Abstract
The nuclear receptor farnesoid X receptor (FXR) acts as a liver protector by regulating normal liver homeostasis. Spontaneously developed liver tumors have been found in FXR-null mice. However, the role of FXR in the tumorigenesis of human hepatocellular carcinoma (HCC) is still poorly understood. In this study, we measured the expression of FXR and its primary target gene, small heterodimer partner, and analyzed the clinical significance of FXR expression in HCC patients. A lentiviral vector that selectively overexpresses FXR was used to investigate the function of FXR in HCC cell proliferation both in vitro and in vivo. Our data showed that in human HCC, FXR expression was significantly reduced and was positively correlated with multiple malignant clinicopathological characteristics. Lentivirus-mediated exogenous FXR expression resulted in a marked increase of small heterodimer partner expression, significant repression of liver cancer cell proliferation, and tumor growth in nude mice. These results suggest that FXR may be of clinical and pharmacological importance as a promising biomarker of HCC.
Keywords: farnesoid X receptor, small heterodimer partner, tumor suppressor, proliferation
human hepatocellular carcinoma (HCC) is one of the most common human malignancies in the world and is prevalent in developing countries. In China, HCC has become the second highest cancer-related cause of death since the 1990s and accounts for 53% of all liver cancer deaths worldwide (12). The high mortality of HCC is due in large part to the lack of good biomarkers for early diagnosis and treatment assessment. Patients are often diagnosed at advanced stages, when most available therapies have only limited efficacy.
The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily of ligand-activated transcription factors and is highly expressed in the liver. FXR controls the expression of various genes involved in bile acid, lipid, and glucose metabolism (21). In recent years, the understanding of the role of FXR in the liver has developed from that as a metabolic regulator to the novel function as a cell protector implicated in liver regeneration (9, 23) and hepatocarcinogenesis (22). In 2007, we first reported that in the absence of FXR, mice displayed spontaneous development of liver tumors (22). In parallel, Kim et al. (10) published very similar observations showing that aged FXR-null mice had a high incidence of liver tumors. However, limited information was available regarding the role of FXR in the development and progression of human HCC.
The nuclear receptor small heterodimer partner (SHP) is an important modulator of metabolic signaling pathways (1, 8, 20) and is a primary FXR target gene. One of the well-characterized mechanisms by which FXR regulates gene expression in the liver is through the induction of SHP. Recent studies (24, 25) have revealed the role of SHP in the inhibition of cellular proliferation. However, combined loss of FXR and SHP expression in human HCC has not been previously reported.
In this study, we measured the expression of FXR and SHP in human HCC specimens and analyzed the association of FXR mRNA levels with clinicopathological features. Correlation of mRNA expression of FXR with SHP in HCC was assessed. To explore the potential function of FXR in HCC, we generated a lentiviral vector that overexpressed FXR and effectively transfected this construct into human liver cancer cells. The effects of FXR on SHP expression, cell proliferation in vitro, and liver cancer cell-derived tumor growth in vivo were investigated. Our results indicate that FXR is a potential candidate biomarker for human HCC.
MATERIALS AND METHODS
Patients and human liver samples.
Cohort 1 samples were from patients of 80 HCC and 20 hepatic hemangiomas at The Liver Center, The First Affiliated Hospital of Fujian Medical University, from between 2009 and 2010. Eighty paired HCC and corresponding peritumoral liver tissues were collected from patients who had undergone curative hepatic resection. Twenty normal liver tissues were obtained from patients undergoing resection for hepatic hemangiomas. Cohort 2 samples, which included 30 paired HCC and corresponding peritumoral liver tissues and 8 normal liver tissues, were collected between 2011 and 2012 from the same hospital as cohort 1. No local or systemic treatment had been conducted for these patients before surgery. Tissue samples were divided into two portions: one portion was immediately snap frozen in liquid nitrogen and stored at −80°C until use, and the other portion was fixed in the 10% neutral formalin for paraffin-embedded blocks. Tumor samples were taken from the non-necrotic peripheral zone of the tumor. Peritumoral liver tissues were taken 3 cm from the tumor. Both tumor and peritumoral samples were histopathologically confirmed. Clinical information was collected from patient records, and the details are shown in Table 1. Tumor stage was determined according to the Barcelona Clinic Liver Cancer (BCLC) staging system (14), and tumor differentiation was graded by the Edmondson grading system (5). This study was approved by the Institute Research Ethics Committee of Fujian Medical University, and informed consent was obtained from each patient according to the committee's regulations.
Table 1.
Correlation of FXR mRNA expression with clinicopathological characteristics in patients with hepatocellular carcinoma
| Variable | Relative FXR mRNA Levels |
|||
|---|---|---|---|---|
| n | Value | t | P | |
| Age, yr | 0.594 | 0.554 | ||
| ≤50 | 36 | 0.443 ± 0.018 | ||
| >50 | 44 | 0.420 ± 0.031 | ||
| Sex | 0.054 | 0.956 | ||
| Male | 70 | 0.453 ± 0.022 | ||
| Female | 10 | 0.450 ± 0.065 | ||
| α-Fetoprotein, ng/ml | −1.866 | 0.066 | ||
| ≤400 | 47 | 0.393 ± 0.025 | ||
| >400 | 33 | 0.572 ± 0.108 | ||
| Tumor size, cm | 2.067 | 0.042 | ||
| ≤5 | 31 | 0.472 ± 0.027 | ||
| >5 | 49 | 0.400 ± 0.022 | ||
| Tumor number | −0.129 | 0.898 | ||
| Single | 63 | 0.445 ± 0.021 | ||
| Multiple | 17 | 0.451 ± 0.046 | ||
| Barcelona Clinic Liver Cancer stage | 2.076 | 0.041 | ||
| 0/A | 13 | 0.507 ± 0.034 | ||
| B/C | 67 | 0.406 ± 0.020 | ||
| Differentiation | 2.128 | 0.037 | ||
| Well/moderate | 46 | 0.491 ± 0.028 | ||
| Poor | 34 | 0.403 ± 0.029 | ||
| Portal vein thrombosis | 0.841 | 0.402 | ||
| Yes | 54 | 0.465 ± 0.025 | ||
| No | 26 | 0.427 ± 0.037 | ||
| Cirrhosis | −0.157 | 0.875 | ||
| Yes | 57 | 0.653 ± 0.020 | ||
| No | 23 | 0.660 ± 0.035 | ||
| Encapsulation | 2.255 | 0.027 | ||
| Complete | 37 | 0.492 ± 0.026 | ||
| None or incomplete | 43 | 0.414 ± 0.023 | ||
Values are means ± SE; n, number of subjects/group.
FXR, farnesoid X receptor.
RNA isolation and quantitative real-time PCR.
Total RNA from liver tissues or cells was extracted with TriPure Isolation Reagent (Roche Applied Science, Mannheim, Germany), and 500 ng of total RNA were reverse transcribed using the Primescript RT reagent kit (Takara Bio, Tokyo, Japan) according to the manufacturer's protocol. Quantitative real-time PCR for the quantification of mRNA was performed using SYBR Premix Ex Taq (Takara Bio, Tokyo, Japan) and an Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA). Expression levels of target genes were normalized to the expression of β-actin. The following specific forward and reverse primers were used: FXR, forward 5′-GATGCCTGTAACAAAGAAGCCCC-3′ and reverse 5′-CACACAGTTGCCCCCGTTTTTAC-3′; SHP, forward 5′-GTGGCTTCAATGCTGTCTGGAG-3′ and reverse 5′-CAGGCTGGTCGGAATGGACTTG-3′; and β-actin, forward 5′-GCGTGACATTAAGGAGAAGC-3′ and reverse 5′-CCACGTCACACTTCATGATGG-3′. The relative amount of mRNA level was calculated as previously described (13).
Immunohistochemistry.
Immunohistochemical staining was performed with the Envision Plus System according to the manufacturer's instructions. Primary antibodies against FXR (R&D Systems, Minneapolis, MN, 1:100), SHP (MBL, Woburn, MA, 1:200), and Ki67 (Maixin Bio, Fuzhou, China, 1:50) were used to detect the corresponding proteins. The results of immunohistochemical staining were analyzed as previously described (19). The proliferative index was calculated as the percentage of Ki67-positive cells.
Western blot analysis.
Forty micrograms of total soluble protein extracted from liver samples or cells were resolved on 10% SDS-polyacrylamide gels and transferred electrophoretically to nitrocellulose membranes. Blots were blocked with 5% milk followed by an overnight incubation with FXR antibody (R&D Systems, 1:300), SHP antibody (Abcam, Cambridge, MA, 1:750), or β-actin antibody (Beyotime, Beijing, China, 1:1,000). Blots were then incubated with horseradish peroxidase-labeled goat anti-mouse IgG secondary antibody (Beyotime) and visualized using enhanced chemiluminescence substrate (Beyotime).
Cell culture and reagents.
HepG2 cells were obtained from the cell bank of the Chinese Academy of Science (Shanghai, China). Huh7 cells were kindly provided by Dr. Xu Lin (Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China). Reagents for cell culture were purchased from HyClone Biochemical Products (Beijing, China). The FXR agonist GW4064 was provided by Dr. Wendong Huang (City of Hope, Duarte, CA). Cells were routinely grown in complete culture medium (high-glucose DMEM supplemented with 10% heat-inactivated BSA and 1% penicillin-streptomycin) at 37°C in a humidified incubator with 5% CO2.
Construction of human FXR recombinant lentivirus and transduction into HepG2 and Huh7 cells.
The human FXR gene was amplified by PCR from plasmid pCR4-TOPO (Open Biosystems, Huntsville, AL). The primer sequences were as follows: forward, 5′-GAGGATCCCCGGGTACCGGTCGCCACCATGGGATCAAAAATGAATCTC-3′; and reverse, 5′-TCACCATGGTGGCGACCGGCTGCACGTCCCAGATTTCAC-3′. PCR fragments and the pGC-FU-GFP lentivirus vector (Genechem, Shanghai, China) were digested with endonuclease AgeI and ligated with T4 DNA ligase to produce the pGC-FU-FXR-GFP vector. Using Lipofectamine 2000 (Invitrogen), 293T cells were cotransfected with the pGC-FU-FXR-GFP vector, pHelper1 vector, and pHelper2 vector for 48 h to generate lentiviral stock. pGC-FU-GFP was used as a negative control. After the determination of the viral titer, lentiviral particles were used to infect HepG2 or Huh7 cells. Cells with green fluorescent protein (GFP) expression were selected to expand cultures for further investigation.
WST-1 cell proliferation assays.
WST-1 assays were used to estimate cell numbers according to the manufacturer's instructions (Roche Applied Science). Briefly, HepG2 or Huh7 cells were stably transfected with LV-FXR-GFP or LV-GFP and then seeded in 96-well plates at a density of 1,000 cells/well. Cells were treated with 2 μM GW4064 for 24 h (2) and WST-1 reagent (1:10) was then added to the cells for 2 h at 37°C. The absorbance value (optical densiometry) of each well was measured at 450 nm. Cells were incubated for 1, 2, 3, and 4 days.
Tumorigenicity in nude mice.
Male BALB/c nu/nu mice (4–6 wk old) were purchased from Shanghai SLAC Laboratory Animal (Shanghai, China). Mice were subcutaneously injected in the flank with 5 × 106 HepG2 cells stably transduced with LV-FXR-GFP or empty vector. Tumor growth was measured every 3 days using vernier calipers. Tumor volume was calculated by the following formula: length × width2 × 0.52 (7). Animals were euthanized on day 21 after implantation, and tumors were harvested for the detection of mRNA and protein expression. All experimental procedures of the animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Animals, with the approval of the Institute Research Ethics Committee of Fujian Medical University [permit no.: SYXK(Min): 2008-0001].
Statistical analysis.
All statistical analyses were performed using SPSS version 17 software. Student's t-test was used to compare qualitative variables. One-way ANOVA was used to compare means of cases among the different groups. The correlation between FXR and SHP expression in HCC was investigated by Pearson's coefficient correlation. P values of <0.05 were considered to be statistically significant.
RESULTS
Downregulation of FXR expression in human HCC tissues.
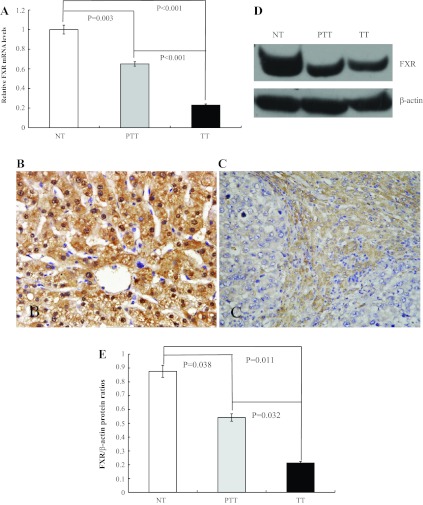
We (22) previously found that FXR- null mice developed spontaneous liver tumors. To determine whether FXR is associated with the tumorigenicity of human HCC, we measured the FXR mRNA levels in human liver tissues, including 20 normal liver and 80 paired HCC and peritumoral liver tissues using quantitative real-time PCR. The results showed that, compared with adjacent noncancerous tissues, FXR expression was downregulated in all tumor samples (Fig. 1A). We also found that FXR mRNA levels in the 80 matched disease groups were significantly lower than those in the 20 normal liver samples (Fig. 1A). We then confirmed our results by further analyzing FXR mRNA expression in another cohort of human liver samples including 8 normal liver and 30 paired HCC and corresponding peritumoral liver tissues (Supplemental Material, Supplemental Fig. S1).1 To further determine FXR protein levels in HCC, we performed immunohistochemistry along with Western blot analysis in 40 paired HCC samples and 10 normal liver samples. The results demonstrated a correlated reduction in FXR protein levels in HCC compared with those in peritumoral liver and normal tissues (Fig. 1, B–E). Our data indicated that diminished FXR expression could be involved in the development and/or progression of human HCC.
Fig. 1.
Farnesoid X receptor (FXR) expression was downregulated in human hepatocellular cancer (HCC) tissues. A: quantitative real-time PCR analysis of FXR mRNA levels in normal liver tissue (NT), peritumoral liver tissue (PTT), and HCC (TT) samples. B and C: immunohistochemical analysis of FXR protein levels in liver tissues. B: hepatocytes in normal liver samples were stained positively for FXR expression in the cytoplasm and nucleus. C: moderate immunoreactivity of FXR was detected in peritumoral liver tissue. Tumor cells from HCC tissue showed no detectable or very weak FXR expression. Magnification: ×400. D: representative images of FXR protein expression by Western blot analysis from 40 HCC and 10 normal liver tissue samples. E: quantification of the Western blot results.
Association between FXR mRNA expression and clinicopathological characteristics of HCC patients.
We next determined the relationship between FXR mRNA expression levels in tumor tissues and the clinicopathological characteristics of 80 HCC patients (Table 1). The results showed no statistical differences of FXR mRNA levels regarding age, sex, serum α-fetoprotein level, tumor number, or presence of portal vein thrombosis and cirrhosis. However, we found that patients with low FXR mRNA levels were prone to have large tumor size (P = 0.042), advanced BCLC stage (P = 0.041), poor differentiation (P = 0.037), and absence of encapsulation (P = 0.027). Thus, low expression of FXR was associated with multiple malignant characteristics of HCC.
Correlation between FXR and SHP in human HCC tissues.
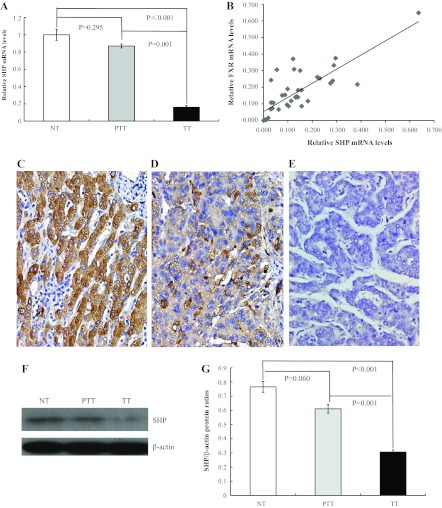
To investigate whether the FXR-SHP pathway plays a role in the tumorigenesis of HCC, we measured SHP mRNA expression in 10 normal livers and 45 paired HCC and peritumoral tissues. Consistent with FXR mRNA levels in HCC, we observed significantly reduced SHP mRNA expression in HCC compared with peritumoral and normal liver tissue samples (Fig. 2A). However, we found no statistically significant differences between peritumoral and normal liver tissue samples. We then analyzed the correlation of gene expression between FXR and SHP in HCC by the Pearson correlation coefficient test. The results showed that the FXR mRNA level was significantly and positively correlated with the SHP mRNA level in HCC (Fig. 2B). SHP protein expression in HCC was also found to be dramatically decreased in HCC by immunohistochemistry (Fig. 2, C–E) and Western blot analysis (Fig. 2, F and G). Thus, suppression of the FXR-SHP pathway may be involved in the tumorigenesis of HCC.
Fig. 2.
Correlation of FXR expression with small heterodimer protein (SHP) in HCC. A: quantitative real-time PCR analysis of SHP mRNA levels in normal liver tissue, peritumoral liver tissue, and HCC liver samples. B: in HCC samples, a significant and positive correlation of FXR and SHP expression was found by the Pearson correlation coefficient test (P < 0.001). C–E: immunoreactivity of SHP in peritumoral tissues (C) was higher compared with that in HCC (D); the negative control is shown in E. Magnification: ×400. F: representative image of SHP protein expression by Western blot analysis. G: quantification of the Western blot results.
LV-FXR-GFP-mediated upregulation of FXR induced SHP expression and inhibited liver cancer cell proliferation in vitro.
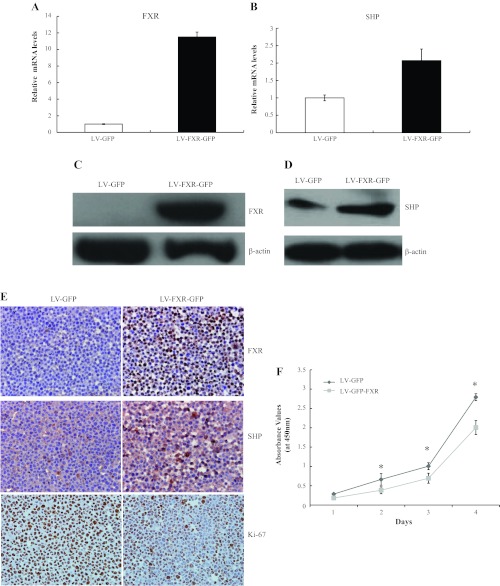
Lentiviral vectors that selectively expressed FXR or negative control were generated and termed LV-FXR-GFP and LV-GFP, respectively. Compared with the control, expression levels of FXR mRNA (Fig. 3A) and FXR protein (Fig. 3, C and E) were dramatically increased in HepG2 cells effectively transduced with LV-FXR-GFP. Consistent with the upregulation of FXR in HepG2 cells with LV-FXR-GFP, expression levels of SHP mRNA (Fig. 3B) and SHP protein (Fig. 3, D and E) were elevated compared with controls. A significant reduction of HepG2 cell proliferation in the LV-FXR-GFP group was observed by WST-1 assays (Fig. 3F) and Ki67 immunoreactivity analysis (Fig. 3E). The Ki67 proliferative index in the LV-FXR-GFP group was reduced by 43% compared with controls. In addition, we also successfully transduced Huh-7 cells with LV-FXR-GFP and observed similar effects of FXR overexpression on SHP expression and cell growth as we did in HepG2 cells (Supplemental Fig. S3). Our data collectively demonstrated that upregulation of FXR elevated SHP expression and inhibited liver cancer cell proliferation in vitro.
Fig. 3.
Overexpression of FXR mediated by LV-FXR-GFP increased SHP expression and inhibited the proliferation of HepG2 cells in vitro. Cultured HepG2 cells were infected with LV-FXR-GFP vector or empty control vector (LV-GFP). A and B: quantitative real-time PCR analysis of FXR mRNA (A) and SHP mRNA (B) expression. C: exogenous expression of FXR protein was detected by Western blot analyis. D: SHP protein was detected by Western blot analysis. E: immunostaining was performed using FXR, SHP, and Ki67 antibodies. Magnification: ×400. F: proliferation of cells transfected with LV-GFP or LV-FXR-GFP was assessed by WST-1. Before the addition of the WST-1, cells were pretreated with 2 μM GW4064 for 24 h. Data are presented for three independent experiments. *P < 0.05.
Suppression of tumor growth and modulation of SHP expression by FXR in a HepG2 xenograft model.
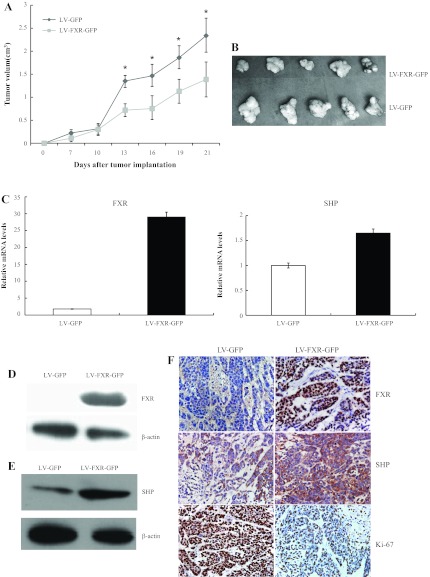
To extend the above findings, in vivo experiments were performed using HepG2 xenograft tumor-bearing mice. Tumor volumes were measured every 3 days until the animals were euthanized. Overexpression of FXR resulted in a significant suppression of tumor growth, as shown by the tumor volumes (Fig. 4, A and B). Quantitative real-time PCR demonstrated that upregulation of FXR elevated SHP mRNA levels (Fig. 4C). Immunohistochemistry and Western blot analysis of FXR and SHP confirmed increased staining in LV-FXR-GFP-transduced tumors compared with controls (Fig. 4, D–F). As expected, Ki67 immunoreactivity varied conversely with the alteration of FXR and SHP staining. The Ki67 proliferative index was reduced by 45% in tumor cells overexpressing FXR and SHP (Fig. 4F). These data suggested that elevated FXR levels increased the expression of SHP and restrained liver tumor growth in vivo.
Fig. 4.
Effect of FXR overexpression on tumorigenicity in nude mice. BALB/c nu/nu mice were subcutaneously injected in the flank with 5 × 106 HepG2 cells stably infected with LV-FXR-GFP or LV-GFP control vector. A: tumor growth curve. Tumor growth was monitored by measuring the tumor volume every 3 days after cell injection. *P < 0.05. B: tumors were harvested and photographed at 21 days postimplantation. Representative tumor images are shown. C–F: tumor samples were subjected to quantitative real-time PCR analysis for FXR and SHP (C), Western blot analysis for FXR (D) and SHP (E), and immunohistochemistry for FXR, SHP and Ki67 (F). Magnification: ×400.
DISCUSSION
In this study, we showed that FXR expression was downregulated at both mRNA and protein levels in human HCC specimens. FXR might be of clinical and pharmacological importance as a HCC biomarker. Low FXR mRNA levels were correlated with large tumor size (P = 0.042), advanced BCLC stage (P = 0.041), poor differentiation (P = 0.037), and absence of encapsulation (P = 0.027). Expression of the FXR downstream gene SHP was also reduced in HCC. FXR mRNA levels were significantly and positively correlated with SHP mRNA levels in HCC. LV-FXR-GFP-mediated exogenous FXR expression upregulated SHP expression and inhibited liver cancer cell proliferation in vitro and tumor growth in vivo. Our data suggested that FXR may function as a tumor inhibitor by suppressing cell proliferation. Loss of FXR expression and suppression of the FXR-SHP pathway may contribute to the development and progression of human HCC.
It has recently been demonstrated that FXR is involved in several human cancers. However, the expression of FXR and its role in the tumorigenesis remain controversial. Lee et al. (11) reported that FXR overexpression in pancreatic cancer tissues with lymphatic metastasis was associated with poor patient survival, and downregulation of FXR was an effective approach for inhibition of pancreatic tumor progression. However, for other cancers, FXR was considered as a tumor suppressor rather than a tumor promoter. Specifically, De Gottardi et al. (4) found that FXR mRNA was decreased in human colorectal adenoma and carcinoma, and expression levels of FXR were inversely related to the degree of the malignancy of colon cancer cell lines. FXR expression has also been shown to be reduced in human intestinal tumors (16) and esophagus adenoma (3).
In 2007, two studies (10, 22) indicated that FXR gene knockout leads to liver injury and irregular regeneration and, finally, to spontaneous liver tumor development in mice. However, the role of FXR in human hepatocarcinogenesis remains unclear. In the present study, we showed that compared with normal liver tissues, FXR expression was dramatically decreased in HCC and adjacent noncancerous tissues. Significant differences were also identified between intratumoral and peritumoral tissues. We then analyzed FXR expression at protein levels and found a correlated reduction in HCC. These findings clearly demonstrate that FXR expression was reduced in human HCC specimens, which indicates that diminished FXR expression might be an important event in tumorigenesis in both mice and humans. Previous studies (9, 21) have suggested an important role of FXR in liver regeneration and repair, which may indirectly suppress liver injury, inflammation, and the consequent hepatcarcinogenesis. However, our findings suggest that FXR may directly inhibit liver cancer cell proliferation and tumor growth. The detailed molecular mechanism by which FXR regulates liver cell proliferation will be our future research effort.
To determine whether there is a reciprocal relationship between the loss of FXR expression and progression of human HCC, we analyzed FXR mRNA levels and their correlation with malignant clinicopathological characteristics in HCC samples. Because larger tumors might have poorer prognosis, we first stratified HCC patients into two subgroups by tumor size with 5 cm as a cutoff value. We observed that FXR mRNA levels were statistically lower in the tumors measuring >5 cm in diameter than in those of ≤ 5 cm. FXR deficiency may increase the susceptibility of the liver to cancer cell proliferation during human HCC tumorigenesis, although the precise mechanism remains unclear. For other clinicopathological features, our data revealed that patients with low FXR mRNA levels tended to have advanced BCLC stage, poor differentiation, and absence of encapsulation. No statistically significant differences were found for FXR mRNA levels regarding age, sex, serum α-fetoprotein level, tumor number, or presence of portal vein thrombosis and cirrhosis. Taken together, downregulation of FXR expression was associated with multiple malignant characteristics of HCC, which might act as a good marker to identify a high-risk subgroup of HCC patients in clinical practice.
As an endogenous receptor of bile acid, the major function of FXR is to maintain bile acid homeostasis and to prevent bile acid-induced liver toxicity (15, 17, 18). SHP is the primary target gene of FXR. Bile acid-activated FXR induces the expression of SHP, which, in turn, inhibits the expression of cytochrome P-450 7A1, the rate-limiting enzyme in bile acid biosynthesis (6). A number of reports have documented that SHP not only acts as metabolic regulator but also functions as a novel tumor suppressor. He et al. (7) identified that epigenetic gene silencing disrupted SHP function by downregulating its expression in human HCC. Zhang Y et al. (24, 25) revealed a role of SHP in the inhibition of cellular proliferation and activation of apoptosis signaling. Our data showed that SHP mRNA and protein levels were significantly decreased in HCC compared with peritumoral tissues, which was consistent with the previous report (7). Correlation analysis determined a significant correlation between the expression of FXR and SHP, and the results showed that FXR mRNA levels were significantly and positively correlated with SHP mRNA levels in HCC. Thus, suppression of the FXR-SHP pathway may promote tumorigenesis in HCC.
To further determine the potential function of FXR in the regulation of liver cancer growth, lentiviral vectors that selectively expressed FXR (LV-FXR-GFP) and control empty vector (LV-GFP) were generated. HepG2 and Huh-7 cells were effectively transduced with the two vectors, and significantly increased FXR mRNA and protein levels were detected in HepG2 and Huh-7 cells infected with LV-FXR-GFP. We then investigated the effect of FXR upregulation on cell proliferation by WST-1 assays. Because FXR is a ligand-activated transcription factor, we added GW4064 to fully activate FXR. The results demonstrated that overexpression of FXR significantly inhibited cell proliferation. We also observed that increased FXR resulted in a dramatic reduction of Ki67-positive nuclei, an index of cell proliferation. To extend our findings in vitro, in vivo experiments were performed using a HepG2 xenograft model in nude mice. As we expected, FXR significantly suppressed tumor growth and inhibited tumor cell proliferation. Consistent with our findings in human HCC samples, upregulation of FXR induced SHP expression both in vitro and in vivo.
Taken together, our study provides evidence demonstrating that FXR expression is significantly reduced in human HCC and is positively correlated with multiple malignant clinicopathological characteristics. Suppression of the FXR-SHP pathway may be involved in the development of HCC. Lentiviral-mediated FXR overexpression inhibits HCC cell growth both in vitro and in vivo. Therefore, FXR may be of clinical and pharmacological importance as a promising HCC biomarker.
GRANTS
This work was supported by National Natural Science Foundation of China Grant 30972927 and Foundation of Fujian Educational Committee Grant JA09111. W. Huang is supported by NCI R01-139158.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.S., C.M., N.L., and M.G. performed experiments; H.S., C.M., J.L., N.L., M.G., X.W., W.H., and X.H. analyzed data; H.S., C.M., N.L., X.W., and X.H. interpreted results of experiments; H.S., C.M., N.L., X.W., and X.H. prepared figures; J.L., A.H., W.H., and X.H. conception and design of research; A.H., W.H., and X.H. edited and revised manuscript; W.H. and X.H. approved final version of manuscript; X.H. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Yuan E. Lian for technical help and Dr. Guangya Wei for assistance in collecting patients' samples and data.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Gastrointestinal and Liver Physiology website.
REFERENCES
- 1. Bavner A, Sanyal S, Gustafsson JA, Treuter E. Transcriptional corepression by SHP: molecular mechanisms and physiological consequences. Trends Endocrinol Metab 16: 478–488, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Chen WD, Wang YD, Zhang L, Shiah S, Wang M, Yang F, Yu D, Forman BM, Huang W. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology 51: 953–962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Gottardi A, Dumonceau JM, Bruttin F, Vonlaufen A, Morard I, Spahr L, Rubbia-Brandt L, Frossard JL, Dinjens WNM, Rabinovitch PS, Hadengue A. Expression of the bile acid receptor FXR in Barrett's esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol Cancer 5: 48, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ, Dufour JF. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci 49: 982–989, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Edmondson HASP. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954: 462–503, 1954 [DOI] [PubMed] [Google Scholar]
- 6. Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6: 517–526, 2000 [DOI] [PubMed] [Google Scholar]
- 7. He N, Park K, Zhang Y, Huang J, Lu S, Wang L. Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology 134: 793–802, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, Wang L. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology 46: 147–157, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28: 940–946, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JY, Lee KT, Lee JK, Lee KH, Jang KT, Heo JS, Choi SH, Kim Y, Rhee JC. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br J Cancer 104: 1027–1037, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li S, Fu H, Wang Y, Tie Y, Xing R, Zhu J, Sun Z, Wei L, Zheng X. MicroRNA-101 regulates expression of the v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene in human hepatocellular carcinoma. Hepatology 49: 1194–1202, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100: 698–711, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res 68: 9589–9594, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3: 543–553, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong WM, Xia Y, Zou QF, Xi T, Shen F, Wang HY, Wu MC. Overexpression of aspartyl-(asparaginyl)-β-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology 52: 164–173, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, Moore DD. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell 2: 721–731, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res 18: 1087–1095, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67: 863–867, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, Huang X, Meng Z, Dong B, Shiah S, Moore DD, Huang W. Significance and mechanism of CYP7a1 gene regulation during the acute phase of liver regeneration. Mol Endocrinol 23: 137–145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Soto J, Park K, Viswanath G, Kuwada S, Abel ED, Wang L. Nuclear receptor SHP, a death receptor that targets mitochondria, induces apoptosis and inhibits tumor growth. Mol Cell Biol 30: 1341–1356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology 48: 289–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]