of patients involved, number of men included, mean or median age, tumor location, histological type, tumor-node-metastasis (TNM) staging, blinded reading, definition of TAM high, anti-cancer treatment(s) during follow up, OS or DFS or both. The main outcomes were tabulated in 2×2 tables showing the TNM staging status, occurrence or not of death or disease during follow-up according to TAM results.
Statistical Analyses
Included studies were divided into three groups for analysis: those with data regarding TNM staging, those regarding OS and those regarding DFS. A study was considered significant when the P for comparing survival distribution between groups with high and low TAM was inferior to 0.05. A study was termed “positive” when a high TAM predicted a late clinical staging or poorer survival, “negative” when a high TAM predicted a early clinical staging or better survival, “indeterminate” when no significant relationship between TAM and clinical staging or survival was found.
For the quantitative aggregation of survival result, impacts of TAM on survival were reported for individual studies by estimating RRs with 95% confidence interval values. We first simply extracted RR and their 95%CI from the original article. If not available, the published data including number of patients at risk and total number of events in each groups from articles were used to estimate RR according to the methods described by Parmer et al [27]. When data were only available in the form of figures, we read Kaplan-Meier curves by Engauge Digitizer version 4.1 (free software down-loaded from http://sourceforge.net) and extracted survival data to reconstruct RRs and its 95%CI. An observed RR>1 indicated worse outcome for the TAM high group relative to TAM low group and would be considered statistically significant if the 95%CI did not overlap 1, with p<0.05. Sensitivity analyses were performed to examine the TAM effect of limiting the evaluation to studies using the 20% cutoff on prognosis. The effect of publication bias on the outcomes was assessed graphically using funnel plots, and funnel plot asymmetry was assessed by Egger's linear regression method. (p<0.05 was considered statistically significant publication bias) [28]. Meta-analyses were carried out by the Stata version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Studies Selection and Characteristics
The initial search algorithm retrieved a total of 3076 references and we evaluated 144 candidate studies in full text. Upon further review, 50 articles were eliminated due to inadequate data for meta-analysis and another 29 articles were out of scope because of evaluating other factors related to TAM (Figure 1). Overall, we identified 55 articles (n = 8693) with TAM measurements in patients with solid tumors.
Figure 1. Flow chart of the literature search and selection of included studies.
The characteristics of included studies are listed in Table 1. The median sample size for all studies was 158 patients (range = 24–1902). The median sample size for staging was 189 patients, that for OS was 164 patients and that for DFS was 202 patients. The total proportion of male subjects was around 45% and that of patients in grade I/II was 43%. All evaluated IHC staining in formalin-fixed paraffin-embedded tissue blocks. The study design was more often a retrospective (N = 25) than a prospective cohort study (N = 5). Only 20% studies had performed blinded reading during evaluating TAM. The median follow-up time for all included studies ranged from 1.84 to 25 years. Of the 55 studies, cut-off value for definition of TAM high only could be retrieved from 32 original articles or by contacting authors.
Table 1. Characteristics of the eligible studies.
| First author (ref) | Year | Study design | n (M/F) | Tumor location | Tumor stage I/II (III/IV) | Median follow-up (y) | Blinded reading | Staining for TAM high | RR estimation | Analysis | Result |
| Gastrointestinal cancer (N = 19) | |||||||||||
| Khorana (29) | 2003 | Prosp. | 131 (69/53) | 131 Colon | 11 (118) | 5 | NR | >2% | Data extrapolated, Survival curve | Stag., OS | Indeterminate |
| Tan (12) | 2005 | Retro. | 60 (35/25) | 60 Colon | 26 (34) | NR | NR | >25% | Data extrapolated, Survival curve | Stag., OS | Negative |
| Bacman (30) | 2007 | NR | 310 (189/121) | 310 Colon | 178 (132) | 7.5 | NR | NR | Data extrapolated | OS | Indeterminate |
| Forssell (15) | 2007 | Retro. | 488 (271/217) | 488 Colon | 207 (254) | NR | NR | NR | Data extrapolated, Reported in text | Stag., OS | Indeterminate, negative |
| Zhou (18) | 2010 | NR | 160 (94/66) | 160 Colon | NR | 5 | Yes | 20% | Data extrapolated, reported in text | Stag., OS | Negative |
| Ishigami (31) | 2003 | Retro. | 97 (72/25) | 97 Stom | 67 (30) | NR | NR | >200 / HPF | Data extrapolated, Survival curve | Stag., OS | Indeterminate, positive |
| Ohno (10) | 2005 | Retro. | 84 (57/27) | 84 Stom | 41 (43) | NR | Yes | >4.7% | Survival curve | DFS | Negative |
| Hass (32) | 2009 | Prosp. | 52 (40/12) | 50 Stom | 39 (13) | 5.9 | NR | NR | Reported in text | DFS | Indeterminate |
| Kawahara (33) | 2010 | NR | 111 (77/34) | 111 Stom | 36 (75) | NR | NR | NR | Reported in text | OS | Positive |
| Osinsky (34) | 2011 | Prosp. | 105 (71/34) | 105 stom | 48 (57) | NR | NR | >23.0% | Reported in text | OS | Positive |
| Zhu (22) | 2008 | Retro. | 105 (96/9) | 105 Liver | 86 (19) | 1.84 | NR | >20% | Data extrapolated, reported in text | Stag., OS, DFS | Indeterminate, positive |
| Li (24) | 2009 | Retro. | 302 (260/42) | 302 Liver | 237 (65) | 4.83 | NR | >20% | Data extrapolated, reported in text | Stag., OS, DFS | Indeterminate, negative |
| Ding (35) | 2009 | Retro. | 107 (87/20) | 107 Liver | 98 (39) | 2.5 | MR | >23% | Reported in text | OS, DFS | Positive |
| Kuang (36) | 2009 | Retro. | 262 (NR) | 206 Liver | 249 (13) | NR | NR | NR | Reported in text | OS, DFS | Positive |
| Ju (23) | 2009 | Retro. | 130 (112/18) | 130 Liver | 66 (64) | 2.65 | NR | >20% | Reported in text | Stag., OS, DFS | Positive |
| Koide (9) | 2002 | Retro. | 56 (42/14) | 56 Esophagus | 30 (26) | NR | NR | NR | Data extrapolated, survival cure | Stag., OS | Indeterminate, negative |
| Guo (19) | 2007 | NR | 137 (103/34) | 137 Esophagus | 86 (51) | NR | NR | >25% | Reported in text | Stag., OS | Indeterminate, positive |
| Kurahara (37) | 2012 | Retro. | 76 (52/24) | 52 Pancreas | 29 (47) | NR | Yes | NR | Survival curve | OS, OSb | Indeterminate |
| Hasita (38) | 2010 | NR | 39 (27/12) | 55 Bile duct | 29 (10) | 3.33 | Yes | NR | Data extrapolated, survival curve | Stag., OS, DFS, Stag.b, OSb, DFSb | Indeterminate |
| Urogenital cancer (N = 20) | |||||||||||
| Leek (39) | 1996 | Retro. | 101 (0/101) | 5 Breast | NR | NR | NR | MD > = 12 | Reported in text | OS, DFS | Positive |
| Toi (40) | 1999 | NR | 229 (0/229) | 229 Breast | NR | 4 | Yes | NR | Survival curve | DFS | Indeterminate |
| Valkovic (41) | 2002 | NR | 97 (0/97) | 97 Breast | 82 (15) | NR | NR | NR | Data extrapolated | Stag. | Indeterminate |
| Bolat (42) | 2006 | Retro. | 78 (0/78) | 78 Breast | 38 (10) | NR | NR | NR | Data extrapolated | Stag. | Indeterminate |
| Mahmound (43) | 2012 | Retro. | 1902 (0/1902) | 1902 Breast | 220 (1682) | NR | NR | NR | Data extrapolated, survival curve | Stag., DFS | Positive, indeterminate |
| Salvesen (44) | 1999 | Prosp. | 60 (0/60) | 60 Endometrium | 50(10) | 11 | NR | NR | Data extrapolated, reported in text | Stag., OS | Positive, indeterminate |
| Hashimoto (45) | 2000 | NR | 109 (0/109) | 109 Endometrium | 56(53) | 4.58 | NR | NR | Data extrapolated | Stag., DFS | Indeterminate |
| Ohno (11) | 2004 | Retro. | 70 (0/70) | 70 Endometrium | NR | NR | NR | MD >10.7 | Survival curve | DFS | Negative |
| Soeda (46) | 2008 | Retro. | 76 (0/76) | 76 Endometrium | 59(17) | 6.83 | NR | NR | Survival curve | OS, DFS | Positive, indeterminate |
| Espinosa (47) | 2010 | NR | 64 (0/64) | 64 Endometrium | 23(26) | NR | NR | NR | Data extrapolated | Stag.b | Indeterminate |
| Lissbrant (48) | 2000 | Retro. | 85 (85/0) | 85 Prostate | 75(10) | NR | NR | > = 0.97% | Survival curve | Stag., OS | Indeterminate |
| Shimura (49) | 2000 | NR | 81 (81/0) | 81 Prostate | 67(13) | 3.9 | NR | NR | Reported in text | DFS | Indeterminate |
| Nonomura (50) | 2011 | NR | 131 (131/0) | 131 Prostate | 30(41) | 2.9 | NR | MD > = 22 | Survival curve | DFS | Positive |
| Heller (51) | 2002 | Retro. | 24 (0/24) | 24 Cervix | 20/4 | NR | NR | NR | Data extrapolated | Stag. | Indeterminate |
| Kawanaka (52) | 2008 | Retro | 73 (0/73) | 73 Cervix | 22/51 | NR | Yes | MD > = 55 | Survival curve | DFS | Indeterminate |
| Hanada (53) | 2000 | NR | 63 (51/12) | 63 Bladder | 42/21 | 5.4 | Yes | MD > = 67 | Data extrapolated, reported in text | Stag., OS | Positive |
| Takayama (54) | 2009 | NR | 41 (38/3) | 41 Bladder | NR | 25 | NR | > = 4 | Reported in text | DFS | Positive |
| Tanaka (55) | 2004 | NR | 89 (0/89) | 89 Ovary | 22/67 | 6.8 | NR | > = 25% | Data extrapolated, reported in text, survival curve | Stag., OS, DFS | Negative, positive, indeterminate |
| Wan (25) | 2009 | NR | 67 (0/67) | 67 Ovary | 0/67 | NR | NR | >20% | Survival curve | OS | Positive |
| Chai (20) | 2008 | NR | 99 (42/57) | 99 Uroth | NR | NR | NR | >20% | Reported in text | OS, DFS | Indeterminate, positive |
| Lung cancer (N = 10) | |||||||||||
| Takanami (56) | 1999 | Retro. | 113 (66/47) | 113 Lung | 61/52 | NR | NR | MD>32 | Survival curve | OS | Positive |
| Chen (57) | 2005 | NR | 41 (27/14) | 41 Lung | 23/18 | NR | NR | MD> = 163 | Survival curve | DFS | Indeterminate |
| Welsh (13) | 2005 | Retro. | 175 (116/59) | 175 Lung | 123/38 | NR | Yes | NR | Data extrapolated | Stag., DFS | Negative |
| Zeni (58) | 2007 | NR | 50 (43/7) | 43 Lung | NR | NR | NR | >16.3% | Reported in text, survival curve | Stag., OS | Positive |
| Kawai (16) | 2008 | NR | 199 (139/60) | 199 Lung | NR | NR | NR | NR | Reported in text | OS | Negative |
| Ohri (17) | 2009 | Retro. | 40 (16/24) | 40 Lung | 34/6 | NR | NR | NR | Survival curve | OS | Negative |
| Al-shibli (59) | 2009 | Retro. | 371 (253/82) | 371 Lung | 303/32 | 8 | NR | > = 25% | Data extrapolated, survival curve | OS, DFS | Indeterminate |
| Ma (26) | 2010 | NR | 50 (40/10) | 50 Lung | 33/17 | NR | NR | >20% | Reported in text | OSa, OSb | Indeterminate |
| Ohtaki (60) | 2010 | Prosp. | 170 (85/85) | 170 Lung | NR | 10.1 | NR | NR | Data extrapolated | Stag., OS | Positive |
| Zhang (61) | 2011 | Retro. | 65 (38/27) | 65 Lung | 38/27 | NR | Yes | TAM counts >102 | Survival curve | OS | Positive |
| Head and neck cancer (N = 4) | |||||||||||
| Liu (21) | 2008 | NR | 112 (93/19) | 112 Oral | 50/62 | NR | NR | >20% | Data extrapolated, survival curve | Stag., OS | Positive |
| Fujii (62) | 2012 | NR | 108 (31/10) | 108 Oral | 46/62 | NR | NR | > = 2/HPF | Data extrapolated, reported in text | Stag., stag.b, OSb | Positive, indeterminate, positive |
| Peng (14) | 2006 | NR | 60 (38/22) | 60 Oral | 15/45 | NR | NR | > = 63.7/HPF | Data extrapolated | OS | Negative |
| Lin (63) | 2011 | NR | 84 (77/7) | 84 Lar | 30/54 | NR | NR | > = 10/HPF | Data extrapolated, reported in text | Stag., OS, DFS | Indeterminate, indeterminate, positive |
| Thyroid cancer (N = 1) | |||||||||||
| Ryder (64) | 2008 | NR | 37 (13/24) | 37 Thyr | 5/32 | NR | Yes | > = 10/HPF | Survival curve | OS | Positive |
| Mesothelioma (N = 1) | |||||||||||
| Burt (65) | 2011 | Retro. | 667 (531/136) | 667 Meso | 64/603 | NR | Yes | NR | Reported in text | OS | Indeterminate |
Of the included studies, 19 studies focused on gastrointestinal cancers, including colorectal cancer (N = 5), gastric cancer (N = 5), liver cancer (N = 5), esophagus cancer (N = 2), pancreatic caner (N = 1) and cholangiocarcinoma (N = 1). 20 studies analyzed the impact of TAM on survival in patients with urogenital cancers, including breast cancer (N = 5), endometrial cancer (N = 5), prostate cancer (N = 3), cervical cancer (N = 2), bladder cancer (N = 2), ovary cancer (N = 2) and urothelial cancer (N = 1). 5 studies mentioned the value of M2-type TAM on survival [26], [37], [38], [47], [62].
Data Synthesis: Clinical Staging
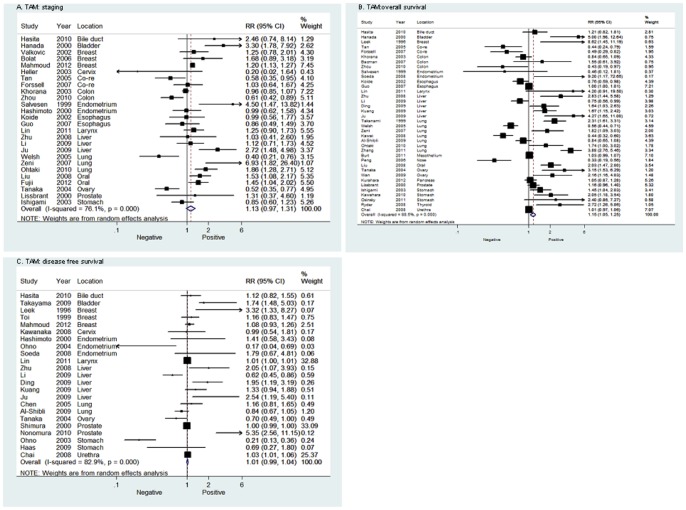
As shown in Figure 2A, the combining data of TAM on clinical staging showed a nonstatistically significant RR of 1.13 (95%CI 0.97–1.31). For sub-group analysis, all of the studies were dispatched into several classes according to the tumor location. Our data showed that high density of TAM was significantly higher in patients with advanced tumor stage (III+IV) than in the patients with early stage (I+II), which occurred in breast cancer [RR = 1.20 (95%CI, 1.14–1.28)], oral cancer [RR = 1.49 (95%CI, 1.17–1.89)] and bladder cancer [RR = 3.30 (95%CI, 1.78–7.92)],whereas 1 article focused on ovary cancer found high density of TAM was associated with early stage [RR = 0.52 (95%CI, 0.36–0.77)]. Other sub-group analysis found no relation between TAM and clinical staging. In studies specific defining TAM positivity as at least 20% positive staining cells in tumor tissue, no significant relation was found between TAM and clinical staging (RR = 1.23 (95%CI, 0.74–2.02). Analysis for M2-type TAM on staging also showed no significant effect [RR = 1.43 (95%CI, 0.70–2.93)].
Figure 2. Forrest plots and meta-analysis of studies evaluating RR of high TAM counts as compared to low counts.
Clinical staging and survival data are reported as (A) staging, (B) overall survival (OS) and (C) disease free survival (DFS).
Data Synthesis: Overall Survival
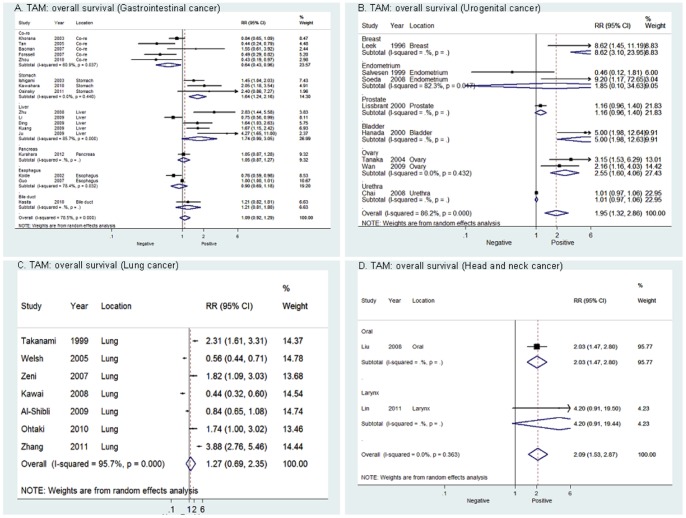
For overall population, high density of TAM was associated with a worse prognosis regarding overall survival (Figure 2B). However, mortality was only 1.15-fold higher in high TAM patients with solid tumor, which showed modest effect. In sub-group analysis (Figure 3), high density of TAM was significantly correlated with poor OS in patients with urogenital cancer [RR = 1.95 (95%CI, 1.32–2.86)], including breast cancer [RR = 8.62 (95%CI, 3.10–23.95)], endometrial cancer [RR = 1.85 (95%CI, 0.10–34.63)], prostate cancer [RR = 1.16 (95%CI, 0.96–1.40)], bladder cancer [RR = 5.00 (95%CI, 1.98–12.63)], ovary cancer [RR = 2.55 (95%CI, 1.60–4.06)] and urothelial cancer [RR = 1.01 (95%CI, 1.32–2.86)]. In addition, gastric cancer and oral cancer showed significant RR between TAM and OS [RR = 1.54 (95%CI 1.24–2.16), 2.03 (95%CI 1.47–2.80)]. However, analysis of studies on colorectal cancer showed that there was a significant correlation between high density of TAM and longer OS [RR = 0.64 (95%CI, 0.43–0.96)]. No significant correlation between TAM and OS was found in other sub-group analysis. Five studies reported that there was no significant correlation between M2-type TAM and OS [RR = 0.98 (95%CI, 0.71–1.35)], one study reported that the M1-type TAM density in the tumor islet is positively associated with extended survival in patients with lung cancer [17]. There was also no difference in the summary estimate of TAM on overall survival when cutoff value was specific to 20% (RR = 1.41 (95%CI, 1.03–2.09).
Figure 3. Forrest plots and meta-analysis of studies evaluating RR of high TAM counts as compared to low counts in different subgroup of tumors.
Clinical staging and survival data are reported as (A) gastrointestinal cancer, (B) urogenital cancer, (C) lung cancer and (D) head and neck cancer.
Data Synthesis: Disease free survival
For the overall population, no significant relation was observed between TAM and DFS [RR = 1.01 (95%CI, 0.99–1.04)](Figure 2C). No significant effect was showed in sub-group analysis. One article [38] with data on M2-type TAM and DFS also showed a non-significant effect [RR = 1.09 (95%CI, 0.77–1.54)]. Analysis for specific 20% cutoff also showed no significant effect (RR = 1.19 (95%CI, 0.78–1.57).
Data Synthesis in sub-groups: clinic-pathological factors
The impact of TAM density on clinic-pathological in patients with different cancer was further analyzed and described in Table 2. Two studies [12], [29] showed a significant correlation between T status and TAM density, nevertheless, no correlation was found between TAM density and lymph node metastasis nor distant metastasis. High density of TAM was also correlated with nonmucinous type of colon cancer. Additionally, two of the studies on gastric cancer described that low density of TAM was significantly correlated with lymph node metastasis [31], [32]. In the breast cancer group, Negative effects of TAM were found not only on TNM stage, but also histological grade, lymph node metastasis, tumor size, vascular invasion and HER-2 status. Similar phenomena were seen in bladder cancer and oral squamous group, and high density of TAM was significantly correlated with TNM stage, T status, lymph node metastasis and distant metastasis. In addition, one study demonstrated that the density of TAM was significantly lower in patients with advanced tumor stage (III/IV) than in patients with early stage (I/II) [55].
Table 2. Meta-analysis of the subgroups based on clinic-pathological factors related to TAM density from the available published studies.
| Cancer type | Clinic-pathological factors | Number of studies | Number of total patients | HR (95% CI) | p-value | Results | Reference |
| Colon cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 2 | 619 | 0.71 (0.35, 1.45) | 0.194 | Indeterminate | (15), (29) | |
| T status (T1/T2 vs. T3/T4) | 2 | 191 | 0.35 (0.13, 0.90) | 0.246 | Positive | (12), (29) | |
| Lymph node metastasis | 2 | 370 | 0.72 (0.13, 4.06) | 0.013 | Indeterminate | (12), (30) | |
| Distant metastasis | 3 | 530 | 0.72 (0.24, 2.11) | 0.005 | Indeterminate | (12), (18), (30) | |
| Histological grade (well/moderate vs. poor) | 3 | 858 | 0.26 (0.06, 1.06) | 0.005 | Indeterminate | (12), (15), (30) | |
| Pathologic classification (mucinous vs. nonmucinous) | 2 | 648 | 2.53 (1.35, 4.73) | 0.675 | Negative | (15), (18) | |
| Gastric cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 1 | 97 | 2.40 (0.98, 5.91) | NR | Indeterminate | (31) | |
| T status (T1/T2 vs. T3/T4) | 1 | 97 | 1.36 (0.54, 3.46) | NR | Indeterminate | (31) | |
| Lymph node metastasis | 2 | 149 | 0.39 (0.19, 0.80) | 0.302 | Positive | (31), (32) | |
| Distant metastasis | 1 | 52 | 1.30 (0.40, 4.28) | NR | Indeterminate | (32) | |
| Histological grade (well/moderate vs. poor) | 1 | 97 | 0.54 (0.22, 1.32) | NR | Indeterminate | (31) | |
| Liver cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 3 | 514 | 1.27 (0.84, 1.93) | 0.702 | Indeterminate | (22), (24), (35) | |
| Vascular invasion (absent vs. present) | 3 | 514 | 1.38 (0.87, 2.21) | 0.410 | Indeterminate | (22), (24), (35) | |
| Tumor differentiation | 2 | 409 | 1.08 (0.70, 1.66) | 0.956 | Indeterminate | (24), (35) | |
| Tumor size (≤5 vs. >5cm) | 2 | 409 | 1.76 (1.19, 2.60) | 0.329 | Negative | (24), (35) | |
| Hepatitis history (No vs. Yes) | 2 | 407 | 1.45 (0.80, 2.60) | 0.456 | Indeterminate | (22), (24) | |
| Liver cirrhosis (No vs. Yes) | 3 | 514 | 1.18 (0.75, 1.88) | 0.886 | Indeterminate | (22), (24), (35) | |
| Fibrous capsule (absent vs. present) | 3 | 514 | 0.83 (0.58, 1.19) | 0.750 | Indeterminate | (22), (24), (35) | |
| Esophagus cancer | |||||||
| T status (T1/T2 vs. T3/T4) | 1 | 56 | 2.73 (0.70, 10.60) | NR | Indeterminate | (9) | |
| Lymph node metastasis | 1 | 56 | 0.75 (0.21, 2.59) | NR | Indeterminate | (9) | |
| Lymphatic invasion (absent vs. present) | 1 | 56 | 3.21 (0.81, 12.8) | NR | Indeterminate | (9) | |
| Venous invasion (absent vs. present) | 1 | 56 | 6.22 (1.48, 26.1) | NR | Negative | (9) | |
| Intrahepatic cholangiocarcinoma | |||||||
| UICC stage (I/II vs. III/IV) | 1 | 55 | 3.31 (0.71, 15.4) | NR | Indeterminate | (38) | |
| Histological grade (well/moderate vs. poor) | 1 | 55 | 5.25 (0.93, 29.7) | NR | Indeterminate | (38) | |
| Lymph node metastasis | 1 | 55 | 1.06 (0.19, 6.05) | NR | Indeterminate | (38) | |
| Vascular invasion (absent vs. present) | 1 | 55 | 2.13 (0.34, 13.2) | NR | Indeterminate | (38) | |
| Tumor size (<4 vs. ≥4cm) | 1 | 55 | 1.38 (0.39, 4.87) | NR | Indeterminate | (38) | |
| Breast cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 1 | 78 | 1.20 (1.14, 1.28) | NR | Negative | (42) | |
| Histological grade (I/II vs. III) | 3 | 2077 | 3.42 (2.71, 4.30) | 0.742 | Negative | (41), (42), (43) | |
| Lymph node metastasis | 3 | 2077 | 1.29 (1.04, 1.62) | 0.604 | Negative | (41), (42), (43) | |
| Tumor size (≤2 vs. >2cm) | 3 | 2077 | 1.43 (1.14, 1.80) | 0.963 | Negative | (41), (42), (43) | |
| Vascular invasion (absent vs. present) | 1 | 1902 | 1.74 (1.35, 2.23) | NR | Negative | (43) | |
| HER-2 status (negative vs. positive) | 1 | 1902 | 2.59 (1.75, 3.85) | NR | Negative | (43) | |
| Endometrial cancer | |||||||
| FIGO stage (I/II vs. III/IV) | 2 | 169 | 2.34 (0.36, 15.39) | 0.021 | Indeterminate | (44), (45) | |
| Lymph node metastasis | 1 | 109 | 0.43 (0.12, 1.53) | NR | Indeterminate | (45) | |
| Myometrial invasion (negative vs. positive) | 1 | 109 | 2.09 (0.65, 6.69) | NR | Indeterminate | (45) | |
| Histological grade (I/II vs. III) | 2 | 169 | 4.34 (0.70, 27.08) | 0.044 | Indeterminate | (44), (45) | |
| Pathologic classification (Endometrioid vs.non-endometrioid) | 1 | 109 | 1.37 (0.41, 4.62) | NR | Indeterminate | (45) | |
| Prostate cancer | |||||||
| T status (T1/T2 vs. T3/T4) | 1 | 85 | 3.30 (1.56, 6.96) | NR | Indeterminate | (48) | |
| Distant metastasis | 1 | 85 | 1.06 (0.25, 4.54) | NR | Indeterminate | (48) | |
| Cervical cancer | |||||||
| FIGO stage (I/II vs. III/IV) | 2 | 97 | 0.68 (0.06, 8.26) | 0.063 | Indeterminate | (51), (52) | |
| Lymph node metastasis | 1 | 24 | 1.75 (0.26, 11.7) | NR | Indeterminate | (51) | |
| Bladder cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 1 | 63 | 5.76 (1.76, 18.9) | NR | Negative | (53) | |
| T status (T1/T2 vs. T3/T4) | 1 | 63 | 17.6 (4,34, 71.1) | NR | Negative | (53) | |
| Distant metastasis | 1 | 63 | 12.4 (2.50, 61.0) | NR | Negative | (53) | |
| Vascular invasion (absent vs. present) | 1 | 63 | 10.8 (1.26, 92.4) | NR | Negative | (53) | |
| Ovary cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 1 | 89 | 0.52 (0.35, 0.77) | NR | Positive | (54) | |
| Lymph node metastasis | 1 | 89 | 0.14 (0.05, 0.35) | NR | Positive | (54) | |
| Tumor differentiation | 1 | 89 | 0.79 (0.34, 1.83) | NR | Indeterminate | (54) | |
| Tumor size (≤5 vs. >5cm) | 1 | 89 | 0.94 (0.41, 2.17) | NR | Indeterminate | (54) | |
| Histological type (serous vs. nonserous) | 1 | 89 | 0.81 (0.34, 1.9) | NR | Indeterminate | (54) | |
| Lung cancer | |||||||
| Pathologic stage (I vs. II/III/IV | 2 | 345 | 1.29 (0.24, 6.78) | <0.001 | Indeterminate | (13), (60) | |
| Tumor differentiation | 1 | 170 | 5.80 (2.99, 11.2) | NR | Negative | (60) | |
| T status (T1/T2 vs. T3/T4) | 1 | 170 | 2.70 (1.40, 5.21) | NR | Negative | (60) | |
| Lymph node metastasis | 1 | 170 | 2.72 (1.27, 5.82) | NR | Negative | (60) | |
| Vessel invasion | 1 | 170 | 3.24 (1.69, 6.24) | NR | Negative | (60) | |
| Oral squamous cell carcinoma | |||||||
| TNM Stage (I/II vs. III/IV) | 2 | 220 | 2.53 (1.46, 4.38) | 0.888 | Negative | (21), (62) | |
| T status (T1/T2 vs. T3/T4) | 2 | 220 | 2.33 (1.34, 4.03) | 0.328 | Negative | (21), (62) | |
| Lymph node metastasis | 2 | 220 | 2.56 (1.46, 4.47) | 0.528 | Negative | (21), (62) | |
| Tumor differentiation | 2 | 220 | 1.32 (0.68, 2.57) | 0.294 | Indeterminate | (21), (62) | |
| Thyroid cancer | |||||||
| TNM Stage (I/II vs. III/IV) | 1 | 37 | 4.31 (0.42, 43.7) | NR | Indeterminate | (64) | |
| Distant metastasis | 1 | 37 | 4.17 (0.66, 26.1) | NR | Indeterminate | (64) | |
Evaluation of publication bias
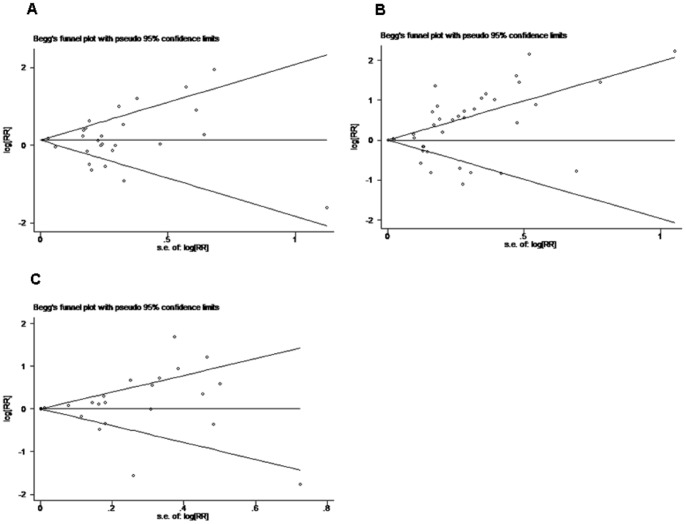
Both Begg's funnel plot and Egger's test were performed to assess the publication bias in all studies evaluating staging, OS, DFS separately, and evaluation was also performed in sub-group analysis. Begg's funnel plot did not reveal any evidence of significant asymmetry in the overall meta-analysis of staging (p = 0.679), OS (p = 0.065) and DFS (p = 0.792)(Figure 4). There was also no indication of publication in Egger's test of staging (p = 0.993), OS (p = 0.058) and DFS (p = 0.357). For sub-group evaluation of publication bias, no significant publication bias was shown from either Egger's or Begg's test (not shown).
Figure 4. Funnel graph for assessment of potential publication bias in studies of TAM density in patients with solid tumor.
(A) Staging, (B) OS, (C) DFS. The funnel graph plots log HR against the standard error of the log HR. The result of the Egger's test showed no statistical significant (p>0.05).
Discussion
So far, a group of original articles and reviews has studied the prognostic significance of TAM in solid tumors, and the presence of both significant and non-significant studies addressing the importance of TAM on survival made it necessary to perform a quantitative aggregation of the survival results. The present result showed that high density of TAM, as detected with immunohistochemistry, was significant associated with worse overall survival in solid tumor, with a global RR of 1.15. As potential bias exists between studies on different tumors, subgroup analysis was also performed, which suggested that high density of TAM was significant associated with OS in patients with gastric cancer [RR = 1.64 (95%CI, 1.24–2.16)], breast cancer [RR = 8.62 (95%CI, 3.10–23.95)], bladder caner [RR = 5.00 (95%CI, 1.98–12.63)], ovarian cancer [RR = 2.55 (95%CI, 1.60–4.06)], oral cancer [RR = 2.03 (95%CI, 1.47–2.80)] and thyroid cancer [RR = 2.72 (95%CI, 1.26–5.86)]. Moreover, there showed positive effect in patients with colorectal cancer [RR = 0.64 (95%CI, 0.43–0.96)]. However, no significant effect was seen between TAM and DFS.
When comparing the results of different types of tumors, several key differences were observed. As mentioned above, although macrophages under certain conditions can kill tumor cells, they can also play potential roles as tumor promoters to secrete a variety of factors that directly stimulate tumor invasion and metastasis. The combing effect of TAM on prognosis in patients with different tumors depends on stimulating factors from two opposite aspects in tumor environments. In this meta-analysis, we reach a conclusion that high TAM infiltration is associated with worsen prognosis in patients with urogenital cancer or gastric cancer, not all cancer type. In other hand, TAM showed antitumorigenic properties in combing 5 studies on colorectal cancer, resulting in improved prognosis.
To further investigate the prognostic value of TAM in different type of cancer, we analyzed the relation between the density of TAM and clinic-pathological factors that was also associated with outcome of cancer patients. As the density of TAM has a negative effect on survival in patients with gastric cancer, breast cancer, bladder cancer, ovary cancer, and oral squamous cell carcinoma, negative effects are also seen in clinic-pathological factors such as TNM staging (breast cancer, bladder cancer and oral squamous cell carcinoma), T status (breast cancer and oral squamous cell carcinoma), lymph node metastasis (breast cancer, bladder cancer and oral squamous cell carcinoma), and distant metastasis (bladder cancer), which contributed to tumor progression and patient survival. Interestingly, there also demonstrated a positive effect of TAM on lymph node metastasis in gastric and ovary cancer, which indicated that high density of TAM was associated with less probability of lymph node metastasis, however, significant negative effect was shown on overall survival. Thus, more studies are needed to clarify this ambivalent phenomenon. Contrary to tumors we mentioned above, our data suggested that an incremental increase in density of TAM improved overall survival in patients with colon cancer, with a homodromous effect on T status. There was also a trend towards lower rate of lymph node metastasis and distant metastasis in TAM rich tumors. In addition, a high density of TAM infiltration was found related to nonmucious type of colon cancer.
The mechanisms behind the oncogenic and anti-tumorigenic effects of TAMs have not been fully elucidated and a great number of studies have focused on explaining these apparently contradictory effects of TAM in different cancer outcome. The functions of TAM in different type of tumors are concerned as the most important determining factor to the prognosis, which are profoundly affected by microenvironmental signals and can range from powerful stimulation of inflammatory responses to induction of immunosuppression [66]. Tumor necrosis factor (TNF)-α, nitric oxide (NO), and monocyte chemoattractant protein (MCP)-1 released from TAM are major intermediate molecules for tumor cell killing [67]–[69], and TAM associated vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) are independent predictor of poor prognostic factor in cancer patients [34]. In addition, a macrophage balance hypothesis between M1 and M2 type macrophages has been proposed and two different macrophage populations range from polarized potent killer M1 cells to alternatively activated M2 macrophages with tumor-promoting capability [70]. However, this study showed no significant relation between the density of two phenotype of TAM and survival of patients. Furthermore, histological classification of the tumor should also be considered as a factor correlated with the function of TAM. A previous study on colon cancer demonstrated that a histologically more malignant phenotype was associated with macrophage infiltration, disorganized matrix deposition, and extensive stromal reaction [71]. In one included study of this meta-analysis, the number of infiltrating TAM was found to be significantly correlated with poor outcome in patients with intestinal type of gastric cancer, but not in patients with diffuse type, indicating that TAM could affect malignant progression and prognosis on the basis of the histological type of gastric cancer [33].
Although the results of meta-analysis are considered as gold standards by authors worldwide [72], potential bias still exists between studies and cannot be completely eliminated. Although Begg's funnel plot and Egger's test were performed in this meta-analysis and found no statistically significant publication bias, results of this study should be interpreted very cautiously and several aspects of importance in this field should be discussed. First of all, we only included studies from which we could extract RR or estimate RR, leading to data inaccessible for data aggregation from studies, which only showed the conclusion on this topic without data presented. Take one excluded study on evaluating the prognostic value of TAM on oral cavity and oropharyngeal squamous cell carcinoma for example, they found that macrophage content was an independent predictor of lymph node metastasis, however, no data was accessible for meta-analysis from this study [73]. Furthermore, considerable attention should be paid to some cancers with few study included in this meta-analysis study. TAM was found associated with worse prognosis in one study on oral cancer [RR = 2.03 (95%CI, 1.47–2.80)] [21] and one study on thyroid cancer [RR = 2.72 (95%CI, 1.26–5.86)] [64]. As meta-analysis could not be performed with such small number of primary studies, more researches are needed in further investigation on these tumors.
Second, macrophages can be identified by cell surface markers, expression of transcriptional factors, the production of cytokines and their functions in vitro [2]. However, we only included literatures evaluating TAM with the use of antibodies to the glycoprotein CD68. Sikert et al quantified TAM by immunohistochemistry with antibodies to PG-M1, KP-1, MRP8, MRP14 and MRP8/14 antigens and found different TAM subpopulation were positively correlated with clinicopathological characteristics in colon cancer [74]. Macrophage differentiation, growth, and chemotaxis are regulated by several growth factors, including colony-stimulating factor (CSF)-1, macrophage chemoattractant protein (MCP)-1 and extracellular matrix protease such as urokinase-type plasminogen activator (uPA) [75]. For example, over expression of CSF is associated with poor prognosis in nongynecological leiomyosarcoma [76]. In breast cancer, TAM density is showed correlated with angiogenesis and poor prognosis [77]–[79]. Ohba et al provide the evidence that uPA has prognostic value in patients with renal cell carcinoma via TAM [80]. Also other factors could be used to evaluate M1 and M2 type macrophages in tumor tissues. As M2 type TAM express high level of interleukin-10 (IL-10) which can be used to discriminate between M1 and M2 macrophages [81], [82], a study assessed IL-10 expression in TAM, and found the high level of IL-10 in TAM significantly correlated with clinical staging and histologic poor differentiation in patients with lung cancer [83]. So, considerable attention should be paid to various kinds of factors related to density of TAM, which might be a potential prognostic marker in solid tumor.
Third, variability in definitions, outcomes, measurements, experimental procedure, and even antibody concentration may contribute to heterogeneity between studies [84]. Multivariate analyses was tried to minimize confounding bias, but the factors controlled for were few and differed between studies. Quality criteria are needed for future studies in this field, and we make the following recommendation: blindly assess the prognostic marker to patient outcome, adequately describe the assay method used for TAM evaluation including antibody concentration and cut-off value staining for TAM high, and precisely define outcome with certain follow-up time. More importantly, in this meta-analysis, some studies have used 20% as the cutoff value, whereas others have chosen score system, mean, median or arbitrary cutoff values, thus cutoff value is a source of considerable interstudy heterogeneity. Although specific synthesis of studies using standardized cutoff value on survival did not differ significantly form the overall result in the total population analysis, conclusions need to be considered cautiously.
In conclusion, it is clear that TAM has protumorigenic as well as antitumorigenic properties in solid tumor. As discussed above, there have been showed a “macrophage balance” on prognosis depending on the microenvironment of the tumor tissue in different type of solid tumor. It may be possible in the future to use or induce activated macrophages to restrain tumor growth and improve patient survival, through altering tumor microenvironment. Moreover, targeted therapies that uniquely strike macrophages may provide innovative therapeutic strategies against tumor progression.
Acknowledgments
We thank all authors whose publications could be included in our meta-analysis, and especially Prof. Liu G (Chinese Evidence-Based Medicine Centre, West China Hospital, Sichuan University, Chengdu, China), Nie Y (West China School of Medicine, Sichuan University, GuoXueXiang 37#, Chengdu, China) for providing us with statistical guidance.
Funding Statement
This work was financially supported by National Natural Science Foundation of China (NSFC 81101729). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bingle L, Brown NJ, Lewis CE (2002) The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196: 254–265. [DOI] [PubMed] [Google Scholar]
- 2. Heusinkveld M, van der Burg SH (2011) Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siveen KS, Kuttan G (2009) Role of macrophages in tumour progression. Immunol Lett 123: 97–102. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555. [DOI] [PubMed] [Google Scholar]
- 5. Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11: 889–896. [DOI] [PubMed] [Google Scholar]
- 6.Virchow R (1863) Aetologie der neoplastichen Geschwulste/ Pathogenie der neoplastischen Geschwulste. Die Krankhaften Geschwulste Berlin: Verlag von August Hirshwald: 57–101.
- 7.Mantovuni BB, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor associated macrophages. Immunol Today 13: p. 2–74. [DOI] [PubMed]
- 8. Mantovuni A (1994) Biology of disease: tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest 71: 5–16. [PubMed] [Google Scholar]
- 9. Koide N, Nishio A, Hiraguri M, Kishimoto K, Nakamura T, et al. (2002) Differences and relationships of thymidine phosphorylase expression in tumor-associated macrophages and cancer cells in squamous cell carcinoma of the esophagus. Dis Esophagus 15: 67–73. [DOI] [PubMed] [Google Scholar]
- 10. Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, et al. (2003) The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 23: 5015–5022. [PubMed] [Google Scholar]
- 11. Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, et al. (2004) Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res 24: 3335–3342. [PubMed] [Google Scholar]
- 12. Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, et al. (2005) Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol 11: 1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, et al. (2005) Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. Journal of Clinical Oncology 23: 8959–8967. [DOI] [PubMed] [Google Scholar]
- 14. Peng J, Ding T, Zheng LM, Shao JY (2006) Influence of tumor-associated macrophages on progression and prognosis of nasopharyngeal carcinoma. Ai Zheng 25: 1340–1345. [PubMed] [Google Scholar]
- 15. Forssell J, Oberg A, Henriksson ML, Senling R, Jung A, et al. (2007) High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 13: 1472–1479. [DOI] [PubMed] [Google Scholar]
- 16. Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, et al. (2008) Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 17. Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P (2009) Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33: 118–126. [DOI] [PubMed] [Google Scholar]
- 18. Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, et al. (2010) The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo SJ, Lin DM, Li J, Liu RZ, Zhou CX, et al. (2007) Tumor-associated macrophages and CD3-zeta expression of tumor-infiltrating lymphocytes in human esophageal squamous-cell carcinoma. Dis Esophagus 20: 107–116. [DOI] [PubMed] [Google Scholar]
- 20. Chai CY, Chen WT, Hung WC, Kang WY, Huang YC, et al. (2008) Hypoxia-inducible factor-1alpha expression correlates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J Clin Pathol 61: 658–664. [DOI] [PubMed] [Google Scholar]
- 21. Liu SY, Chang LC, Pan LF, Hung YJ, Lee CH, et al. (2008) Clinicopathologic significance of tumor cell-lined vessel and microenvironment in oral squamous cell carcinoma. Oral Oncol 44: 277–285. [DOI] [PubMed] [Google Scholar]
- 22. Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, et al. (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26: 2707–2716. [DOI] [PubMed] [Google Scholar]
- 23. Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, et al. (2009) Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol 131: 498–510. [DOI] [PubMed] [Google Scholar]
- 24. Li YW, Qiu SJ, Fan J, Gao Q, Zhou J, et al. (2009) Tumor-infiltrating macrophages can predict favorable prognosis in hepatocellular carcinoma after resection. J Cancer Res Clin Oncol 135: 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan T, Liu JH, Zheng LM, Cai MY, Ding T (2009) Prognostic significance of tumor-associated macrophage infiltration in advanced epithelial ovarian carcinoma. Ai Zheng 28: 323–327. [PubMed] [Google Scholar]
- 26. Ma J, Liu L, Che G, Yu N, Dai F, et al. (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 28. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM (2003) Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer 97: 960–968. [DOI] [PubMed] [Google Scholar]
- 30. Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, et al. (2007) TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: a retrospective study. BMC Cancer 7: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, et al. (2003) Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res 23: 4079–4083. [PubMed] [Google Scholar]
- 32. Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, et al. (2009) Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 9: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawahara A, Hattori S, Akiba J, Nakashima K, Taira T, et al. (2010) Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncol Rep 24: 405–415. [DOI] [PubMed] [Google Scholar]
- 34. Osinsky S, Bubnovskaya L, Ganusevich I, Kovelskaya A, Gumenyuk L, et al. (2011) Hypoxia, tumour-associated macrophages, microvessel density, VEGF and matrix metalloproteinases in human gastric cancer: Interaction and impact on survival. Clinical and Translational Oncology 13: 133–138. [DOI] [PubMed] [Google Scholar]
- 35. Ding T, Xu J, Wang F, Shi M, Zhang Y, et al. (2009) High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol 40: 381–389. [DOI] [PubMed] [Google Scholar]
- 36. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, et al. (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 206: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurahara H, Takao S, Kuwahata T, Nagai T, Ding Q, et al.. (2012) Clinical Significance of Folate Receptor beta-expressing Tumor-associated Macrophages in Pancreatic Cancer. Ann Surg Oncol. [DOI] [PubMed]
- 38. Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, et al. (2010) Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci 101: 1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, et al. (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56: 4625–4629. [PubMed] [Google Scholar]
- 40. Toi M, Ueno T, Matsumoto H, Saji H, Funata N, et al. (1999) Significance of thymidine phosphorylase as a marker of protumor monocytes in breast cancer. Clin Cancer Res 5: 1131–1137. [PubMed] [Google Scholar]
- 41. Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, et al. (2002) Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch 440: 583–588. [DOI] [PubMed] [Google Scholar]
- 42. Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, et al. (2006) Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res 25: 365–372. [PubMed] [Google Scholar]
- 43. Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, et al. (2012) Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 65: 159–163. [DOI] [PubMed] [Google Scholar]
- 44. Salvesen HB, Akslen LA (1999) Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer 84: 538–543. [DOI] [PubMed] [Google Scholar]
- 45. Hashimoto I, Kodama J, Seki N, Hongo A, Miyagi Y, et al. (2000) Macrophage infiltration and angiogenesis in endometrial cancer. Anticancer Res 20: 4853–4856. [PubMed] [Google Scholar]
- 46. Soeda S, Nakamura N, Ozeki T, Nishiyama H, Hojo H, et al. (2008) Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma. Gynecol Oncol 109: 122–128. [DOI] [PubMed] [Google Scholar]
- 47. Espinosa I, Jose Carnicer M, Catasus L, Canet B, D'Angelo E, et al. (2010) Myometrial invasion and lymph node metastasis in endometrioid carcinomas: tumor-associated macrophages, microvessel density, and HIF1A have a crucial role. Am J Surg Pathol 34: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 48. Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, et al. (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17: 445–451. [DOI] [PubMed] [Google Scholar]
- 49. Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, et al. (2000) Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res 60: 5857–5861. [PubMed] [Google Scholar]
- 50. Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, et al. (2011) Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int 107: 1918–1922. [DOI] [PubMed] [Google Scholar]
- 51. Heller DS, Hameed M, Cracchiolo B, Wiederkehr M, Scott D, et al. (2003) Presence and quantification of macrophages in squamous cell carcinoma of the cervix. Int J Gynecol Cancer 13: 67–70. [DOI] [PubMed] [Google Scholar]
- 52. Kawanaka T, Kubo A, Ikushima H, Sano T, Takegawa Y, et al. (2008) Prognostic significance of HIF-2alpha expression on tumor infiltrating macrophages in patients with uterine cervical cancer undergoing radiotherapy. J Med Invest 55: 78–86. [DOI] [PubMed] [Google Scholar]
- 53. Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, et al. (2000) Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7: 263–269. [DOI] [PubMed] [Google Scholar]
- 54. Takayama H, Nishimura K, Tsujimura A, Nakai Y, Nakayama M, et al. (2009) Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J Urol 181: 1894–1900. [DOI] [PubMed] [Google Scholar]
- 55. Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T (2004) Upregulation of bikunin in tumor-infiltrating macrophages as a factor of favorable prognosis in ovarian cancer. Gynecol Oncol 94: 725–734. [DOI] [PubMed] [Google Scholar]
- 56. Takanami I, Takeuchi K, Kodaira S (1999) Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology 57: 138–142. [DOI] [PubMed] [Google Scholar]
- 57. Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, et al. (2005) Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol 23: 953–964. [DOI] [PubMed] [Google Scholar]
- 58. Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, et al. (2007) Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J 30: 627–632. [DOI] [PubMed] [Google Scholar]
- 59. Al-Shibli Ibrahim K, Al-Saad S, Donnem T, Persson M, Bremnes R, et al. (2009) The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Virchows Archiv 455: S85. [DOI] [PubMed] [Google Scholar]
- 60. Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, et al. (2010) Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol 5: 1507–1515. [DOI] [PubMed] [Google Scholar]
- 61. Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, et al. (2011) Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol 28: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 62.Fujii N, Shomori K, Shiomi T, Nakabayashi M, Takeda C, et al.. (2012) Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med. [DOI] [PubMed]
- 63. Lin JY, Li XY, Tadashi N, Dong P (2011) Clinical significance of tumor-associated macrophage infiltration in supraglottic laryngeal carcinoma. Chin J Cancer 30: 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA (2008) Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer 15: 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, et al. (2011) Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer 117: 5234–5244. [DOI] [PubMed] [Google Scholar]
- 66. Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 67. Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, et al. (1992) Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256: 225–228. [DOI] [PubMed] [Google Scholar]
- 68. Bonta IL, Ben-Efraim S (1993) Involvement of inflammatory mediators in macrophage antitumor activity. J Leukoc Biol 54: 613–626. [DOI] [PubMed] [Google Scholar]
- 69. Nesbit M, Schaider H, Miller TH, Herlyn M (2001) Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 166: 6483–6490. [DOI] [PubMed] [Google Scholar]
- 70. Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, et al. (2008) Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol 83: 1136–1144. [DOI] [PubMed] [Google Scholar]
- 71. Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, et al. (2005) Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol 207: 147–155. [DOI] [PubMed] [Google Scholar]
- 72. Stewart LA, Parmar MK (1993) Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 341: 418–422. [DOI] [PubMed] [Google Scholar]
- 73. Marcus B, Arenberg D, Lee J, Kleer C, Chepeha DB, et al. (2004) Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer 101: 2779–2787. [DOI] [PubMed] [Google Scholar]
- 74. Sickert D, Aust DE, Langer S, Haupt I, Baretton GB, et al. (2005) Characterization of macrophage subpopulations in colon cancer using tissue microarrays. Histopathology 46: 515–521. [DOI] [PubMed] [Google Scholar]
- 75. Liguori M, Solinas G, Germano G, Mantovani A, Allavena P (2011) Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers 3: 3740–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Espinosa I, Edris B, Lee CH, Cheng HW, Gilks CB, et al. (2011) CSF1 expression in nongynecological leiomyosarcoma is associated with increased tumor angiogenesis. Am J Pathol 179: 2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Valkovic T, Lucin K, Krstulja M, Dobi-Babic R, Jonjic N (1998) Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res Pract 194: 335–340. [DOI] [PubMed] [Google Scholar]
- 78. Ueno T, Toi M, Saji H, Muta M, Bando H, et al. (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 6: 3282–3289. [PubMed] [Google Scholar]
- 79. Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, et al. (2009) Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 125: 1276–1284. [DOI] [PubMed] [Google Scholar]
- 80. Ohba K, Miyata Y, Kanda S, Koga S, Hayashi T, et al. (2005) Expression of urokinase-type plasminogen activator, urokinase-type plasminogen activator receptor and plasminogen activator inhibitors in patients with renal cell carcinoma: correlation with tumor associated macrophage and prognosis. J Urol 174: 461–465. [DOI] [PubMed] [Google Scholar]
- 81. Avila-Moreno F, Lopez-Gonzalez JS, Galindo-Rodriguez G, Prado-Garcia H, Bajana S, et al. (2006) Lung squamous cell carcinoma and adenocarcinoma cell lines use different mediators to induce comparable phenotypic and functional changes in human monocyte-derived dendritic cells. Cancer Immunol Immunother 55: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH (2006) Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol 79: 285–293. [DOI] [PubMed] [Google Scholar]
- 83. Wang R, Lu M, Zhang J, Chen S, Luo X, et al. (2011) Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res 30: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Simon R, Altman DG (1994) Statistical aspects of prognostic factor studies in oncology. Br J Cancer 69: 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]