Abstract
Transforming growth factor (TGF)-β family members exert strong effects on restoration of liver mass after injury. Bone morphogenetic proteins (BMPs) are members of the TGF-β family and are found in the liver, suggesting that these proteins may play a role in liver regeneration. We examined BMP signaling in the liver during hepatectomy. We found that BMP4 is constitutively expressed in the peribiliary stroma and endothelial cells of the liver and that expression is decreased after hepatectomy. Mice driven to maintain BMP4 expression in the liver display inhibited hepatocyte proliferation and restoration of liver mass after hepatectomy, suggesting that reduced BMP4 is necessary for normal regeneration. Consistent with this finding, hepatocyte-specific deletion of the BMP receptor activin receptor-like kinase 3 (Alk3) enhances regeneration and reduces phosphorylation of SMAD1/5/8, a transducer of BMP signaling. In contrast to experiments in wild-type mice, maintaining BMP4 levels has no effect on liver regeneration in hepatocyte-specific Alk3 null mice, providing evidence that BMP4 signals through Alk3 to inhibit liver regeneration. Consistent with these findings, the BMP4 antagonist Noggin enhances regeneration. Furthermore, high-dose BMP4 inhibits proliferation of primary hepatocytes and HepG2 cells in culture. These findings elucidate a new, potentially clinically relevant paradigm in which a constitutively expressed paracrine inhibitory factor plays a critical role in liver regeneration.
Keywords: injury, repair, SMAD, adeno-associated virus 8
complex and highly choreographed, the homeostatic response of the liver to loss of mass or injury requires a panoply of molecules involved in diverse processes, including cytokine signaling [suppressor of cytokine signaling (SOCS3), IL-6, and TNF] (11, 30, 32, 44), transcription (NF-κB and STAT3) (8), growth [EGF and transforming growth factor (TGF)-β] (18, 31), glucose metabolism (4), bile acid metabolism (16), and platelet signaling involving extracellular nucleotides and serotonin (3, 23).
Conspicuously absent from our understanding is how the response is initiated, maintained, and terminated. In particular, what is it about partial hepatectomy that leads to the molecular signaling cascade that controls the regenerative process? Answering this fundamental question would allow us to determine how these signals are altered in disease states and suggest therapeutic options.
One possible mechanism is the chalone hypothesis, in which a constitutively expressed inhibitor of mitosis prevents increases in liver size through a negative-feedback loop (5). In this scenario, injury leads to loss of liver mass and decreased total production of the chalone. Recovery of mass increases chalone production and terminates regeneration.
There are many proteins that specifically inhibit liver regeneration, but their physiological role remains unclear. For example, TGF-β and its family member activin impair liver regeneration (31, 33), and follistatin, an activin antagonist, enhances regeneration (20). Interestingly, TGF-β1 synthesis in the liver starts 2–3 h after partial hepatectomy and remains elevated for 72 h (17), TGF-β1 plasma levels rise shortly after two-thirds hepatectomy and remain elevated for several hours (26, 38), and activin-βA is increased by 12 h after partial hepatectomy in rats (36). It is difficult to reconcile these early increases after partial hepatectomy with a role as an endogenous inhibitor of hepatocyte proliferation. There are other mitoinhibitory factors that are likely involved in liver regeneration, including Tob1, CCAAT/enhancer binding protein-α, and p21 (1, 12, 14). These molecules do not fit the chalone hypothesis, as they are not secreted signaling molecules, and p21 is induced after partial hepatectomy.
To find candidate inhibitory factors, we performed a chip-based screen of the regenerating liver. Gene ontogeny analysis demonstrated that transcription of BMP family members was highly regulated after partial hepatectomy (28), and we hypothesized that BMPs, as members of the TGF-β family, might function as constitutively expressed repressors of regeneration. Consistent with this hypothesis, bone morphogenetic proteins (BMPs) are expressed in the adult liver, and receptors for BMPs are present on adult hepatocytes (41, 42). These include a type I receptor [e.g., activin receptor-like kinase 3 (Alk3)], which is recruited to the signaling complex and transduces signal via SMAD phosphorylation (2, 27,40). We found that BMP4 plays a critical role as a constitutively expressed paracrine inhibitor of liver regeneration and that manipulation of this pathway may have therapeutic potential.
EXPERIMENTAL PROCEDURES
Mice.
BMP4-lacZ knock-in mice were a kind gift of Dr. Brigid Hogan (21). Genotyping was performed as described. C57B/6 mice were obtained from Jackson Laboratories or produced as part of our own colony.
Adeno-associated virus 8 vector production: subcloning and packaging.
A previously described adeno-associated virus (AAV) vector plasmid bearing a hepatocyte-specific murine urinary protein (MUP) promoter (15) was used as a starting point for the vectors derived for these studies. A mouse Noggin cDNA plasmid was provided by Richard Harland and amplified using the primers 5′-AAAATCTAGACCATGGAGCGCTGCCCCAG-3′ and 5′-AAAAGTCGACCTAGCAGGAACACTTAC-3′. The resultant 700-bp fragment was then cloned into the AAV-MUP vector plasmid, also called pAMP, between the promoter and an SV40 sequence providing an intron and poly(A) functions. Similarly, a mouse BMP4 cDNA plasmid was obtained from American Type Culture Collection and amplified using the primers 5′-AAAATCTAGACCATGGACTGTTATTATGC-3′ and 5′-AAAAGTCGACTCAGCGGCATCCACACC-3′. This 1,270-bp PCR product was also cloned into pAMP. The integrity of each gene was confirmed after amplification and cloning by directional sequencing. Both vectors were then packaged in AAV8 using a hybrid AAV8 capsid plasmid with type 2 AAV rep functions (AAV2/8) obtained from the Gene Therapy Center at the University of Pennsylvania. Preps were titered by real-time PCR. Typical titers were 1012 DNase-resistant particles/ml. An AAV2-MUP-enhanced green fluorescent protein (eGFP) vector plasmid was constructed as a negative control. Both vectors were cross-packaged in AAV8 via triple-plasmid transfection into AAV-293 cells (Stratagene). Helper virus was not used. Briefly, each AAV vector plasmid, an AAV2/8 rep/cap plasmid providing AAV2 replicase and AAV8 capsid functions, and a third plasmid encoding adenovirus helper functions, pHelper (Stratagene), were cotransfected into 293 cells at a molar ratio of 1:1:1. Cells were harvested 48 h posttransfection. Cell pellets were resuspended in DMEM, and intracellular virus particles were released by three consecutive freeze-thaw cycles and then centrifuged at 13,000 rpm for 10 min to remove particulates. Vector stocks were stored at −80°C and titered by real-time PCR using a sequence detection system (ABI Prism 7700, Perkin-Elmer Applied Biosystems). Titers were 3.21 × 1012, 2.76 × 1012, and 3.13 × 1012 DNase-resistant particles/ml for MUP-Noggin-AAV8, MUP-BMP4-AAV8, and MUP-eGFP-AAV8, respectively.
Two-thirds hepatectomy.
All surgeries were performed according to the National Institutes of Health guidelines for the humane treatment of laboratory animals and with approval of the Harvard or Vanderbilt Institutional Animal Care and Use Committee. Mice were anesthetized with ketamine (60 mg/kg; Hospira) and xylazine (7 mg/kg; Phoenix Pharmaceuticals). A transverse incision was made inferior to the xiphoid process, which was excised. The median and left lateral lobes of the liver were eviscerated and ligated. At tissue recovery, mice were anesthetized and weighed. Livers were excised, rinsed, blotted, and weighed. Sections were snap-frozen in liquid nitrogen, fixed in 10% neutral buffered formalin, or preserved in RNAlater (Qiagen).
Virus injections.
AAV8 viruses were administered via tail veins, as described previously (15). Two-thirds hepatectomy was performed 2 wk after virus injection. Excised livers were rinsed in PBS and snap-frozen in liquid nitrogen or fixed in 10% neutral buffered formalin.
Immunohistochemistry.
Bromodeoxyuridine was injected intraperitoneally 2 h prior to euthanization. Staining was performed on fixed, paraffin-embedded liver sections. Proliferation index was quantified as the percentage of labeled hepatocyte nuclei that stained among at least three high-power fields (×400 magnification).
RNA isolation and real-time RT-PCR.
Total RNA was purified from ∼30 mg of liver tissue preserved in RNAlater with the RNeasy Mini Kit (Qiagen). RNA (1 μg) was reverse-transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The ABI 7700 sequence detector (Applied Biosystems) was used for all real-time PCR. cDNA template was diluted 1:5 and amplified using TaqMan gene expression assays (Applied Biosystems) under standard conditions. Gene expression levels were normalized to GAPDH using the comparative cycle threshold method. Data were analyzed using ABI sequence detector software (Applied Biosystems).
Protein sample preparation and Western blot analysis.
Whole cell liver lysates were prepared by homogenization of 50 mg of frozen liver tissue in lysis buffer (50 mM Tris, 100 mM NaCl, 5 mM EDTA, 10% glycerol, and 0.5% NP-40) containing phosphatase inhibitor (Pierce) and protease inhibitor cocktail (Roche). Samples were sonicated for 30 s and clarified by centrifugation. Protein concentration was determined with the DC assay kit (Bio-Rad). Protein was prepared as follows: liver tissue was homogenized in hypotonic buffer (10 mM Tris, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.1% NP-40) containing protease inhibitor cocktail, and cells were allowed to swell on ice for 10 min. Nuclei were pelleted, and the supernatant containing the cytoplasmic fraction was cleared. Nuclei were resuspended and lysed with 1% NP-40, and the supernatant was collected. Protein concentration was determined with a bicinchoninic acid kit (Pierce). Protein was denatured and separated by SDS-PAGE in 4–12% or 4–20% Novex Tris-glycine precast gels (Invitrogen) and transferred to nitrocellulose. All antibody dilutions were made in 5% milk in TBS buffer. The primary antibodies were rabbit anti-SMAD1/5/8 (Santa Cruz Biotechnology), mouse monoclonal anti-actin (Sigma), rabbit anti-histone H3 (Millipore), mouse monoclonal anti-BMP4 (Chemicon International), and rabbit anti-Noggin (Abcam). Horseradish peroxidase-conjugated species-specific secondary antibodies were obtained from Jackson ImmunoResearch, Santa Cruz Biotechnology, or Promega.
In some cases, membranes were stripped with 1× stripping buffer (Boston Bioproducts) according to the manufacturer's instructions, washed extensively, and then reblocked and reprobed as described above. Each lane in each blot represents a different animal; solid lines denote different blots.
Primary hepatocyte culture.
Primary hepatocytes were cultured as previously described (34). Briefly, mice were anesthetized, and the portal vein was cannulated. The liver was perfused with preperfusion solution for 20 min and then with collagenase solution. The liver was then mechanically dissociated in MEM-1. Cells were filtered, collected, washed, resuspended, and plated. For the proliferation assay, cells were grown for 4 days in serum-free medium with one change and with growth factors as indicated [recombinant human hepatocyte growth factor (HGF), recombinant human EGF, and/or recombinant human BMP4 (R & D Systems)]. CyQUANT (Invitrogen) was used to measure proliferation according to the manufacturer's instructions.
HepG2 culture.
Cells were obtained from the American Type Culture Collection and grown in DMEM + penicillin-streptomycin + l-glutamine (4 mM) + 10% fetal bovine serum. In a manner similar to that used to prepare primary hepatocytes, cells were plated into serum-free medium and grown for 4 days with the appropriate growth factor and one medium change, and CyQUANT proliferation assay was performed.
RESULTS
BMP4 is constitutively expressed in stromal cells of the liver.
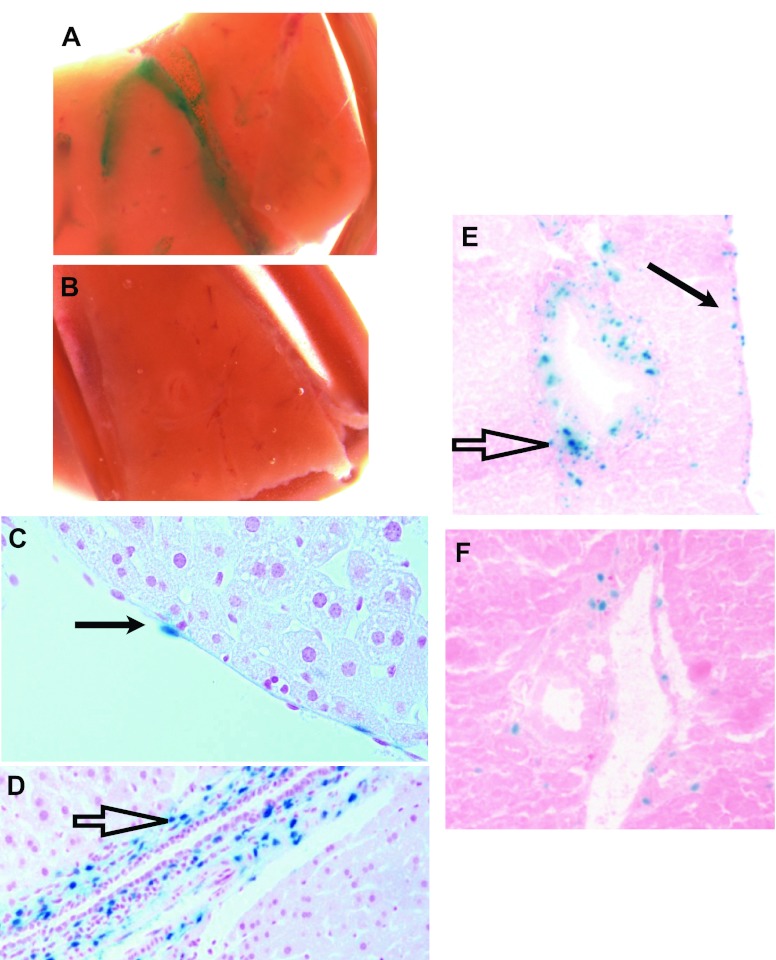
On the basis of a preliminary screen suggesting that BMP signaling is inhibited after two-thirds hepatectomy (28), we sought to localize production of BMP4 in the liver. This information is critical to understand cell-cell communication. We obtained mice expressing lacZ under the control of the regulatory elements of BMP4 (BMP4-lacZ knock-in mice). These mice provide an accurate and sensitive method for detection of BMP4 promoter activity (22). Mice containing this allele demonstrated β-galactosidase activity in a vascular and biliary pattern in whole-mount livers (Fig. 1, A and B). To localize this activity to individual cells, we next examined sections. Endothelial cells (Fig. 1C) and periductal stromal cells (Fig. 1D), most likely smooth muscle cells and fibroblasts, demonstrated β-galactosidase activity. Note that the activity in the nucleus occurs as a result of a nuclear localization sequence added to the lacZ. In contrast, wild-type mice not containing the knock-in demonstrate no green staining in sections (data not shown). Over time, after two-thirds hepatectomy, these cells continued to express lacZ, although the total number of cells decreased (Fig. 1, E and F). These results establish that the BMP4 promoter is active in nonparenchymal liver tissue.
Fig. 1.
Bone morphogenetic protein (BMP4) is expressed in nonparenchymal liver cells and decreases after partial hepatectomy. A: β-galactosidase activity (green stain in a vascular and biliary pattern) in whole-mount tissue from mice containing the BMP4-lacZ knock-in after addition of 5-bromo-4-chloroindolyl-β-d-galactopyranoside (X-Gal). B: no staining in control mice without the BMP4-lacZ knock-in. C and D: endothelial cell staining (solid arrow) and peribiliary stromal staining (open arrow) in a section stained with X-Gal. E and F: decrease in number of cells that stain with X-Gal from the time of hepatectomy (E) to 48 h after hepatectomy (F); arrows as described in C and D. Images are representative of findings from ≥3 different animals.
BMP4 is constitutively expressed in the quiescent liver and decreased after hepatectomy.
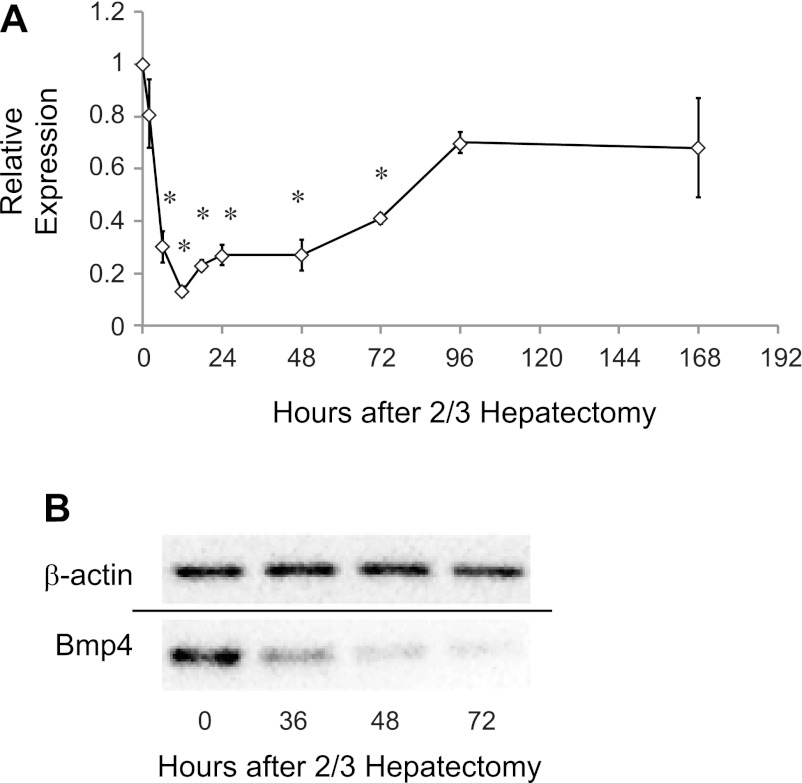
We next performed real-time PCR to determine how BMP4 expression responds to two-thirds hepatectomy. BMP4 expression decreases to <20% of basal levels by 12 h after two-thirds hepatectomy and then returns toward prehepatectomy levels by 7 days after hepatectomy (Fig. 2A). To determine whether protein levels similarly decreased, we performed Western blot analysis for BMP4 at intervals after hepatectomy (Fig. 2B). Consistent with the real-time PCR data, BMP4 levels again showed a sustained decrease compared with basal levels up to 72 h after two-thirds hepatectomy. Neither BMP4 mRNA nor protein expression changed in sham-operated mice (data not shown). Taken together, these results demonstrate that decreased BMP4 protein production is likely due to decreases in mRNA.
Fig. 2.
BMP4 mRNA and protein are decreased after hepatectomy. A: real-time PCR demonstrates steady decrease in mRNA for BMP4 after two-thirds hepatectomy to <20% of its prehepatectomy value by 12 h. Levels remain low and then trend toward normal 7 days after injury (n = 4–7). *P = 0.002 at 6, 24, and 48 h; *P = 0.001 at 12, 18, and 72 h. B: Western blot reveals that BMP protein expression follows mRNA expression and decreases after hepatectomy by 72 h.
Decreased BMP4 is necessary for normal liver regeneration.
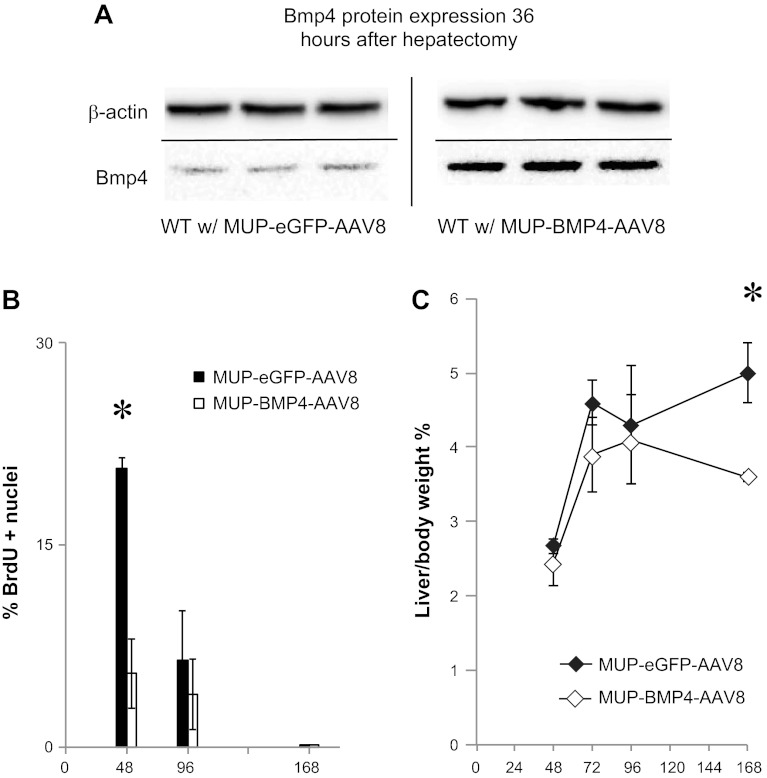
The presence of BMP4 as a constitutively expressed TGF-β family member that decreases after hepatectomy suggests a possible function as an inhibitor of hepatocyte proliferation. To test whether decreased BMP4 is necessary for normal liver regeneration, we set up an experiment in which BMP4 expression was artificially maintained after regeneration. BMP4 was cloned into an AAV backbone containing a MUP promoter and then packaged in AAV8 to yield MUP-BMP4-AAV8. The MUP promoter confers hepatocyte-specific expression to AAV8, and the virus causes minimal liver injury with no effect on liver regeneration (15). In mice receiving this virus, BMP4 protein levels in the liver were maintained 36 h after two-thirds hepatectomy (Fig. 3A). These mice demonstrated inhibition of hepatocyte proliferation 48 h after two-thirds hepatectomy compared with eGFP-containing virus (MUP-eGFP-AAV8; Fig. 3B). Furthermore, liver mass was not restored as quickly in mice receiving MUP-BMP4-AAV8 as in control mice that received MUP-eGFP-AAV8 (Fig. 3C). In fact, in MUP-BMP4-AAV8-infected mice, no increase in liver mass was observed at 3–7 days after hepatectomy, consistent with a role for BMP4 as an inhibitor of liver regeneration.
Fig. 3.
Maintenance of BMP4 expression after hepatectomy inhibits hepatocyte proliferation. A: Western blot demonstrates that murine urinary protein (MUP)-BMP4-adeno-associated virus (AAV8) infection maintains BMP4 protein levels after hepatectomy. WT, wild-type. B: wild-type mice infected with MUP-BMP4-AAV8 demonstrate decreased hepatocyte proliferation compared with control MUP-enhanced green fluorescent protein (eGFP)-AAV8-infected mice at 48 h after hepatectomy (n = 3–5). *P = 0.002. There is no significant difference in hepatocyte proliferation at 96 h or 7 days (168 h) after hepatectomy. Results are shown as percentage of hepatocytes that stain for bromodeoxyuridine (BrdU) among 3 high-power fields. C: infection with MUP-BMP4-AAV8 inhibits restoration of liver mass after two-thirds hepatectomy as long as 168 h after hepatectomy (n = 3–5). *P = 0.002. Solid lines denote different blots or deletion of lanes in a single blot.
BMP signaling during liver regeneration is mediated by Alk3.
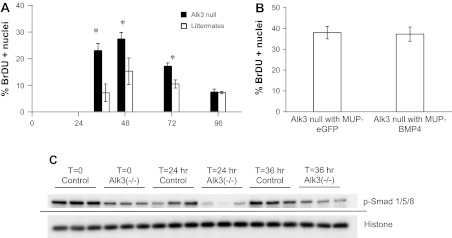
Of the type I BMP receptors, Alk2 and Alk3, but not Alk6, are expressed in human liver (41). To determine if BMP signals are transduced by Alk3, we used floxed Alk3 mice (gift of Yuji Mishina) mated to Alfp-cre mice (gift of Klaus Kaestner), denoted Alfp-cre;Alk3flox/flox, to generate mice null for Alk3. Deletion of Alk3 in the liver of these mice was confirmed using PCR (data not shown). Alk3 null mice demonstrated more rapid hepatocyte proliferation beginning at 36 h after two-thirds hepatectomy and higher peak levels than littermates without the Alfp-cre;Alk3flox/flox genotype (Fig. 4A).
Fig. 4.
BMP4 inhibits hepatocyte proliferation in an activin receptor-like kinase 3 (Alk3)-dependent manner. A: liver-specific deletion of Alk3 results in enhanced hepatocyte proliferation after hepatectomy (n = 3–5). *P = 0.01 at 36 h. *P = 0.02 at 48 and 72 h. B: MUP-BMP4-AAV8 has no effect on hepatocyte proliferation in liver-specific null mice. C: Western blot demonstrates decreased SMAD1/5/8 phosphorylation (p-SMAD) in liver-specific Alk3 null mice. Histone H3 is used as loading control. Solid line denotes a separate blot. Alk3 null mice are genotype Alfp-cre+;alk3flox/flox; littermates with genotypes other than Alfp-cre+;alk3flox/flox were used as controls.
If BMP4 acts via Alk3, it would be expected that BMP4, which inhibits regeneration in normal mice, would have no effect on liver-specific Alk3 null mice. To test this hypothesis, the MUP-BMP4-AAV8 virus was administered to liver-specific Alk3 null mice. This virus had no effect on hepatocyte proliferation 48 h after hepatectomy (Fig. 4B), evidence that BMP4 is acting via Alk3 during liver regeneration.
BMP4 signal transduction is dependent on SMAD phosphorylation. To determine the effect of loss of Alk3 on SMAD signaling, we performed Western blot analysis on samples from liver-specific Alk3 null mice and littermate controls. As expected, in mice lacking Alk3, SMAD phosphorylation was reduced 24 h after hepatectomy (Fig. 4C). This finding supports the notion that SMAD signaling is decreased in these mice and that decreases in SMAD signaling are associated with decreased hepatocyte proliferation after hepatectomy.
Blockade of BMP signaling enhances liver regeneration.
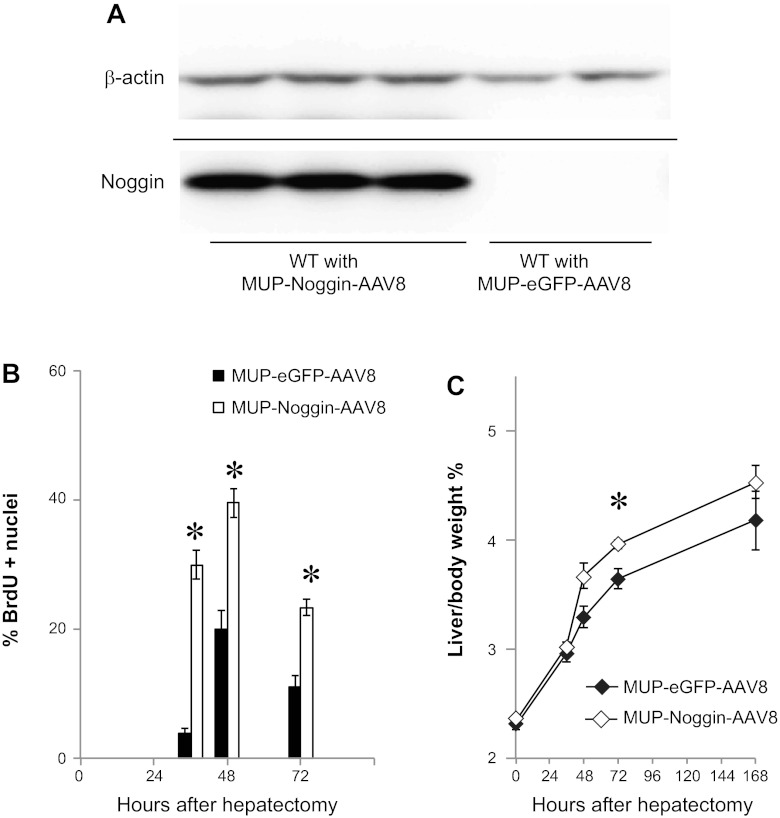
To determine whether modulation of BMP signaling might be used to enhance liver regeneration, we constructed an AAV8 vector to express the BMP antagonist Noggin (MUP-Noggin-AAV8) (43). Western blot analysis established that Noggin protein was expressed in mice at 2 wk after infection with MUP-Noggin-AAV8, but not with MUP-eGFP-AAV8 control virus (Fig. 5A). To determine the effect on restoration of liver mass after injury, virus was injected, and 2 wk later two-thirds hepatectomy was performed. Animals were examined for hepatocyte proliferation. By 36 h and continuing through 72 h after two-thirds hepatectomy, hepatocyte proliferation was enhanced in mice receiving MUP-Noggin-AAV8 (Fig. 5B) compared with mice receiving control virus. As a result, mice receiving the virus exhibited more rapid restoration of liver mass by 72 h. By 7 days after two-thirds hepatectomy, this difference was no longer statistically significant (Fig. 5C). These results suggest that BMP signaling acts as a limit on hepatocyte proliferation and that inhibition of BMP might be of therapeutic value in enhancing proliferation.
Fig. 5.
Expression of Noggin in the liver increases restoration of liver mass after two-thirds hepatectomy. A: Western blot demonstrates Noggin expression in mice infected with MUP-Noggin-AAV8. B: infection with MUP-Noggin-AAV8 increases hepatocyte proliferation at 36–72 h after two-thirds hepatectomy, as measured by BrdU staining (n = 3–5). *P < 0.001 at 36 h. *P = 0.005 at 48 and 72 h. C: infection with MUP-Noggin-AAV8 enhances restoration of liver mass after two-thirds hepatectomy (n = 3–5). P = 0.07 at 48 h. *P = 0.03 at 72 h. Solid line denotes a separate blot.
BMP4 inhibits hepatocyte and HepG2 proliferation.
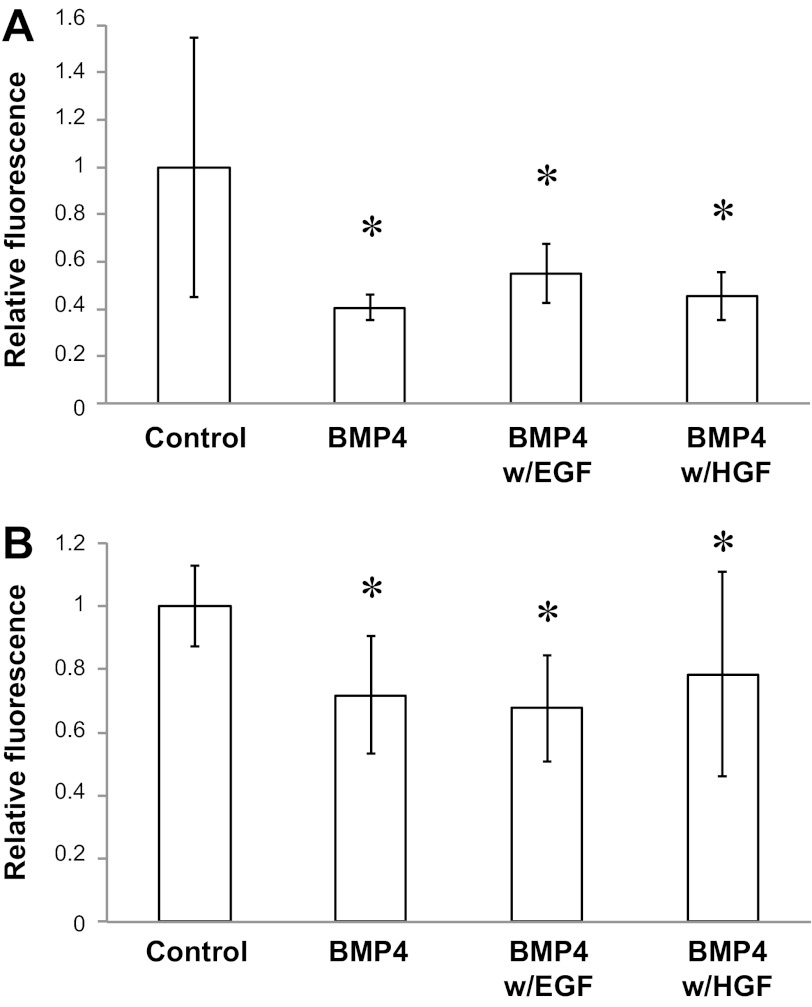
To validate our in vivo studies, we determined the effect of BMP4 on hepatocyte proliferation in primary culture and in HepG2 cells. In both cases, 20 μg/ml of BMP4 led to decreases in hepatocyte proliferation (Fig. 6). This effect was partially rescued by EGF, but not by HGF, in primary cell cultures and was not rescued by EGF or HGF in HepG2 cultures.
Fig. 6.
BMP4 inhibits proliferation in vitro. A: CyQUANT cell proliferation assay using primary hepatocyte cultures. Hepatocyte proliferation is inhibited by BMP4 and partially rescued by EGF, but not hepatocyte growth factor (HGF) (n = 5–25). BMP4 (20 μg/ml) alone resulted in 0.4 fluorescence unit compared with control (P = 0.02). Addition of EGF to BMP4 (300 ng/ml) caused an increase to 0.55 fluorescence unit (P = 0.04 vs. BMP4). Addition of HGF to BMP4 (200 ng/ml) resulted in 0.45 fluorescence unit (P = 0.035 vs. control). B: CyQUANT cell proliferation assay using HepG2 cultures. HepG2 proliferation is inhibited by BMP4 and not rescued by EGF or HGF (n = 6–30). BMP4 (20 μg/ml) alone resulted in 0.71 fluorescence unit (P < 0.01 vs. control). Addition of EGF to BMP4 (300 ng/ml) resulted in 0.67 fluorescence unit (P < 0.01 vs. control). Addition of HGF to BMP4 (200 ng/ml) resulted in 0.78 fluorescence unit (P < 0.01 vs. control).
DISCUSSION
The prodigious ability of the liver to regenerate is well known. How the liver senses injury and initiates regeneration is a fundamental unanswered question. Using microarrays, we originally identified inhibited BMP signaling after two-thirds hepatectomy. We focused on BMP4, as it decreases after hepatectomy and receptors for BMP4, specifically Alk2 and Alk3, are present on hepatocytes (41). In addition, other TGF-β family members are known to inhibit hepatocyte proliferation. In this report, we demonstrate that BMP4 is constitutively expressed in the stromal cells of the liver, and we provide evidence that it is a secreted signaling molecule that serves an antiproliferative role.
These findings suggest that an important mechanism of control of liver regeneration is a paracrine loop in which BMP4 expressed by stromal cells negatively regulates hepatocyte growth. This is the first demonstration of this type of control mechanism and potentially explains how the liver might sense its size. An important recent report recognized the importance of liver sinusoidal endothelial cells in promoting hepatocyte proliferation after hepatectomy (9). Our results suggest that this paracrine control may be multifaceted and include antiproliferative signals as well.
As an inhibitory molecule, it is tempting to speculate that injury or loss of tissue mass would decrease the amount of BMP4. In this way, the simple act of partial hepatectomy or injury diminishes BMP signaling, creating a proliferative pressure for hepatocytes. In some models of liver regeneration in which a small amount of liver is removed, most hepatocyte proliferation occurs near the cut surface, in contrast to the diffuse proliferation found after larger resection (20a). This finding suggests that local mechanisms play an important part in the regenerative response. It is tempting to postulate that small resections might be enough to decrease local BMP4 concentrations, whereas larger resections might affect concentrations throughout the liver and, therefore, affect the entire organ.
Our findings that BMP4 is constitutively expressed in the liver are consistent with reports that BMP4 is expressed in pig and rat liver (10, 24), although localization has not been performed. Similarly, BMP4 has been found in arterial endothelial cells (7). These data validate our finding of BMP4 expression in the nonparenchymal liver cells.
BMP signaling is complex, and there are illustrations in the literature of different family members mediating antagonistic effects or the same ligand producing different effects in similar tissues. For example, in the murine inner medullary collecting duct, BMP7 stimulates, while BMP2 suppresses, branching morphogenesis (29). In vitro data using BMPs in the liver show that, in zebrafish hepatocyte cultures, BMP2 enhances hepatocyte proliferation, whereas another report demonstrates that BMP2 inhibits proliferation in the hepatoma cell line Huh7 (19, 42).
A number of possible mechanisms may explain this. Ligand-binding affinity may be important, as BMP7, but not BMP4 or BMP2, can bind to activin receptors and transduce signals via Alk2 (13, 37). Alternative signal transduction mechanisms may also play a role. For example, Chiu et al. (6) distinguish two distinct and antagonistic signal transduction pathways for BMP4: 1) an inhibitory, SMAD-dependent pathway and 2) a stimulatory, MEK/ERK-dependent pathway.
Our results, therefore, may help shed light onto this complex schema. First, our finding that BMP4 inhibits liver regeneration in an in vivo model contrasts with another report that BMP7 is an endocrine factor expressed in the kidney that enhances liver regeneration (35). One possible explanation is that BMP7 is preferentially acting through Alk2 (a stimulatory pathway), whereas BMP4 is preferentially acting through Alk3 (an inhibitory pathway): increased BMP4 with our MUP-BMP4-AAV8 virus leads to increased SMAD phosphorylation and inhibition of proliferation. This hypothesis is supported by and consistent with our experiments showing enhanced liver regeneration in liver-specific Alk3 null mice, as well as our data that loss of Alk3 inhibits SMAD phosphorylation. This explanation is also consistent with our findings that Noggin stimulates liver regeneration, since Noggin preferentially binds to BMP4 compared with BMP7 (25).
Our in vitro findings that BMP4 inhibits hepatocyte proliferation in primary cultures and HepG2 cultures, although supporting our in vivo data, contrasts with other findings that BMP4 at much lower doses enhances the proliferation of HepG2 cells (6). We hypothesize that, at higher doses of BMP4, preferential activation of the SMAD-dependent pathway overcomes the MEK/ERK pathway.
BMP signaling in the liver is critical for iron metabolism. Hepatocyte-specific SMAD4 null mice demonstrate markedly abnormal iron homeostasis (39). How iron handling affects liver regeneration is not known, however, and this was not investigated in the current study.
The discovery of a paracrine, constitutively expressed secreted signaling molecule that inhibits restoration of liver mass after injury offers new mechanistic insight into how the liver senses injury. A mechanism such as this would be activated simply by removal of a section of liver. Furthermore, as a secreted signaling molecule, BMP4 or other family members may be particularly amenable to pharmacological intervention. A number of human syndromes and diseases of the liver could be treated with pharmacological agents to enhance regeneration. These include 1) acute liver failure, 2) small-for-size syndrome after liver resection or transplantation, when hepatic mass is insufficient for adequate liver function, and 3) chronic liver disease, in which regenerative mechanisms ultimately fail.
GRANTS
This work was supported in part by National Institutes of Health Grants DK-64648 (to S. J. Karp) and HL-007734 (to K. Ho), the Beth Israel Deaconess Medical Center (to S. J. Karp), the Julie Henry Fund at Beth Israel Deaconess Medical Center, and the Warren Fellowship of Harvard Medical School (to K. Ho).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.D., M.D., E.T., and S.J.K. are responsible for conception and design of the research; N.D., R.Z., K.R., K.J.H., X.R., P.K., and S.J.K. performed the experiments; N.D., R.Z., K.R., K.J.H., X.R., P.K., E.T., and S.J.K. analyzed the data; N.D., R.Z., K.R., K.J.H., X.R., E.T., and S.J.K. interpreted the results of the experiments; N.D., R.Z., K.R., and S.J.K. prepared the figures; N.D. and S.J.K. drafted the manuscript; N.D., R.Z., K.R., and S.J.K. edited and revised the manuscript; N.D., R.Z., K.R., K.J.H., M.D., X.R., P.K., E.T., and S.J.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Michael Sirover and Simon Robson for critical review of the manuscript.
REFERENCES
- 1. Albrecht JH, Poon RY, Ahonen CL, Rieland BM, Deng C, Crary GS. Involvement of p21 and p27 in the regulation of CDK activity and cell cycle progression in the regenerating liver. Oncogene 16: 2141–2150, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250: 231–250, 2002 [PubMed] [Google Scholar]
- 3. Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in liver transplantation: effects of extracellular nucleotides on graft vascular injury, rejection and metabolism. Front Biosci 13: 2588–2603, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bucher ML, Swaffield MN. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci USA 72: 1157–1160, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bullough WS. The chalones: a review. Natl Cancer Inst Monogr 38: 5–16, 1973 [PubMed] [Google Scholar]
- 6. Chiu CY, Kuo KK, Kuo TL, Lee KT, Cheng KH. The activation of MEK/ERK signaling pathway by bone morphogenetic protein 4 to increase hepatocellular carcinoma cell proliferation and migration. Mol Cancer Res 10: 415–427, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Corriere MA, Rogers CM, Eliason JL, Faulk J, Kume T, Hogan BL, Guzman RJ. Endothelial BMP4 is induced during arterial remodeling: effects on smooth muscle cell migration and proliferation. J Surg Res 145: 142–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274: 1379–1383, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468: 310–315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan J, Shen H, Sun Y, Li P, Burczynski F, Namaka M, Gong Y. Bone morphogenetic protein 4 mediates bile duct ligation induced liver fibrosis through activation of SMAD1 and ERK1/2 in rat hepatic stellate cells. J Cell Physiol 207: 499–505, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Feingold KR, Soued M, Grunfeld C. Tumor necrosis factor stimulates DNA synthesis in the liver of intact rats. Biochem Biophys Res Commun 153: 576–582, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Flodby P, Barlow C, Kylefjord H, Ahrlund-Richter L, Xanthopoulos KG. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein-α. J Biol Chem 271: 24753–24760, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 28: 491–498, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Ho KJ, Do NL, Otu HH, Dib MJ, Ren X, Enjyoji K, Robson SC, Terwilliger EF, Karp SJ. Tob1 is a constitutively expressed repressor of liver regeneration. J Exp Med 207: 1197–208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho KJ, Bass CE, Kroemer AH, Ma C, Terwilliger E, Karp SJ. Optimized adeno-associated virus 8 produces hepatocyte-specific Cre-mediated recombination without toxicity or affecting liver regeneration. Am J Physiol Gastrointest Liver Physiol 295: G412–G419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236 2006 [DOI] [PubMed] [Google Scholar]
- 17. Jakowlew SB, Mead JE, Danielpour D, Wu J, Roberts AB, Fausto N. Transforming growth factor-β (TGF-β) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-β. Cell Regul 2: 535–548, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones DE, Jr, Tran-Patterson R, Cui DM, Davin D, Estell KP, Miller DM. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am J Physiol Gastrointest Liver Physiol 268: G872–G878, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Kan NG, Junghans D, Belmonte JCI. Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. FASEB J 23: 3516–3525, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kogure K, Zhang YQ, Kanzaki M, Omata W, Mine T, Kojima I. Intravenous administration of follistatin: delivery to the liver and effect on liver regeneration after partial hepatectomy. Hepatology 24: 361–366, 1996 [DOI] [PubMed] [Google Scholar]
- 20a. Koniaris LG, McKillop IH, Schwartz SI, Zimmers TA. Liver regeneration. J Am Coll Surg 197: 634–659, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kulessa H, Hogan BL. Generation of a loxP flanked BMP4loxP-lacZ allele marked by conditional lacZ expression. Genesis 32: 66–68, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. BMP4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13: 424–436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Li M, Chen Q, Sun G, Shi X, Zhao Q, Zhang C, Zhou J, Qin N. Characterization and expression of bone morphogenetic protein 4 gene in postnatal pigs. Mol Biol Rep 37: 2369–2377, 2010 [DOI] [PubMed] [Google Scholar]
- 25. McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12: 1438–1452, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michalopoulos GK, DeFrances M. Liver regeneration. Adv Biochem Eng Biotechnol 93: 101–134, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Moustakas A, Souchelnytskyi S, Heldin CH. SMAD regulation in TGF-β signal transduction. J Cell Sci 114: 4359–4369, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Otu HH, Naxerova K, Ho K, Can H, Nesbitt N, Libermann TA, Karp SJ. Restoration of liver mass after injury requires proliferative and not embryonic transcriptional patterns. J Biol Chem 282: 11197–11204, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Piscione TD, Yager TD, Gupta IR, Grinfeld B, Pei Y, Attisano L, Wrana JL, Rosenblum ND. BMP-2 and OP-1 exert direct and opposite effects on renal branching morphogenesis. Am J Physiol Renal Physiol 273: F961–F975, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med 205: 91–103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russell WE, Coffey RJ, Jr, Ouellette AJ, Moses HL. Type β transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci USA 85: 5126–5130, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 29: 403–411, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Schwall RH, Robbins K, Jardieu P, Chang L, Lai C, Terrell TG. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology 18: 347–356, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Shiota M, Inagami M, Fujimoto Y, Moriyama M, Kimura K, Sugano T. Cold acclimation induces zonal heterogeneity in gluconeogenic responses to glucagon in rat liver lobule. Am J Physiol Endocrinol Metab 268: E1184–E1191, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto H, Yang C, LeBleu VS, Soubasakos MA, Giraldo M, Zeisberg M, Kalluri R. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J 21: 256–264, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Takamura K, Tsuchida K, Miyake H, Tashiro S, Sugino H. Activin and activin receptor expression changes in liver regeneration in rat. J Surg Res 126: 3–11, 2005 [DOI] [PubMed] [Google Scholar]
- 37. ten Dijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, Heldin CH, Miyazono K. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene 8: 2879–2887, 1993 [PubMed] [Google Scholar]
- 38. Ueda S, Yamanoi A, Hishikawa Y, Dhar DK, Tachibana M, Nagasue N. Transforming growth factor-β1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab Invest 83: 1595–1603, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab 2: 399–409, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGFβ signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood 111: 5195–5204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu CP, Ji WM, van den Brink GR, Peppelenbosch MP. Bone morphogenetic protein-2 is a negative regulator of hepatocyte proliferation downregulated in the regenerating liver. World J Gastroenterol 12: 7621–7625, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86: 599–606, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Zimmers TA, McKillop IH, Pierce RH, Yoo JY, Koniaris LG. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology 38: 326–334, 2003 [DOI] [PubMed] [Google Scholar]