Abstract
The continued presence of gonadotropin-releasing hormone (GnRH) neurons is required for a healthy reproductive lifespan, but factors that maintain postnatal GnRH neurons have not been identified. To begin to understand these factors, we investigated whether 1) fibroblast growth factor (FGF) signaling and 2) interactions with the opposite sex are involved in the maintenance of the postnatal GnRH system. A transgenic mouse model (dnFGFR mouse) with the targeted expression of a dominant-negative FGF receptor (dnFGFR) in GnRH neurons was used to examine the consequence of FGF signaling deficiency on postnatal GnRH neurons. Male dnFGFR mice suffered a significant loss of postnatal GnRH neurons within the first 100 days of life. Interestingly, this loss was reversed after cohabitation with female, but not male, mice for 300–550 days. Along with a rescue in GnRH neuron numbers, opposite-sex housing in dnFGFR males also increased hypothalamic GnRH peptide levels, promoted a more mature GnRH neuronal morphology, facilitated litter production, and enhanced testicular morphology. Last, mice hypomorphic for FGFR3 exhibited a similar pattern of postnatal GnRH neuronal loss as dnFGFR males, suggesting FGF signaling acts, in part, through FGFR3 to enhance the maintenance of the postnatal GnRH system. In summary, we have shown that FGF signaling is required for the continued presence of postnatal GnRH neurons. However, this requirement is not absolute, since sexual interactions can compensate for defects in FGFR signaling, thereby rescuing the declining GnRH system. This suggests the postnatal GnRH system is highly plastic and capable of responding to environmental stimuli throughout adult life.
Keywords: fibroblast growth factor, gonadotropin-releasing hormone, neuroendocrine system, sexual behavior, social interaction
gonadotropin-releasing hormone (GnRH) secretion from the brain drives sexual maturation and adult reproduction in all vertebrates (53). The proper maturation and maintenance of GnRH neurons, which activate the hypothalamic-pituitary-gonadal (HPG) axis, are vital to the reproductive success of vertebrates. Many signaling molecules, including neurotransmitters, neurotransmitter receptors, neuropeptides, and chemoattractants/chemorepellents, organize the prenatal development of GnRH neurons into a functional network (54). In rodents, the number of postnatal GnRH neurons remains essentially unchanged once the network is formed (29). It is presently unclear what factors contribute to the continued differentiation and survival of postnatal GnRH neurons after the system is fully established.
Prenatally, GnRH neurons depend on fibroblast growth factor (FGF) signaling for the proper specification of their cell fate (7). There is currently no direct evidence for a neurotrophic role of FGF signaling in postnatal GnRH neurons, but two forms of FGF receptors, FGFR1 and FGFR3, are present on postnatal GnRH neurons (24), and FGF signaling promotes the survival and differentiated morphology of immortalized GnRH neurons in culture (57). These findings raise the possibility of an FGF signaling involvement in the postnatal GnRH system. Of further interest, the soma size or number of adult GnRH neurons can be rapidly altered by social and environmental cues in several vertebrate species (4, 13, 35, 38, 52). This, along with reports that aging male mice housed with or exposed to females exhibit improved gonadal function and fertility compared with males housed alone or with other males (17, 18, 30, 47, 61), suggest that environmental stimuli may also enhance the long-term health of the GnRH system via unknown mechanisms to lengthen the animal's reproductive lifespan.
The goals of the present study are to examine whether a healthy postnatal GnRH system requires FGF signaling and if environmental cues interact with FGF signaling to contribute to the long-term health and function of the postnatal GnRH system. To examine the contribution of FGF signaling, a line of transgenic mice with attenuated FGF responsiveness specifically in their GnRH neurons [dominant-negative FGF receptor (dnFGFR) mice] is used. These mice express a dominant-negative FGFR (dnFGFR) in their GnRH neurons to disrupt the function of FGFRs 1 and 3 (56). These animals are initially fertile but later exhibit delayed puberty. They also produce smaller litters and exhibit early termination of litter production by ∼100 days (56). We examine 1) if the GnRH system is compromised in postnatal dnFGFR mice, 2) if defects in the GnRH system and reproductive function in dnFGFR mice can be rescued by environmental cues such as opposite-sex interaction, and 3) which specific FGFR mediate the postnatal maintenance of GnRH neurons. Overall, our data not only support a novel role of FGF signaling in the health of the postnatal GnRH system, they also suggest environmental inputs, such as interaction with the opposite sex, can override the deficit in FGF signaling to reactivate an aging GnRH system.
MATERIALS AND METHODS
Transgenic Animals
dnFGFR mice expressing a dominant-negative FGFR (dnFGFR), specifically in GnRH neurons (MGI:3588566), were previously described (56). In these mice, the expression of a transgene that encodes a truncated murine FGFR1 lacking the tyrosine kinase domain was targeted to GnRH neurons using the rat GnRH promoter (Fig. 1). These mice were derived from the mating of C57BL/6J × DBA/2J hybrid mice and their offspring. FGFR3 knockout (KO) mice (Swiss-Webster/C57BL/6) were generated previously (9) and obtained from Jackson Laboratories (Bar Harbor, ME). Because 48% of homozygous FGFR3 KO die between birth and 21 days of age, only heterozygous FGFR3 KO (FGFR3+/−) were used for comparisons with wild-type (WT) siblings. Mice were housed in our animal facility under a 12:12-h light-dark cycle. Food and water were provided ad libitum. Day of birth was designated as postnatal day (PND) 0. All animal procedures were in compliance with protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado.
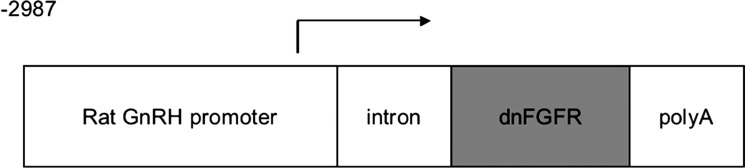
Fig. 1.
The construct used to generate dominant-negative fibroblast growth factor receptor (dnFGFR) mice. A 2.3-kb fragment of the rat gonadotropin-releasing hormone (GnRH) promoter (−2987 to −1172 appended to −441 to +104) was used to drive the expression of a dnFGFR, which is the IIIc variant of FGFR1 lacking the intracellular tyrosine kinase domain. A rabbit β-globin intron (intron) and human growth hormone polyadenylation signal (polyA) were placed upstream and downstream of dnFGFR, respectively, to facilitate transgene expression and processing.
Housing Treatments
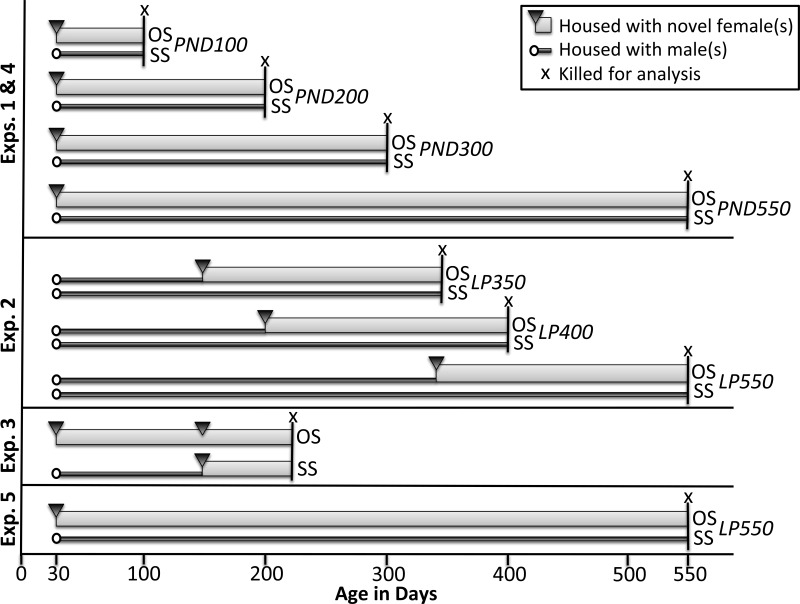
Housing treatments for all experiments below are illustrated in Fig. 2.
Fig. 2.
Illustration of housing treatments in experiments 1–5. The initiation and duration of opposite-sex (OS) and same-sex (SS) housing in experiments 1–5 are depicted. Thin dark gray bars indicate the duration of SS housing, and the thicker light gray bars indicate the duration of OS housing. Experiments were initiated by housing postnatal day (PND) 30 wild-type (WT) and dnFGFR males with novel age-matched female(s) (black triangle) or male(s) (white circle). Animals were killed for analysis at the end of the housing treatments (indicated by “x”). LP, late paired.
Experiment 1. Determine if the GnRH system and testicular morphology are defective in dnFGFR mice under different housing conditions.
WT and dnFGFR males were weaned on PND20 and temporarily maintained in same-sex (SS) groups. On PND30, dnFGFR males were housed in one of two configurations: 1) SS groups of four to five age- and genotype-matched males or 2) opposite-sex (OS) groups of one male and one to three age- and genotype-matched females. OS groups were allowed to breed and raise pups. The majority of WT males were SS housed, but some were OS housed to ensure that housing configuration had no effect on WT males. Adult mice were killed at the following four time points, each including a range of age: 1) PND100 (age range = 100–150 days), 2) PND200 (age range = 200–250 days of age), 3) PND300 (age range = 300–350 days), and 4) PND550 (age range = 520–620 days). In addition, male WT and dnFGFR pups were killed within 24 h of birth (PND0) to assess differences in GnRH neuron numbers. Brains were collected from all five age groups for GnRH immunocytochemistry (ICC) and from PND200 and PND550 groups for the measurement of GnRH peptide and mRNA. Last, testes were collected from the PND550 group for histological analysis and Cyp17a1 ICC. The PND100 and -200 age groups were chosen to examine changes during the period of optimal fecundity in male mice (21). The PND300 age group was selected based on possible reproductive differences between WT and dnFGFR mice, as this was the approximate age of reproductive senescence in dnFGFR females (56). Last, the 550-day time period was chosen to examine reproductively aged males (47).
Experiment 2. Determine if OS housing later in life rescues the GnRH system in dnFGFR males.
Male WT and dnFGFR mice, originally SS housed after weaning, were subject to OS or SS housing on PND150, PND200, or PND350 for 186 days and then killed for the measurement of hypothalamic GnRH peptide by a GnRH radioimmunoassay (RIA). Because these mice had undergone OS or SS treatment for ∼200 days, they were termed late paired (LP) 350, LP400, or LP550 at the time of death. The 186-day cohabitation period was chosen based on our pilot study showing PND150 dnFGFR males subject to OS housing for 6 mo was sufficient to restore their GnRH content.
Experiment 3. Determine if OS housing enhances several reproductive parameters.
WT and dnFGFR males, originally SS housed after weaning, were subject to OS or SS housing on PND30. On PND150, they were paired with novel virgin WT females that were 45–55 days old. Pairs were allowed to breed, and several breeding parameters, including time between litters, number of pups born, and number of pups survived, were recorded. All animals were paired for 4 mo to ensure sufficient time for collecting reproductive data. At the end of the experiment, mice were killed, and gonads and seminal vesicles were weighed. The anogenital distance of a subgroup of SS- or OS-housed dnFGFR males was also measured with a caliper on PND225. This value was normalized for the body mass to gauge their genital development.
Experiment 4. Determine if FGF signaling deficiency is associated with morphological defects in GnRH neurons and if OS housing could rescue these defects.
GnRH neuronal morphology was previously reported to correlate with GnRH neuronal maturation during pubertal transition (11) and thus could be used to examine the rescue of functional defects in the GnRH system by OS housing. WT and dnFGFR males were weaned on PND20 and SS housed until death at PND35. GnRH ICC was conducted on these brains to ascertain prepubertal differences in GnRH neuronal morphology between genotypes. Additionally, GnRH ICC from PND100 and PND200 WT and dnFGFR males from experiment 1 were used for morphological analysis of GnRH neurons after puberty.
Experiment 5. Determine if FGFR3 deficiency is associated with a postnatal loss of GnRH neurons.
FGFR3+/− mice and their WT siblings were housed in SS groups containing three to five animals. Animals were killed at PND0 and PND550, which correspond to the first and last time points of experiment 1, and GnRH neuron number was determined using ICC. For the analysis of the FGFR3+/− mice, mixed-sex animals of ∼1:1 sex ratio were used for all genotype and age groups for comparison with previous data (6, 7).
Tissue Collection
For GnRH ICC, adult animals were anesthetized with pentobarbital sodium and perfused intracardially with 20 ml of heparinized saline followed by 40 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were removed and postfixed in the same fixative for 2 h then cryoprotected in 30% sucrose. Brains from PND0 mice were removed and immersion fixed overnight in 4% paraformaldehyde at 4°C and cryoprotected in 30% sucrose. For GnRH RIA and quantitative reverse-transcription polymerase chain reaction (qPCR), mice were killed by rapid decapitation. Brains were removed from the skull and blocked to isolate the preoptic area (POA; for qPCR) and the hypothalamic (for GnRH RIA) fragments. The POA fragment was generated by an anterior cut at the caudal border of the olfactory bulbs, a posterior cut at 1 mm caudal to the optic chiasm, and a dorsal cut to remove the cortex. The hypothalamic fragment was generated from the remaining brain by a posterior cut at the rostral border of the mammillary body and another dorsal cut to remove the cortex. Tissues were rapidly frozen on dry ice and stored at −70°C.
GnRH ICC, Neuron Count, and Morphological Analysis
GnRH ICC was conducted on free-floating 60-μm frozen sections (for adult brains) and 20-μm thaw-mounted frozen sections (for PND0 brains) using a rabbit anti-GnRH polyclonal antibody LR-5 (a gift from Dr. R. Benoit, Montréal General Hospital, Montréal, Québec, Canada) as described previously (56). LR-5 was previously validated for specificity to GnRH1 (48). For the adult mice, GnRH neuronal counts and morphological assessments were performed on all 20 sections rostral (1.2 mm) and all 40 sections caudal (2.4 mm) to the organum vasculosum of the lamina terminalis (OVLT). For the PND0 mice, GnRH neuronal counts were performed on all 90 sections rostral (1.8 mm) and all 40 sections caudal (0.8 mm) to the OVLT. Only immunopositive cells with nuclei and intact cellular morphology were scored as described previously (56). GnRH neuron morphology was classified according to Cottrell et al. (11) and included the following three categories: unipolar (one process), bipolar (two processes at opposite ends), and complex (2 processes on the same end of the soma, 3 or more processes, or bifurcating primary dendrites). All slides were coded to conceal their identity before they were scored. To normalize the difference in GnRH neuron number between WT and dnFGFR mice, the data were presented as percentages of GnRH neurons exhibiting each morphological type.
GnRH RIA
Hypothalamic GnRH peptide levels were measured by a GnRH RIA previously described using an antiserum specific for GnRH1 (R1245, obtained from Dr. Terry Nett, Colorado State University) (41). All samples were assayed in five serially diluted doses to ensure parallelism with the standard curve. The detection limit was 1 pg/tube. The intra- and interassay coefficients of variation were 6.6 ± 2.4 and 8.9 ± 1.5%, respectively.
RNA Isolation, cDNA Synthesis, and Quantification of GnRH Transcript
Total RNA from the frozen POA fragment was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The quality of RNA was verified by gel electrophoresis. First-strand cDNA was synthesized from 1 μg of total RNA using the SuperScriptIII First-Strand Synthesis SuperMix (Invitrogen). qPCR of GnRH transcript was performed using the FastStart DNA Master SYBR Green I kit (Roche Pharmaceuticals, Indianapolis, IN) as previously described (3). Absolute quantification was performed using the full-length mouse GnRH cDNA subcloned into PGEM T Easy vector as the standard. Primer sequences were 5′-CTGCTGACTGTGTGTTTGGAAGG (forward) and 5′-CCTGGCTTCCTCTTCAATCA (reverse). All amplifications were performed for 40 cycles with an annealing temperature of 60°C.
Seminal Vesicle and Gonadal-Somatic Index
At the time of death, testes and seminal vesicles were removed from males in experiment 3, and their wet mass was determined. Gonadal-somatic index and seminal vesicle somatic index (SVSI) were determined by dividing the mass of the respective organs by the body mass.
Testicular Histology and Analysis
Testes were removed from the nonperfused PND550 males from experiment 1, immersion-fixed overnight in Bouin's Fixative, dehydrated, and embedded in paraffin. For each testis, 12-μm sections were cut, starting from the equator. Approximately 50 sections for each animal were collected, mounted onto gelatin-coated slides, and stained with hematoxylin and eosin. For histological analysis, four sections per animal were scored. Each section was divided into the following five areas: top, bottom, right, left, and middle. Within each area, four randomly selected seminiferous tubules (ST) were measured for ST diameter and luminal diameter using a calibrated ocular micrometer. Because most ST were elliptical in shape, each diameter measurement consisted of the average of two scores: one taken at the widest and one at the narrowest part of the ST. Luminal ratio was calculated by dividing the average luminal diameter by average ST diameter of the same ST. Luminal ratios of all STs from a single animal were averaged to obtain the value for that animal.
Testicular Cyp17a1 ICC and Analysis
Testes from PND550 males of experiment 1 were also used for Cyp17a1 ICC. Testes were fixed and paraffin embedded as described above for testicular histology. Approximately 10 sections from each animal were collected, mounted on slides, and processed for ICC. A polyclonal antibody specific for the Cyp17a1 (sc-46081; Santa Cruz Biotechnology, Santa Cruz, CA), an enzyme needed for androgen biosynthesis, was used to label Leydig cells as previously described (51). After dehydration and coverslipping, sections were photographed under identical camera settings. The outlines of two evenly spaced sections from each animal were manually traced, and their optical density (OD) was measured using NIH ImageJ while controlling for background color and threshold levels (15, 16). OD measurements of two sections from a single animal were averaged to obtain a value for that animal.
Statistical Analysis
The Student's t-test or one-way ANOVA followed by Tukey's post hoc test were used to determine differences among groups. Occasional outliers (no more than one per group) were removed when their values were outside 1.5 times the interquartile range (19, 39, 58). Two-way ANOVA was used to determine genotype and age effects and the interaction between genotype and age. When data failed the homoscedasticity test, they were log10 transformed or analyzed by the Kruskal-Wallis test followed by Dunn's post hoc test. All data were presented as means ± SE.
RESULTS
Experiment 1. Determine if the GnRH System and Testicular Morphology are Defective in dnFGFR Mice in Different Housing Conditions
GnRH neuronal counts.
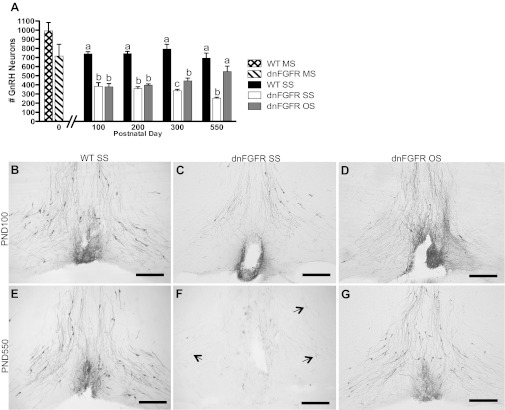
At birth (PND0), the total number of GnRH neurons in the brains of dnFGFR mice did not differ significantly from WT (Fig. 3A). In the post-PND0 age groups investigated, two-way ANOVA revealed a significant effect of genotype [F(2,62) = 132.8, P < 0.0001] and genotype × age interaction [F(6,62) = 3.74, P < 0.01], but not age [F(3,62) = 0.34, P = 0.79], on GnRH neuron number (Fig. 3A). In the absence of an age effect between PND100 and -550 (Fig. 3), and in the presence of a significant genotype × age interaction, we analyzed the effect of genotype and housing within each age group using one-way ANOVA followed by Tukey's post hoc test. At PND100 and -200, both dnFGFR SS and OS groups had significantly less GnRH neurons than the WT SS group (P < 0.001), but they were not different from each other (P > 0.05). Compared with SS housing, OS housing in dnFGFR mice significantly increased GnRH neurons at PND300 (P < 0.05) and -550 (P < 0.001), with the number of GnRH neurons no longer different from the WT SS group at PND550 (Fig. 3A). Overall, these results indicated that a significant loss of GnRH neurons in dnFGFR mice occurred between PND0 and PND100, and there was a positive effect of OS in restoring GnRH neuron number in dnFGFR males (Fig. 3, A and B–G).
Fig. 3.
GnRH neuron number in OS- and SS-housed WT and dnFGFR males. A: GnRH neuron numbers were quantified in PND0 WT and dnFGFR male pups housed in mixed-sex (MS) configuration and in older WT and dnFGFR males that were SS or OS housed from PND30 to various ages. There were no differences between WT and dnFGFR MS pups at PND0 (n = 3–5/group; Student's t-test). From PND100 to PND550, dnFGFR SS males had significantly fewer GnRH neurons than WT SS males; OS in dnFGFR males significantly increased GnRH neuron numbers at PND300 and -550 but not earlier. Letter differences above bars represent significant differences (P < 0.05) within each age group (one-way ANOVA followed by Tukey's test; n = 4–7/group). B–G: representative photomicrographs of GnRH-immunoreactive (ir) neurons at the level of the organum vasculosum of the lamina terminalis (OVLT). At PND100, SS-housed WT males (B) had visibly more GnRH neurons than SS-housed (C) and OS-housed (D) dnFGFR males. At PND550, OS housing in dnFGFR males (G) visibly increased the number of GnRH neurons to a level higher than dnFGFR SS males (F) and comparable to WT SS males (E). Black arrows indicate lightly stained GnRH-ir soma in the SS-housed PND550 dnFGFR males (F). Scale bar = 200 μm.
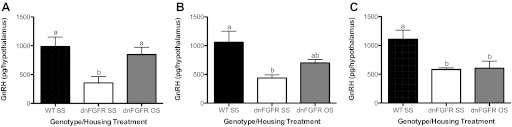
Hypothalamic GnRH peptide and transcript levels.
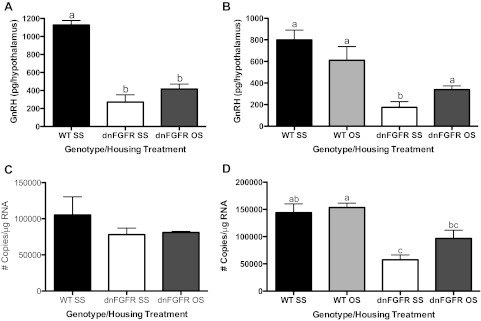
GnRH peptide and transcript were measured in two age groups, PND200 (Fig. 4, A and C) and PND550 (Fig. 4, B and D), to examine if these two parameters paralleled changes in GnRH neuronal count. One-way ANOVA followed by post hoc test revealed that, at PND200, the dnFGFR SS group had significantly lower hypothalamic GnRH peptide levels than the WT SS group (P < 0.001), and OS housing of dnFGFR males did not change this trend (P < 0.001) (Fig. 4A). No differences were detected in GnRH transcript levels among groups at PND200 (Fig. 4C). By PND550, OS housing significantly elevated hypothalamic GnRH peptide levels in dnFGFR males compared with their SS-housed counterpart (P < 0.05) but had no effect on WT mice (Fig. 4B). OS housing did not alter GnRH transcript levels in dnFGFR or WT mice compared with their respective SS-housed counterparts but elevated dnFGFR mice to levels comparable to the WT SS group (Fig. 4D).
Fig. 4.
GnRH peptide and transcript levels in OS- and SS-housed males. WT and dnFGFR males were SS or OS housed from PND30 to PND200 or PND550. Levels of hypothalamic GnRH peptide (A and B) and preoptic area (POA) GnRH transcript (C and D) of PND200 males (A and C) and PND550 males (B and D) were determined. Letter differences above bars represent significant differences (P < 0.05, one-way ANOVA followed by Tukey's test; n = 4–6/group).
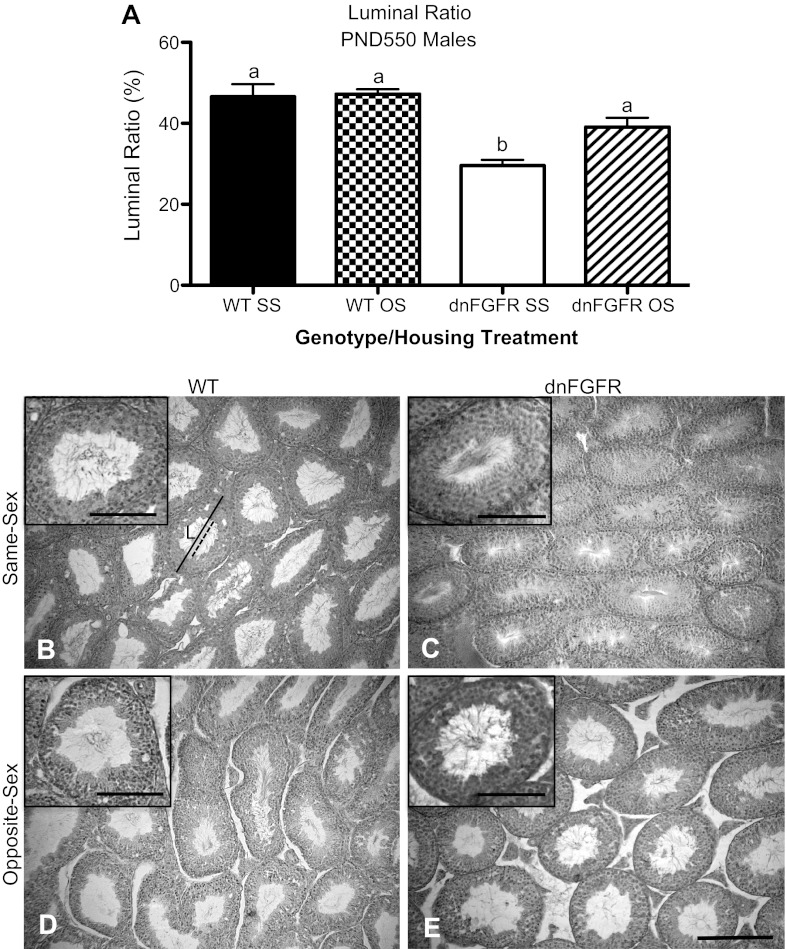
Testicular histology.
The size of ST lumen has been correlated with fertility (1, 12, 49, 50). To determine if OS housing affected testicular histology, we examined the ST luminal ratio of PND550 WT and dnFGFR males that were SS or OS housed. We found that dnFGFR SS males had a significantly smaller luminal ratio than the other three groups (P < 0.001 for dnFGFR SS vs. WT SS and WT OS; P < 0.05 for dnFGFR SS vs. dnFGFR OS; Fig. 5A). OS housing significantly increased the luminal ratio in dnFGFR mice but had no effect on this parameter in WT mice. The reduced luminal ratio in the dnFGFR SS group was largely due to smaller luminal diameters in the dnFGFR SS mice (Fig. 5, B–E). Although the dnFGFR SS group possessed markedly fewer ST with open lumen, mature spermatozoa were still present in the ST when the lumen was open (Fig. 5C, inset), suggesting spermatogenesis was not completely halted. In all groups, mature spermatozoa were observed in all ST with open lumen (Fig. 5, B–E, insets).
Fig. 5.
Seminiferous tubule (ST) luminal ratio in OS- and SS-housed WT and dnFGFR males at PND550. A: WT and dnFGFR males were SS or OS housed from PND30 to PND550. ST luminal ratio was significantly lower in the SS-housed dnFGFR males compared with the other three groups. Letter differences above bars represent significant differences (P < 0.05, one-way ANOVA followed by Tukey's test; n = 4–5/group). B–E: representative photomicrographs of testicular histology from SS (B and C)- or OS (D and E)-housed WT (B and D) and dnFGFR (C and E) males. Average ST diameter was determined by measuring the widest dimension of randomly selected ST (solid line) and averaging it with the narrowest dimension of the same tubule. Luminal diameters were determined similarly by measuring the lumen (L) of the same ST (dashed line). The ST luminal ratio was calculated as a percentage of the mean ST diameter to mean luminal diameter of the same ST. Scale bar = 200 μm. Representative ST with open lumen are magnified in insets in B–E to show the presence of mature spermatozoa. Scale bars for insets = 100 μm.
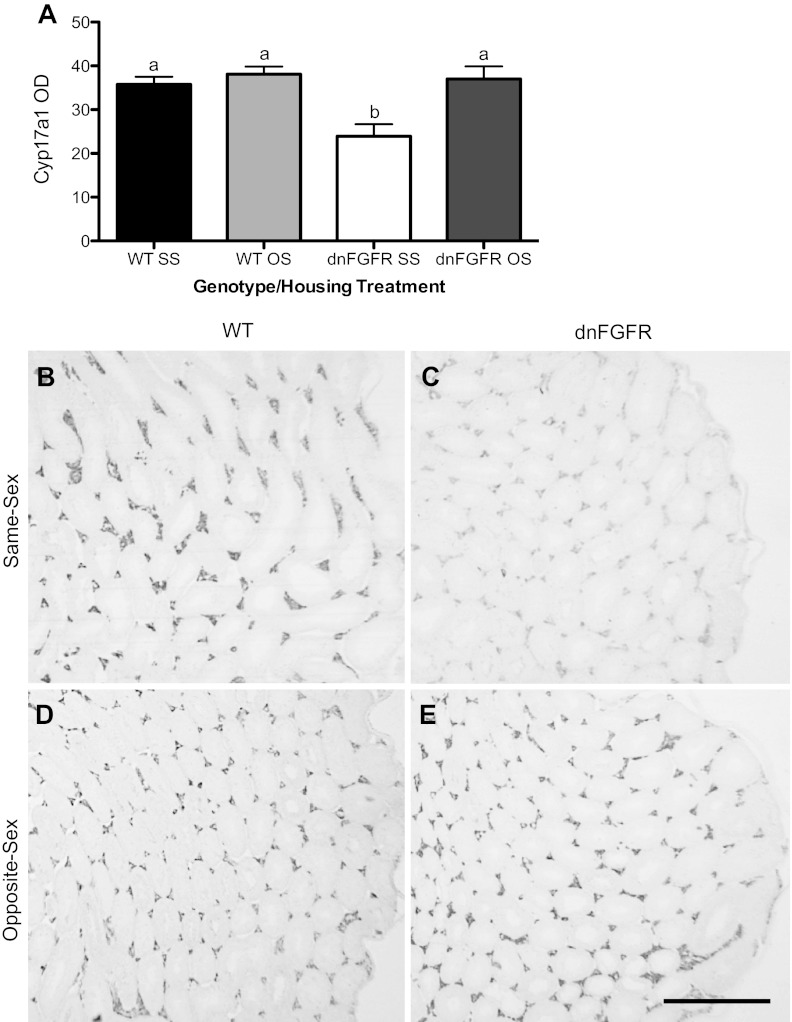
Cyp17a1 ICC.
Cyp17a1, an enzyme responsible for the 17α-hydroxylation of progesterone and pregnenolone, is needed for the biosynthesis of Δ4 and Δ5 androgens. In the testes, Cyp17a1 is upregulated by luteinizing hormone (LH) and present only in Leydig cells (33). In PND550 testes, dnFGFR SS males had significantly lower Cyp17a1 staining intensity (measured in OD) compared with all other groups (P < 0.05 vs. WT SS and P < 0.01 vs. WT OS and dnFGFR OS; Fig. 6A). OS housing significantly increased Cyp17a1 OD measurements in dnFGFR mice but had no effect on WT mice (Fig. 6, A and B–E).
Fig. 6.
Testicular Cyp17a1 immunostaining in OS- and SS-housed WT and dnFGFR males at PND550. WT and dnFGFR males were SS- or OS-housed from PND30 to PND550. A: testicular Cyp17a1 immunostaining [optical density (OD)] was significantly lower in SS-housed dnFGFR males compared with the other three groups. Letter differences above bars represent significant differences (P < 0.05, one-way ANOVA followed by Tukey's test; n = 4–5/group). B–E: representative photomicrographs of testicular Cyp17a immunocytochemistry (ICC) in SS (B and C)- or OS (D and E)-housed WT (B and D) and dnFGFR (C and E) males. Scale bar = 500 μm.
Experiment 2. Determine if OS Housing Later in Life Rescues the GnRH System in dnFGFR Males
It was unclear whether OS housing after puberty could still restore the declining GnRH system in dnFGFR males. To test this, we paired postpubertal dnFGFR males at various ages with females. OS housing significantly elevated hypothalamic GnRH peptide above SS levels when dnFGFR males were paired with females at PND150 (P < 0.05; Fig. 7A), but not later (Fig. 7, B and C).
Fig. 7.
GnRH peptide levels in males paired with females after puberty. dnFGFR males were OS or SS housed at PND150 (A), PND200 (B), or PND300 (C) and killed after ∼200 days. Thus, these mice were termed late paired (LP) 350, LP400, and LP550, respectively. OS housing significantly increased hypothalamic GnRH peptide levels only if initiated by PND150. Letter differences above bars indicate significant differences (P < 0.05, one-way ANOVA followed by Tukey's test; n = 4–5/group).
Experiment 3. Determine if OS Housing Enhances Several Reproductive Parameters
To determine if the rescue of the GnRH system by OS housing correlated with improved reproductive function, we monitored the breeding success and reproductive organ mass of dnFGFR and WT males under different housing conditions. Because data from experiment 1 indicated that OS housing at PND30 was beneficial to the GnRH system (Fig. 3), the same pairing regimen was used in this experiment (Table 1). OS housing did not improve any of the reproductive parameters in WT mice but significantly shortened the interval between first and second litters and elevated SVSI in dnFGFR mice compared with their SS counterparts (Table 1). There were no significant differences in litter size or litter survival between the genotypes/housing groups (data not shown). Last, OS housing significantly increased the anogenital distance-to-body mass ratio in PND225 dnFGFR males (0.58 ± 0.019, n = 4 for the SS group; 0.70 ± 0.015, n = 3 for the OS group; P < 0.01 by Student's t-test) but had no effect on the body mass alone (data not shown).
Table 1.
Reproductive parameters in WT and dnFGFR males subject to OS or SS housing at PND30
| Genotype/Housing Treatment | Time to First Litter, days | Time Between First and Second Litters, days | GSI (Testes Mass/Body Mass) | SVSI (SV Mass/Body Mass) |
|---|---|---|---|---|
| WT SS | 46.2 ± 9.8a | 22.6 ± 1.3a | 0.0090 ± 0.0006a | 0.0117 ± 0.0005a,b |
| n | 6 | 5 | 6 | 6 |
| WT OS | 32.8 ± 7.4a | 21.8 ± 1.6a | 0.0090 ± 0.0006a | 0.0125 ± 0.0002a |
| n | 5 | 5 | 5 | 4 |
| dnFGFR SS | 44.6 ± 15.3a | 33.8 ± 4.5b | 0.0081 ± 0.0004a | 0.0104 ± 0.0003b |
| n | 5 | 4 | 5 | 6 |
| dnFGFR OS | 27.6 ± 3.3a | 21.8 ± 1.2a | 0.0083 ± 0.0003a | 0.0129 ± 0.0005a |
| n | 5 | 5 | 6 | 5 |
Values are presented as means ± SE; n, no. of mice. WT, wild type; dnFGFR, dominant-negative fibroblast growth factor receptor; OS, opposite sex; SS, same sex; PND, postnatal day; GSI, gonadal-somatic index; SVSI, seminal vesicle somatic index; SV, seminal vesicle. At PND150, all males were paired with a novel virgin WT female (45–55 days old), allowed to breed for 120 days, and killed for the measurement of GSI or SVSI. Letter differences indicate significant differences between column values, P < 0.05 (one-way ANOVA followed by Tukey's post hoc test).
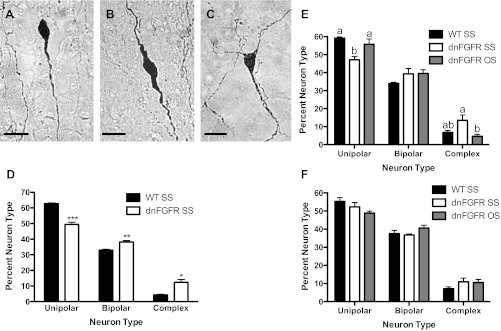
Experiment 4. Determine if FGF Signaling Deficiency is Associated with Morphological Defects in GnRH Neurons and if OS Housing Could Rescue These Defects
Representative examples of unipolar, bipolar, and complex neurons are presented in Fig. 8, A–C. In general, fewer unipolar and more complex neurons represent a state of sexual immaturity (11). To establish if inherent morphological defects in GnRH neurons existed in prepubertal dnFGFR males, we quantified the percentages of GnRH neurons within each morphological category in SS-housed PND35 WT and dnFGFR males (Fig. 8D). SS-housed prepubertal dnFGFR mice possessed fewer unipolar (P < 0.0001) and more bipolar (P < 0.001) and complex (P < 0.01) GnRH neurons, reflecting a more immature morphology. Next, we examined if OS housing facilitated the maturation of GnRH neuronal morphology in postpubertal dnFGFR males (Fig. 8, E and F). Indeed, in PND100 dnFGFR males, OS housing increased the percentage of unipolar neurons (P < 0.05) and reduced that of complex neurons (P < 0.05) compared with the SS-housed counterparts (Fig. 8E), suggesting a maturational effect. However, by PND200, all morphological differences between genotypes had disappeared, and OS housing no longer had an effect on GnRH neuronal morphology in dnFGFR males (Fig. 8F).
Fig. 8.
GnRH neuronal morphology in SS- and OS-housed WT and dnFGFR males. GnRH neuronal morphology was classified into the following three categories: unipolar (A), bipolar (B), and complex (C). Scale bar = 20 μm. Percent GnRH neurons in each category was quantified in WT and dnFGFR males that were SS housed from weaning to PND35 (D) or in older males that were SS or OS housed from weaning to PND100 (E) and PND200 (F). For PND35, *P < 0.01, **P < 0.001, and ***P < 0.0001 compared with WT in each category (Student's t-test; n = 6–8/group). For PND100 and -200, letter differences above bars represent P < 0.05 within the same morphological category (one-way ANOVA followed by Tukey's test; n = 5–7/group).
Experiment 5. Determine if FGFR3 Deficiency is Associated with a Postnatal Loss of GnRH Neurons
The expression of dnFGFR could disrupt the function of multiple FGFRs, including FGFR1 and FGFR3 (59). Both FGFR1 and -3 are present on postnatal GnRH neurons (24) and may contribute to the postnatal maintenance of GnRH neurons. Previous studies showed that FGFR1+/− hypomorphic mice did not exhibit a postnatal decline in GnRH neurons (6, 7). Thus, we examined if FGFR3+/− mice suffered an age-dependent decline in GnRH neuron number similar to dnFGFR mice. At PND0, GnRH neurons in FGFR3+/− mice (370.5 ± 66.1 neurons, n = 4) did not differ significantly from that of WT mice (362.0 ± 58.0 neurons, n = 5, Student's t-test). However, at PND550, a significant decrease (P < 0.05, Student's t-test) was seen in FGFR3+/− mice (321.8 ± 11.4 neurons, n = 6) compared with WT (438.8 ± 45.7 neurons, n = 4). The generally lower numbers of GnRH neurons in the FGFR3+/− mice compared with the dnFGFR mice and their controls may be due to strain differences, as shown previously (6).
DISCUSSION
Two novel observations emerge from the current study. First, FGF signaling, possibly acting via FGFR3, is needed to maintain the postnatal GnRH system. Second, environmental stimulation, such as OS cohabitation, restores the declining postnatal GnRH system in FGF-deficient animals. These results collectively suggest that the postnatal maintenance of an established neuroendocrine system over time is not static. Its loss or restoration may be a plastic process highly responsive to environmental cues.
FGF signaling is crucial for the prenatal development of the GnRH system (6–8, 23, 24, 55–57). In particular, FGF8 acting through FGFR1 is necessary for the proper genesis of GnRH neurons in the olfactory placode (7). The role of FGF signaling in GnRH system development is further supported by the observation that hypogonadotropic hypogonadism is causally linked to mutations in FGFR1 and FGF8 (14, 43, 44). Interestingly, individuals harboring identical FGF gene mutations may exhibit a wide spectrum of reproductive phenotypes, from absent or delayed puberty to normal reproductive function (43). These findings suggest that the reproductive phenotype of FGF signaling defective humans can also be influenced by poorly understood nongenetic (environmental and epigenetic) factors. Indeed, the GnRH system of several vertebrate species has been shown to respond acutely to environmental factors (4, 13, 35, 38, 52). For example, cichlid fish show rapid activation of the GnRH system (mRNA expression in GnRH neurons) in response to social status (38), and breeding stimuli increase the number of GnRH-immunoreactive (ir) neurons in amphibians (4) and birds (35, 52). Furthermore, in the musk shrew, a mammal in which puberty is induced by mating, the exposure of prepubertal females to males increases the number of GnRH-ir neurons (13). However, these documented effects are rapid and differ from the long-term beneficial effects seen in the current study.
Our data are the first to provide strong evidence for a role of FGF signaling in the postnatal maintenance of the GnRH system. It is presently unclear why the majority of GnRH neuronal loss in dnFGFR mice occurs within the first 100 days. One possibility is that this period encompasses the pubertal transition during which a robust activation of the GnRH promoter takes place (26). Because the expression of dnFGFR is coupled to the activity of the GnRH promoter, dnFGFR may be expressed at a higher level during this period, leading to a greater disruption of FGF signaling. The maximum loss of GnRH neurons in dnFGFR males seen in this study is ∼65%. One might question why this level of loss could impact reproduction, since only 12% or fewer GnRH neurons are sufficient to drive puberty and maintain fertility (27, 31). It is important to note that dnFGFR mice display not only reduced GnRH neuron numbers but also reduced axon targeting to the median eminence (24) and defects in morphological remodeling (this study). In this regard, the cumulative functional defect in their GnRH system is likely to extend beyond the reduction of GnRH neurons alone.
The mechanisms underlying the OS housing-mediated rescue of the GnRH system are not entirely clear, but the following conclusions can be drawn. First, OS housing treatment needs to be initiated within a critical window (by PND150; Fig. 7A) to be effective; thus, the mechanism of long-term GnRH system rescue by OS housing may be distinct from the activational effects reported previously (4, 13, 35, 38, 52). Second, the fact that OS housing could restore GnRH neuron number in dnFGFR mice suggests that the postnatal loss of GnRH neurons in these mice is likely due to a decline in GnRH production rather than the death of GnRH neurons. There is some evidence of adult GnRH neurogenesis in response to sexual interactions in birds (5), but it is unlikely that OS housing could promote the full developmental array of GnRH neurons, including neurogenesis and migration to the proper neuroanatomical locale, in adulthood. As such, OS housing likely increases GnRH neurons by promoting GnRH synthesis of dedifferentiated GnRH neurons. Third, OS housing rescues the GnRH system predominantly by increasing GnRH peptide and less so by altering GnRH transcript (Figs. 4 and 7A), suggesting enhanced translation as a mechanism for promoting GnRH synthesis (26). Fourth, although OS housing needs to be initiated during a critical period, the duration of OS housing is also important, since dnFGFR males subject to shorter OS housing do not benefit from this treatment (Fig. 3). Last, OS housing does not benefit WT animals; rather, it benefits only dnFGFR mice with a suboptimal GnRH system. As such, we surmise that the benefits offered by environmental cues may surface only when the GnRH system is declining due to disease or aging.
Although previous studies have documented that OS housing enhances male fertility and delays male reproductive aging (2, 30, 47), the specific cues responsible for the OS housing-mediated rescue of GnRH neurons in the present study are unclear. Pheromones in female mouse urine increase circulating LH levels, presumably via the activation of GnRH neurons, in the exposed male conspecifics (36, 37). However, this response is transient and becomes blunted with repeated exposures to the same female (10). The transient nature of this response argues against the importance of pheromones alone in the long-term maintenance of the GnRH system in OS-housed males. Instead, we favor the hypothesis that OS housing generates a socially enriched environment through diverse cues provided by mating, copulation, and pup rearing. These cues may include a combination of olfactory, tactile, and visual signals. An enriched environment has been shown to enhance the production of neurotrophic factors, including Fgf2, in the rodent brain to improve the affective state of the animals (42). As such, we hypothesize that certain neurotrophic factors may be upregulated in the POA/hypothalamic region of OS-housed dnFGFR males to rescue the deteriorating GnRH system.
In addition to GnRH neuronal counts and peptide levels, we validate the beneficial effects of OS housing using three other criteria: GnRH neuronal morphology, testicular histology, and several reproductive parameters. With regard to the former, the postnatal changes in GnRH neuronal morphology have been correlated with the functional maturation of the GnRH system (11, 62). During puberty, dendritic pruning increases the proportion of unipolar neurons and decreases that of complex neurons (11, 62). dnFGFR mice have a more juvenile distribution of GnRH neuronal morphology, suggesting that FGF signaling is involved in the dendritic remodeling of mature GnRH neurons. In dnFGFR males, OS housing induces a significant shift of GnRH neuronal morphology toward a more mature distribution by PND100. This indicates that OS housing is not merely important for the rescue of number of GnRH neurons; it also participates in their anatomical remodeling, which correlates with GnRH neurons' ability to receive excitatory inputs (11, 62). The differences between the genotypes disappeared by PND200, suggesting the remodeling defect in dnFGFR mice represents only a delay, not an absence, of dendritic pruning.
The OS housing-mediated rescue of the GnRH system in dnFGFR males is also accompanied by an increase in the ST luminal ratio (Fig. 5), anogenital distance-to-body mass ratio (results), and several reproductive parameters (Table 1). Reduced ratio of lumen to ST volume occurs just after spermiation in normal mice (49, 60), but typically in a regionalized fashion since spermiation occurs in waves along the ST (28). In this regard, a uniform and consistent reduction of luminal ratio in aged, SS-housed dnFGFR males likely indicates abnormal ST function. A reduction in ST lumen size has also been reported in rodents with reduced androgens (50), exposed to reproductive toxins (1, 49), and with androgen receptor gene knocked out (12). Although mature spermatozoa are visible in the ST of SS-housed dnFGFR males, the majority of their ST has close lumen with no spermatozoa, suggesting spermatogenesis may be significantly compromised. Thus, increased luminal ratio by OS housing in dnFGFR males (Fig. 5) serves as a correlate for the restoration of testicular function. Seminal vesicle size and anogenital distance, both androgen-dependent traits (22, 32), are also restored by OS housing, suggesting an increase in androgen production accompanies OS housing. Further supporting this notion, OS housing increases immunostaining of Cyp17a1, an LH-dependent enzyme (33) that catalyzes the formation of intermediates in androgen biosynthesis pathways. The ultimate functional test of GnRH system restoration is enhanced offspring production. The large variability in the lag to first litter (Table 1) for all genotypes and housing conditions may be related to the use of virgin females. However, once the females are experienced, OS housing shortened the lag between the first and second litters by 12 days in dnFGFR males (Table 1), providing the most important proof of the functional restoration of the GnRH system.
Because both FGFRs 1 and 3 are present on GnRH neurons, we determine in our last experiment which receptor is likely responsible for maintaining the postnatal GnRH system. We show a decline in GnRH neurons of FGFR3+/− KO mice, implying that FGFR3 is the receptor mediating this maintenance. The postnatal involvement of FGFR1 is unlikely, since FGFR1+/− mice were born with ∼60% less GnRH neurons than WTs at PND0 (7), with no further age-dependent decline of GnRH neurons (6). These data support an interesting dichotomy of the role of FGFR1 in the prenatal development, and FGFR3 in the postnatal maintenance, of the GnRH system.
To summarize, we have shown that FGF signaling, possibly acting through FGFR3, is needed for the postnatal maintenance of GnRH neurons. However, sexual interactions in the form of OS housing and during a critical period can compensate for FGF signaling deficiencies to restore the declining GnRH system, testicular function, and fecundity. Our findings demonstrate that sexual interactions can have effects beyond the transient activation of the HPG axis shown previously (20, 25, 34, 40, 45, 46) to have a long-term beneficial impact on the GnRH system. In this regard, the postnatal GnRH system is a highly plastic and resilient system able to override serious preexisting defects by responding to environmental cues.
GRANTS
This research was funded by National Institutes of Health Grants 5R01HD-04263 (P.-S. Tsai) and 1F32AG-037257 (J. R. Rochester).
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: J.R.R., W.C.C., and P.-S.T. conception and design of research; J.R.R. and T.H. performed experiments; J.R.R. analyzed data; J.R.R., W.C.C., and P.-S.T. interpreted results of experiments; J.R.R. prepared figures; J.R.R. drafted manuscript; J.R.R. and P.-S.T. edited and revised manuscript; J.R.R. and P.-S.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Scott I. Kavanaugh for technical assistance.
REFERENCES
- 1. Bormann CL, Smith GD, Padmanabhan V, Lee TM. Prenatal testosterone and dihydrotestosterone exposure disrupts ovine testicular development. Reprod 142: 167–173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bronson FH. Relative effects of exercise, diet, and female stimulation on sexual aging of male mice. J Gerontol 37: 555–559, 1982 [DOI] [PubMed] [Google Scholar]
- 3. Brooks LR, Chung WC, Tsai PS. Abnormal hypothalamic oxytocin system in fibroblast growth factor 8-deficient mice. Endocrine 38: 174–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burmeister SS, Wilczynski W. Social signals regulate gonadotropin-releasing hormone neurons in the green treefrog. Brain Behav Evol 65: 26–32, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng MF, Alexander K, Zhou S, Bonder E, Chuang LS. Newborn GnRH neurons in the adult forebrain of the ring dove. Horm Behav 60: 94–104, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Chung WC, Matthews TA, Tata BK, Tsai PS. Compound deficiencies in multiple fibroblast growth factor signalling components differentially impact the murine gonadotrophin-releasing hormone system. J Neuroendocrinol 22: 944–950, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung WC, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology 149: 4997–5003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung WC, Tsai PS. Role of fibroblast growth factor signaling in gonadotropin-releasing hormone neuronal system development. Front Horm Res 39: 37–50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12: 390–397, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Coquelin A, Bronson FH. Release of luteinizing hormone in male mice during exposure to females: habituation of the response. Science 206: 1099–1101, 1979 [DOI] [PubMed] [Google Scholar]
- 11. Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 147: 3652–3661, 2006 [DOI] [PubMed] [Google Scholar]
- 12. De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101: 1327–1332, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dellovade TL, Rissman EF. Gonadotropin-releasing hormone-immunoreactive cell numbers change in response to social interactions. Endocrinology 134: 2189–2197, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pecheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33: 463–465, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety 29: 307–319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donner NC, Montoya CD, Lukkes JL, Lowry CA. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37: 645–661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drori D, Folman Y. Effects of Cohabitation on the Reproductive System, Kidneys and Body Composition of Male Rats. J Reprod Fertil 8: 351–359, 1964 [DOI] [PubMed] [Google Scholar]
- 18. Drori D, Folman Y. The sexual behaviour of male rats unmated to 16 months of age. Anim Behav 15: 20–24, 1967 [DOI] [PubMed] [Google Scholar]
- 19. Falagas ME, Rizos M, Bliziotis IA, Rellos K, Kasiakou SK, Michalopoulos A. Toxicity after prolonged (more than four weeks) administration of intravenous colistin (Abstract). BMC Infect Dis 5: 1, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox CA, Ismail AA, Love DN, Kirkham KE, Loraine JA. Studies on the relationship between plasma testosterone levels and human sexual activity. J Endocrinol 52: 51–58, 1972 [DOI] [PubMed] [Google Scholar]
- 21. Franks LM, Payne J. The influence of age on reproductive capacity in C57BL mice. J Reprod Fertil 21: 563–565, 1970 [DOI] [PubMed] [Google Scholar]
- 22. Fujii T. Roles of age and androgen in the regulation of sex accessory organs. Adv Sex Horm Res 3: 103–137, 1977 [PubMed] [Google Scholar]
- 23. Gill JC, Moenter SM, Tsai PS. Developmental regulation of gonadotropin-releasing hormone neurons by fibroblast growth factor signaling. Endocrinology 145: 3830–3839, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Gill JC, Tsai PS. Expression of a dominant negative FGF receptor in developing GNRH1 neurons disrupts axon outgrowth and targeting to the median eminence. Biol Reprod 74: 463–472, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: when it occurs and why. Front Neuroendocrinol 30: 460–469, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Gore AC, Ho A, Roberts JL. Translational efficiency of gonadotropin-releasing hormone messenger ribonucleic acid is negatively regulated by phorbol ester in GT1–7 cells. Endocrinology 136: 1620–1625, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 149: 597–604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636: 1–15, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging 7: 45–48, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Huber MH, Bronson FH. Recovery of sexual activity with experience in aged male mice. Exp Aging Res 6: 385–391, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Krieger DT, Perlow MJ, Gibson MJ, Davies TF, Zimmerman EA, Ferin M, Charlton HM. Brain grafts reverse hypogonadism of gonadotropin releasing hormone deficiency. Nature 298: 468–471, 1982 [DOI] [PubMed] [Google Scholar]
- 32. Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148: 4927–4936, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA 101: 17294–17299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science 189: 1104–1106, 1975 [DOI] [PubMed] [Google Scholar]
- 35. Mantei KE, Ramakrishnan S, Sharp PJ, Buntin JD. Courtship interactions stimulate rapid changes in GnRH synthesis in male ring doves. Horm Behav 54: 669–675, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maruniak JA, Bronson FH. Gonadotropic responses of male mice to female urine. Endocrinology 99: 963–969, 1976 [DOI] [PubMed] [Google Scholar]
- 37. Maruniak JA, Coquelin A, Bronson FH. The release of LH in male mice in response to female urinary odors: characteristics of the response in young males. Biol Reprod 18: 251–255, 1978 [DOI] [PubMed] [Google Scholar]
- 38. Maruska KP, Levavi-Sivan B, Biran J, Fernald RD. Plasticity of the reproductive axis caused by social status change in an african cichlid fish. I. Pituitary gonadotropins. Endocrinology 152: 281–290, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mugford ST, Mallon EB, Franks NR. The accuracy of Buffon's needle: a rule of thumb used by ants to estimate area. Behav Ecol 12: 655–658, 2001 [Google Scholar]
- 40. Nyby JG. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Front Neuroendocrinol 29: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pak TR, Lynch GR, Tsai PS. Testosterone and estrogen act via different pathways to inhibit puberty in the male Siberian hamster (Phodopus sungorus). Endocrinology 142: 3309–3316, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci 29: 6379–6387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pitteloud N, Meysing A, Quinton R, Acierno JS, Jr, Dwyer AA, Plummer L, Fliers E, Boepple P, Hayes F, Seminara S, Hughes VA, Ma J, Bouloux P, Mohammadi M, Crowley WF., Jr Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol 254–255: 60–69, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117: 457–463, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Purvis K, Haynes NB. Effect of the odour of female rat urine on plasma testosterone concentrations in male rats. J Reprod Fertil 53: 63–65, 1978 [DOI] [PubMed] [Google Scholar]
- 46. Rose RM, Berstein IS, Gordon TP. Consequences of social conflict on plasma testosterone levels in rhesus monkeys. Psychosom Med 37: 50–61, 1975 [DOI] [PubMed] [Google Scholar]
- 47. Schmidt JA, Oatley JM, Brinster RL. Female mice delay reproductive aging in males. Biol Reprod 80: 1009–1014, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sergeeva A, Jansen HT. Neuroanatomical plasticity in the gonadotropin-releasing hormone system of the ewe: seasonal variation in glutamatergic and gamma-aminobutyric acidergic afferents. J Comp Neurol 515: 615–628, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Sharpe RM. Possible role of elongated spermatids in control of stage-dependent changes in the diameter of the lumen of the rat seminiferous tubule. J Androl 10: 304–310, 1989 [DOI] [PubMed] [Google Scholar]
- 50. Sharpe RM, Kerr JB, McKinnell C, Millar M. Temporal relationship between androgen-dependent changes in the volume of seminiferous tubule fluid, lumen size and seminiferous tubule protein secretion in rats. J Reprod Fertil 101: 193–198, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Sherrill JD, Sparks M, Dennis J, Mansour M, Kemppainen BW, Bartol FF, Morrison EE, Akingbemi BT. Developmental exposures of male rats to soy isoflavones impact Leydig cell differentiation. Biol Reprod 83: 488–501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stevenson TJ, Ball GF. Anatomical localization of the effects of reproductive state, castration, and social milieu on cells immunoreactive for gonadotropin-releasing hormone-I in male European starlings (Sturnus vulgaris). J Comp Neurol 517: 146–155, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Tobet SA, Bless EP, Schwarting GA. Developmental aspect of the gonadotropin-releasing hormone system. Mol Cell Endocrinol 185: 173–184, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology 147: 1159–1165, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Tsai PS, Brooks LR, Rochester JR, Kavanaugh SI, Chung WC. Fibroblast growth factor signaling in the developing neuroendocrine hypothalamus. Front Neuroendocrinol 32: 95–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol 19: 225–236, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Tsai PS, Werner S, Weiner RI. Basic fibroblast growth factor is a neurotropic factor in GT1 gonadotropin-releasing hormone neuronal cell lines. Endocrinology 136: 3831–3838, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24: 747–753, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Werner S, Weinberg W, Liao X, Peters KG, Blessing M, Yuspa SH, Weiner RL, Williams LT. Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. EMBO J 12: 2635–2643, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wing TY, Christensen AK. Morphometric studies on rat seminiferous tubules. Am J Anat 165: 13–25, 1982 [DOI] [PubMed] [Google Scholar]
- 61. Wu D, Gore AC. Sexual experience changes sex hormones but not hypothalamic steroid hormone receptor expression in young and middle-aged male rats. Horm Behav 56: 299–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ybarra N, Hemond PJ, O'Boyle MP, Suter KJ. Spatially selective, testosterone-independent, remodeling of dendrites in gonadotropin-releasing hormone (GnRH) neurons prepubertally in male rats. Endocrinology 152: 2011–2019, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]