Abstract
Elevated plasma triglyceride (TG) levels contribute to an atherogenic dyslipidemia that is associated with obesity, diabetes, and metabolic syndrome. Numerous models of obesity are characterized by increased central nervous system (CNS) neuropeptide Y (NPY) tone that contributes to excess food intake and obesity. Previously, we demonstrated that intracerebroventricular (icv) administration of NPY in lean fasted rats also elevates hepatic production of very low-density lipoprotein (VLDL)-TG. Thus, we hypothesize that elevated CNS NPY action contributes to not only the pathogenesis of obesity but also dyslipidemia. Here, we sought to determine whether the effects of NPY on feeding and/or obesity are dissociable from effects on hepatic VLDL-TG secretion. Pair-fed, icv NPY-treated, chow-fed Long-Evans rats develop hypertriglyceridemia in the absence of increased food intake and body fat accumulation compared with vehicle-treated controls. We then modulated CNS NPY signaling by icv injection of selective NPY receptor agonists and found that Y1, Y2, Y4, and Y5 receptor agonists all induced hyperphagia in lean, ad libitum chow-fed Long-Evans rats, with the Y2 receptor agonist having the most pronounced effect. Next, we found that at equipotent doses for food intake NPY Y1 receptor agonist had the most robust effect on VLDL-TG secretion, a Y2 receptor agonist had a modest effect, and no effect was observed for Y4 and Y5 receptor agonists. These findings, using selective agonists, suggest the possibility that the effect of CNS NPY signaling on hepatic VLDL-TG secretion may be relatively dissociable from effects on feeding behavior via the Y1 receptor.
Keywords: brain, obesity dyslipidemia, hepatic lipid metabolism
despite the fact that clinicians have become increasingly adept at treating classical cardiovascular risk factors (i.e., hypertension, smoking, and cholesterol) (24), cardiovascular disease remains one of the leading causes of deaths worldwide (20), potentially due to the parallel diabetes and obesity epidemics (39). The dyslipidemia associated with diabetes and obesity consists of elevated very low-density lipoprotein (VLDL)-triglyceride (TG) together with small, dense, low-density lipoprotein cholesterol (LDL-C) and reduced high-density lipoprotein cholesterol (HDL-C) levels (22, 23, 26) and is an increasingly recognized component of cardiovascular risk, morbidity, and mortality (26).
Models of obesity/diabetes dyslipidemia suggest that increased visceral fat mass and insulin resistance lead to elevated adipocyte lipolysis, which increases free fatty acid (FFA) delivery to liver, where it is efficiently cleared, reesterified to TG, and loaded onto a nascent apolipoprotein B (apoB) particle, ultimately resulting in VLDL maturation and secretion (32, 49). This process is ordinarily suppressed by integrated hepatic insulin action. With a rise in VLDL-TG in the circulation, cholesteryl ester transfer protein exchanges cholesterol esters from HDL and LDL particles with TGs from VLDL, ultimately lowering HDL-C levels and facilitating the accumulation of atherogenic LDL-C particles (reviewed in Refs. 1 and 22).
In the context of integrated energy homeostasis, short-term energy stores are provided by glucose and glycogen, whereas long-term needs are met by adipose and liver-derived lipids, a process that is tightly regulated by the central nervous system (CNS). Neuropeptide Y (NPY)-expressing neurons are widely distributed in the CNS, concentrated in the mediobasal hypothalamus, important in the regulation of feeding and energy homeostasis, and increasingly recognized as having a role in lipid homeostasis (59). NPY is a potent orexigenic peptide and, when delivered by chronic infusion directly into the brain of rats and mice, has been observed to promote hyperphagia, obesity, dyslipidemia, and metabolic syndrome (48, 65), similar to that of the leptin-deficient ob/ob mouse (25, 31, 71) and the genetically leptin-resistant fa/fa Zucker fatty rat (16, 43). These genetic models of obesity are characterized by high NPY mRNA and peptide levels in the hypothalamus, secondary to the absence of negative feedback regulation by leptin (9, 28, 54, 56). Rodent models of diet-induced obesity (made obese by feeding a highly palatable diet) and streptozotocin-induced diabetes (insulin deficient), which are more typical of human diabetes, are also characterized by elevated CNS NPY tone (57, 68).
Positive energy balance and obesity pathogenesis are thought to result from defects in inhibitory feedback signaling to the CNS (including neuronal insulin and leptin resistance and impaired nutrient sensing) (44, 45). We hypothesized that increases in NPY tone within these neural circuits may contribute to dyslipidemia in addition to obesity. We demonstrated previously that intracerebroventricular (icv) administration of NPY directly into the third ventricle of lean, fasted, wild-type rats increases hepatic VLDL-TG secretion independent of food intake (59). Peripherally administered NPY had no such effect, and taken together these findings suggest that NPY-regulated neural circuits may be involved in the regulation of TG metabolism in the liver (59).
NPY is a 36-amino acid neuropeptide member of the pancreatic polypeptide family that includes peptide YY (PYY) and pancreatic polypeptide (PP) (29). NPY affects a wide variety of physiological functions via the activation of a population of distinct G protein-coupled NPY receptor subtypes: Y1, Y2, Y4, and Y5 (29, 35). All NPY receptor subtypes are expressed in the hypothalamus (18, 60). The effects of NPY on feeding and energy homeostasis are thought to be mediated largely by hypothalamic Y1 and Y5 receptors (reviewed in Ref. 29). Y2 receptors, having affinity for both NPY and PYY (35), act in an inhibitory manner on both orexigenic NPY as well as anorexigenic proopiomelanocortin (POMC) neurons in the arcuate nucleus (ARC) (21). The hypothalamic Y4 receptor is highly selective for PP over NPY or PYY (35) and is thought to mediate anorexigenic effects by decreasing hypothalamic NPY (2). Yet the receptor subtype(s) involved in the central NPY regulation of lipoprotein metabolism is not well understood, nor is the relative effect of a given receptor on feeding vs. lipoprotein metabolism. In genetic models in which the NPY Y1, Y2, Y4, or Y5 receptor has been deleted on an ob/ob background (a rodent model of severe hypertriglyceridemia), the effect on TGs is not reported, except for the Y2 receptor deletion having no effect (37, 46, 52, 53).
Our study employed two approaches, the first determining whether CNS NPY retains the effect on hepatic VLDL-TG secretion chronically (3 days; twice daily icv administration) in the absence of increased food intake and fat mass and the second determining whether different CNS NPY receptors mediate feeding vs. VLDL-TG regulatory effects. We evaluated the effect of selective icv NPY Y1, Y2, Y4, and Y5 receptor agonists on both feeding and hepatic VLDL-TG secretion in lean, fasted rats to determine which receptor subtype(s) is involved in the central NPY regulation of lipoprotein metabolism vs. feeding. We ultimately sought to determine whether the effects on feeding vs. lipids overlap or are dissociable since this might yield novel structure/function insight and/or therapeutic implications in obesity and metabolic syndrome.
MATERIALS AND METHODS
Animal studies.
Male Long-Evans rats (HsdBlu:LE) weighing 250–274 g were purchased from Harlan (Indianapolis, IN), maintained under temperature- and humidity-controlled conditions with a 12:12-h light-dark cycle (lights on at 6 AM), and given free access to water and a standard rodent chow diet (5001; 3.02 kcal/g, 58% carbohydrate, 28.5% protein, 13.5% fat; Lab Diet, Richmond, IN). Study protocols were approved by the Institutional Animal Care and Use Committee of the Tennessee Valley Veterans Affairs (VA) Healthcare System.
Surgical preparation.
Rat surgeries were performed under inhalational isofluorane anesthesia, and Buprenex (0.05 mg/kg body wt) was administered postoperatively to mitigate pain and distress. Animals were prepared with stereotaxic implantation of a stainless-steel guide cannula (22 gauge; Plastics One, Roanoke, VA) into the third cerebral ventricle (40) and a sterile MicroRenathane catheter (R-CAC-M37-R; Brain Tree Scientific, Braintree, MA) implanted into the carotid artery, as described (59).
Intracerebroventricular infusions.
Studies were performed 5–7 days after surgery, when food intake and body weight curves returned to a presurgery trajectory. Recombinant NPY and a selective agonist for the Y2 receptor, human (h)PYY-(3-36), were purchased from GenScript (Piscataway, NJ). Selective agonists for the Y1 receptor, [F7, P34]-NPY, and the Y5 receptor, [Ala31, Aib32]-NPY, were synthesized as described previously (13, 58). A selective agonist for Y4, human PP (hPP), was purchased from Tocris Bioscience (Ellisville, MO). All receptor agonists were dissolved in 0.9% normal saline and freshly prepared. All icv compounds were administered in a 2-μl volume over a time period of 1 min.
Chronic NPY pair-feeding study.
Recombinant NPY (1 nmol) was administered icv twice daily (8 AM and 5 PM) over 3 days in rats matched for body weight. To control for the orexigenic effects of NPY, the caloric intake of pair-fed NPY-treated rats was carefully matched to that of the icv vehicle (Veh; saline)-treated control rats. Plasma TG and cholesterol levels were measured daily from blood collected by tail prick. At the end of the study (day 3) 4-h-fasted rats were given either icv NPY (1 nmol) or Veh, and following a 120-min postinjection period, trunk blood was collected at study termination. Body weight and body composition using an EchoMRI-700 NMR spectrometer (Echo Medical Systems, Houston, TX) to determine lean and fat mass were measured at study termination.
NPY receptor agonist selectivity and dosing.
The NPY EC50 values as measured in vitro are 2.6, 5.1, 814, and 4.9 nM for the Y1, Y2, Y4, and Y5 receptor subtypes, respectively (35). All peptide ligands used in our studies are selective compounds for their respective NPY receptor subtypes and bind with subnanomolar affinity (35). The Y1 selective peptide [F7, P34]-NPY has >3,000-fold selectivity for the Y1 receptor over that of either the Y2 or Y5 receptor (58). For Y2 receptor activation, we used hPYY-(3–36), which has a 181-, >1,000-, and fivefold greater affinity for the Y2 receptor than for Y1, Y4, and Y5 receptor subtypes, respectively (69). To activate the Y4 receptor, we used hPP that has a 2.2-, >56-, and 1.4-fold greater affinity for the Y4 receptor than for Y1, Y2, and Y5 receptor subtypes, respectively (35, 38). We also used a Y5 agonist, [Ala31, Aib32]-NPY, which has >77-, 12-, and >77-fold selectivity for the Y5 receptor than for the Y1, Y2, and Y4 receptor subtypes, respectively (35, 38). The dose of agonist utilized for each receptor subtype was estimated on the basis of in vitro receptor potencies relative to NPY (35, 38, 58, 69) for food intake and lipid studies. The dosages chosen for icv treatment were NPY (1 nmol), Y1 agonist [F7, P34]-NPY (1 nmol), Y2 agonist hPYY-(3–36) (1 nmol), Y4 agonist hPP (1 nmol), and Y5 agonist [Ala31, Aib32]-NPY (2 nmol).
Food intake studies.
We assessed food intake responses to agonists of each receptor subtype compared with vehicle control, 12-h-fasted, and NPY-treated animals. Rats that had surgically implanted icv cannulae and were matched for body weight were studied. We administered the icv compounds NPY (1 nmol), [F7, P34]-NPY (1 nmol), hPYY-(3–36) (1 nmol), hPP (1 nmol), [Ala31, Aib32]-NPY (2 nmol), or Veh (saline) to ad libitum chow-fed rats at 10 AM (lights on at 6 AM) and measured 2-h food intake postinjection.
Tyloxapol (lipid production) experiments.
For lipid production studies, rats with surgically implanted icv cannulae and carotid catheters were matched for body weight and fasted, with free access to water from 6 to 10 AM. We confirmed previously that 4-h-fasted rats are in a postabsorptive state and that chylomicrons do not contribute to the observed changes in TGs (59). A baseline blood sample was drawn through the carotid catheter, and then plasma TG clearance was blocked by an intravenous infusion of tyloxapol (300 mg/kg body wt) that at the dosage used potently inhibits lipoprotein lipase (LPL) activity (42, 55). Thirty minutes after tyloxapol infusion, icv compounds or Veh were injected at time 0 min. Also, at 0 min and at 30-min intervals, 200 μl of blood was collected in a tube with 2 μl of 50 mM EDTA (59). The TG secretion rate was determined as the slope of the concentration of plasma TGs over time using linear regression analysis (calculated from time 0 to 120 min).
Lipoprotein fractionation.
Fast protein liquid chromatography (FPLC) analysis was performed as described previously (59). Plasma samples (150 μl) were size fractionated on a Superose-6 300 GL column (GE Healthcare Biosciences, Little Chalfont, UK), and 300-μl column fractions were collected in a 96-well plate.
Lipid assays.
Lipids were assayed from plasma with enzymatic colorimetric assays using the following reagent kits for TG and total cholesterol from Raichem (San Diego, CA): nonesterified FFA from Wako Diagnostics (Richmond, VA) and glycerol from Sigma-Aldrich (St. Louis, MO).
Plasma hormones and metabolites.
Plasma levels of insulin and glucagon were quantified using radioimmunoassays from Millipore (Billerica, MA); all assays were performed by the Vanderbilt Diabetes Center Hormone Assay & Analytical Services Core (Nashville, TN). Blood glucose concentration was measured using a glucometer from Roche Diagnostics (Accu-Chek Advantage, Indianapolis, IN) on trunk blood at study termination.
Western blot analysis.
Liver proteins were extracted and protein concentrations determined using Pierce BCA Protein Kit (Thermoscientific, Rockford, IL). For immunoblotting, liver protein extracts were separated on a 4–12% Bis-Tris XT gel using a Bio-Rad XT Criterion System (Bio-Rad, Hercules, CA), followed by electrophoretic transfer of proteins to nitrocellulose membranes. Primary antibodies used at a 1:1,000 dilution for immunodetection were as follows: microsomal TG transfer protein (MTP; sc-33116), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-25778), and actin (sc-1616) purchased from Santa Cruz Biotechnology (Santa Cruz, CA); acetyl-CoA carboxylase (ACC; 3662), phosphorylated ACC (Ser79; 3661), and fatty acid synthase (FAS; 3180) antibodies purchased from Cell Signaling Technology (Beverly, MA); heat shock protein 70 (HSP70; ADI-SPA-816) purchased from Enzo Life Sciences (Framingdale, NY); and apoB 100/48 (kindly provided by Larry Swift; Vanderbilt University, Nashville, TN) (61). Horseradish peroxidase-conjugated secondary antibodies used at a 1:7,500 dilution for immunodetection were donkey anti-goat IgG purchased from Santa Cruz Biotechnology and anti-rabbit IgG purchased from Promega (Madison, WI). Western blots were analyzed by densitometry utilizing Image J Software (National Institutes of Health).
RNA isolation and quantitative RT-PCR.
Liver samples were homogenized, and mRNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). From these RNA samples, cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). RT-PCR was performed with IQ SYBR Green Supermix, as described previously (51). Quantification results for each RNA of interest were normalized to the housekeeping gene ribosomal protein L13a (RPL13a), and for comparative analysis RNA ratios of the treatment group were normalized to the Veh control group. Primer sets designed to detect and amplify stearoyl-CoA desaturase-1 (SCD-1) [as described previously (41)] and RPL13a (forward primer: 5′-TACTCTGGAGGAGAAACGGAAG-3′ and reverse primer: 5′-GCCTGTTTCCTTAGCCTCAA-3′) were used.
Statistical analysis.
Data are presented as means ± SE. Two-group comparisons were performed using Student's t-test (unpaired, 2-tailed) and three-group (or more) comparisons by one-way ANOVA with Bonferroni's posttest analysis. Treatment vs. time was compared by two-way repeated-measures ANOVA with Bonferroni's posttest analysis. All analyses were performed using GraphPad Prism, version 5.04, 2010 (GraphPad Software, San Diego, CA). Differences with P < 0.05 were considered statistically significant.
RESULTS
Chronic NPY treatment increases plasma TG levels independent of food intake, positive energy balance, and increased body adiposity.
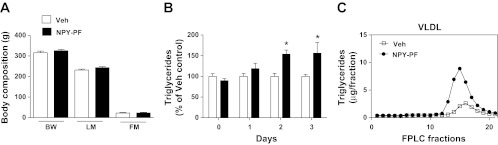
Previously, we demonstrated that icv administration of NPY into the third ventricle of lean rats increases hepatic VLDL-TG secretion independent of feeding, as the animals were denied access to food just prior to and during experimental procedures (59). Because exogenous administration of NPY to a lean animal can drive the development of obesity and metabolic syndrome (48, 65), we sought to determine whether a more chronic NPY administration increases plasma TGs independent of its orexigenic effects to increase food intake and body adiposity. NPY was administered icv (1 nmol) twice daily over 3 days in chow-fed rats. Importantly, to prevent NPY-induced hyperphagia, the food intake of pair-fed NPY-treated rats was calorically matched to that of the Veh-treated controls. At the end of the study (day 3), 4-h-fasted rats were given either icv NPY (1 nmol) or Veh, and trunk blood was collected at study termination (120 min postinjection). Pair-feeding successfully matched the body composition of NPY-treated rats relative to Veh-treated animals, resulting in no differences in body weight, lean mass, or fat mass at study termination (Fig. 1A). However, we found that chronic NPY injections in pair-fed rats resulted in an ∼50% increase in plasma TGs by treatment days 2 and 3 (P < 0.01, n = 6/group; Fig. 1B) with no observed alteration in total plasma cholesterol levels [P = not significant (NS); Table 1]. The effect of chronic NPY treatment to increase plasma TG over time was dependent on the treatment [F(1,22) = 7.99, P < 0.01]. The changes in TGs observed with chronic NPY treatment in pair-fed rats (P < 0.05; Table 1) were not due to elevated adipocyte lipolysis, as we did not observe any changes in FFA or glycerol at study termination (P = NS; Table 1), consistent with our previous findings (59). Although glucagon levels were significantly elevated compared with Veh treatment (P < 0.05; Table 1), insulin levels only trended higher (P = 0.09; Table 1). Individual plasma samples were size-fractionated by FPLC and quantified for TG content. As observed previously with an acute injection of icv NPY in lean fasted rats, chronic NPY treatment in pair-fed rats increased TG content in VLDL-containing fractions (fractions 10–20; Fig. 1C).
Fig. 1.
Chronic neuropeptide Y (NPY) treatment induces hypertriglyceridemia independent of positive energy balance. A: the food intake of chronic intracerebroventricular (icv) NPY-treated (3 days, twice daily; 1 nmol), pair-fed (NPY-PF), chow-fed rats (black bars) was calorically matched to that of the icv vehicle (Veh; open bars)-treated rats (n = 6/group), and the effects on body weight (BW), lean mass (LM), and fat mass (FM) are illustrated. Data are presented as means ± SE and were analyzed by Student's t-test (unpaired, 2-tailed); P = not significant (NS) for NPY-PF vs. Veh comparisons. B: daily plasma TG levels (%Veh control) from blood collected by tail prick of NPY-PF (black bars) and Veh-treated (open bars) rats are shown. Data are presented as means ± SE and were analyzed by 2-way repeated-measures ANOVA with Bonferroni's posttest analysis. *P < 0.01 for NPY-PF vs. Veh comparisons. The effect of chronic NPY treatment to increase plasma TG over time was dependent upon treatment [F(1,22) = 7.99, P < 0.01]. C: the TG content of fast protein liquid chromatography (FPLC) fractions of size-fractionated individual plasma samples from NPY-PF (●) and Veh-treated rats (□) as measured on study day 3 are illustrated for comparison of fractions 10–20, with the size range corresponding to VLDL.
Table 1.
Effects of chronic NPY administration to pair-fed chow-fed rats at 120 min post-icv injection on glucoregulatory hormones and metabolites
| Veh | NPY-PF | |
|---|---|---|
| TG, mg/dl | 80.5 ± 5.0 | 156 ± 32* |
| Cholesterol, mg/dl | 97.6 ± 10.0 | 83.8 ± 3.6 |
| FFA, mmol/l | 0.31 ± 0.03 | 0.23 ± 0.02 |
| Glycerol, mg/dl | 10.4 ± 1.8 | 15.6 ± 4.7 |
| Insulin, ng/ml | 1.04 ± 0.10 | 2.09 ± 0.50 |
| Glucagon, pg/ml | 63.1 ± 4.4 | 107 ± 14* |
Data are means ± SE (n = 6/group). NPY, neuropeptide Y; icv, intracerebroventricular; Veh, vehicle; NPY-PF, NPY-pair-fed; TG, triglyceride; FFA, free fatty acid.
P < 0.05 for NPY-PF vs. Veh comparisons.
Selective activation of NPY receptor subtypes induces hyperphagia in lean, ad libitum chow-fed rats.
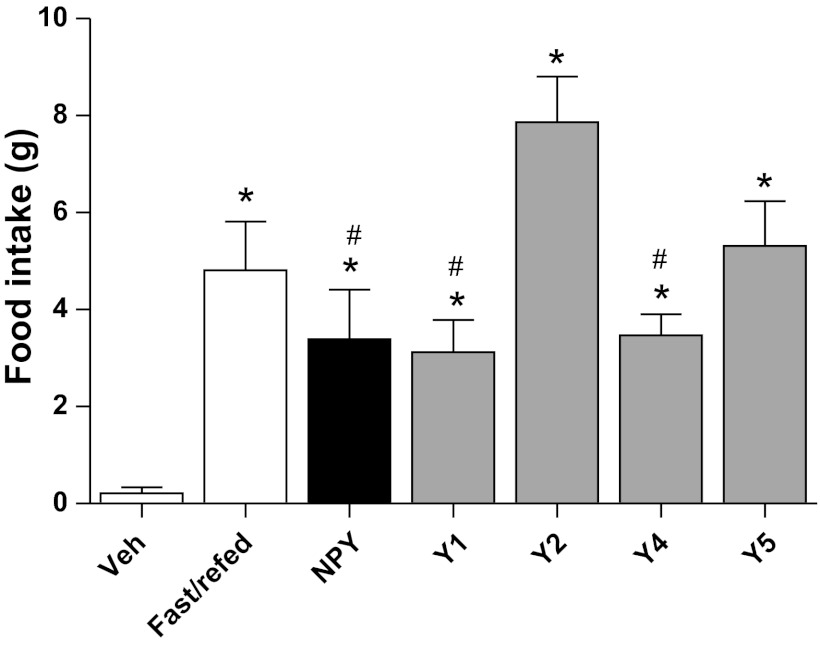
We first sought to identify an icv dosage of NPY that would lead to a physiologically relevant feeding response. Lean, ad libitum chow-fed rats were given either icv NPY (1 nmol) or Veh, and the 2-h feeding response postinjection was compared with the 2-h refeeding response of 12-h-fasted rats. As expected, both icv NPY treatment and 12 h of fasting potently induced hyperphagia compared with Veh treatment (Fig. 2). We found that 1 nmol NPY given icv induced the same 2-h feeding response as observed after a 12-h fast (P = NS; Fig. 2). All of the selective NPY receptor agonists, Y1 ([F7, P34]-NPY; 1 nmol), Y2 [hPYY-(3–36), 1 nmol], Y4 (hPP; 1 nmol), and Y5 ([Ala31, Aib32]-NPY; 2 nmol), induced hyperphagia in ad libitum chow-fed rats relative to Veh (Fig. 2). Therefore, all of the selective receptor agonists (Y1, Y2, Y4, and Y5) induced a 2-h feeding response similar in magnitude to 12 h of fasting (P = NS; Fig. 2). Of particular note, the Y2 receptor agonist, at a dose equivalent to NPY (1 nmol), stimulated feeding above all of the other compounds except for the Y5 agonist (Fig. 2). Collectively, these data confirm that we used physiologically relevant doses (1–2 nmol) of each selective receptor agonist for our food intake and lipid studies.
Fig. 2.
Effects of central nervous system (CNS)-administered NPY receptor subtype agonists on 2-h food intake. Food intake was measured in lean, ad libitum chow-fed rats for 2-h after they received an icv injection of Veh (n = 13), NPY (1 nmol; n = 4), or a selective NPY receptor subtype agonist for Y1 ([F7, P34]-NPY, 1 nmol), Y2 [human peptide YY-(3–36), 1 nmol], Y4 (human pancreatic polypeptide, 1 nmol), or Y5 ([Ala31, Aib32]-NPY, 2 nmol) (n = 4–6/group). These 2-h food intake measurements compared with a 2-h refeeding response of a 12-h-fasted lean rat are shown (fast/refed; n = 3). Data are presented as means ± SE and were analyzed by 1-way ANOVA. *Significant differences (P < 0.05), as tested with Bonferroni's posttest, for comparisons relative to Veh; and #significant differences (P < 0.05) for comparisons relative to Y2 receptor agonist.
A Y1 receptor agonist increases plasma TG levels to the greatest extent relative to other NPY receptor subtype agonists.
In corroboration with our previous findings (59), NPY increased the hepatic TG production rate significantly [6.4 ± 0.5 mg·dl−1·min−1 for NPY (n = 5) vs. 3.5 ± 0.1 mg·dl−1·min−1 for Veh (n = 26), P < 0.0001; Fig. 3, A and E]. We found that the Y1 receptor agonist [F7, P34]-NPY (1 nmol) also increased hepatic TG production rate to a similar extent [6.7 ± 0.4 mg·dl−1·min−1 for Y1 (n = 7) vs. Veh, P < 0.0001; Fig. 3, B and E]. The Y2 receptor agonist hPYY-(3–36) (1 nmol) stimulated TG production 1.5-fold [5.1 ± 0.3 mg·dl−1·min−1 for Y2 (n = 7) vs. Veh, P < 0.01; Fig. 3, C and E] and was less potent than the Y1 receptor agonist (Y1 vs. Y2, P < 0.01; Fig. 3E). Neither the Y4, hPP (1 nmol), nor Y5 [Ala31, Aib32]-NPY (2 nmol) receptor agonists increased the rate of hepatic TG production beyond that of Veh treatment (P = NS; Fig. 3, D and E). The ability of NPY and its Y1 and Y2 receptor agonists to increase TG production over time was treatment dependent [F(6,50) = 11.75, P < 0.0001]. Collectively, our results suggest that although both Y1 and Y2 receptor agonists regulate plasma TG levels and food intake, an NPY signal mediated through a Y1 receptor increases hepatic TG production more potently, whereas one mediated through the Y2 receptor has a greater effect on food intake (compare Figs. 2 and 3E).
Fig. 3.
Effect of each CNS NPY receptor subtype (Y1, Y2, Y4, and Y5) on hepatic triglyceride (TG) production. A–D: plasma TG levels after an intra-arterial injection of tyloxapol (at time −30 min), and then at time 0 min, icv injections of NPY (1 nmol; ●) or Veh (□) (A), Y1 receptor agonist ([F7, P34]-NPY, 1 nmol; ▲) or Veh (□) (B), Y2 receptor agonist [human peptide YY-(3–36), 1 nmol; ◆] or Veh (□) (C), Y4 receptor agonist (human pancreatic polypeptide, 1 nmol; ●), or Y5 receptor agonist ([Ala31, Aib32]-NPY, 2 nmol; ◇) or Veh (□) (D) were administered to lean 4-h-fasted rats (NPY, n = 5; NPY receptor agonists, n = 4–7/group; Veh, n = 26). Data are means ± SE, and significant differences (*P < 0.05) for treatment vs. Veh were determined by 2-way repeated-measures ANOVA (A–D). The ability of NPY and its Y1 and Y2 receptor agonists to increase TG production over time was treatment dependent [F(6,50) = 11.75, P < 0.0001]. E: TG production rates calculated from the change in plasma TG levels over time after icv treatment of either NPY or NPY receptor agonists (Y1, Y2, Y4, and Y5) compared with Veh in lean 4-h-fasted rats. Data are means ± SE. *Statistical significance, as determined by 1-way ANOVA with Bonferroni's posttest analysis (P < 0.05), for comparisons relative to Veh; #statistical significance for the receptor agonist comparison of Y1 relative to Y2.
Intracerebroventricularly administered Y1 receptor agonist enhances hepatic secretion of TGs in the form of VLDL-lipoprotein.
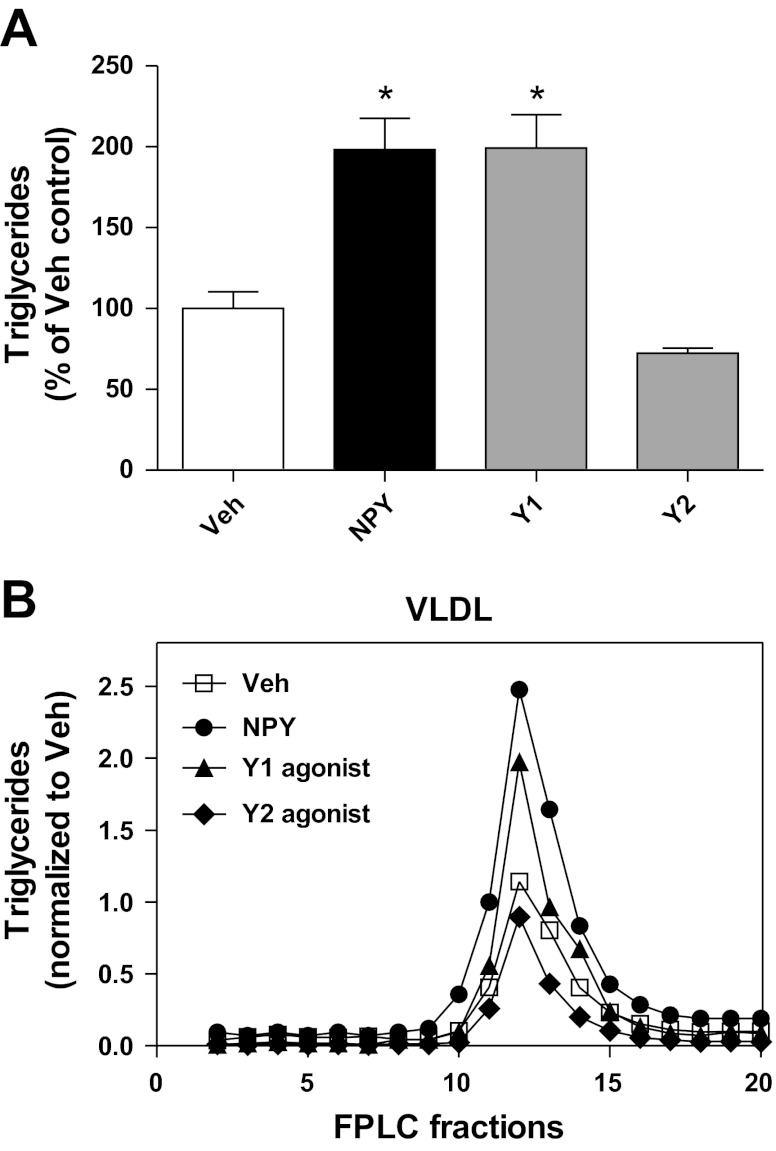
Since both Y1 and Y2 receptor agonists increased hepatic TG production, we next confirmed that modulation of CNS NPY signaling via these receptor subtypes increases TGs in the VLDL fraction. To avoid any nonspecific effects from the use of tyloxapol during the measurement of TG production rates, we performed these experiments in the absence of tyloxapol. NPY (1 nmol), Y1 receptor agonist (1 nmol), Y2 receptor agonist (1 nmol), and Veh were given icv, and then trunk blood was collected at study termination (120 min postinjection). NPY increased plasma TGs by ∼200% (NPY vs. Veh, P < 0.001, n = 6–13/group; Fig. 4A). Similarly, the Y1 receptor agonist recapitulated the NPY effect by doubling plasma TG levels (Y1 receptor agonist vs. Veh, P < 0.001, n = 6–13/group; Fig. 4A), whereas the Y2 receptor agonist had no effect on plasma TGs in the absence of tyloxapol (Y2 receptor agonist vs. Veh, P = NS, n = 6–13/group; Fig. 4A). Pooled plasma samples were size-fractionated by FPLC, and TG content was quantified in each column fraction. NPY treatment increased TG content in column fractions (10–20) that corresponded in size to that of VLDL (Fig. 4B), consistent with our previous findings (59). Similarly, the Y1 receptor agonist increased TG content in these fractions, whereas the Y2 receptor agonist had little effect (Fig. 4B). Neither treatment with NPY nor the agonists for Y1 or Y2 receptor subtypes altered total plasma cholesterol levels (data not shown).
Fig. 4.
Effects of CNS NPY receptor subtypes Y1 and Y2 on hepatic VLDL-TG secretion. A and B: NPY (1 nmol), Veh, or receptor agonists for either Y1 ([F7, P34]-NPY, 1 nmol) or Y2 [human peptide YY-(3–36), 1 nmol] were given icv in the absence of tyloxapol to 4-h-fasted lean rats (n = 6–13/group), and trunk blood was collected at 120 min postinjection. Each receptor subtype agonist was tested in a separate cohort of animals, and each cohort was matched to its own Veh control group, so plasma TG levels of each treatment group were normalized to their matched Veh controls. A: plasma TG levels of each treatment group (%Veh control) are shown. Data are means ± SE. *Statistical significance (P < 0.05), as determined by 1-way ANOVA with Bonferroni's posttest analysis, for all comparisons relative to Veh. B: the TG content of FPLC fractions after size fractionation of pooled plasma samples is illustrated. Column fractions 10–20, with the size range corresponding to VLDL, are illustrated to allow comparisons between the different icv treatment groups: Veh (□), NPY (●), Y1 receptor agonist (▲), and Y2 receptor agonist (◆).
Increased CNS NPY Y1 or Y2 receptor signaling did not alter markers of adipocyte lipolysis or glucoregulatory hormones.
Current models suggest that increased adipocyte lipolysis during states of fasting or insulin resistance leads to increased substrate (FFA and glycerol) delivery to the liver for greater VLDL-TG secretion (6, 32, 49). Thus, we sought to determine whether CNS NPY signaling via the Y1 or Y2 receptor increases lipolysis. Extending our previous findings (59) as well as others (12), icv NPY did not activate markers of lipolysis, FFA, and glycerol nor Y1 or Y2 receptor agonists (P = NS; Table 2).
Table 2.
Effects of CNS NPY and agonists for Y1 and Y2 receptor subtypes at 120 min post-icv injection on glucoregulatory hormones and metabolites
| NPY |
Y1 Agonist |
Y2 Agonist |
||||
|---|---|---|---|---|---|---|
| Veh | NPY | Veh | Y1 | Veh | Y2 | |
| FFA, mmol/l | 0.33 ± 0.03 | 0.39 ± 0.05 | 0.26 ± 0.04 | 0.25 ± 0.02 | 0.30 ± 0.04 | 0.37 ± 0.02 |
| Glycerol, mg/dl | 10.0 ± 0.8 | 11.4 ± 0.3 | 14.9 ± 2.2 | 16.9 ± 1.4 | 10.2 ± 2.2 | 6.2 ± 1.2 |
| Insulin, ng/ml | 1.1 ± 0.2 | 1.5 ± 0.3 | 2.6 ± 0.4 | 3.8 ± 0.9 | 3.7 ± 0.3 | 3.8 ± 0.2 |
| Glucagon, pg/ml | 79 ± 7 | 76 ± 4 | 77 ± 10 | 76 ± 6 | 76 ± 11 | 86 ± 12 |
| Blood glucose, mg/dl | 137 ± 5 | 145 ± 6 | 150 ± 5 | 159 ± 6 | 135 ± 4 | 152 ± 4* |
Data are means ± SE (n = 6-7/group). CNS, central nervous system.
P < 0.05 for icv treatment vs. Veh comparison.
Intracerebroventricular infusion of NPY influences glucose metabolism and sensitivity to insulin in fasted rats (12, 36, 63, 64); therefore, we determined whether CNS NPY signaling via either the Y1 or Y2 receptor alters glucoregulatory hormones. The Y2 receptor agonist increased plasma glucose levels by 13% (P < 0.05; Table 2), whereas neither the NPY nor Y1 receptor agonist had any effect on glucose concentration (P = NS; Table 2). Intracerebroventricularly administered NPY, Y1, and Y2 receptor agonists did not alter plasma insulin or glucagon levels (P = NS; Table 2).
CNS NPY signaling via the Y1 receptor modulates liver SCD-1 mRNA levels.
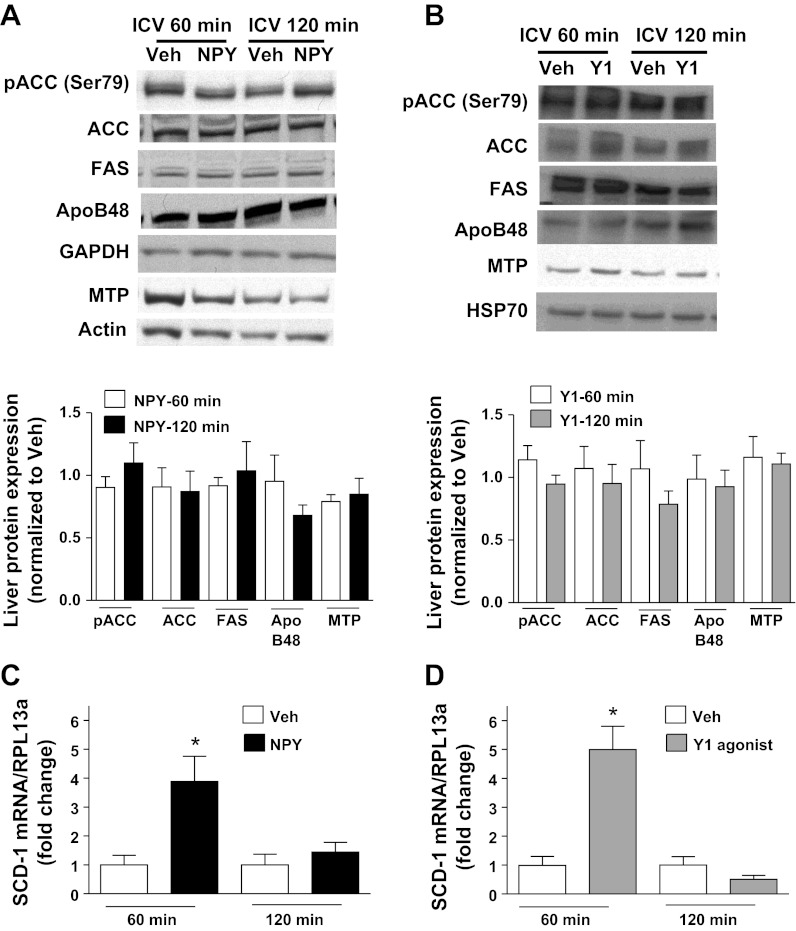
Hypothalamic signaling via several hormones (especially leptin) and metabolites regulates hepatic SCD-1 gene expression (17, 34, 66, 67, 70), and thus liver expression of SCD-1 is a robust marker of known hypothalamic-liver regulatory pathways. First, we investigated whether increased CNS NPY signaling via the Y1 receptor modulated relevant liver regulatory targets. Lean, 4-h-fasted rats were given either icv NPY (1 nmol), the Y1 receptor agonist (1 nmol), or Veh, and at either 60 or 120 min postinjection, trunk blood and liver samples were collected. Similar to our previous findings at 120 min post-icv injection (Fig. 4A), we found at 60 min post-icv injection that treatment with either NPY or the Y1 receptor agonist doubles plasma TG levels with respect to Veh (NPY: 256 ± 60% vs. Veh: 100 ± 11%, P < 0.01; Y1 receptor agonist: 171 ± 21% vs. Veh: P < 0.01; n = 5–11/group). We then quantified expression levels of key lipid metabolic markers involved in VLDL assembly and secretion. At both 60 and 120 min, neither NPY nor the Y1 receptor agonist altered levels of phosphorylated ACC (Ser79), ACC, FAS, hepatic apoB-48, or MTP (P = NS, n = 5–7/group; Fig. 5, A and B), consistent with our previous findings (59). CNS NPY did induce SCD-1 mRNA levels fourfold relative to Veh (P < 0.05, n = 5/group; Fig. 5C), whereas the Y1 receptor agonist increased SCD-1 mRNA levels fivefold (P < 0.01, n = 5–6/group; Fig. 5D). Thus, signaling via the Y1 receptor recapitulates CNS NPY regulation of hepatic SCD-1 gene expression, which is a known marker of hypothalamic-hepatic metabolic regulation whose gene product is involved in lipid metabolism.
Fig. 5.
CNS NPY signaling via the Y1 receptor induces hepatic stearoyl-CoA desaturase-1 (SCD-1) gene expression. A and B: representative immunoblots of key lipid metabolic markers involved in VLDL assembly and secretion are shown. Protein extracts prepared from livers of 4-h-fasted lean rats (n = 5–7/group) isolated 60 or 120 min after icv injection of NPY (1 nmol) or Veh (A) or Y1 receptor agonist ([F7, P34]-NPY, 1 nmol) or Veh (B) were immunoblotted to detect levels of phosphorylated acetyl-CoA carboxylase (ACC; Ser79), ACC, fatty acid synthase (FAS), apolipoprotein B-48 (apoB-48), and microsomal TG transfer protein (MTP). Western blots were analyzed by densitometry (normalized to Veh control) for icv NPY treatment at 60 (open bars) and 120 min (black bars) postinjection (A) and icv Y1 receptor agonist treatment at 60 (open bars) and 120 min (gray bars) postinjection (B). Densitometry results were corrected relative to those of the protein loading control GAPDH, actin, or heat shock protein 70 (HSP70). C and D: RNA were isolated from livers of 4-h-fasted rats that were obtained 60 or 120 min after treatment with icv NPY (black bars) or Veh (open bars) (C) or ICV Y1 receptor agonist (gray bars) or Veh (open bars) (D) and were assessed by quantitative RT-PCR for changes in SCD-1 mRNA levels. SCD-1 mRNA levels, normalized to the reference RNA ribosomal protein L13a (RPL13a), are shown. For comparative analysis, RNA ratios were normalized to the Veh control. Data are means ± SE and were analyzed by Student's t-test (unpaired, 2-tailed). *Significant difference (P < 0.05) between icv treatment and Veh.
DISCUSSION
An atherogenic dyslipidemia characterized in part by elevated plasma TG levels is the major lipid abnormality associated with obesity, diabetes, and metabolic syndrome (22, 23, 26). Although peripheral factors (visceral adiposity and insulin resistance) clearly contribute to this disorder (32, 49), we hypothesized that increased CNS NPY action contributes to both the pathogenesis of obesity and the pathogenesis of obesity dyslipidemia. Here, we sought to determine whether the effects of NPY on feeding and/or weight gain are dissociable from the effects on hepatic VLDL-TG secretion. We employed two approaches, the first one asking whether NPY retains the effect on hepatic VLDL-TG secretion chronically in the absence of increased food intake and weight gain and the second one asking whether different NPY receptors mediate feeding vs. lipid effects. We found that chronic (3 days) icv injections of NPY in pair-fed animals compared with Veh-treated controls to maintain identical body composition also led to hypertriglyceridemia, which suggests that hyperphagia and accumulation of excess adiposity are not required for this effect. Moreover, using selective NPY receptor agonists, we demonstrate, within the limits of our model, that central NPY signaling via the Y1 receptor predominantly regulates effects on hepatic lipoprotein production. The Y2 receptor agonist modestly stimulated VLDL-TG production, albeit at a dose that had a profound effect on feeding. Neither the Y4 nor the Y5 agonist had an effect on plasma TGs. Conversely, all agonists stimulated feeding, with the NPY Y2 receptor agonist having a much more robust effect on feeding than TG production. In contrast, at the same dose used as the NPY Y2 receptor agonist, the NPY Y1 receptor agonist had a greater effect on TG production than feeding. Thus, we postulate that NPY regulates feeding and lipoprotein metabolism partially via separate NPY receptor systems and/or mechanisms.
Whereas our study and others (27, 62) reveal that central administration of the NPY Y2 receptor agonist PYY-(3–36) stimulates feeding under some conditions, Batterham et al. (8) reported that this Y2 receptor agonist has an inhibitory effect on food intake when administered by direct intra-arcuate injection or via peripheral administration. Because the inhibitory Y2 receptor is found on both the ARC NPY and POMC neurons, this adds an additional layer of complexity to the regulation of the NPY/POMC neural circuit by Y2 agonists. Both endogenous and exogenous Y2 agonist action in the ARC is context dependent, as elegantly described by Ghamari-Langroudi et al. (21). An additional consideration is that icv administration of the Y2 agonist may result in its dispersion to other hypothalamic and nonhypothalamic regions. Finally, the potential activation of the Y5 receptor by PYY-(3–36) could also explain the increase in food intake.
A strength of our study was that we matched test compounds for potency on feeding behavior (similar to a 12-h fast), with the exception of the Y2 receptor agonist. A weakness is that we utilized only a single dosage of each test compound. It is conceivable that, at significantly higher or lower doses, opposite and/or differential effects on feeding relative to VLDL-TG secretion may have been observed. Indeed a “U-shaped” curve, as well as exquisite dose dependency of the effects of several neuropeptides, has been noted (47, 50). Thus, this study does not reveal whether dose response effects of NPY Y1 receptor signaling on feeding resemble or differ from those on VLDL-TG secretion.
We reported previously that the NPY Y5 receptor agonist BWX-46 increased VLDL-TG secretion in lean, fasted rats (59), a finding not replicated here using [Ala31, Aib32]-NPY (Fig. 3, D and E). However, the NPY Y5 receptor agonist [Ala31, Aib32]-NPY (2 nmol icv) employed in our current study has greater than 77-fold selectivity for the Y5 than for the Y1 receptor (35, 38), whereas BWX-46 (12 nmol icv) employed in our previous study has less Y5 selectively and greater cross-reactivity with the Y1 receptor (3). Thus, at the dose used in our previous study (59), it appears likely that BWX-46 activated both the NPY Y1 and Y5 receptors, resulting in the observed increase in VLDL-TG production.
Elevated hypothalamic NPY tone (and a reduction of POMC tone) is associated with obesity and diabetes in both rodent models (57, 68) and humans (4, 5), likely due to defects in inhibitory feedback signals to the CNS (i.e., insulin and leptin resistance) (44, 45). Because we show that Y1 receptor activation has a greater effect than activation of the Y2 receptor on lipoprotein metabolism, it informs of potential structure-function relationships of the NPY-regulated neural circuitry involved. There is a reportedly close physical localization and apparent functional relationship between NPY Y1 and Y2 receptors in neurons found within the ARC, the lateral hypothalamic area, the dorsomedial hypothalamic nucleus, and the paraventricular nucleus (PVN), whereas the ventromedial hypothalamic nucleus (VMN) contains only Y1 receptor-positive neurons (60). Furthermore, a study by Chee et al. (15) reports that NPY inhibits the excitatory (anorexigenic) outflow between the VMN and ARC POMC neural circuitry via the activation of the Y1 receptor subtype in the VMN. Moreover, studies in VMN-lesioned rats, which recapitulate a state of elevated NPY tone, have elevated plasma TGs (10), even as early as 10 days postoperatively, together with decreased plasma FFA and glucose levels (30). Finally, perfused livers from VMN-lesioned rats secrete more TGs than controls (30). Altogether, these data suggest that the VMN is a potential hypothalamic site in which NPY may regulate lipoprotein metabolism via selective activation of the Y1 receptor. Of course, our initial studies reported here employing icv injections cannot localize the effect. Thus, future studies will involve selective inhibition of the Y1 receptor in PVN compared with VMN with microinjection techniques in an obese, hypertriglyceridemic rodent model characterized by elevated NPY tone (i.e., fa/fa Zucker fatty rat). Collectively, these findings may lend plausibility that the brain is a potential therapeutic target to treat obesity dyslipidemia.
Our finding that the NPY Y1 receptor is most robustly coupled to lipoprotein metabolism is consistent with the conclusions from a genetic association study in severely obese human subjects matched for body mass index in which those individuals with the endogenous CC haplotype (relative to the TT/CT polymorphism) of the untranslated region of the NPY Y1 receptor gene had elevated fasting serum triglycerides and significantly lower high-density lipoprotein cholesterol concentrations (11). It is not yet clear whether this haplotype correlates with a relative gain of NPY Y1 receptor function, but we would hypothesize in the context of our findings that the CC haplotype is a relatively hyperfunctional allele and thus would confer increased TGs in the setting of obesity.
Several studies have investigated the effect of global deletion of the NPY Y1, Y2, Y4, and Y5 receptors on the background of the ob/ob mouse, which is a leptin-deficient, obese rodent model characterized by elevated CNS NPY tone and severe hypertriglyceridemia. Unfortunately, these studies report only on the effect of this genetic manipulation on energy homeostasis and not on whether deletion of the various NPY receptors attenuates hypertriglyceridemia, except for the Y2 receptor, which was noted to have no effect (37, 46, 52, 53). Because all of the NPY receptor subtypes are expressed both centrally and peripherally (except for the brain-specific Y5 receptor) (35), it would be interesting to determine whether brain-specific deletion of the Y1 receptor in the obese ob/ob mouse would attenuate hypertriglyceridemia.
Current models suggest that increased adipocyte lipolysis during states of fasting or insulin resistance leads to increased substrate (FFA and glycerol) delivery to liver, which can increase hepatic VLDL-TG secretion (32, 49). The observation that increased CNS NPY signaling via the Y1 receptor doubled VLDL-TGs, whereas plasma FFA and glycerol levels were unchanged, suggests that adipocyte lipolysis was not increased and thus would not account for the NPY-stimulated increase in hepatic VLDL-TG production. Moreover, we observed this same effect in rats given chronic NPY treatments under pair-fed conditions, leading to the doubling of VLDL-TG secretion independent of changes in adipocyte lipolysis. Although CNS Y2 receptor signaling did not alter markers of adipocyte lipolysis, the Y2 receptor agonist surprisingly had no effect on plasma TGs in the absence of tyloxapol (Fig. 4, A and B). That the Y2 receptor agonist did increase VLDL-TG secretion modestly in the presence of tyloxapol (Fig. 3, C and E) suggests that Y2 receptor activation may also have an effect to enhance TG clearance perhaps through the modulation of adipose tissue LPL activity (33).
SCD-1 catalyzes the desaturation of palmitic and stearic acids to palmitoleic and oleic acids, and its expression is known to be regulated by CNS leptin (17, 66), glucose (34), and melanocortin action (67) in the same hypothalamic feeding circuits engaged by NPY. Whereas leptin suppresses SCD-1 [and NPY tone (56)], we have observed a robust induction of hepatic SCD-1 expression in response to CNS NPY treatment. This effect is recapitulated by Y1 receptor activation, providing further evidence that NPY signaling via the Y1 receptor is mechanistically involved in hepatic lipid metabolism.
Provision of oleic acid or modulation of SCD-1 activity changes VLDL production rate by increasing triglyceride loading in the late maturation phase (17, 34). Presently, we demonstrate that the elevation in hepatic VLDL-TG secretion by increased CNS NPY signaling via the Y1 receptor is associated with a rapid (within 60 min) induction of hepatic SCD-1 gene expression, which would suggest that SCD-1 activation may contribute to changes in VLDL-TG secretion. This is supported by previous findings in which hypothalamic glucose (34) and glycine (70) metabolism reduced hepatic SCD-1 mRNA and inhibited hepatic VLDL-TG secretion. Although the definitive mechanistic link between hepatic SCD-1 and the alteration of VLDL-TG secretion by CNS NPY signaling remains to be determined, changes in the formation rate of hepatic oleic acid may be an important mediator (34).
Our results show that neither the NPY nor the Y1 agonist influenced the level of key de novo lipogenic enzymes, phosphorylated ACC (Ser79), ACC, or FAS. Hepatic apoB is an essential component of liver-derived VLDL; if lipid is not loaded by MTP, apoB becomes a target for proteasomal degradation (19). In contrast to humans, the rat produces predominantly apoB-48 instead of apoB-100 in the liver (14). Our results show that there were no significant changes in hepatic tissue levels of apoB-48 detectable by Western blot from increased CNS NPY and Y1 receptor signaling (Fig. 5, A and B), although this method is not as sensitive as radiolabeling methods. Similarly, we found that MTP, which plays a pivotal role in VLDL maturation and secretion of triglyceride-rich lipoproteins (7, 19), was unaltered by NPY and Y1 receptor agonist treatment (Fig. 5, A and B). Collectively, these data suggest that CNS NPY acts primarily via the Y1 receptor to increase plasma TGs and likely does so by altering the late maturation step of VLDL assembly and secretion.
In conclusion, we demonstrate, within the limits of our model, that the effects of NPY on feeding and/or weight gain are relatively dissociable from the effects on hepatic VLDL-TG secretion. Three days of twice daily icv NPY injections in pair-fed animals compared with Veh-treated controls to maintain identical caloric intake and body composition led to hypertriglyceridemia. CNS NPY signaling via the Y1 receptor predominantly regulates effects on hepatic lipoprotein production, whereas the activation of the Y2 receptor has a greater effect on feeding. Altogether, these findings raise the possibility that NPY regulates feeding and lipoprotein metabolism partially via separate NPY receptor systems and/or mechanisms.
GRANTS
This work was supported by a VA Merit Review Award. J. M. Rojas received a National Research Service Award from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; 1F31-DK-089906). J. M. Stafford was supported by a VA Career Development Award. DRTC was supported by NIDDK Grant DK-020593.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.R., J.M.S., R.L.P., and K.D.N. did the conception and design of the research; J.M.R., J.M.S., and S.S. performed the experiments; J.M.R., J.M.S., R.L.P., and K.D.N. analyzed the data; J.M.R., J.M.S., R.L.P., and K.D.N. interpreted the results of the experiments; J.M.R. prepared the figures; J.M.R. drafted the manuscript; J.M.R., J.M.S., R.L.P., A.G.B.-S., and K.D.N. edited and revised the manuscript; J.M.R., R.L.P., and K.D.N. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Maxine Turney, Leena George, and Christopher Emfinger for excellent technical support. The Vanderbilt Diabetes Research and Training Center (DRTC) Hormone Assay & Analytical Services Core performed the described hormone assays.
REFERENCES
- 1. Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 28: 1225–1236, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Balasubramaniam A, Joshi R, Su C, Friend LA, James JH. Neuropeptide Y (NPY) Y2 receptor-selective agonist inhibits food intake and promotes fat metabolism in mice: combined anorectic effects of Y2 and Y4 receptor-selective agonists. Peptides 28: 235–240, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Balasubramaniam A, Sheriff S, Zhai W, Chance WT. Bis(31/31′){[Cys(31), Nva(34)]NPY(27–36)-NH(2)}: a neuropeptide Y (NPY) Y(5) receptor selective agonist with a latent stimulatory effect on food intake in rats. Peptides 23: 1485–1490, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Baranowska B, Wolińska-Witort E, Martyńska L, Chmielowska M, Mazurczak-Pluta T, Boguradzka A, Baranowska-Bik A. Sibutramine therapy in obese women—effects on plasma neuropeptide Y (NPY), insulin, leptin and beta-endorphin concentrations. Neuro Endocrinol Lett 26: 675–679, 2005 [PubMed] [Google Scholar]
- 5. Baranowska B, Wolinska-Witort E, Wasilewska-Dziubinska E, Roguski K, Martynska L, Chmielowska M. The role of neuropeptides in the disturbed control of appetite and hormone secretion in eating disorders. Neuro Endocrinol Lett 24: 431–434, 2003 [PubMed] [Google Scholar]
- 6. Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab 91: 1446–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bartels ED, Lauritsen M, Nielsen LB. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes 51: 1233–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Beck B, Burlet A, Bazin R, Nicolas JP, Burlet C. Elevated neuropeptide Y in the arcuate nucleus of young obese Zucker rats may contribute to the development of their overeating. J Nutr 123: 1168–1172, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Bernardis LL, Schnatz JD. Localization in the ventromdedial hypothalamic nuclei of an area affecting plasma triglyceride and cholesterol levels. J Neurovisc Relat 32: 90–103, 1971 [DOI] [PubMed] [Google Scholar]
- 11. Blumenthal JB, Andersen RE, Mitchell BD, Seibert MJ, Yang H, Herzog H, Beamer BA, Franckowiak SC, Walston JD. Novel neuropeptide Y1 and Y5 receptor gene variants: associations with serum triglyceride and high-density lipoprotein cholesterol levels. Clin Genet 62: 196–202, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Bruinstroop E, Pei L, Ackermans MT, Foppen E, Borgers AJ, Kwakkel J, Alkemade A, Fliers E, Kalsbeek A. Hypothalamic neuropeptide Y (NPY) controls hepatic VLDL-triglyceride secretion in rats via the sympathetic nervous system. Diabetes 61: 1043–1050, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cabrele C, Langer M, Bader R, Wieland HA, Doods HN, Zerbe O, Beck-Sickinger AG. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J Biol Chem 275: 36043–36048, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Cartwright IJ, Higgins JA. Quantification of apolipoprotein B-48 and B-100 in rat liver endoplasmic reticulum and Golgi fractions. Biochem J 285: 153–159, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chee MJ, Myers MG, Jr, Price CJ, Colmers WF. Neuropeptide Y suppresses anorexigenic output from the ventromedial nucleus of the hypothalamus. J Neurosci 30: 3380–3390, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cleary MP, Vasselli JR, Greenwood MR. Development of obesity in Zucker obese (fafa) rat in absence of hyperphagia. Am J Physiol Endocrinol Metab 238: E284–E292, 1980 [DOI] [PubMed] [Google Scholar]
- 17. Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297: 240–243, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Fetissov SO, Kopp J, Hokfelt T. Distribution of NPY receptors in the hypothalamus. Neuropeptides 38: 175–188, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 277: 17377–17380, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J 31: 642–648, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Ghamari-Langroudi M, Colmers WF, Cone RD. PYY3–36 inhibits the action potential firing activity of POMC neurons of arcuate nucleus through postsynaptic Y2 receptors. Cell Metab 2: 191–199, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 106: 453–458, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 14, Suppl 1: 41S–49S, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA 293: 1868–1874, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269: 543–546, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24: 683–689, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Iyengar S, Li DL, Simmons RM. Characterization of neuropeptide Y-induced feeding in mice: do Y1–Y6 receptor subtypes mediate feeding? J Pharmacol Exp Ther 289: 1031–1040, 1999 [PubMed] [Google Scholar]
- 28. Jang M, Romsos DR. Neuropeptide Y and corticotropin-releasing hormone concentrations within specific hypothalamic regions of lean but not ob/ob mice respond to food-deprivation and refeeding. J Nutr 128: 2520–2525, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Kamiji MM, Inui A. Neuropeptide y receptor selective ligands in the treatment of obesity. Endocr Rev 28: 664–684, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Karakash C, Hustvedt BE, Lovo A, Le Marchand Y, Jeanrenaud B. Consequences of ventromedial hypothalamic lesions on metabolism of perfused rat liver. Am J Physiol Endocrinol Metab Gastrointest Physiol 232: E286–E293, 1977 [DOI] [PubMed] [Google Scholar]
- 31. Kennedy AJ, Ellacott KL, King VL, Hasty AH. Mouse models of the metabolic syndrome. Dis Model Mech 3: 156–166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kissebah AH, Alfarsi S, Adams PW, Wynn V. Role of insulin resistance in adipose tissue and liver in the pathogenesis of endogenous hypertriglyceridaemia in man. Diabetologia 12: 563–571, 1976 [DOI] [PubMed] [Google Scholar]
- 33. Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13: 803–811, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med 13: 171–180, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Lindner D, Stichel J, Beck-Sickinger AG. Molecular recognition of the NPY hormone family by their receptors. Nutrition 24: 907–917, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Marks JL, Waite K. Intracerebroventricular neuropeptide Y acutely influences glucose metabolism and insulin sensitivity in the rat. J Neuroendocrinol 9: 99–103, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med 4: 718–721, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Merten N, Lindner D, Rabe N, Römpler H, Mörl K, Schöneberg T, Beck-Sickinger AG. Receptor subtype-specific docking of Asp6.59 with C-terminal arginine residues in Y receptor ligands. J Biol Chem 282: 7543–7551, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52: 227–231, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Otway S, Robinson DS. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol 190: 321–332, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 13: 18–19, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Picardi PK, Calegari VC, Prada Pde O, Moraes JC, Araújo E, Marcondes MC, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology 149: 3870–3880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296: E1003–E1012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pralong FP, Gonzales C, Voirol MJ, Palmiter RD, Brunner HR, Gaillard RC, Seydoux J, Pedrazzini T. The neuropeptide Y Y1 receptor regulates leptin-mediated control of energy homeostasis and reproductive functions. FASEB J 16: 712–714, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Raposinho PD, Broqua P, Pierroz DD, Hayward A, Dumont Y, Quirion R, Junien JL, Aubert ML. Evidence that the inhibition of luteinizing hormone secretion exerted by central administration of neuropeptide Y (NPY) in the rat is predominantly mediated by the NPY-Y5 receptor subtype. Endocrinology 140: 4046–4055, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Raposinho PD, Pierroz DD, Broqua P, White RB, Pedrazzini T, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol Cell Endocrinol 185: 195–204, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Riches FM, Watts GF, Naoumova RP, Kelly JM, Croft KD, Thompson GR. Hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 studied with a stable isotope technique in men with visceral obesity. Int J Obes Relat Metab Disord 22: 414–423, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Rioux KP, Le T, Swain MG. Decreased orexigenic response to neuropeptide Y in rats with obstructive cholestasis. Am J Physiol Gastrointest Liver Physiol 280: G449–G456, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Rojas JM, Printz RL, Niswender KD. Insulin detemir attenuates food intake, body weight gain and fat mass gain in diet-induced obese Sprague-Dawley rats. Nutr Diab 1: e10, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sainsbury A, Schwarzer C, Couzens M, Herzog H. Y2 receptor deletion attenuates the type 2 diabetic syndrome of ob/ob mice. Diabetes 51: 3420–3427, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev 16: 1077–1088, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanacora G, Kershaw M, Finkelstein JA, White JD. Increased hypothalamic content of preproneuropeptide Y messenger ribonucleic acid in genetically obese Zucker rats and its regulation by food deprivation. Endocrinology 127: 730–737, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Schotz MC, Scanu A, Page IH. Effect of triton on lipoprotein lipase of rat plasma. Am J Physiol 188: 399–402, 1957 [DOI] [PubMed] [Google Scholar]
- 56. Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, Weigle DS. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45: 531–535, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Sipols AJ, Baskin DG, Schwartz MW. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 44: 147–151, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Söll RM, Dinger MC, Lundell I, Larhammer D, Beck-Sickinger AG. Novel analogues of neuropeptide Y with a preference for the Y1-receptor. Eur J Biochem 268: 2828–2837, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Stafford JM, Yu F, Printz R, Hasty AH, Swift LL, Niswender KD. Central nervous system neuropeptide Y signaling modulates VLDL triglyceride secretion. Diabetes 57: 1482–1490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stanić D, Mulder J, Watanabe M, Hökfelt T. Characterization of NPY Y2 receptor protein expression in the mouse brain. II. Coexistence with NPY, the Y1 receptor, and other neurotransmitter-related molecules. J Comp Neurol 519: 1219–1257, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Swift LL. Role of the Golgi apparatus in the phosphorylation of apolipoprotein B. J Biol Chem 271: 31491–31495, 1996 [PubMed] [Google Scholar]
- 62. Tschöp M, Castañeda TR, Joost HG, Thöne-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature 430: 1 p following 165; discussion 162 p following 165, 2004 [DOI] [PubMed] [Google Scholar]
- 63. van den Hoek AM, van Heijningen C, Schroder-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes 57: 2304–2310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van den Hoek AM, Voshol PJ, Karnekamp BN, Buijs RM, Romijn JA, Havekes LM, Pijl H. Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes 53: 2529–2534, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Vettor R, Zarjevski N, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B. Induction and reversibility of an obesity syndrome by intracerebroventricular neuropeptide Y administration to normal rats. Diabetologia 37: 1202–1208, 1994 [DOI] [PubMed] [Google Scholar]
- 66. Warne JP, Alemi F, Reed AS, Varonin JM, Chan H, Piper ML, Mullin ME, Myers MG, Jr, Corvera CU, Xu AW. Impairment of central leptin-mediated PI3K signaling manifested as hepatic steatosis independent of hyperphagia and obesity. Cell Metab 14: 791–803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Wiedmer P, Chaudhary N, Rath M, Yi CX, Ananthakrishnan G, Nogueiras R, Wirth EK, Kirchner H, Schweizer U, Jonas W, Veyrat-Durebex C, Rohner-Jeanrenaud F, Schürmann A, Joost HG, Tschöp MH, Perez-Tilve D. The HPA axis modulates the CNS melanocortin control of liver triacylglyceride metabolism. Physiol Behav 105: 791–799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilding JP, Gilbey SG, Mannan M, Aslam N, Ghatei MA, Bloom SR. Increased neuropeptide Y content in individual hypothalamic nuclei, but not neuropeptide Y mRNA, in diet-induced obesity in rats. J Endocrinol 132: 299–304, 1992 [DOI] [PubMed] [Google Scholar]
- 69. Wyss P, Stricker-Krongrad A, Brunner L, Miller J, Crossthwaite A, Whitebread S, Criscione L. The pharmacology of neuropeptide Y (NPY) receptor-mediated feeding in rats characterizes better Y5 than Y1, but not Y2 or Y4 subtypes. Regul Pept 75–76: 363–371, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Yue JT, Mighiu PI, Naples M, Adeli K, Lam TK. Glycine normalizes hepatic triglyceride-rich VLDL secretion by triggering the CNS in high-fat fed rats. Circ Res 110: 1345–1354, 2012 [DOI] [PubMed] [Google Scholar]
- 71. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994 [DOI] [PubMed] [Google Scholar]