Abstract
Patients with irritable bowel syndrome (IBS) with diarrhea (IBS-D) carrying human leukocyte antigen (HLA)-DQ2/8 genotypes benefit from gluten withdrawal. Our objective was to compare gastrointestinal barrier function, mucosal inflammation, and transit in nonceliac IBS-D patients and assess association with HLA-DQ2/8 status. In 45 IBS-D patients who were naive to prior exclusion of dietary gluten, we measured small bowel (SB) and colonic mucosal permeability by cumulative urinary lactulose and mannitol excretion (0–2 h for SB and 8–24 h for colon), inflammation on duodenal and rectosigmoid mucosal biopsies (obtained in 28 of 45 patients), tight junction (TJ) protein mRNA and protein expression in SB and rectosigmoid mucosa, and gastrointestinal and colonic transit by validated scintigraphy. SB mucosal biopsies were stained with hematoxylin-eosin to assess villi and intraepithelial lymphocytes, and immunohistochemistry was used to assess CD3, CD8, tryptase, and zonula occludens 1 (ZO-1); colonic biopsy intraepithelial lymphocytes were quantitated. Associations of HLA-DQ were assessed using Wilcoxon's rank-sum test. Relative to healthy control data, we observed a significant increase in SB permeability (P < 0.001), a borderline increase in colonic permeability (P = 0.10), and a decrease in TJ mRNA expression in rectosigmoid mucosa in IBS-D. In HLA-DQ2/8-positive patients, ZO-1 protein expression in the rectosigmoid mucosa was reduced compared with that in HLA-DQ2/8-negative patients and colonic transit was slower than in HLA-DQ2/8-negative patients. No other associations with HLA genotype were identified. There is abnormal barrier function (increased SB permeability and reduced mRNA expression of TJ proteins) in IBS-D relative to health that may be, in part, related to immunogenotype, given reduced ZO-1 protein expression in rectosigmoid mucosa in HLA-DQ2/8-positive relative to HLA-DQ2/8-negative patients.
Keywords: gluten, celiac, lactulose, mannitol, immunogenotype
the relationship of celiac disease, gluten sensitivity, and irritable bowel syndrome (IBS) is complex. While guidelines suggest screening for celiac disease in patients with functional chronic diarrhea (FD) or IBS with diarrhea (IBS-D), there is little evidence supporting that recommendation. In Olmsted County, MN, overall prevalence of positive tissue transglutaminase (TTG) serology was 4%; celiac disease did not explain the presence of IBS or dyspepsia (22). In a four-site study conducted from the United States, the prevalence of celiac disease among patients with nonconstipated IBS was similar to that in controls, ∼0.4% (10). In a cohort of Italian patients with IBS, 25% had food hypersensitivity on the basis of excretion of fecal eosinophilic cationic protein during oral food challenge with cow's milk proteins followed by wheat proteins (9), the latter providing the equivalent to 100 g of bread.
Patients with IBS-D or FD may be intolerant to gluten without celiac disease. This was first documented in 1981 (12); until recently, few studies investigated gluten intolerance as a factor contributing to IBS-D or FD. Celiac disease is associated with specific human leukocyte antigen (HLA) DQ genotypes. Some patients with IBS-D who experience gluten intolerance may benefit from a gluten-free diet (GFD), especially if they are HLA-DQ2/8-positive and secrete IgG tissue transglutaminase (TTG) antibody (TGA) and anti-gliadin (AGA) IgG (37); IgG AGA antibodies in serum were positive in 56.4% of gluten-sensitive patients and 81.2% of celiac patients. Thus, for those who were HLA-DQ2- and IgG TGA-positive, the response to gluten withdrawal occurred in 60% compared with 12% in those who were HLA-DQ2/8- and IgG TGA-negative (37). This suggests immunogenetic predisposition to gluten intolerance among a subset of patients with IBS-D or FD in the absence of celiac disease. In a double-blind, randomized, placebo-controlled, rechallenge trial in patients with nonceliac IBS who were symptomatically controlled on a GFD, 68% of the patients who were randomly assigned to gluten treatment compared with 40% in the placebo group had symptoms that were not adequately controlled. In this study, there were no differences in individuals with or without HLA-DQ2/8, and the mechanisms for symptom generation were not elucidated (4).
The mechanisms underlying gluten intolerance in IBS patients are unclear; however, HLA genotype may facilitate abnormal functions in response to dietary gluten. In HLA-DQ8 transgenic mice sensitized to gluten, gliadin exposure (in contrast to negative and positive controls) resulted in CD3 and CD4 lymphocyte and macrophage infiltration of villi and increased contractile responses of smooth muscle to electrical field stimulation and carbachol (35). It is plausible that the link between gluten or gliadin and inflammation may be the increase in intestinal permeability, which is well established in celiac disease and involves binding to the chemokine 7-transmembrane G protein-coupled receptor CXCR3, leading to recruitment of the adapter protein MyD88, with subsequent zonulin release and increased permeability (21). Increased small bowel (SB) or colonic permeability is reported in patients with IBS (reviewed in Ref. 29); it is unknown whether barrier function in IBS-D is associated with HLA-DQ status.
Our hypothesis was that, among IBS-D patients who were TTG-negative, were eating a diet containing gluten, and were naive to manipulation of dietary gluten, HLA-DQ2/8-positive status was associated with higher SB and colonic permeability and faster colonic transit than HLA-DQ2/8-negative patients. The primary aim was to compare gastric emptying, SB and colonic transit, SB and colonic barrier function, and SB and colonic mucosal inflammation in a prospective cohort of patients with IBS-D on a gluten-containing diet [as documented by a food questionnaire administered by a registered dietitian (D.J.)] who were HLA-DQ2/8-positive or -negative. A secondary aim was to compare intestinal permeability and tight junction (TJ) protein expression in IBS-D patients and healthy controls.
METHODS
Participants and Negative Screening for Celiac Disease
Forty-five IBS-D patients (43 women and 2 men) were identified as having Rome II symptom criteria of IBS (16) on the basis of a comprehensive and validated bowel disease questionnaire, which included a somatic symptom listing, including headaches, stiffness, back pain, and joint pains, among 16 symptoms [scored on a scale of 0–4 for frequency and bothersomeness (32)]. Participants were recruited from April 2010 to December 2011. The protocol was approved by the Institutional Review Board of the Mayo Clinic.
Patients were not selected on the basis of a prior documentation of clinical response to a GFD. Patients had negative serum TTG and anti-endomysial antibody test if TTG was positive or equivocal. TTG IgA and IgG were measured by commercial enzyme immunoassay kits (human recombinant anti-TTG IgA kit, INOVA, San Diego, CA).
The main exclusion criteria were as follows: serum TTG IgA- or IgG-positive or medical record evidence of SB biopsy suggestive of celiac disease, use of tobacco products within the past 6 mo or nonsteroidal anti-inflammatory drugs or aspirin within the past week (since they may affect intestinal permeability), and bleeding disorders or medications that increase risk of bleeding from mucosal biopsies. For 2 days prior to studies, patients were instructed to avoid ingestion of artificial sweeteners such as Splenda (sucralose) and NutraSweet (aspartame), foods containing lactulose or mannitol, and medications that can affect gastrointestinal (GI) transit. A dietary questionnaire verified adequate gluten intake by participants to ensure that they were not on a GFD prior to enrollment.
HLA Genotyping
DNA was extracted from peripheral blood for HLA typing of DR and DQ alleles. HLA-DQ2 and HLA-DQ8 were determined using six HLA-tagging single-nucleotide polymorphisms (26).
Measurement of SB and Colonic Permeability
As in prior studies (8, 29), lactulose (1,000 mg) and mannitol (200 mg) (catalog nos. L7877 and M8429, respectively, Sigma-Aldrich, St. Louis, MO) were used to determine the urine sugar excretions at different times as markers of SB and colonic mucosal permeability after oral ingestion of the sugars in aqueous solution. Urine was collected every 30 min for the first 2 h (when the participant was able to provide a specimen, and cumulated for the entire 2 h), every 2 h for the next 6 h, and from 8 to 24 h. The total volume of each collection was measured, and an aliquot from each collection was obtained to estimate the total content of each sugar for the different time intervals. The urine aliquot was stored at −20°C until it was thawed for analysis.
Participants ingested standardized meals during the first 8 h. Specifically, 500 ml of water were given 30 min after sugar administration to aid in the collection of urine. A breakfast of egg, toast, and water was given after 2 h, and a lunch of chicken, potato, and water was offered after 6 h. Water was allowed ad libitum throughout the day.
We estimated cumulative and ratio excretions of the two sugars at 0–2 h and 8–24 h for SB and colonic mucosal permeability, respectively, on the basis of recent validation studies (29). Urinary saccharide concentrations were measured by high-performance liquid chromatography-tandem mass spectrometry. Details of this method are described elsewhere (8); the assay was adapted from the method of Lostia et al. (23). The cumulative excretion was calculated as concentration of sugar (μg/ml) × total urine volume (ml). The lactulose-to-mannitol ratio was calculated as 0.2 × (cumulative excretion of lactulose)/(cumulative excretion of mannitol).
SB and colonic permeability of IBS-D patients in this cohort were compared with the permeability of 12 healthy subjects acquired in a contemporaneous study in our laboratory using the same method of measurement (29).
SB and Colonic Mucosal Morphology
The SB biopsies (obtained in 28 of 45 IBS-D patients who consented to upper and lower endoscopy with biopsies conducted solely for research purposes) were stained with hematoxylin-eosin to assess villous architecture, villous-to-crypt ratio, and intraepithelial lymphocytes (IELs) and immunohistochemistry for CD3, CD8, tryptase, and zonula occludens 1 (ZO-1). Section orientation was controlled by counting cells in areas with three adjacent villi in SB biopsies and three adjacent crypts in colonic biopsies viewable for their entire length. CD3, CD8, and tryptase stain were assessed quantitatively. ZO-1 staining was assessed using a semiquantitative score: percentage of positive cells, intensity of the stain (on a scale of 1–3), and percentage of cells × intensity (20). Biopsy analyses were assessed by one experienced GI pathologist (T.S.) who was blinded to each patient's HLA-DQ genotype.
In addition, rectosigmoid colon mucosal biopsies were obtained from the same 28 patients and assessed for number of IELs.
Immunohistochemical staining of 5-μm-thick paraffin sections was performed using a commercial kit (EnVision+, Dako, Carpinteria, CA). The primary antibodies were CD3, CD8, and tryptase (Dako) and ZO-1 (Invitrogen, Camarillo, CA). Slides were deparaffinized and rehydrated to water. Heat-induced epitope retrieval was performed in a citrate buffer target retrieval solution (pH 6.0; Dako) in a water bath for 20 min. Endogenous peroxidase activity was blocked for 10 min at room temperature in 3% H2O2, and the slides were rinsed in running tap water. Nonspecific protein binding sites were blocked by application of 5% (vol/vol) dilution of normal goat serum to slides for 10 min at room temperature. The serum was blotted off, and the sections were subsequently incubated with the primary antibodies for 30 min at room temperature. After processing according to the manufacturer's protocols, 3,3′-diaminobenzidine was used as the final chromogen, and nuclei were counterstained with Mayer's hematoxylin.
Quantitation of TJ Proteins by Real-Time PCR
We used real-time PCR to quantitate TJ proteins in rectosigmoid colon biopsy samples from 28 IBS-D patients and 16 healthy subjects collected in a prior study (1). Rectosigmoid colon biopsy samples were submerged in RNAlater solution (Ambion, Austin, TX) and stored at −80°C. After tissue was homogenized in RNeasy lysis buffer (RLT buffer, Qiagen, Valencia, CA), RNA was extracted (RNeasy mini kit, Qiagen). cDNA synthesis was performed using 0.2 μg of total RNA with the High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). TaqMan gene expression assays were carried out in triplicate for each gene on a real-time PCR system (Prism 7900HT, Applied Biosystems, Foster City, CA) according to the manufacturer's instructions using the comparative cycle threshold (ΔΔCT) method for relative quantification. The following Assays-on-Demand were applied: ZO-1 (Hs01551861_m1), occludin (OCLN, Hs00170162_m1), claudin (CLDN-1, Hs00221623_m1), and GAPDH (Hs03929097_g1). The expression of each gene was normalized to the endogenous control (i.e., GAPDH) and compared with the expression in 16 healthy subjects who underwent rectosigmoid mucosal biopsies in a prior study (1). All mRNA expressions were assayed in triplicate, and the mean value was used for the statistical analysis. All assays were performed in the same laboratory within a period of 1 mo by one technologist (P.C.).
Measurement of Gastric Emptying and SB and Colonic Transit With Scintigraphy
Procedure.
As in prior studies (8, 20, 23), 1 mCi of 99mTc-sulfur colloid was added to two raw eggs during the scrambling-and-cooking process (14). The scrambled eggs were served on one slice of bread with 240 ml of skim milk. The total calorie content of the meal was 296 kcal, and macronutrient composition was 32% protein, 35% fat, and 33% carbohydrate. To evaluate colonic transit (5, 15), 111In adsorbed on activated charcoal particles was delivered to the colon by a methacrylate-coated, delayed-release capsule (5), which was ingested at 6 AM after an overnight fast. When the capsule emptied from the stomach (on images obtained every 30 min while the patient was fasting), all participants received the radiolabeled egg meal at time 0.
Anterior and posterior gamma camera images were obtained immediately after radiolabeled meal ingestion, hourly through 8 h, and then at 24 and 48 h after radiolabeled meal ingestion. Two standardized meals were ingested at 4 h (530-kcal chicken meal) and 8 h (750-kcal roast beef sandwich, which included 2 slices of bread).
A variable region-of-interest program was used to measure isotope counts in each region and, thereby, derive a transit measurement. Geometric means of counts in anterior and posterior gastric regions of interest were used (after correction for radioisotope decay and tissue attenuation) to estimate the proportion of 99mTc emptied from the stomach at each time point or filling of the colon at 6 h and the proportion of 111In in each colonic region at specified times. Gastric emptying was measured over 4 h after meal ingestion. Filling of the colon at 6 h serves as a valid surrogate for SB transit (14). The performance characteristics of these tests are published elsewhere (14, 15).
Transit data analysis.
The gastric emptying end point of analysis was half time (t1/2) for solids, which was estimated by linear interpolation of the data at each time point. SB transit time was assessed indirectly by the colonic filling at 6 h. Overall colonic transit was summarized as the colonic geometric center (GC) at specified times. The GC is the weighted average of counts in the colonic regions (ascending, transverse, descending, and rectosigmoid) and stool, weighted 1–5, respectively. At any time, the proportion of counts in each colonic region is multiplied by its weighting factor as follows: (%ascending × 1 + %transverse × 2 + %descending × 3 + %rectosigmoid × 4 + %stool × 5)/100 = GC. Thus, a higher GC reflects a faster colonic transit.
Statistical Analysis
The primary end points were colonic GC at 24 h (GC24) and urine mannitol excretion at 0–2 h and 8–24 h. Secondary end points were gastric emptying t1/2 (min), colonic filling at 6 h (CF6, %), GC at 48 h (GC48), ascending colon (AC) t1/2, urine lactulose at 8–24 h, lactulose-to-mannitol ratio at 0–2 h and 8–24 h, and mucosal inflammation. The association of HLA-DQ status with end points was assessed using Wilcoxon's rank-sum test.
Data from a separate group of normal healthy volunteers were used to compute fold change levels for each of the IBS-D patients. Because the GAPDH expression was different in the healthy volunteers and IBS patients (despite use of the same method), we adjusted the values for the proteins of interest (ZO-1, occludin, and claudin) relative to the GAPDH in each subject; we then computed the adjusted fold changes in IBS-D patients relative to the overall mean fold change in the healthy volunteers for each of the three proteins. These fold changes (ratios) were tested against a hypothesized value of 1 for the total set of IBS-D patients using the signed-rank test, and the association of fold changes with HLA status in the IBS-D patients was assessed using Wilcoxon's rank-sum test. ZO-1 protein intensities of staining grades (range 1–3) were documented in each individual as proportions in SB and rectosigmoid mucosa, and the distributions of those proportions were compared using Fisher's exact test.
The transit results for the entire group of IBS-D patients were compared with the results of a cohort of 170 healthy controls (118 women and 52 men) previously reported from our laboratory using identical measurements of transit (20). The GC24, GC48, and CF6 mean values in the healthy controls were 2.44, 3.62, and 50.0%, respectively. The differences between the corresponding data in IBS-D patients and these values (IBS-D patient − healthy control mean values) were compared using a one-sample signed-rank test, since the distributions of the deltas were not normal. Age and gender distributions were generally similar for the comparisons of permeability in the IBS-D patient and control (29) groups (both P > 0.19). While the healthy controls used for the transit comparison were, on average, ∼9 yr younger (24) than the IBS-D patients, previous studies showed no significant effect of age between 18 and 65 yr on GI or colonic transit (14). The two groups were predominantly female (96% in IBS-D and 69% in healthy controls).
Statistical Power
Permeability.
Table 1 shows excretion of mannitol and lactulose in healthy volunteers in recent studies conducted using the same methods in our laboratory (29). On the basis of these data, we elected to study 48 patients to have ∼80% power to detect an ∼30% difference in the two groups on the basis of HLA-DQ genotype (Table 2).
Table 1.
Excretion of mannitol and lactulose
| Time Period | Mannitol | Lactulose |
|---|---|---|
| Cumulative excretion, mg | ||
| 0–2 h | 48 ± 17 | 3.7 ± 1.0 |
| 2–24 h | 210 ± 70 | 45 ± 22 |
| 8–24 h, mg/h | 4.9 ± 3.1 | 1.6 ± 1.0 |
Values are means ± SD. Data are from a recent study conducted in healthy volunteers (29).
Table 2.
Sample size assessment for excretion of mannitol and lactulose: effect sizes detectable with ∼80% power for two groups based on HLA-DQ genotype
| Response | Difference Detectable (80% Power) |
Effect Size |
||
|---|---|---|---|---|
| n = 12 | n = 24* | n = 12 | n = 24* | |
| Mannitol excretion | ||||
| 0–2 h | 21 mg/h | 14 mg/h | 44% | 29% |
| Cumulative 2–24 h | 84 mg | 58 mg | 40% | 28% |
| Lactulose excretion | ||||
| Cumulative 2–24 h | 27 mg | 19 mg | 60% | 42% |
Difference detectable is based on a 2-sample t-test (2-sided α = 0.05). Effect size is difference as percentage of overall mean.
Detectable for overall main effect of diet.
Transit.
The same sample size of ∼24 per group was anticipated to have 80% power to detect a clinically meaningful colonic transit difference in the two groups separated by genotype, as shown in Table 3 (31).
Table 3.
Sample size assessment for transit data: effect sizes detectable with ∼80% power for two groups based on HLA-DQ genotype
| Response | Mean | SD | COV, % | Effect Size, % Detectable |
|
|---|---|---|---|---|---|
| n = 12/group | n = 24/group* | ||||
| Colon GC24 | 3.53 | 0.87 | 25 | 30 | 20 |
| Ascending colon t1/2 h | 14.9 | 9.2 | 62 | 74 | 51 |
Data are from our studies performed in IBS-D patients (Ref. 30).
GC24, geometric center at 24 h; t1/2, half time; COV, coefficient of variation.
Overall main effect of human leukocyte antigen (HLA) genotype.
RESULTS
Characterization of Prior Dietary Gluten Intake and Extraintestinal Symptoms
The diet questionnaire demonstrated that none of the patients followed a GFD prior to the study. The number of gluten-containing servings per day ranged from 1 to 15 (mean 3.10 ± 0.46). Only two patients were ingesting 15 servings of gluten-containing foods per day; 90% of participants ingested 1–4.4 servings of gluten-containing foods per day. Given the tight distribution of the data, we do not perceive that an analysis based on the level of consumption of gluten would be meaningful to assess whether there were differences between low and high consumers of gluten in this cohort of patients who do not have celiac disease or prior evidence of intolerance to gluten.
The mean somatic symptom score in the entire patient cohort was 0.422 ± 0.295 (SD). The frequency and bothersomeness of these somatic symptoms were not significantly associated with HLA-DQ2/8-positive and -negative patients (P = 0.60).
SB and Colonic Permeability
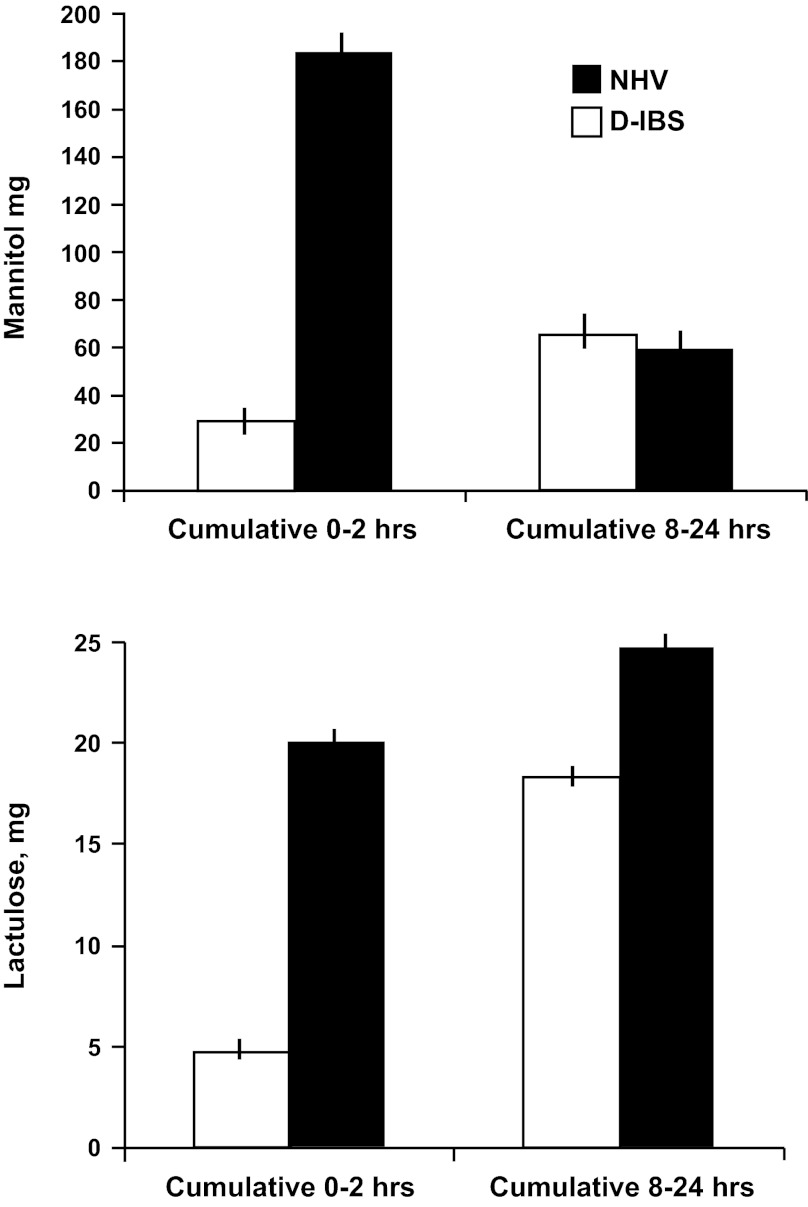
When compared with healthy volunteers recently studied in our laboratory (29), the overall permeability measurements of the IBS-D patients showed increased SB permeability (P < 0.001; Fig. 1) and no changes in colonic permeability (Fig. 1); these observations were based on urine mannitol and lactulose excretion (both P < 0.001). Urine lactulose-to-mannitol excretion ratio in the 8–24 h collection, corresponding to colonic permeability, was higher in IBS-D patients than in healthy controls (P = 0.106).
Fig. 1.
Comparisons in irritable bowel syndrome (IBS) with diarrhea (IBS-D) and health of small bowel permeability [0–2 h cumulative excretion of mannitol and lactulose (both P < 0.001)] and colonic permeability [8–24 h cumulative excretion of mannitol (P = not significant) and lactulose (P = not significant)] permeability. Lactulose-to-mannose excretion ratio for the 8–24 h collection showed a numerical difference that was not significant (P = 0.106). Values are means ± SE. NHV, normal healthy volunteers.
There was no association of urine mannitol or lactulose excretion or lactulose-to-mannitol excretion ratios at 0–2 h or 8–24 h with HLA status (Table 4).
Table 4.
Demographics, permeability, and transit in HLA-DQ2/8-positive and -negative IBS-D patients
| HLA-DQ2/8-Negative (n = 22) | HLA-DQ2/8-positive (n = 23) | P Value* | |
|---|---|---|---|
| Age, yr | 44.5 ± 2.5 | 41.5 ± 3.0 | NS |
| BMI, kg/m2 | 30.7 ± 2.2 | 30.3 ± 1.7 | NS |
| Cumulative urine mannitol, mg | |||
| 0–2 h | 33.8 ± 2.6 | 32.5 ± 5.9 | NS |
| 8–24 h | 80.9 ± 13.0 | 76.2 ± 16.6 | NS |
| Cumulative urine lactulose, mg | |||
| 0–2 h | 2.6 ± 0.2 | 8.5 ± 5.2 | NS |
| 8–24 h | 34.6 ± 5.7 | 29.2 ± 6.8 | NS |
| Urine lactulose-to-mannitol ratio | |||
| 0–2 h | 0.017 ± 0.002 | 0.03 ± 0.009 | NS |
| 8–24 h | 0.10 ± 0.014 | 0.12 ± 0.042 | NS |
| Gastric emptying t1/2, min | 113.9 ± 7.2 | 130.5 ± 5.4 | 0.030 |
| CF6, % | 60.0 ± 5.8 | 66.5 ± 4.8 | NS |
| Overall colonic transit | |||
| GC24 | 3.48 ± 0.25 | 2.78 ± 0.25 | 0.031 |
| GC48 | 4.78 ± 0.09 | 4.01 ± 0.19 | 0.008 |
| Ascending colon emptying t1/2, h | 9.95 ± 1.29 | 11.46 ± 1.88 | NS |
Values are means ± SE. Normal values (means ± SE) are as follows: 121.7 ± 1.7 min (n = 319) for gastric emptying t1/2 (Ref. 6), 50 ± 2.4% (n = 167) for colonic filling at 6 h (CF6, small bowel transit) (Ref. 24), 2.4 ± 0.1 (n = 167) for GC24 (Ref. 24), and 3.6 ± 0.1 (n = 151) for GC48 (Ref. 24).
Test for association with HLA status (Wilcoxon's rank-sum test). NS, not significant.
TJ Protein mRNA Expression in SB and Colonic Mucosa
There was statistically significant decreased mRNA expression of ZO-1 and occludin (both P < 0.001), but not claudin proteins, in rectosigmoid biopsies of IBS-D patients compared with those from normal healthy controls (Table 5). There was no significant association of the expression of the three TJ proteins with HLA-DQ2/8 status (positive vs. negative).
Table 5.
mRNA expression in colonic mucosa of healthy controls and IBS-D patients
| Healthy Controls (n = 16) | IBS-D Patients |
|||
|---|---|---|---|---|
| All (n = 25) | HLA-DQ2/8-positive (n = 12) | HLA-DQ2/8-negative (n = 13) | ||
| Colon ZO-1 | 1.00 ± 0.024 | 0.960 ± 0.046* | 0.978 ± 0.027 | 0.943 ± 0.055† |
| Colon claudin | 1.00 ± 0.018 | 1.003 ± 0.020 | 0.997 ± 0.023 | 1.009 ± 0.015 |
| Colon occludin | 1.00 ± 0.016 | 0.931 ± 0.025* | 0.936 ± 0.017 | 0.926 ± 0.031 |
Values (means ± SD) are expressed as fold change relative to mean in normal controls.
ZO-1, zonula occludens 1.
Significantly different from 1.0 (P < 0.001, by signed-rank test).
Association with HLA status (P = 0.097, by Wilcoxon's rank-sum test).
SB and Colonic Mucosal Morphology and ZO-1 Protein Expression
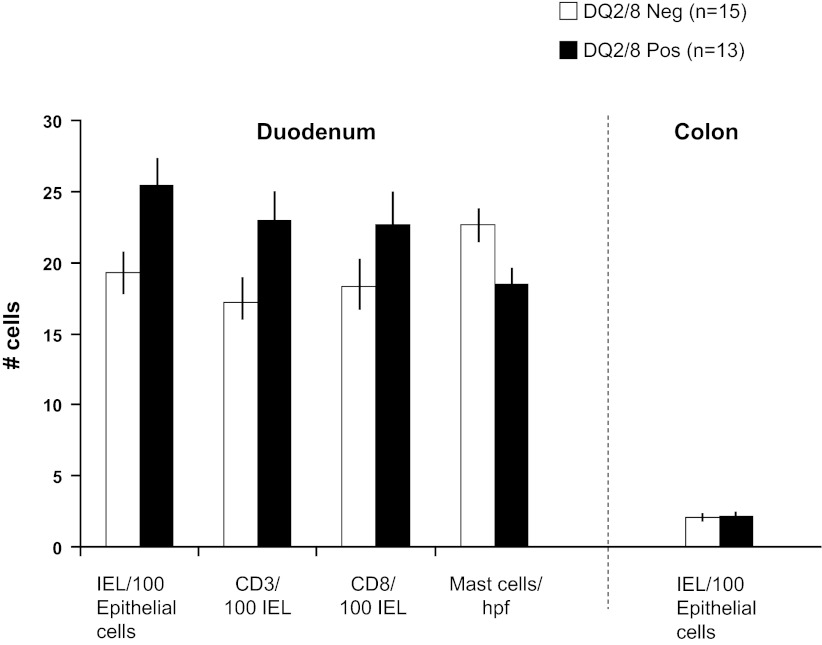
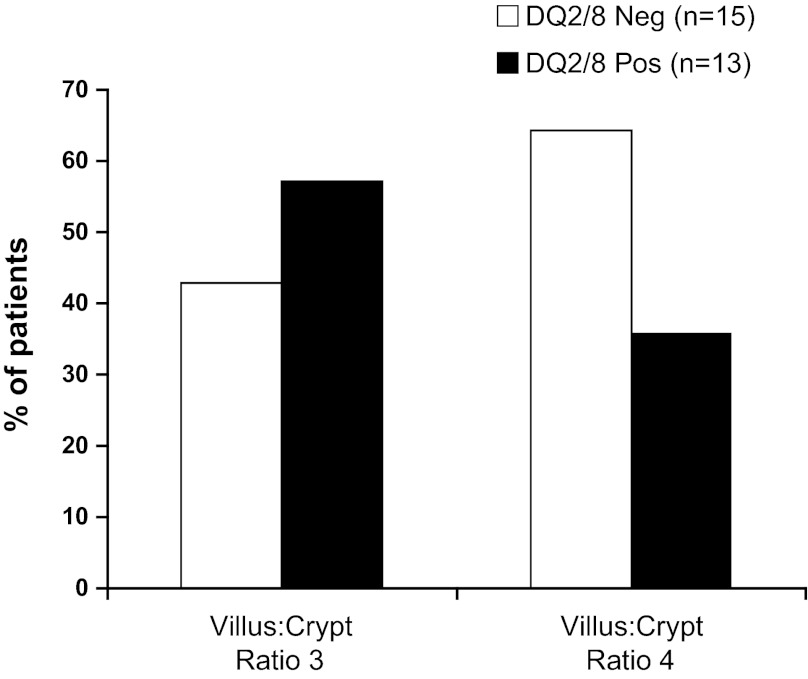
There were no significant associations in numbers of IELs, CD3 and CD8 cells, and tryptase-positive (mast) cells with HLA genotype status in the SB mucosa or IELs in the colonic mucosa in the IBS-D patients (Fig. 2). Villus-to-crypt ratios confirmed the absence of celiac disease in all participants who showed ratios of 3:1 or 4:1. There was no association of the percentages of patients with ratios of 3:1 or 4:1 according to HLA-DQ status (Fig. 3).
Fig. 2.
Morphology of duodenal and colonic biopsies according to human leukocyte antigen (HLA)-DQ status. Data are presented as a quantitative assessment of intraepithelial lymphocytes (IELs) in duodenum and colon and immunohistochemistry in duodenal mucosa. hpf, High-power field. There were no statistically significant differences according to HLA-DQ status. Values are means ± SE.
Fig. 3.
Duodenal biopsy villus-to-crypt ratio according to HLA-DQ status. Data are presented as percentage of patients with the specific villus-to-crypt ratio. There were no statistically significant differences in the proportion of patients with crypt-to-villus ratios of 3:1 or 4:1 according to HLA-DQ status.
In view of the difference in mRNA expression of ZO-1 and occludin in rectosigmoid mucosa in the IBS-D patients, we compared the morphological expression of ZO-1 protein in SB and rectosigmoid biopsies in the two HLA subgroups (Table 6). There was a borderline significant difference in the ZO-1 intensity score in the SB mucosa (P = 0.06), with lower intensity in HLA-DQ2/8-positive patients. There was a significantly lower ZO-1 intensity score (P = 0.01) and borderline significant %cell × intensity score (P = 0.07) in the rectosigmoid biopsies in HLA-DQ2/8-positive patients. The observation of reduced ZO-1 protein expression in rectosigmoid mucosa is consistent with the reduced ZO-1 mRNA expression in the same tissue.
Table 6.
ZO-1 immunohistochemistry and semiquantitative assessment of cell number and intensity of staining in small bowel and rectosigmoid mucosa in HLA-DQ2/8-positive and -negative groups
| IBS-D Patients |
Overall P Value | ||
|---|---|---|---|
| HLA-DQ2/8-positive (n = 13) | HLA-DQ2/8-negative (n = 15) | ||
| Small bowel | |||
| ZO-1, %cells | 60 ± 6.6 | 70.7 ± 5.0 | 0.23 |
| ZO-1 intensity score, no. of patients | |||
| Grade 1 | 3 | 1 | 0.06 |
| Grade 2 | 5 | 6 | |
| Grade 3 | 5 | 8 | |
| ZO-1, %cells × intensity | 141.5 ± 25.7 | 182.7 ± 20.6 | 0.24 |
| Rectosigmoid | |||
| ZO-1, %cells | 50.8 ± 6.7 | 63.1 ± 4.1 | 0.21 |
| ZO-1 intensity score, no. of patients | |||
| Grade 1 | 6 | 1 | 0.01 |
| Grade 2 | 4 | 8 | |
| Grade 3 | 2 | 4 | |
| ZO-1, %cells × intensity | 95.0 ± 21.8 | 146.2 ± 17.1 | 0.07 |
GI and Colonic Transit
The (mean) differences (IBS-D patient − healthy control values) were 0.67% (n = 44, P = 0.002), 0.75% (n = 43, P < 0.0001), and 13.3% (n = 45, P = 0.0009) for GC24, GC48, and CF6, respectively, consistent with larger values in IBS-D patients, i.e., accelerated transit relative to the previously published data in controls (24).
Gastric emptying, as well as colonic transit at 24 and 48 h, was slower in HLA-DQ-positive than HLA-DQ-negative patients (Table 4). The other transit end points used to assess SB and ascending colon transit were not significantly associated with HLA-DQ status (Table 4).
DISCUSSION
In this study, we evaluated the influence of HLA-DQ2/8 genotypes on mucosal barrier function, mucosal inflammation, and GI transit in unselected IBS-D patients. Diet assessment of each participant confirmed that all the patients were ingesting gluten in the weeks prior to entry to the study.
We confirmed our prior observations (29) and other reports in the literature (17, 25, 28) that IBS-D patients have increased SB permeability compared with healthy controls. While significantly increased colonic permeability was not observed, the cumulative urine excretion of lactulose at 8–24 h was increased (P = 0.10). Consistent with this finding, IBS-D patients had decreased mRNA expression of ZO-1 and occludin compared with healthy volunteers, but HLA type did not appear to influence TJ mRNA expression, mucosal permeability, or SB mucosal morphology in this cohort of IBS-D patients. On the other hand, ZO-1 protein expression was generally lower in HLA-DQ2/8-positive patients (borderline in SB and significant in rectosigmoid mucosa).
The roles of HLA-DQ status and potential immunogenetic mechanisms in symptom generation in IBS-D patients are controversial. Evidence in favor of immunogenetic mechanisms include the observation that, in HLA-DQ8 transgenic mice sensitized to gluten, gliadin exposure led to increased contraction of smooth muscle to electrical field stimulation and carbachol (35). However, this model is not strictly applicable to our study, since our patients, who had adequate gluten exposure in their diets, were not preselected for evidence of gluten intolerance. On the other hand, the diarrhea in IBS-D patients may be the result of other factors, such as alterations in gut permeability, inflammation, or microbial flora. HLA genes in association with the gut microbiome may determine the immune environment and confer susceptibility to autoimmune arthritis (18).
Our current observations confirm reports in the literature on increased intestinal permeability and reduced barrier function in IBS-D patients and gluten-sensitive patients. Patients with postinfectious IBS-D had greater intestinal permeability (17), suggesting that symptom generation reflects disruption of the SB mucosal integrity. On the other hand, Sapone et al. (30) reported decreased intestinal permeability in patients with nonceliac gluten sensitivity; it is worth noting that the patients included in our study were not selected on the basis of gluten sensitivity. Alterations in jejunal mucosal TJ signaling have been described in IBS-D, but the relationship with HLA-DQ status was not previously investigated (25). In the current study, IBS-D patients had decreased mRNA expression of two TJ proteins, ZO-1 and occludin, consistent with prior studies (25, 28). A recent study from Martinez et al. (25) identified significant correlation between ZO proteins, mast cell activation, and clinical symptoms. Piche et al. (28) also demonstrated that, overall, IBS patients had decreased ZO-1 and occludin mRNA expression in colonic biopsies. Whereas there were no differences in TJ protein mRNA expression according to HLA type in our current study, ZO-1 protein expression data suggest that HLA type may influence alterations in intestinal permeability to sugars, but larger studies are warranted. The potential interaction between gliadin and HLA genotype is being assessed in a randomized, controlled trial of gluten-containing vs. gluten-free diets (34). Overall, these data are suggestive of the hypothesis that the reduced expression of TJ structures may mediate increased permeability and might be a target for treatment with larazotide, which has been shown to regulate epithelial TJs in vitro in IEC-6 and Caco-2 cells (19). In vivo, larazotide inhibited gliadin-induced macrophage accumulation in the intestine and preserved normal TJ structure in gliadin-sensitized HLA-HCD4/DQ8 double-transgenic mice (19).
Recent in vitro studies have demonstrated that gliadin interacts with the chemokine receptor CXCR3, leading to increased zonulin release and increased intestinal permeability (21), possibly through activation of the innate or adaptive immune system, leading to an inflammatory response (11). This may provide an alternative HLA-independent mechanism to explain the increased permeability in IBS-D. The putative mechanisms whereby increased permeability and reduced TJ expression might mediate IBS-D symptoms are increased water flux, resulting in looser-consistency bowel movements, and visceral hypersensitivity, as demonstrated by Bertiaux-Vandaële et al. (3), who observed association of lower occluding expression with more severe abdominal pain.
A low-grade inflammatory state may have a role in symptom development, such as pain, in IBS patients, as demonstrated by changes in CD3-positive cell count population and mast cell numbers, and an increase in serotonin release (2, 13, 27, 38). In our study, HLA-DQ2/8 genotype was not associated with degree of inflammation in SB or rectosigmoid colon mucosa. Although this was not a primary end point, our study was sufficiently powered to detect a clinically meaningful difference in the inflammatory cell infiltrate in the two groups on the basis of HLA-DQ status. The quantitative histological data pertaining to SB CD3, CD8, and (tryptase-positive) mast cells (no./high-power field) were normally distributed, and the coefficients of variation were 53%, 57%, and 32%, respectively. Therefore, even with the total of 28 biopsies (13 from HLA-DQ2/8-positive and 15 from HLA-DQ2/8-negative patients), the effect sizes in SB inflammatory cells that could be detected between the two groups with 80% power (at α = 0.05) were 48% for CD3 lymphocytes, 50% for CD8 lymphocytes, and 31% for mast cells. Given the small numbers of inflammatory cells in patients with IBS-D, the absolute numbers of cells are relatively small; therefore, the study had adequate power to detect such a difference, if it was present.
Patients with IBS-D had accelerated colonic transit compared with healthy controls examined with the same method that has been established in our laboratory for over 20 years. We previously showed that ∼45% of patients with symptom phenotype of IBS-D have accelerated transit (7); we did not investigate the relationship to HLA-DQ status in that study. The observation that HLA-DQ2/8-positive patients had slower colonic transit at 24 and 48 h was unexpected. The clinical significance of the slower transit in HLA-DQ2/8-positive patients is unclear, given the relatively small sample size and the multiplicity of transit end points, some of which (e.g., SB transit and ascending colon emptying time) were not different according to HLA-DQ status. In a recent study in IBS-D patients who had previously responded to gluten withdrawal, there was no difference in symptom generation after gluten exposure on the basis of HLA-DQ2/8 status (4). A preliminary report of a randomized, controlled trial of GI and colonic transit in IBS-D patients exposed to gluten (34) showed no significant difference in SB or colonic transit with a GFD compared with a gluten-containing diet. Our overall assessment is that the significant association between HLA genotype and overall colonic transit may be a chance finding and requires replication.
A potential pitfall of our study is that patient participants were not selected on the basis of a history suggestive of gluten sensitivity or a prior response to gluten withdrawal. Hence, it is conceivable that our results cannot be generalized to patients with gluten intolerance by history or response to gluten withdrawal; these patients might be more likely to recognize gluten as an antigen that leads to increased inflammation. Other pitfalls are the sample size, which was too small to appraise heterogeneity within the HLA group, such as homozygotes, heterozygotes, complex heterozygotes, or DQ8-positive only, and the use of staining intensity to appraise the morphological changes in ZO-1 protein expression. This approach is highly dependent on labeling techniques; therefore, our observation suggesting no differences according to HLA status may not have functional relevance.
In summary, in this prospective cohort of IBS-D patients who were not selected on the basis of prior response to a GFD, carriers of HLA-DQ2/8 actually had slower colonic transit than HLA-DQ2/8-negative patients. We confirmed intestinal mucosal barrier abnormalities in gluten-ingesting IBS-D patients, but HLA-DQ2/8 immunogenotype was not associated with alterations in SB or colonic permeability or mucosal inflammation, although genotype was associated with ZO-1 protein expression in rectosigmoid mucosa. Overall, the mucosal barrier data are consistent with the hypothesis that gluten sensitivity (without celiac disease) may represent a separate entity in the spectrum of IBS-D patients. Consistent with this hypothesis, we have presented the preliminary results of the effects of a diet-intervention, randomized, controlled study of gluten exclusion on symptoms (bowel function), intestinal barrier functions (permeability and TJ expression), and morphology in IBS-D patients, and we have demonstrated deleterious effects of gluten, which were aggravated in HLA-DQ2/8-positive patients (33, 34).
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1RC1-DK-086182 and R01-DK-092179 to M. Camilleri.
DISCLOSURES
J. A. Murray has received grants from Alba Therapeutics for clinical trials with the drug larazotide.
AUTHOR CONTRIBUTIONS
M.I.V.-R., T.S., J.O., P.C., J.L., D.J.E., D.J., M.R., and D.R. performed the experiments; M.I.V.-R., M.C., and A.R.Z. interpreted the results of the experiments; M.I.V.-R. and M.C. prepared the figures; M.I.V.-R. and M.C. drafted the manuscript; M.I.V.-R., M.C., J.A.M., and A.R.Z. edited and revised the manuscript; M.I.V.-R., M.C., T.S., J.A.M., and A.R.Z. approved the final version of the manuscript; M.C. is responsible for conception and design of the research; D.D.B. and A.R.Z. analyzed the data.
ACKNOWLEDGMENTS
We thank Cindy Stanislav for excellent secretarial assistance.
REFERENCES
- 1. Aerssens J, Camilleri M, Talloen W, Thielemans L, Göhlmann HW, Van Den Wyngaert I, Thielemans T, De Hoogt R, Andrews CN, Bharucha AE, Carlson PJ, Busciglio I, Burton DD, Smyrk T, Urrutia R, Coulie B. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 194–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol 106: 2165–2173, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 106: 508–514, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med 38: 1807–1810, 1997 [PubMed] [Google Scholar]
- 6. Camilleri M, Iturrino J, Bharucha AE, Burton DD, Shin A, Jeong ID, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 6: 772–781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Camilleri M, Nadeau A, Lamsam J, Linker-Nord S, Ryks M, Burton D, Sweetser S, Zinsmeister AR, Singh R. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 22: e15–e26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroccio A, Brusca I, Mansueto P, Soresi M, D'Alcamo A, Ambrosiano G, Pepe I, Iacono G, Lospalluti ML, La Chiusa SM, Di Fede G. Fecal assays detect hypersensitivity to cow's milk protein and gluten in adults with irritable bowel syndrome. Clin Gastroenterol Hepatol 9: 965–971, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Cash BD, Rubenstein JH, Young PE, Gentry A, Nojkov B, Lee D, Andrews AH, Dobhan R, Chey WD. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology 141: 1187–1193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cinova J, Palova-Jelinkova L, Smythies LE, Cerná M, Pecharová B, Dvorák M, Fruhauf P, Tlaskalová-Hogenová H, Smith PD, Tucková L. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol 27: 201–209, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 81: 192–194, 1981 [PubMed] [Google Scholar]
- 13. Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De Ponti F, Corinaldesi R, Barbara G. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 106: 1290–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther 16: 1781–1790, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Deiteren A, Camilleri M, Bharucha AE, Burton D, McKinzie S, Rao AS, Zinsmeister AR. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil 22: 415–423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drossman DA, Corazziari E, Talley N.(Editors). Functional bowel disorders and functional abdominal pain. In: The Functional Gastrointestinal Disorders (2nd ed.). McLean, VA: Degnon Associates, 2000 [Google Scholar]
- 17. Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101: 1288–1294, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA, White BA, Taneja V. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible *0401 mice but not arthritis-resistant *0402 mice. PLos One 7: e36095, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gopalakrishnan S, Durai M, Kitchens K, Tamiz AP, Somerville R, Ginski M, Paterson BM, Murray JA, Verdu EF, Alkan SS, Pandey NB. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides 35: 86–94, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Guan Y, Watson AJ, Marchiando AM, Bradford E, Shen L, Turner JR, Montrose MH. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am J Physiol Cell Physiol 300: C1404–C1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135: 194–204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Locke GR, 3rd, Murray JA, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Celiac disease serology in IBS and dyspepsia: a population-based case-control study. Mayo Clin Proc 79: 476–482, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Lostia AM, Lionetto L, Principessa L, Evangelisti M, Gamba A, Villa MP, Simmaco M. A liquid chromatography/mass spectrometry method for the evaluation of intestinal permeability. Clin Biochem 41: 887–892, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil 22: 293-–e82., 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martínez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, Sánchez A, Guilarte M, Antolín M, de Torres I, González-Castro AM, Pigrau M, Saperas E, Azpiroz F, Santos J. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol 107: 736–746, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Monsuur AJ, de Bakker PI, Zhernakova A, Pinto D, Verduijn W, Romanos J, Auricchio R, Lopez A, van Heel DA, Crusius JB, Wijmenga C. Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLos One 3: e2270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park JH, Rhee PL, Kim HS, Lee JH, Kim YH, Kim JJ, Rhee JC. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol 21: 71–78, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol 301: G919–G928, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI, Esposito P, Ferraraccio F, Cartenμ M, Riegler G, de Magistris L, Fasano A. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 9: 23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sweetser S, Camilleri M, Linker Nord SJ, Burton DD, Castenada L, Croop R, Tong G, Dockens R, Zinsmeister AR. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in females with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol 296: G1299–G1306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 65: 1456–1479, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Vazquez-Roque M, Camilleri M, Murray JA, Smyrk TC, O'Neill J, Carlson P, Lamsam J, Eckert D, Burton D, Janzow D, Zinsmeister AR. Effects of gluten-free (GFD) and gluten-containing diet (GCD) on gastrointestinal and colonic transit, permeability and small bowel inflammation in irritable bowel syndrome with diarrhea (IBS-D): a randomized study (Abstract). Gastroenterology 142: S133, 2012 [Google Scholar]
- 35. Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, McKay DM, Murray JA. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol 294: G217–G225, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, Fiorini E, Caio G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol 46: 680–685, 2012 [DOI] [PubMed] [Google Scholar]
- 37. Wahnschaffe U, Schulzke JD, Zeitz M, Ulrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 5: 844–850, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Weston AP, Biddle WL, Bhatia PS, Miner PB., Jr Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci 38: 1590–1595, 1993 [DOI] [PubMed] [Google Scholar]