Abstract
The essential requirement for copper in early development is dramatically illustrated by Menkes disease, a fatal neurodegenerative disorder of early childhood caused by loss-of-function mutations in the gene encoding the copper transporting ATPase ATP7A. In this study, we generated mice with enterocyte-specific knockout of the murine ATP7A gene (Atp7a) to test its importance in dietary copper acquisition. Although mice lacking Atp7a protein within intestinal enterocytes appeared normal at birth, they exhibited profound growth impairment and neurological deterioration as a consequence of copper deficiency, resulting in excessive mortality prior to weaning. Copper supplementation of lactating females or parenteral copper injection of the affected offspring markedly attenuated this rapid demise. Enterocyte-specific deletion of Atp7a in rescued pregnant females did not restrict embryogenesis; however, copper accumulation in the late-term fetus was severely reduced, resulting in early postnatal mortality. Taken together, these data demonstrate unique and specific requirements for enterocyte Atp7a in neonatal and maternofetal copper acquisition that are dependent on dietary copper availability, thus providing new insights into the mechanisms of gene-nutrient interaction essential for early human development.
Keywords: copper, micronutrient, mineral, nutrition, transporter
the importance of micronutrient acquisition in the perinatal period is illustrated by recent data highlighting the worldwide prevalence of maternal micronutrient deficiencies and the detrimental effects of such deficiencies on pregnancy outcome (1). The consequences of maternal nutrient deprivation were most clearly demonstrated in the Dutch Famine Study, revealing that inadequate nutrient availability during human gestation directly increases the likelihood of birth defects (30). Further studies have established the now well-recognized link between maternal folate supplementation and the suppression of neural tube defects and the role of maternal iron deficiency on neonatal brain development (3, 15). Despite considerable epidemiological data indicating the benefits of nutrient supplementation, we understand very little regarding either the mechanisms of micronutrient metabolism during this critical time period or the genetic factors that predispose to these abnormal developmental phenotypes under conditions of suboptimal nutrition.
Copper is an essential micronutrient that plays well-defined roles in cellular respiration, iron metabolism, neurotransmitter production, pigment formation, peptide amidation, and connective tissue biosynthesis (34). Extensive clinical and experimental data reveal that a lack of copper has detrimental effects on gestation and perinatal development (12), a finding dramatically illustrated in patients with Menkes disease, an inherited disorder of copper metabolism that is typically lethal during early childhood arising from loss-of-function mutations in the ATP7A gene (10). The ATP7A protein is a ubiquitously expressed copper pump with roles that include copper transport into secretory compartments for the metallation of copper-dependent enzymes and the export of copper across the plasma membrane. Menkes patients exhibit symptoms consistent with hypoactivity of copper-dependent enzymes, including severe mental retardation associated with neurodegeneration and seizures, reduced melanin pigmentation, and impaired extracellular matrix formation. Mutations in ATP7A can also manifest as occipital horn syndrome, a connective tissue disorder arising from copper deficiency in which patients are spared the neurological symptoms of Menkes disease (31). More recently, certain partial loss-of-function mutations in ATP7A were shown to cause a distal motor neuropathy known as X-linked spinal muscular atrophy type 3 (SMAX3) (13, 39). Although it is unknown how such allelic variants of the ATP7A gene give rise to diseases that differentially affect specific organs, these disorders clearly underscore the need to elucidate the tissue-specific roles of the ATP7A protein.
Recent studies have revealed a critical role for the enterocyte plasma membrane copper importer, Ctr1, in intestinal copper uptake and neonatal survival, indicating a key role for this organ in ensuring adequate copper is available for postnatal development (25). Although studies have shown that ATP7A protein is expressed in the enterocyte (23), it is unclear the extent to which growth and development during the postnatal period is dependent on copper acquisition via ATP7A expression in the intestine. This question cannot be adequately addressed in Menkes patients or in traditional mouse models of Menkes disease (e.g., mottled mice), in part because the phenotypic consequences of ATP7A mutation originate in utero (11, 33), and loss-of-function mutations in mouse Atp7a are embryonic lethal (14, 24, 36). In this study, we generated enterocyte-specific Atp7a knockout mice to critically elucidate the requirement for Atp7a activity in dietary copper transport across the intestine. We find that the copper demands of pregnancy and perinatal growth require Atp7a expression in the maternal and neonatal enterocyte, respectively, highlighting the important contribution of dietary copper malabsorption to Menkes disease and the biology of gene-nutrient interactions relevant to the developmental pathophysiology of copper metabolism.
MATERIALS AND METHODS
Animals.
All animal husbandry and euthanasia procedures were approved by the Animal Care and Use Committee of the University of Missouri. All mice were on the C57BL6 strain background. Mice were housed in vented cages with 12-h light-dark cycle, and food and water were provided ad libitum. Picolab diet 5053 (13 ppm Cu) was provided to mice at weaning and Picolab diet 5058 (17 ppm Cu) was provided during pregnancy and lactation (PMI International, St. Louis, MO).
Conditional knockout of Atp7a.
Heterozygous floxed Atp7afl/+ females with LoxP sites flanking exon 11 generated previously in our laboratory (36) were used to generate enterocyte-specific knockout of Atp7a by crossing with B6.SJL-Tg(Vil-cre)997Gum/J males that were purchased from the Jackson Laboratory (16). The allelic status of the Atp7a gene in offspring was determined by PCR analysis of tail genomic DNA by using the primer pairs P1 (5′-GACAATACTACACTGACCATATTCA-3′) and P2 (5′-GTTCCACAGAAACTATATGCCTGGG-3′), as previously described (36). The Cre transgene was detected by PCR of tail DNA using primers CreF (5′-GATCGCTGCCAGGATATACG-3′) and CreR (5′-AATCGCCATCTTCCAGCAG-3′). Sex determination was performed by PCR as described previously (2).
SDS-PAGE and Western blot analysis.
Tissue samples were homogenized in ice-cold PBS at pH 7.4, and protein lysates were prepared by sonicating cell pellets in lysis buffer containing 2.5 mM Tris·HCl (pH 7.4), 2% SDS, 1% Triton X-100, 1 mM EDTA, and Complete protease inhibitor (Roche Molecular Biochemicals, Indianapolis, IN). Protein lysates were fractionated by 7.5% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 1% casein solution and incubated in blocking buffer at 4°C overnight with a rabbit anti-Atp7a antibody raised against the last 33 amino acids of the COOH-terminus (1:5,000 dilution) (36) or mouse β-actin antibody (1:5,000) (abcam, Cambridge, MA). Horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (1:5,000) were used as secondary antibodies, and blots were developed by using the SuperSignal West Pico Substrate according to the manufacturer's instructions (Pierce, Rockford, IL).
Tissue metal measurements.
Tissues or whole fetuses were wet digested with HNO3 (Trace Metal grade; Fisher Scientific, Pittsburgh, PA) and analyzed for total copper and iron content by flame atomic absorption spectroscopy (model 1100B, Perkin-Elmer, Waltham, MA) (28).
Enzyme assays.
Hemoglobin concentrations in whole blood were determined spectrophotometrically as metcyanohemoglobin (29), and serum ceruloplasmin activity was measured by the oxidation of o-dianisidine as previously described (28). An in-gel colorimetric l-3,4-dihydroxyphenylalanine (l-DOPA) oxidase assay was used to detect tyrosinase activity in skin lysates as described previously (27). Cytochrome-c oxidase activity was measured in total brain lysates by using a cytochrome-c oxidase assay kit according to the manufacturer's instructions (CYTOCOX1 kit; Sigma, St. Louis, MO).
Microscopy.
For immunofluorescence microscopy, tissues were dissected and snap frozen in isopentane and stored at −80°C for later use. Cryosections of 10-μm thickness were prepared and fixed in ice-cold acetone for 5 min, washed several times with Tris-buffered saline (TBS) containing 1% Tween-20 (TBST), and blocked for 2 h in 1% casein solution in TBS. Sections were then incubated with rabbit anti-Atp7a antibody (1:3,000) overnight at 4°C, washed three times with TBST and then incubated for 1 h at room temperature with Alexa Fluor 488 goat anti-rabbit IgG (1:4,000) (Invitrogen, Grand Island, NY). Where indicated, endothelial cells were detected by using mouse anti-CD31 antibody (1:3,000; Novus Biologicals, Littleton, CO) followed by Alexa 568-conjugated donkey anti-mouse antibodies (1:5,000; Invitrogen). For histological analysis of tissues, anesthetized mice were perfused with 4% paraformaldehyde in PBS via intracardiac injection. Tissues were removed, postfixed overnight in 4% paraformaldehyde at 4°C, dehydrated overnight in 70% ethanol, and embedded in paraffin. Sections of 4 μm were prepared and stained with hematoxylin and eosin.
Copper treatments.
Parenteral copper rescue of P6 pups involved intraperitoneal injection with 10 μg CuCl2 per gram body weight dissolved in 0.9% NaCl solution (saline). Saline alone was injected as a negative control. Intraperitoneal injection of 100 μg CuCl2 in 0.9% NaCl solution was used to inject pregnant Atp7aint/int mice at embryonic day 17 (E17). Where indicated, copper was supplemented to the drinking water of lactating dams as 100 μg/ml copper acetate. Diets were otherwise identical.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism 5.0. Unpaired t-test was used to compare values (means ± SE) between wild-type and mutant mice as described in the figure legends. P < 0.05 was considered statistically significant.
RESULTS
Deletion of Atp7a in intestinal enterocytes results in systemic copper deficiency and high neonatal mortality.
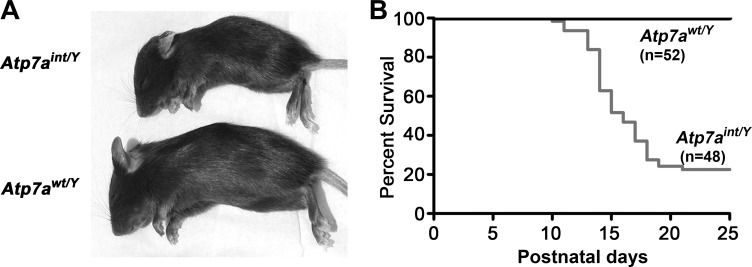
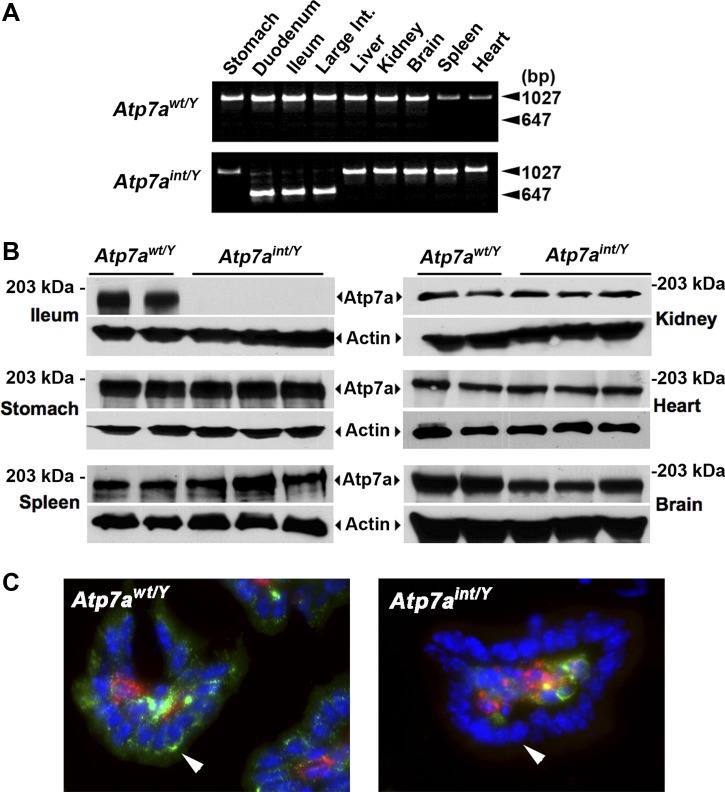
To generate mice in which Atp7a deletion was restricted to intestinal epithelial cells, heterozygous floxed female mice (Atp7afl/+) were crossed with males expressing Cre recombinase from the Villin gene promoter within enterocytes along the entire intestinal tract (16). The resulting hemizygous Cre-positive male progeny, Atp7afl/Y;VilCre+, hereafter referred to as Atp7aint/Y, were born at the expected Mendelian frequency and during the first week of birth were indistinguishable from Atp7afl/Y;VilCre− littermate controls, hereafter designated Atp7awt/Y. However, by the second week, ∼80% of Atp7aint/Y mice had developed severe growth impairment relative to Atp7awt/Y littermates (P16 weight: 4.7 ± 0.6 vs. 7.5 ± 0.59 g; means ± SE; P < 0.01) and a high mortality prior to weaning (Fig. 1, A and B). PCR genotyping to assess the Atp7a allele status of Atp7aint/Y mice indicated that exon 11 was excised with high efficiency throughout the intestine, with no detectable excision in stomach, liver, kidney, brain, spleen, and heart (Fig. 2A). Western blot analysis confirmed a loss of Atp7a protein restricted to the intestine of Atp7aint/Y mice (Fig. 2B). Immunofluorescence microscopy of duodenal cryosections with anti-Atp7a antibodies revealed robust staining of Atp7a protein within perinuclear and vesicular compartments of the enterocytes of Atp7awt/Y mice, which was absent in the knockout Atp7aint/Y mice (Fig. 2C). Atp7a expression was detected in CD31-positive endothelial cells of the Atp7aint/Y mice indicating that deletion of Atp7a did not occur in the microvasculature of the microvilli (Fig. 2C).
Fig. 1.
Conditional deletion of Atp7a in enterocytes. A: Atp7awt/Y (wild type) and Atp7aint/Y (intestinal knockout) male siblings at postnatal day P16. B: Kaplan-Meier postnatal survival curve for the Atp7awt/Y and mutant Atp7aint/Y offspring.
Fig. 2.
Tissue-specific knockout of Atp7a in enterocytes. A: PCR analysis of tissue genomic DNA from Atp7awt/Y and Atp7aint/Y siblings at P16. Note the smaller PCR product corresponding to the deleted allele is restricted to intestinal tissue of the mutant. Int., intestine. B: Atp7a protein levels in the ileum of Atp7awt/Y (n = 2) and Atp7aint/Y (n = 3) littermates at P16 were detected by immunoblotting with anti-Atp7a antibody at 1:5,000 dilution. Actin was detected as a loading control. C: immunofluorescence detection of Atp7a protein within cryosections of duodenum of Atp7awt/Y and Atp7aint/Y mice at P16. Atp7a protein (green) was probed with rabbit anti-Atp7a antibodies and detected by use of Alexa 488-conjugated goat anti-rabbit antibodies. Endothelial cells (red) were probed with mouse anti-CD31 antibodies and detected by use of Alexa 568-conjugated donkey anti-mouse antibodies. Nuclei (blue) were labeled with the 4,6-diamidino-2-phenylindole (DAPI) stain. Note the absence of Atp7a protein in the perinuclear region epithelial cells of microvilli in mutant mice in contrast to wild type (arrowheads). As expected, the Atp7a protein was expressed in the centrally located CD31-positive endothelial cells in the microvilli of both wild-type and mutant mice.
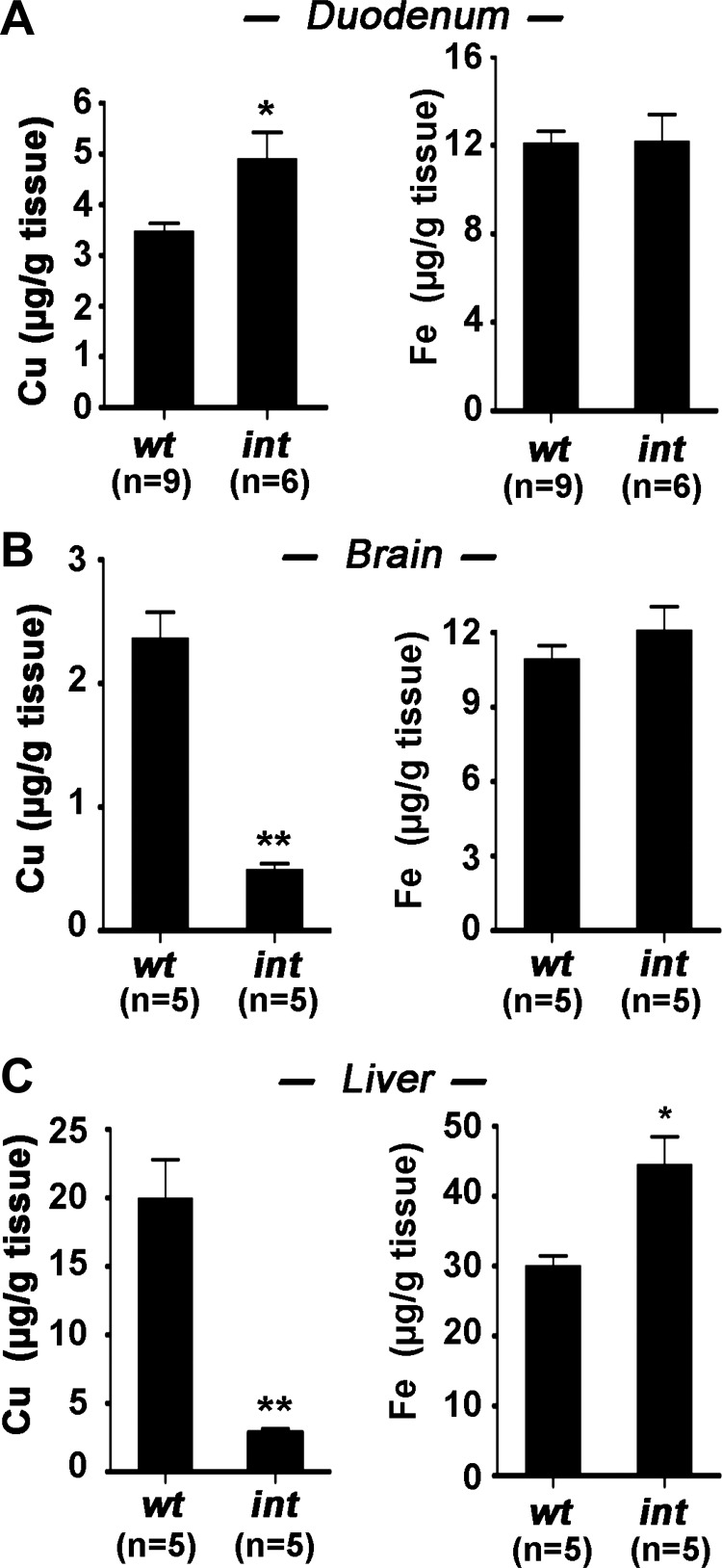
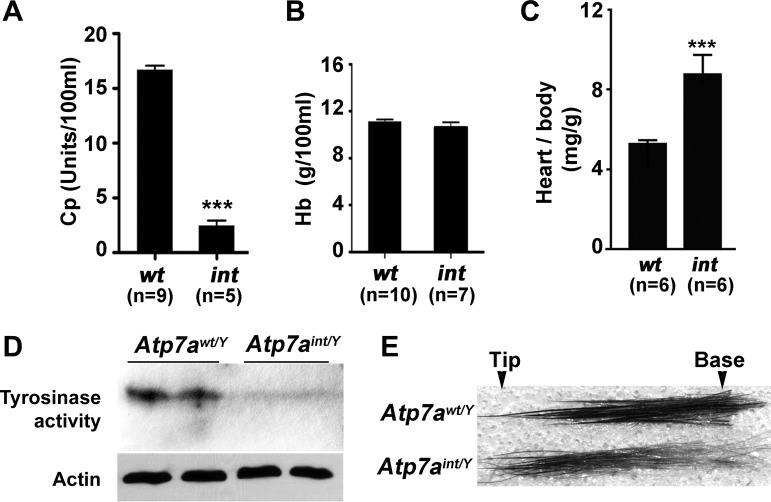
Copper levels were significantly elevated in the intestine of Atp7aint/Y mice at day P16 relative to wild-type mice (Fig. 3A), whereas copper levels in brain and liver were markedly lower (Fig. 3, B and C), consistent with a peripheral copper deficiency arising from intestinal entrapment of copper. Livers from these Atp7aint/Y mice contained 25% higher iron levels than wild-type mice (Fig. 3C), which may be a consequence of the aceruloplasminemia (9), since compared with wild-type mice the serum of Atp7aint/Y mice exhibited hypoactivity of the copper-dependent ferroxidase ceruloplasmin (Fig. 4A). However, the Atp7aint/Y mice also exhibited normal hemoglobin levels (Fig. 4B), and normal iron levels were present in the intestine and brain of Atp7aint/Y mice, indicating that dietary iron absorption was not affected by Atp7a deletion in the enterocyte (Fig. 3, A and B). Other phenotypes consistent with a systemic copper deficiency in the Atp7aint/Y mice included cardiac hypertrophy (Fig. 4C) and reduced activity of skin tyrosinase, a melanin-producing copper-dependent enzyme (Fig. 4D). Moreover, there was a progressive decline in melanin production in the Atp7aint/Y mice as evidenced by a marked reduction in pigmentation toward the base of the hair shaft (i.e., new growth), consistent with a gradual depletion of copper stores during early postnatal growth (Fig. 4E).
Fig. 3.
Copper and iron concentrations in different tissues. Copper and iron was measured in small intestine (duodenum) (A), brain (B), and liver (C) of Atp7awt/Y (wt) and Atp7aint/Y (int) mice at day P16 using atomic absorption spectroscopy. Mean values are expressed as micrograms metal per gram of wet tissue weight ± SE. *P < 0.05, **P < 0.01. All Atp7aint/Y mice were exhibiting symptoms of copper deficiency.
Fig. 4.
Atp7aint/Y mice exhibit signatures of Cu deficiency. A: copper-dependent ceruloplasmin (Cp) activity in the plasma of Atp7awt/Y and Atp7aint/Y mice at day P16. Values are expressed as means ± SE; ***P < 0.001. B: blood hemoglobin (Hb) levels (mean ± SE) in Atp7awt/Y and Atp7aint/Y mice at day P16. No significant differences. C: cardiac hypertrophy in Atp7aint/Y mice at P16. Values are expressed as average ratio of heart to body weight ± SE; ***P < 0.001. D: tyrosinase activity is reduced in the skin of Atp7aint/Y mice at P16. In-gel oxidation of l-3,4-dihydroxyphenylalanine (l-DOPA) was used to assay tyrosinase activity in 10 μg of skin lysates fractionated by use of nonreducing SDS-PAGE. Immunoblot detection of actin was used to indicate protein loading. E: microscopic analysis of hair taken from the flanks of Atp7awt/Y and Atp7aint/Y mice at P16. Note the progressive loss of pigmentation toward the base of the hair in Atp7aint/Y mice consistent with a depletion of copper stores during neonatal growth.
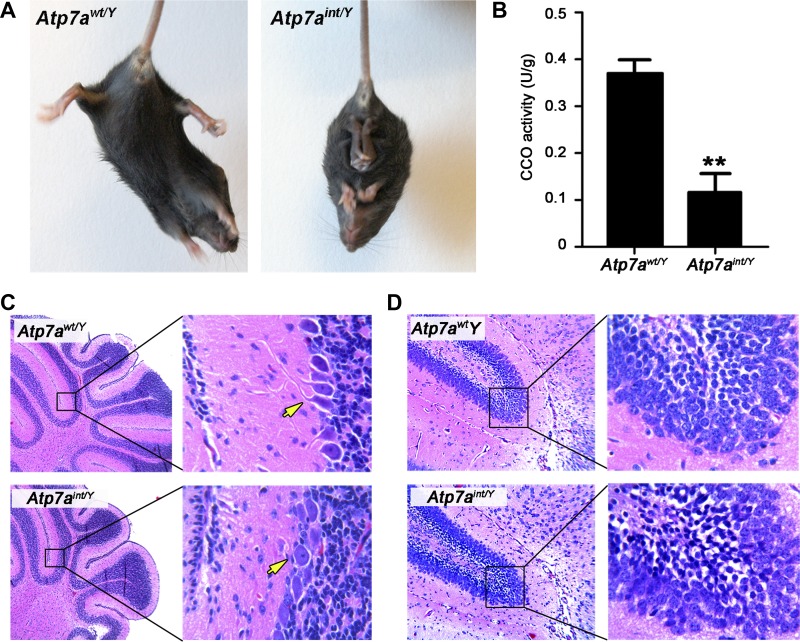
At ∼2 wk of age, Atp7aint/Y mice had developed prominent neurological defects that initially presented as abnormal limb clasping upon tail elevation (Fig. 5A), but then progressed to severe ataxia and seizure (Supplemental Video S1; supplemental material for this article is available online at the Journal website). The activity of copper-dependent cytochrome-c oxidase was significantly reduced in whole brain lysates of Atp7aint/Y mice (Fig. 5B), and regional neuropathology consistent with a defect in brain development was observed in these animals including a lack of arborization of Purkinje cell dendrites in the cerebellum (Fig. 5C) and the presence of pyknotic nuclei in the granule cell neurons of the dentate gyrus (Fig. 5D).
Fig. 5.
Neurological symptoms of copper deficiency in Atp7aint/Y mice at P16. A: Atp7aint/Y mice exhibit clasping of the hind limbs upon elevation by the tail in contrast to the normal splaying of hind legs in Atp7awt/Y mice. B: cytochrome-c oxidase (CCO) activity in whole brain extracts of Atp7awt/Y and Atp7aint/Y mice at P16 (n = 3). Data are shown as units/g protein (mean ± SE). **P < 0.01. C: neuropathology in the cerebellum of Atp7aint/Y mice. Paraffin-embedded whole brain sections were stained with hematoxylin and eosin. Dendritic arborization in the Purkinje cell layer is readily observed in the cerebellum of control Atp7awt/Y mice (arrow), which is lacking in the Atp7aint/Y cerebellum. D: hematoxylin and eosin staining of the granular cell layer of the dentate gyrus reveals numerous darkly stained pyknotic nuclei in Atp7aint/Y mice, which are absent in Atp7awt/Y mice.
Rescue of Atp7aint/Y mice by copper supplementation.
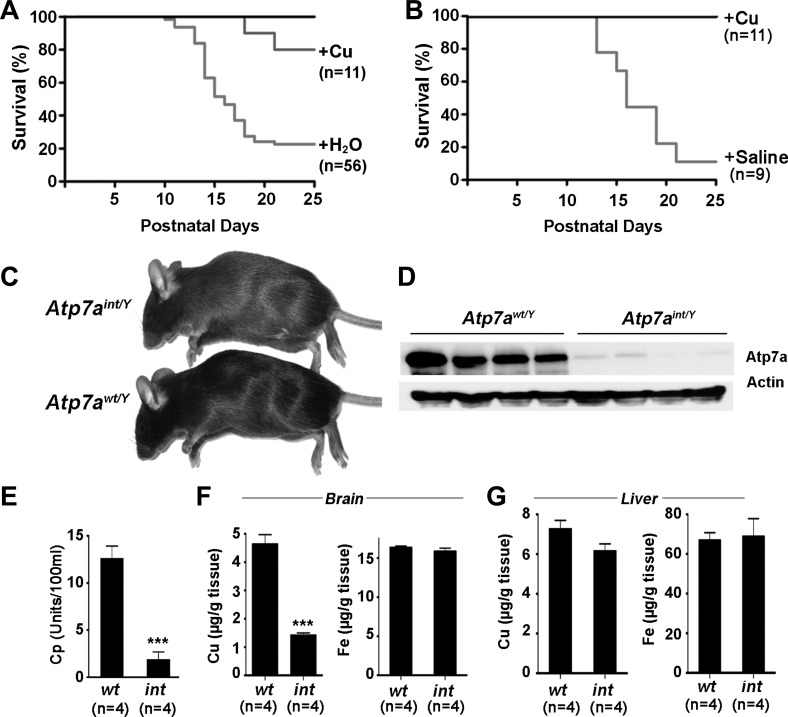
Despite these striking neurological findings, marked growth impairment, and early mortality in Atp7aint/Y mice, ∼20% of these mutants survived beyond weaning, which was not attributable to variances in the effectiveness of Cre recombinase-mediated Atp7a gene deletion (Wang Y and Petris MJ, unpublished observations). These observations suggested the possible existence of an Atp7a-independent pathway of intestinal copper egress in neonatal mice that may partially compensate for the loss of Atp7a. Consistent with this postulate, when copper was added to the maternal drinking water at parturition to enrich copper in the maternal milk there was a marked abrogation of the growth impairment and neurological phenotype in the Atp7aint/Y offspring, resulting in a fourfold increase in survival (Fig. 6A). This was associated with a 25% increase in copper concentration in the stomach contents of pups suckled from copper-supplemented dams compared with pups suckled from dams on deionized water (2.10 ± 0.07 vs. 2.75 ± 0.13 μg/g; means ± SE; N = 6; P < 0.001), suggesting that relatively small increases in copper consumption are sufficient to bypass the requirement for Atp7a in the enterocyte.
Fig. 6.
Rescue of Atp7aint/Y mice with lactational or parenteral copper supplementation. A: rescue of Atp7aint/Y pups by postnatal copper supplementation of lactating dams. Kaplan-Meier survival curve of Atp7aint/Y mice that suckled from dams whose drinking water was either supplemented with 100 μg/ml copper acetate (+Cu) or left untreated (+H2O) from the day of parturition until weaning. B: rescue of Atp7aint/Y pups by a single parenteral copper injection. Kaplan-Meier survival curve of Atp7aint/Y pups given a single intraperitoneal (IP) injection of either 10 μg CuCl2/g body wt (+Cu) in normal saline or saline alone at P6. C: a mature Atp7aint/Y mutant mouse and Atp7awt/Y littermate at day P60 after receiving a copper injection at P6. Note the slight hypopigmentation of the Atp7aint/Y coat. D: immunoblot analysis of Atp7a protein in the ileum of the copper-rescued mutant Atp7aint/Y and control Atp7awt/Y mice at P60 (n = 4). Both control and mutant mice were given a single IP copper injection at P6. E: ceruloplasmin (Cp) activity (mean ± SE) in the plasma at day P60 of the copper-rescued mutant Atp7aint/Y and control Atp7awt/Y mice. ***P < 0.001. F and G: copper concentrations (mean ± SE) in brain (F) and liver (G) of rescued Atp7aint/Y and control Atp7awt/Y mice at day P60. ***P < 0.001.
Further evidence of a critical role for Atp7a in copper transport across the intestinal tract was the finding that complete rescue with long-term survival of Atp7aint/Y mice could also be attained by the administration of a single intraperitoneal injection of CuCl2 in saline (10 μg copper/g total body wt) between postnatal days P5 and P7 (Fig. 6B). Although adult copper-rescued Atp7aint/Y mice were of normal size (Fig. 6C) and exhibited no evidence of cardiac hypertrophy or neuropathology, this phenotypic correction was not the result of reestablishment of Atp7a expression in the intestine (Fig. 6D). Furthermore, a subtle hypopigmentation of the fur of the copper-rescued Atp7aint/Y mice suggested an underlying copper deficiency (Fig. 6C), and this was confirmed by low serum ceruloplasmin activity (Fig. 6E) and strikingly low copper levels in the brain (Fig. 6F), despite normal hepatic copper content (Fig. 6G). Additional studies suggested that the minimal amount of injected copper during the perinatal period required for long-term survival of Atp7aint/Y mice was 4 μg copper/g body wt. Taken together, these findings reveal that relatively small variations in copper availability underscore dramatic phenotypic differences in Atp7aint/Y mice and suggest a critical role for Atp7a in acquiring the minimum threshold of copper essential for neonatal growth and development.
Maternal expression of intestinal Atp7a is essential to produce viable offspring.
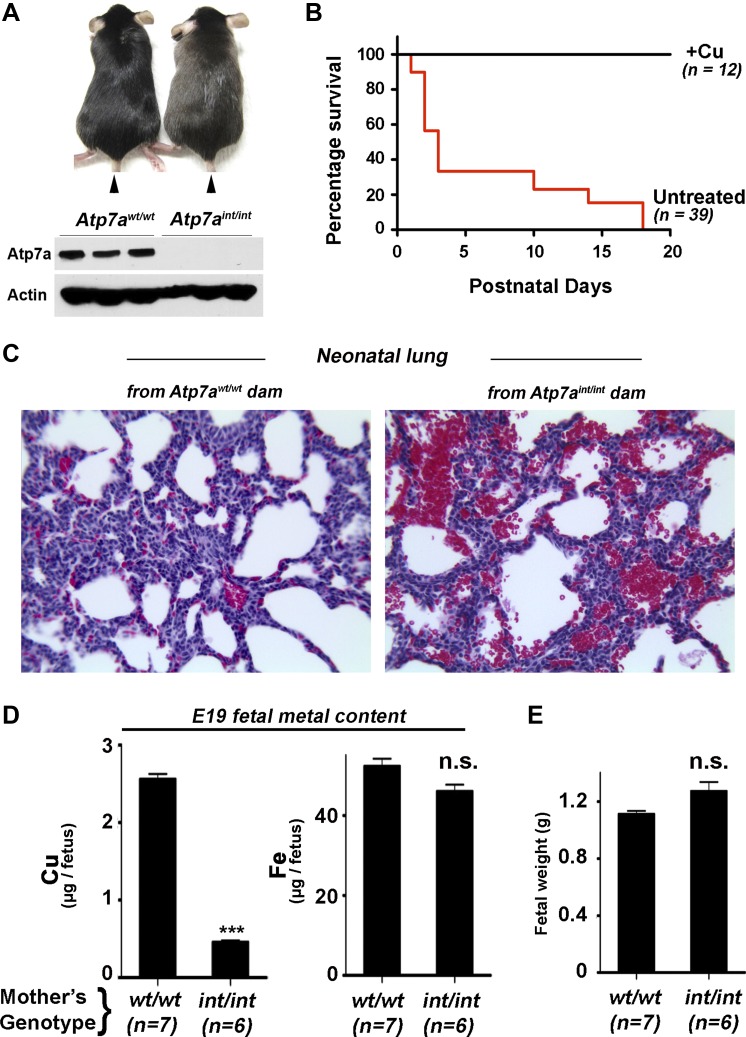
We next determined whether maternal expression of Atp7a in the enterocyte was required for successful gestation. Copper-dependent rescue of male Atp7aint/Y mice permitted matings resulting in homozygous female Atp7aint/int pups, and these offspring exhibited severe defects in growth and viability that were identical to those observed in the male Atp7aint/Y mice. As with the males, female Atp7aint/int pups were rescued by a single copper injection given at P6, and at P60 they exhibited the same phenotypic characteristics as rescued males such as hypopigmentation (Fig. 7A), low serum ceruloplasmin activity and low brain copper levels (Wang Y and Petris MJ, unpublished observations). When mated with wild-type C57BL6 males, these Atp7aint/int females produced fully developed live-born offspring at full term that were of normal weight; however, 70% of these pups died suddenly within 3 days of birth with 100% mortality prior to weaning (Fig. 7B). Upon examination of the internal organs of P3 mice, bleeding in the lungs was noted and confirmed histologically by the abundance of red blood cells in the alveolar sacs (Fig. 7C), suggesting that pulmonary asphyxia was a likely cause of sudden death in these mice. Importantly, pups born to Atp7aint/int females could be fully rescued by intraperitoneal (IP) copper injection of pregnant Atp7aint/int females at E17 followed by IP copper injection of the offspring at day P5 (Fig. 7B; +Cu). These observations reveal a necessary role for Atp7a for intestinal copper acquisition during late stage pregnancy when maternal copper transfer across the placenta peaks in mammals (20). Consistent with this role was the finding that fetuses harvested from pregnant Atp7aint/int females just prior to parturition (E19) contained less than 20% of the copper content of the same-aged fetuses derived from wild-type females (Fig. 7D), despite a normal weight (Fig. 7E). These findings demonstrate that Atp7a expression in the maternal enterocyte is necessary to ensure late-term copper mobilization to the developing fetus for postnatal growth and development.
Fig. 7.
Perinatal mortality in offspring from female mice with enterocyte-specific Atp7a deletion. A: comparison of copper-rescued Atp7aint/int and Atp7awt/wt female mice at P60. Atp7aint/int and Atp7awt/wt pups were given a single copper injection as described in Fig. 4. Note the slight hypopigmentation of the Atp7aint/int mutant compared with the control. Bottom: Atp7a protein levels in the ileum of Atp7aint/int and Atp7awt/wt female mice at P60 after perinatal copper injection. B: high perinatal mortality of pups born to Atp7aint/int and rescue by copper injection. A high degree of mortality within 3 days parturition is observed for pups born to Atp7aint/int dams (untreated). In the copper-treated group (+Cu), pregnant Atp7aint/int dams were injected at E17 with 100 μg of CuCl2 and pups born to these dams were subsequently injected with 10 μg CuCl2/g body wt at P6. C: pulmonary hemorrhage in pups born to Atp7aint/int or Atp7awt/wt dams. Lung sections stained with hematoxylin and eosin reveal abundant red blood cells within the alveolar sacs of P5 offspring born to Atp7aint/int dams, which are absent in pups born to Atp7awt/wt dams. D: Cu and Fe levels (mean ± SE) in the E19 fetuses of pregnant Atp7aint/int and Atp7awt/wt mice; ***P < 0.0001 Student's t-test. E: mean fetal weights in pregnant Atp7aint/int were not significantly different (n.s.) from those of Atp7awt/wt mice at E19.
DISCUSSION
The data in this study demonstrate an essential role for enterocyte Atp7a in perinatal copper homeostasis. Affected mice developed growth failure, severe neurological disease prior to weaning, reduced copper content, and diminished copper-dependent enzyme activities in peripheral organs compared with wild-type mice, data that are supportive of a critical role for Atp7a in copper transport across the intestinal mucosa. The finding that parenteral copper prevented the onset of severe ataxia and seizure, restored normal growth, and permitted long-term survival of Atp7aint/Y mice confirmed this essential functional role. Moreover, that Atp7aint/Y mice could be rescued by small increases in milk copper content in lactating dams provided evidence of a secondary pathway of copper acquisition that under these conditions was adequate to permit survival; however, the identity of this pathway is currently unknown. We speculate that Atp7a-mediated transport across of the gastric mucosa might function as a minor secondary pathway for copper acquisition, which is consistent with robust expression of Atp7a protein in the stomach (Fig. 2B) and with previous studies in rats showing the existence of a pathway for copper absorption across the gastric mucosa (6, 35). Another possibility is that the Atp7b protein, a copper exporter that is weakly expressed in the enterocyte (37), might partially compensate for the loss of Atp7a. However, our preliminary investigations found no evidence that Atp7b abundance was altered in mice lacking intestinal Atp7a (Wang Y and Petris MJ, unpublished observations). Whether small differences in this alternative pathway account for the 20% survival in Atp7aint/Y mice that did not receive copper supplementation is unknown; however, this survival trait was not heritable, suggesting that it may be due to stochastic events (Wang Y and Petris MJ, unpublished observations).
Our studies of pregnant female mice also revealed enterocyte-specific requirements for Atp7a in meeting critical steps in the perinatal period. Although the copper starvation imposed by a lack of Atp7a in the enterocytes of pregnant Atp7aint/int females was not sufficient to prevent embryogenesis, it was nonetheless restrictive to copper-dependent processes that occurred late in fetal development and early postnatal growth such as alveolar maturation, a process that requires copper-dependent lysyl oxidase (8, 19). Moreover, the finding that late-term fetuses from affected mothers were severely copper deficient, despite a normal size and morphology, provided further evidence that late gestational development rather than embryogenesis was sensitive to the loss of Atp7a in the maternal enterocyte. Since the most active transfer of copper across the placenta to the fetus occurs during the last trimester of pregnancy in mammals (20, 21), these observations suggest that maternal expression of Atp7a in the enterocyte is necessary to meet this demand, a conclusion that was also supported by the finding that copper supplementation of Atp7aint/int females at day E17 of pregnancy could prevent sudden perinatal death of the offspring.
The severe neurological disease in Atp7aint/Y mice underscores the critical role of copper in brain development. It is likely that unique mechanisms have evolved to preserve this function in the face of genetic or environmental copper deficiency as evidenced by recent observations in a zebrafish model of Menkes disease that reveal an adaptive hierarchy of copper metabolism where the brain and notochord are preferentially preserved when copper availability is limited (18, 22). This hierarchy of copper distribution preferential to the brain is also observed in Menkes disease patients with residual ATP7A activity who have minimal central nervous system pathology despite systemic copper deficiency (10). Although the precise mechanisms underlying this hierarchy remain unknown, observations in the developing zebrafish embryo suggest that as copper becomes limiting, tissues expressing higher levels of Atp7a are advantaged (22), a finding supported by recent studies demonstrating a critical role for expression of Atp7a in the choroid plexus in the preservation of neurological function in Menkes disease (4, 5). Other studies have indicated that the copper-deficient brain is resistant to copper repletion in postweanling rats, arguing that the mechanisms of hierarchical copper allocation to the brain are functionally restricted to early neonatal development (26), a suggestion that is consistent with our observation that copper deficiency persisted in the brain of adult Atp7aint/Y mice despite normalization of hepatic copper levels.
Although elucidation of the molecular basis of Menkes disease has provided valuable insights into the mechanisms of copper metabolism, the availability of effective therapies in this disorder remains problematic. That intestinal deletion of Atp7a in mice produces a phenotype resembling that of Menkes disease patients that can be rescued by small increases in parenteral copper reinforces the idea that the prevention of neurodegeneration in patients with Menkes disease may be possible with therapeutic interventions that result in a modest increase in brain copper availability within a specific developmental window (17). The genetic models described here provide testable paradigms to define the spatiotemporal requirements for Atp7a-mediated copper transport together with further focus on the structural features of Atp7a that determine efficiency of copper transport (7), the role of other micronutrients such as iron and zinc that are known to influence this process (32), and the role of the microbiome in modulating copper absorption and metabolism (38). Such approaches will provide unique insights into the complex interplay of genes, environment and nutrition in development and disease and may permit the elaboration of novel therapeutic approaches to prevent or ameliorate neurodegenerative disease in affected patients.
GRANTS
This work was supported by National Institutes of Health Grants DK59893 and DK093386 to M. J. Petris, and DK44464 to J. D. Gitlin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.W., J.R.P., G.A.W., J.D.G., and M.J.P. conception and design of research; Y.W., S.Z., V.H., and J.R.P. performed experiments; Y.W., J.R.P., J.D.G., and M.J.P. analyzed data; Y.W., J.R.P., and M.J.P. interpreted results of experiments; Y.W. and M.J.P. prepared figures; Y.W. drafted manuscript; Y.W., J.D.G., and M.J.P. edited and revised manuscript; Y.W., J.R.P., J.D.G., and M.J.P. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of our laboratories for helpful comments throughout this project.
REFERENCES
- 1. Christian P. Micronutrients, birth weight, survival. Annu Rev Nutr 30: 83–104, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques 38: 702, 704, 706, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 327: 1832–1835, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Donsante A, Johnson P, Jansen LA, Kaler SG. Somatic mosaicism in Menkes disease suggests choroid plexus-mediated copper transport to the developing brain. Am J Med Genet A 152A: 2529–2534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donsante A, Yi L, Zerfas PM, Brinster LR, Sullivan P, Goldstein DS, Prohaska J, Centeno JA, Rushing E, Kaler SG. ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Mol Ther 19: 2114–2123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fields M, Craft N, Lewis C, Holbrook J, Rose A, Reiser S, Smith JC. Contrasting effects of the stomach and small intestine of rats on copper absorption. J Nutr 116: 2219–2228, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Gourdon P, Liu XY, Skjorringe T, Morth JP, Moller LB, Pedersen BP, Nissen P. Crystal structure of a copper-transporting PIB-type ATPase. Nature 475: 59–64, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Hamza I, Faisst A, Prohaska J, Chen J, Gruss P, Gitlin JD. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci USA 98: 6848–6852, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96: 10812–10817, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol 7: 15–29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Donsante A, Liew CJ, Sato S, Patronas N. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med 358: 605–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwik-Uribe CL, Rucker RB. Effect of copper deficiency on prenatal development and pregnancy outcome. Am J Clin Nutr 67: 1003S–1011S, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Kennerson ML, Nicholson GA, Kaler SG, Kowalski B, Mercer JF, Tang J, Llanos RM, Chu S, Takata RI, Speck-Martins CE, Baets J, Almeida-Souza L, Fischer D, Timmerman V, Taylor PE, Scherer SS, Ferguson TA, Bird TD, De Jonghe P, Feely SM, Shy ME, Garbern JY. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet 86: 343–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levinson B, Packman S, Gitschier J. Deletion of the promoter region in the Atp7a gene of the mottled dappled mouse. Nature Genet 16: 224–225, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64: S34–S43; discussion S72–S91, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–33283, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci 30: 317–337, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Madsen EC, Gitlin JD. Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism. PLoS Genet 4: e1000261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509, 2002 [DOI] [PubMed] [Google Scholar]
- 20. McArdle HJ, Andersen HS, Jones H, Gambling L. Copper and iron transport across the placenta: regulation and interactions. J Neuroendocrinol 20: 427–431, 2008 [DOI] [PubMed] [Google Scholar]
- 21. McArdle HJ, Erlich R. Copper uptake and transfer to the mouse fetus during pregnancy. J Nutr 121: 208–214, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab 4: 155–162, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Monty JF, Llanos RM, Mercer JF, Kramer DR. Copper exposure induces trafficking of the Menkes protein in intestinal epithelium of ATP7A transgenic mice. J Nutr 135: 2762–2766, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Mototani Y, Miyoshi I, Okamura T, Moriya T, Meng Y, Yuan Pei X, Kameo S, Kasai N. Phenotypic and genetic characterization of the Atp7a(Mo-Tohm) mottled mouse: a new murine model of Menkes disease. Genomics 87: 191–199, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4: 235–244, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J Nutr 134: 1984–1988, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Petris MJ, Strausak D, Mercer JF. The Menkes copper transporter is required for the activation of tyrosinase. Hum Mol Genet 9: 2845–2851, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Prohaska JR. Changes in Cu,Zn-superoxide dismutase, cytochrome c oxidase, glutathione peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr 121: 355–363, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Prohaska JR. Changes in tissue growth, concentrations of copper, iron, cytochrome oxidase and superoxide dismutase subsequent to dietary or genetic copper deficiency in mice. J Nutr 113: 2048–2058, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: the story of the Dutch Famine Study. Am J Epidemiol 147: 213–216, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Tang J, Robertson S, Lem KE, Godwin SC, Kaler SG. Functional copper transport explains neurologic sparing in occipital horn syndrome. Genet Med 8: 711–718, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Thiele DJ, Gitlin JD. Assembling the pieces. Nat Chem Biol 4: 145–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tonnesen T, Horn N, Sondergaard F, Mikkelsen M, Boue J, Damsgaard E, Heydorn K. Measurement of copper in chorionic villi for first-trimester diagnosis of Menkes' disease. Lancet 1: 1038–1039, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Turski ML, Thiele DJ. New roles for copper metabolism in cell proliferation, signaling, and disease. J Biol Chem 284: 717–721, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vancampen DR, Mitchell EA. Absorption of Cu-64, Zn-65, Mo-99, and Fe-59 from ligated segments of the rat gastrointestinal tract. J Nutr 86: 120–124, 1965 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Zhu S, Weisman GA, Gitlin JD, Petris MJ. Conditional knockout of the Menkes disease copper transporter demonstrates its critical role in embryogenesis. PloS One 7: e43039, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weiss KH, Wurz J, Gotthardt D, Merle U, Stremmel W, Fullekrug J. Localization of the Wilson disease protein in murine intestine. J Anat 213: 232–240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yi L, Donsante A, Kennerson ML, Mercer JF, Garbern JY, Kaler SG. Altered intracellular localization and valosin-containing protein (p97 VCP) interaction underlie ATP7A-related distal motor neuropathy. Hum Mol Genet 21: 1794–1807, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.