Abstract
Vascular access dysfunction causes morbidity in hemodialysis patients. This study examined the generation and pathobiological significance of superoxide anion in a rat femoral arteriovenous fistula (AVF). One week after AVF creation, there was increased production of superoxide anion accompanied by decreased total superoxide dismutase (SOD) and Cu/Zn SOD activities and induction of the redox-sensitive gene heme oxygenase-1. Immunohistochemical studies of nitrotyrosine formation demonstrated that peroxynitrite, a product of superoxide anion and nitric oxide, was present in increased amounts in endothelial and smooth muscle cells in the AVF. Because uncoupled NOS isoforms generate superoxide anion, and NOS coupling requires tetrahydrobiopterin (BH4) as a cofactor, we assessed NOS uncoupling by determining the ratio of BH4 to dihydrobiopterin (BH2); the BH4-to-BH2 ratio was markedly attenuated in the AVF. Because Src is a vasculopathic signaling species upstream and downstream of superoxide anion, such expression was evaluated; expression of Src and phosphorylated Src was both markedly increased in the AVF. Expression of NADPH oxidase (NOX) 1, NOX2, NOX4, cyclooxygenase (COX) 1, COX2, p47phox, and p67phox was all unchanged, as assessed by Western analyses, thereby suggesting that these proteins may not be involved in increased production of superoxide anion. Finally, administration of tempol, a superoxide anion scavenger, decreased neointima formation in the juxta-anastomotic venous segment and improved AVF blood flow. We conclude that the AVF exhibits increased superoxide anion generation that may reflect the combined effects of decreased scavenging by SOD and increased generation by uncoupled NOS, and that enhanced superoxide anion production promotes juxta-anastomotic stenosis and impairs AVF function.
Keywords: oxidant stress, nitric oxide synthase, tetrahydrobiopterin, vascular access dysfunction, hemodialysis
effective chronic hemodialysis requires a functional vascular access, and of the available modalities, the surgically fashioned native arteriovenous fistula (AVF) is widely regarded as preferable to the arteriovenous graft or the tunneled central venous catheter (1, 3, 12, 18, 31, 32). However, even for the AVF, the functional outcome is poor: ∼50% of AVFs never adapt sufficiently such that they can support hemodialysis and, of the AVFs that adequately develop into functional vascular accesses, attrition of function subsequently occurs in substantial numbers. Such AVF failure and dysfunction contribute substantially to the hospitalization of hemodialysis patients and their morbidity and mortality and, along with dysfunction of other vascular accesses, incur over a billion dollars annually in health care costs; understanding the pathobiological basis for vascular access dysfunction is thus a pressing issue in the care of patients on maintenance hemodialysis. Either for primary or secondary AVF failure, substantial evidence indicates that neointimal hyperplasia at the juxta-anastamotic venous segment is one of the major underlying pathobiological processes (1, 3, 12, 18, 31, 32).
We recently described a femoral AVF model in the rat that recapitulates the salient features of the human hemodialysis AVF, including marked increases in AVF blood flow, the development of neointimal hyperplasia in the juxta-anastomotic veins, and the upregulation of vasculopathic genes (5). To explore the pathogenesis of these changes, the present study drew upon the finding observed in models of vascular injury that increased generation of superoxide anion can instigate vasculopathic effects (10). The present study thus examined the generation and pathobiological significance of superoxide anion in the juxta-anastamotic venous segment in this femoral AVF model.
MATERIALS AND METHODS
The femoral AVF in the rat.
All studies were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic and performed in accordance with National Institutes of Health guidelines. The surgical creation of a peripheral AVF for these studies was performed in Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225–275 grams using an end-to-side anastomosis of the femoral artery to the femoral vein, as described for our previous study (5). At time points between 1 and 8 wk after the placement of the AVF, rats were killed, and femoral veins were harvested for histological, immunohistochemical, and biochemical analyses. Assessment of blood flow through the arterial limb of the AVF and in the femoral artery of sham-operated rats was performed using a perivascular flow probe (Transonic Systems, Ithaca, NY) as described previously (5). In some studies, the effect of scavenging of superoxide anion on AVF function and histology was investigated in rats treated with a low (1 mmol/l) (28, 29) or high (6 mmol/l) (9, 33) dose of tempol (Sigma, St. Louis, MO) in the drinking water for up to 8 wk.
Superoxide anion production.
Intracellular superoxide anion levels in venous segments were quantified using an assay that employs dihydroethidium as a probe and HPLC/fluorescence analysis (Beckman Coulter, Brea, CA), as previously described for our studies (7, 25). Data were quantified using a 2-hydroxyethidium standard from the reaction between dihydroethidium and Fremy's salt (36) and normalized against tissue protein levels.
Tetrahydrobiopterin/dihydrobiopterin levels.
Biopterin levels were determined in the venous limb of the AVF and in sham-operated femoral veins employing a RP-HPLC method described in our prior studies. Tetrahydrobiopterin (BH4) levels were calculated as the difference in biopterin after differential oxidation in acid [which converts both BH4 and 7,8-dihydrobiopterin (BH2) to biopterin] and base (which converts only 7,8-BH2 to biopterin) conditions (6, 8).
Superoxide dismutase, catalase, and glutathione peroxidase activities.
Total and Cu/Zn superoxide dismutase (SOD) activities were determined in venous lysates using an assay kit (catalog no. 706002; Cayman Chemical, Ann Arbor, MI) and were normalized for tissue protein content. Cu/Zn SOD activity was calculated as activity that was inhibited by potassium cyanide (125 μM). Catalase and glutathione peroxidase activities were determined in venous lysates as previously described (27).
Heme oxygenase-1 mRNA expression by quantitative real-time reverse transcription-PCR.
Heme oxygenase-1 (HO-1) mRNA expression was determined as in our previous studies (5). cDNA was synthesized by reverse transcription (Transcriptor First Strand cDNA Synthesis Kit; Roche Applied Science, Indianapolis, IN) from total RNA extracted from rat venous tissue using the TRIzol method (Invitrogen, Carlsbad, CA) with purification with an RNeasy Mini Kit (Qiagen, Valencia, CA). Quantitative real-time PCR analysis for HO-1 mRNA and 18S rRNA was performed employing TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) for each target. HO-1 protein expression was also assessed by immunohistochemical staining as described below.
Western analysis.
Western analyses were performed as described in our previous studies (17, 26). Primary antibodies employed for these analyses include the following: cyclooxygenase (COX) 1, COX2, p67phox, Src, and phosphorylated (p)-Src (catalog nos. 4841, 4892, 3923, 2108, and 2113, respectively; Cell Signaling Technologies, Danvers, MA); NADPH oxidase (NOX) 1 (catalog no. AV46818; Sigma); NOX2 and NOX4 (catalog nos. ab80508 and ab41886; Abcam, Cambridge, MA); and p47phox (catalog no. 45-096; ProSci, Poway, CA).
Immunohistochemical staining.
Immunohistochemical staining for HO-1 and nitrotyrosine was performed on formalin-fixed, paraffin-embedded venous sections using a method described in our previous study (16). Following antigen retrieval (10 mmol/l citrate, pH 6.0, 98°C, 20 min), rabbit polyclonal primary antibodies for HO-1 (catalog no. ADI-SPA-895; Enzo Life Sciences, Farmingdale, NY) and nitrotyrosine (catalog no. 06-284; EMD Millipore, Billerica, MA) were employed along with a secondary antibody conjugated to a horseradish peroxidase-labeled polymer (EnVision+, HRP, rabbit, catalog no. K4003; Dako, Carpinteria, CA). Visualization was achieved using diaminobenzidine (DAB+, catalog no. K3468; Dako) as a chromogen and utilizing hematoxylin for counterstaining.
Histological evaluation of the AVF.
In addition to qualitative assessment of the venous segment of the AVF (J. P. Grande), a semiquantitative analysis was performed to determine the extent of neointimal hyperplasia and thrombus formation in the AVF (J. P. Grande). Hematoxylin- and eosin-stained sections, cut at 250, 500, 750, and 1,000 μm from the most proximal point of the arteriovenous anastomosis, were evaluated to determine whether neointimal hyperplasia and thrombus formation occupied >75% of venous vessel cross-sectional area on at least one of the four levels from the anastomosis.
Statistics.
Data are expressed as means ± SE. The Student's t-test for parametric data and the Mann-Whitney test for nonparametric data were used for comparisons between groups. AVF patency was assessed using Fisher's exact test. Results were considered significant for P < 0.05.
RESULTS
Superoxide anion generation in the AVF.
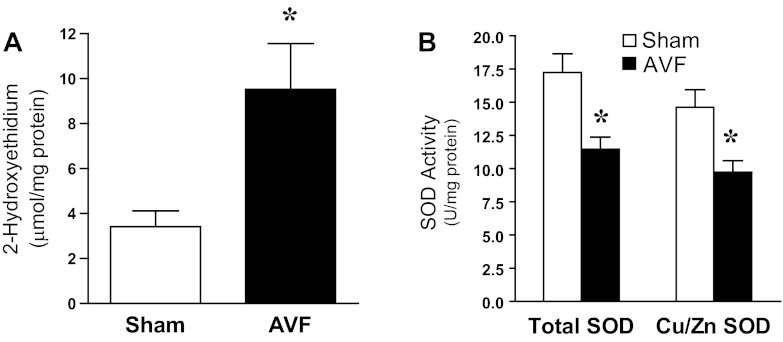
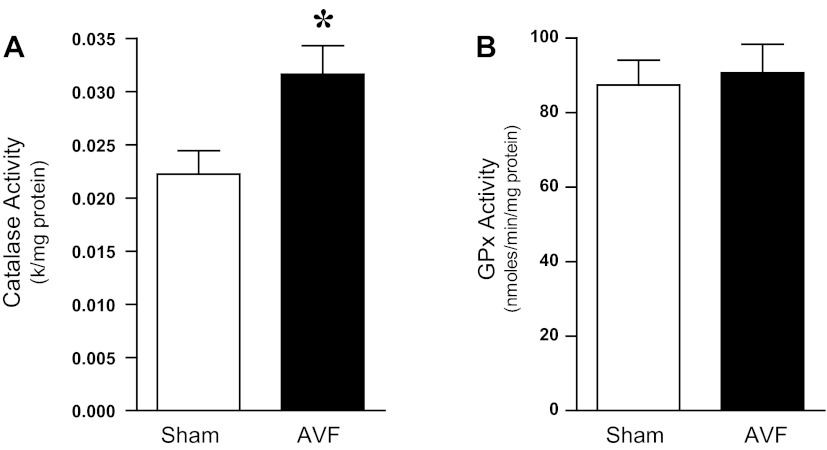
One week after the creation of the femoral AVF, generation of superoxide anion was significantly increased in the juxta-anastomotic venous segment in the AVF compared with such production in veins from the sham-operated group (Fig. 1A). To determine whether such increased generation of superoxide anion may reflect impaired superoxide anion scavenging activity, measurements of total SOD and Cu/Zn SOD activity were undertaken in the venous segment. As shown in Fig. 1B, both activities were significantly decreased in the AVF compared with the venous segment in the sham-operated group, thereby raising the possibility that decreased scavenging of superoxide anion may underlie the observed increased generation of this oxidant. The relative specificity of these changes in SOD was underscored by the fact that other oxidant (hydrogen peroxide)-scavenging enzyme activity was either significantly increased, as occurs for catalase, or unchanged, as occurs for glutathione peroxidase (Fig. 2).
Fig. 1.
Superoxide anion production and superoxide dismutase (SOD) activity in the venous limb of the rat arteriovenous fistula (AVF) and in the femoral vein of sham-operated (sham) rats 1 wk after AVF creation. A: superoxide anion production as determined by 2-hydroxyethidium in the venous limb of the rat AVF and in sham femoral veins. *P < 0.05 vs. sham; n = 9 and 11 rats for sham and AVF groups, respectively. B: total SOD activity and Cu/Zn SOD activity measured in the venous limb of the rat AVF and in sham femoral veins. *P < 0.01 vs. sham; n = 10 in each group.
Fig. 2.
Hydrogen peroxide-scavenging enzyme activity in the venous limb of the rat AVF and in the femoral vein of sham rats. Catalase (A) and glutathione peroxidase (GPx, B) activities were assessed in venous lysates 1 wk after AVF creation. *P < 0.01 vs. sham; n = 10 in each group.
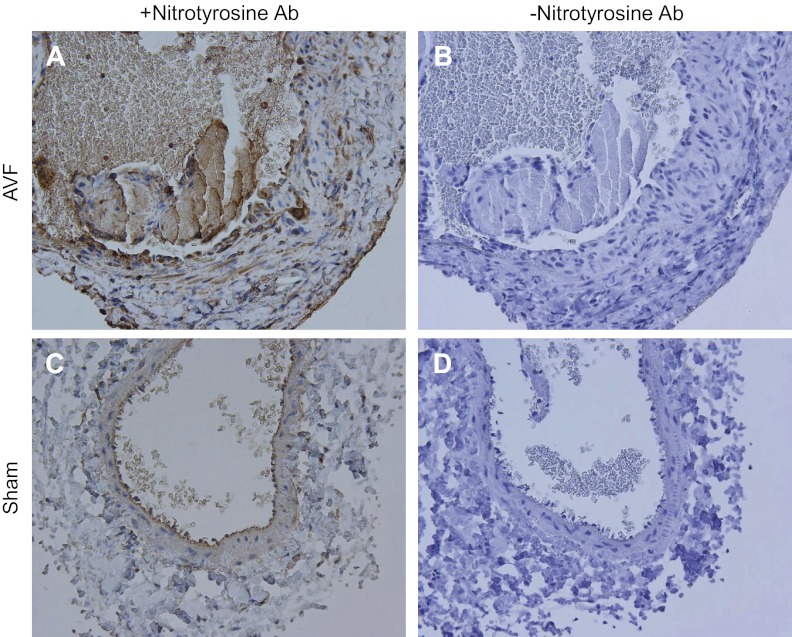
Studies were then undertaken of the distal effects of superoxide anion, including, first, its interaction with nitric oxide to form peroxynitrite and, second, its capacity to upregulate redox-sensitive genes by virtue of its oxidizing effects. Peroxynitrite is a reactive nitrogen species with established vasculopathic effects. We thus assessed production of peroxynitrite by immunohistochemical analyses of nitrotyrosine formation in the AVF. As shown in Fig. 3, there was increased expression of nitrotyrosine in the endothelium, smooth muscle cells, adventitia, and in infiltrating leukocytes in the AVF; weaker staining was restricted to the endothelial region of the venous segment in the sham-operated group, which may possibly reflect nonspecific staining of the elastic lamina.
Fig. 3.
Immunohistochemical (IHC) localization of nitrotyrosine expression in the venous limb of the rat AVF. IHC staining for nitrotyrosine (+Nitrotyrosine Ab) in the venous limb of the AVF (A) and in the sham femoral vein (C) was performed 2 wk after AVF creation. Incubations with nonimmune rabbit IgG in the AVF and sham vein (−Nitrotyrosine Ab, B and D, respectively) displayed no such staining. Specific nitrotyrosine staining, localizing to the endothelium, infiltrating cells, smooth muscle cells, and adventitia, is present in the AVF. In contrast, limited nitrotyrosine staining in the sham vein is confined to the endothelial region and may reflect nonspecific staining of the elastic lamina.
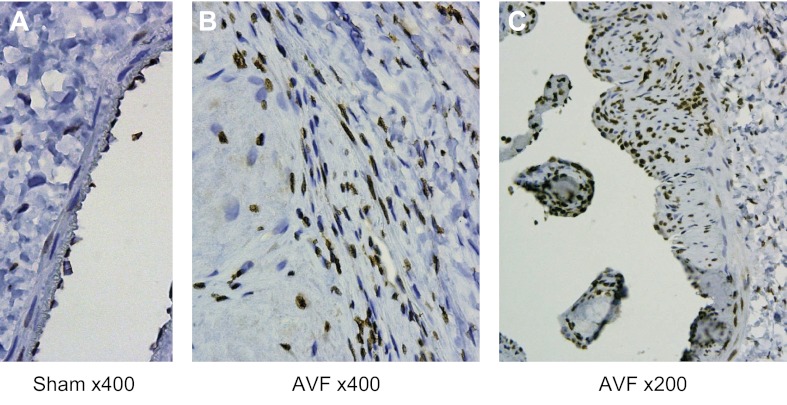
HO-1, a redox-sensitive gene, was markedly induced (mRNA by RT-PCR: 1.1 ± 0.2 vs. 8.2 ± 1.8 standardized units, n = 8 in each group, P < 0.01) concomitantly with increased superoxide anion generation at 1 wk; these findings corroborate the occurrence of oxidative stress in the venous segment at this time point. Immunohistochemical analyses undertaken at a later time point (when neointimal hyperplasia is substantially developed in this AVF model) localized such expression to the endothelial cells, smooth muscle cells, and infiltrating leukocytes (Fig. 4).
Fig. 4.
IHC localization of heme oxygenase-1 (HO-1) expression in the venous limb of the rat AVF. IHC staining for HO-1 in the venous limb of the AVF [×400 (B) and ×200 (C)] and in the sham femoral vein [×400 (A)] was performed 4 wk after AVF creation. In the AVF, staining for HO-1 localizes to endothelial and smooth muscle cells, as well as infiltrating leukocytes while, in the sham vein, endothelial cell staining is present.
BH4/BH2 content in the AVF.
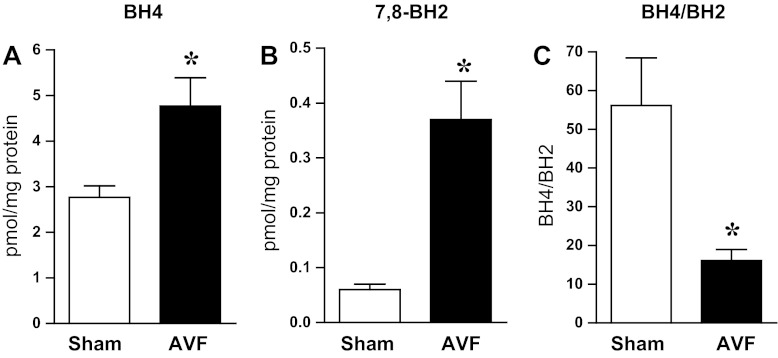
In the injured vasculature, increased generation of superoxide anion may originate from the uncoupling of NOS isoforms. Effective coupling of NOS isoforms requires adequate amounts of BH4, and the extent to which the BH4-to-BH2 ratio is diminished reflects the propensity toward uncoupling of NOS (19). We thus measured BH4 and BH2 and calculated the BH4/BH2 ratio. Although both BH4 and BH2 were increased in the AVF, the increment in BH2 greatly outstripped that which occurred in BH4 and, as demonstrated in Fig. 5, the BH4/BH2 ratio was markedly diminished in the juxta-anastomotic venous segment in the AVF.
Fig. 5.
Tetrahydrobiopterin (BH4) and 7,8-dihydrobiopterin (BH2) content and BH4-to-BH2 ratio in the venous limb of the rat AVF and in the femoral vein of sham rats. BH4 and BH2 content and the BH4/BH2 ratio were assessed in the venous limb of the rat AVF and in sham femoral veins 1 wk after AVF creation. *P < 0.01 vs. sham; n = 9–10 in each group.
Redox-related signaling in the AVF.
In addition to the uncoupling of NOS isoforms, we assessed other potential sources for increased generation of superoxide anion. As assessed by Western analyses, expression of NOX1, NOX2, NOX4, COX1, COX2, p47phox, and p67phox was all unchanged, thereby suggesting that these proteins may not be involved in increased production of superoxide anion (data not shown). Pronounced upregulation of Src, however, was observed. Src-dependent signaling promotes vascular generation of superoxide anion, which, in turn, can activate Src (21). We thus evaluated Src, as well as p-Src, the active moiety. As demonstrated in Fig. 6, there was marked upregulation of Src (1.5 ± 0.2 vs. 5.2 ± 0.8 standardized units, n = 8 and 7, respectively, P < 0.05) as well as p-Src (0.6 ± 0.2 vs. 3.6 ± 1.3 standardized units, n = 8 and 7, respectively, P < 0.05), thereby indicating that a vasculopathic signaling species that is upstream and downstream of superoxide anion is induced in the AVF.
Fig. 6.
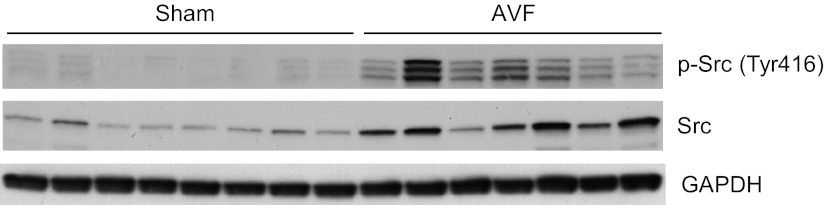
Expression of Src and phosphorylated (p)-Src in the venous limb of the rat AVF and in the femoral vein of sham rats. Western analysis of Src and p-Src protein expression in the venous limb of the AVF and in sham femoral veins 1 wk after AVF creation. Each lane represents a venous sample obtained from one rat in that group. Equivalency of loading was assessed by immunoblotting for GAPDH, the latter used to normalize densitometric assessments of Src and p-Src expression.
Pathobiological significance of increased superoxide anion in the AVF.
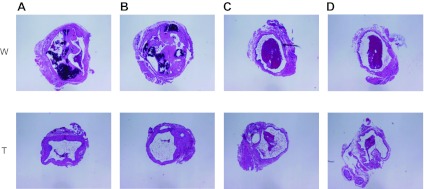
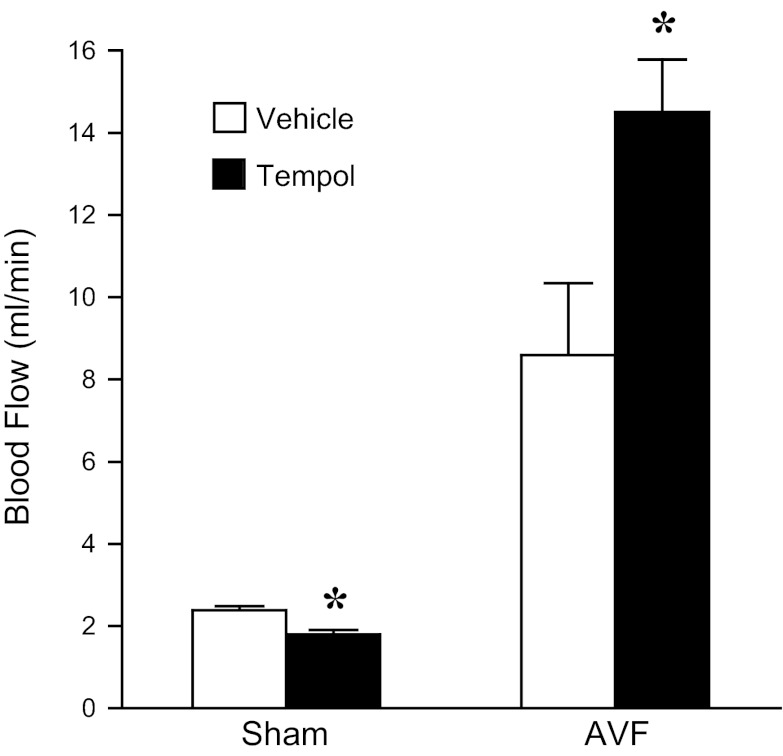
To determine the pathophysiological significance of increased generation of superoxide anion in the AVF, we examined the effect of tempol, an effective scavenger of superoxide anion. Administration of low-dose tempol reduced histological injury, as indicated by qualitative assessment of the extent of neointimal hyperplasia and vascular calcification in the venous segment of the AVF. Semiquantitative analyses involving four sections at 250-μm intervals from the anastomotic site (Fig. 7) revealed that tempol markedly reduced the percentage of AVFs exhibiting >75% loss of patency in at least one of the four sections (80 vs. 33%, n = 10 and 12, respectively, P < 0.05). At this dose of tempol, the reduction in histological injury was not accompanied by a significant improvement in AVF blood flow (data not shown). However, when high-dose tempol was administered, blood flow through the AVF was significantly improved (Fig. 8).
Fig. 7.
Effect of low-dose tempol on the histology of the venous limb of the rat AVF. Shown are representative histological sections of the venous segment of the AVF in water-treated (W) and tempol-treated (T, 1 mM in drinking water) rats, which were taken at 250 (A), 500 (B), 750 (C), and 1,000 (D) μm from the AVF anastomosis; these studies were performed 5 wk after AVF creation. The AVF vein, at this time point, exhibits neointima formation with papillary projections into the lumen, leukocytic infiltration within the hyperplastic neointima, the presence of luminal thrombi, and areas of calcifications.
Fig. 8.
Effect of high-dose tempol on blood flow in the rat AVF. In rats drinking water with tempol (6 mM) or standard drinking water (vehicle), blood flow was assessed in the arterial limb of the AVF or femoral artery of sham rats using a perivascular flow probe and undertaken 8 wk after creation of the AVF. *P < 0.05 vs. vehicle-treated rats with similar surgical treatment.
DISCUSSION
Our present findings demonstrate increased generation of superoxide anion in the juxta-anastomotic veins in the AVF. Such augmented generation of superoxide anion may reflect the combined effects of impaired scavenging in conjunction with increased production. Evidence of the former is provided by substantially decreased total SOD, as well as Cu/Zn SOD activities. The relative specificity of this decrease in SOD activity was reflected by the fact that the oxidant-scavenging activities for the oxidant downstream of superoxide anion, hydrogen peroxide, were either unchanged (glutathione peroxidase) or increased (catalase).
In health, NOS isoforms are coupled such that nitric oxide is generated; such coupling necessitates adequate amounts of BH4 relative to the amount of NOS proteins that are present (19). The uncoupling of NOS leads to the generation of superoxide anion rather than nitric oxide. As the BH4/BH2 ratio is progressively diminished, the propensity for the uncoupling of NOS increases. Our data demonstrate a striking diminution in the BH4/BH2 ratio, thereby raising the possibility that uncoupling of NOS isoforms contributes to increased generation of superoxide anion. This reduction in the BH4/BH2 ratio assumes greater significance with regard to the uncoupling of NOS in light of our prior observations demonstrating marked induction of endothelial NOS and inducible NOS proteins in the venous segment of the AVF (5): increased amounts of NOS proteins in the AVF, as observed previously, may be expected to exaggerate the uncoupling effect incurred by the markedly diminished BH4/BH2 ratio, as observed in the present study.
The fold increment in the BH2 content in the venous segment of the AVF markedly exceeded the fold increment in the BH4 content, thereby leading to a substantially diminished BH4/BH2 ratio. Such lowering in the BH4/BH2 ratio may also reflect, at least in part, the oxidation of BH4 to BH2 by ambient oxidative stress. This premise is based on the original observation by Milstein and Katusic that peroxynitrite effectively oxidizes BH4 (24), an observation that has now been abundantly confirmed. In this regard, oxidant-mediated conversion of BH4 to BH2 may provide a positive feedback loop for continued oxidant generation in the AVF along the following lines: decreased SOD activity in the AVF, as demonstrated by our findings, impairs the scavenging of superoxide anion, thereby increasing vascular content of superoxide anion; increased superoxide anion promotes increased amounts of peroxynitrite in the AVF, the latter confirmed by our immunohistochemical studies; such increased peroxynitrite content oxidizes BH4, thereby fostering the uncoupling of NOS; in turn, uncoupled NOS generates superoxide anion and fuels an ongoing cycle of oxidant generation.
We suggest that a bidirectional relationship exists between NOS uncoupling, on the one hand, and endothelial injury and dysfunction, on the other. Uncoupling of NOS impairs the generation of NO, and such NO deficiency promotes endothelial dysfunction and injury. Additionally, endothelial injury occurring in the venous limb of the AVF, induced by any one of a number of pathways [for example, hemodynamic stress, inflammation, etc. (5, 31)], may alter the biochemical machinery that determines vascular biopterin content, specifically, levels of BH4 and BH2. Such alterations may lead to a diminished BH4/BH2 ratio, thereby predisposing to the uncoupling of NOS. We thus posit that a bidirectional relationship between NOS uncoupling and endothelial injury/dysfunction, hinged on the BH4/BH2 ratio, is an important consideration in the pathobiology of the AVF.
Increased generation of superoxide anion may also reflect Src-dependent mechanisms. Src was markedly induced in the venous segment of the AVF and, in other settings, Src is known to mediate increased vascular production of superoxide anion (21). Interestingly, Src is downstream as well as upstream of superoxide anion, since oxidants promote the phosphorylation, and thus the activation, of Src (21). Activation of Src by oxidants is significant in that the Src signaling instigates endothelial and inflammatory processes relevant to neointimal hyperplasia (14, 20, 30). Based on these considerations, we speculate that the observed activation of Src in the AVF may be germane to the augmented generation of superoxide anion and its attendant vasculopathic effects in the AVF. Of note, not only was Src activated, as indicated by increased expression of p-Src, but expression of total Src was induced in the AVF. While the basis for induction of Src was not examined in our study, we speculate that processes that are present in the venous limb of the AVF, for example, hemodynamic stress, inflammation, and oxidant stress, may be germane to the Src induction we observed in the AVF.
In our studies, there was increased expression of nitrotyrosine in the endothelium, smooth muscle cells, adventitia, and in infiltrating leukocytes in the AVF. Because these cellular sites all possess the capability of generating superoxide anion as well as nitric oxide, it is possible that such increased expression of nitrotyrosine may represent cell-specific, autocrine production of nitrotyrosine. However, it is possible that a paracrine mechanism for such production may also occur since superoxide anion and/or nitric oxide, diffusing from cells where they are generated, may promote the production of nitrotyrosine in neighboring and distant cells.
To determine the functional significance of such generation of superoxide anion, we examined the effect of tempol, an effective scavenger of superoxide anion. The low-dose tempol regimen reduced neointimal hyperplasia and calcification, which, on semiquantitative analysis, led to greater patency of the venous lumen of the AVF. Such amelioration in histological injury was accompanied by improved function, as reflected by augmentation in AVF blood flow on the high-dose tempol regimen. These beneficial effects of tempol on AVF histological venous injury and AVF blood flow lead us to conclude that the augmented generation of superoxide anion observed in the AVF promotes neointimal hyperplasia and related lesions that impair patency and, ultimately, blood flow through the AVF.
Our findings are relevant to several aspects of the growing literature on the significance of the redox status of veins in health and disease. First, evidence of oxidative stress exists in human AVFs (34). Second, diseased veins (such as varicose veins) exhibit increased generation of superoxide anion (11). Third, saphenous veins cultured ex vivo exhibit increased superoxide anion production and intimal hyperplasia, both of which are blocked by antioxidant strategies (15). Fourth, the vein interposition graft model in vivo exhibits increased superoxide anion and intimal hyperplasia, and pharmacological agents that reduce intimal hyperplasia also reduce superoxide anion production (13, 22, 35); in this model, superoxide anion and venous hyperplasia are both attenuated in mice with endothelial-specific, GTP cyclohydrolase 1 overexpression and accompanying increased vascular content of BH4 (2). Finally, statin therapy, which may protect saphenous vein grafts from atherosclerosis after coronary bypass grafting, effectively inhibits superoxide anion generation in cultured saphenous veins (4).
A final consideration is the marked induction of HO-1 we observed in the juxta-anastomotic venous segment of the AVF. We have previously demonstrated that HO-1 is induced in the murine AVF model and that HO-1−/− mice exhibit premature failure of the AVF (17). Additionally, the functional outcomes of AVFs are worse in patients with polymorphisms in the HO-1 gene that lead to decreased HO activity (23). The present findings demonstrating that induction of HO-1 occurs in the AVF in rats complement our prior observations demonstrating such induction in the murine AVF model; based on the prior findings in mice and humans (17, 23), we suggest that this upregulation of HO-1 may be an adaptive response initiated by oxidative stress in the AVF.
In summary, the present study is the first analysis in an AVF model to demonstrate increased generation of superoxide anion, to elucidate the biochemical processes that underlie such heightened generation, and to determine the pathobiological significance of generation of this oxidant. In the course of these studies, we uncover marked upregulation of Src in the AVF. We suggest that our findings open up novel therapeutic strategies for the prevention of hemodialysis AVF dysfunction and failure that involve the scavenging of superoxide anion. Additionally, administration of BH4 or agents that promote the synthesis of BH4 is under current study as a therapy for vascular diseases (19), and such a therapeutic approach may be considered as a strategy in the prevention and/or treatment of AVF dysfunction. Finally, specific pharmacological strategies are emerging for the inhibition of Src signaling, and AVF dysfunction may also be considered as a potential therapeutic target for such agents.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-70124 and DK-47060.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Conception and design of research: MVT, ZSK, KAN. Performed experiments: MVT, LVD, AJC, MCH, AWA. Analyzed data: MVT, LVD, JPG, AJC, MCH, AWA, ZSK, KAN. Interpreted results of experiments: MVT, LVD, JPG, AJC, MCH, AWA, ZSK, KAN. Prepared figures: MVT, LVD, AJC. Drafted manuscript: MVT, AJC, KAN. Edited and revised manuscript: KAN, ZSK. Approved final version of manuscript: MVT, LVD, JPG, AJC, MCH, AWA, ZSK, KAN.
ACKNOWLEGMENTS
We gratefully acknowledge the secretarial expertise of Tammy Engel in preparation of the manuscript.
REFERENCES
- 1. Agarwal A, Segal MS. Intimal exuberance: veins in jeopardy. Am J Pathol 162: 1759–1761, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali ZA, Bursill CA, Douglas G, McNeill E, Papaspyridonos M, Tatham AL, Bendall JK, Akhtar AM, Alp NJ, Greaves DR, Channon KM. CCR2-mediated antiinflammatory effects of endothelial tetrahydrobiopterin inhibit vascular injury-induced accelerated atherosclerosis. Circulation 118: S71–S77, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Allon M. Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C, Marinou K, Sfyras N, Economopoulos G, Casadei B, Channon KM, Stefanadis C. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation 122: S66–S73, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Croatt AJ, Grande JP, Hernandez MC, Ackerman AW, Katusic ZS, Nath KA. Characterization of a model of an arteriovenous fistula in the rat: the effect of l-NAME. Am J Pathol 176: 2530–2541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. d'Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ Res 92: 88–95, 2003 [DOI] [PubMed] [Google Scholar]
- 7. d'Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol 301: H2227–H2234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. d'Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase. Hypertension 55: 998–1004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fortepiani LA, Zhang H, Racusen L, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension 41: 640–645, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15: 1583–1606, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guzik B, Chwala M, Matusik P, Ludew D, Skiba D, Wilk G, Mrowiecki W, Batko B, Cencora A, Kapelak B, Sadowski J, Korbut R, Guzik TJ. Mechanisms of increased vascular superoxide production in human varicose veins. Pol Arch Med Wewn 121: 279–286, 2011 [PMC free article] [PubMed] [Google Scholar]
- 12. Hakim R, Himmelfarb J. Hemodialysis access failure: a call to action. Kidney Int 54: 1029–1040, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Hattori K, Yamanouchi D, Banno H, Kobayashi M, Yamamoto K, Kajikuri J, Itoh T, Komori K. Celiprolol reduces the intimal thickening of autogenous vein grafts via an enhancement of nitric oxide function through an inhibition of superoxide production. J Vasc Surg 46: 116–123, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hu G, Minshall RD. Regulation of transendothelial permeability by Src kinase. Microvasc Res 77: 21–25, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Joddar B, Reen RK, Firstenberg MS, Varadharaj S, McCord JM, Zweier JL, Gooch KJ. Protandim attenuates intimal hyperplasia in human saphenous veins cultured ex vivo via a catalase-dependent pathway. Free Radic Biol Med 50: 700–709, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Juncos JP, Grande JP, Kang L, Ackerman AW, Croatt AJ, Katusic ZS, Nath KA. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol 22: 43–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanwar YS. Functional duality of progenitor cells influxing into arteriovenous fistula during its neoangiogenesis. Am J Physiol Renal Physiol 293: F468–F469, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 30: 48–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim MP, Park SI, Kopetz S, Gallick GE. Src family kinases as mediators of endothelial permeability: effects on inflammation and metastasis. Cell Tissue Res 335: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knock GA, Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal 15: 1531–1547, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Kodama A, Komori K, Hattori K, Yamanouchi D, Kajikuri J, Itoh T. Sarpogrelate hydrochloride reduced intimal hyperplasia in experimental rabbit vein graft. J Vasc Surg 49: 1272–1281, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Lin CC, Yang WC, Lin SJ, Chen TW, Lee WS, Chang CF, Lee PC, Lee SD, Su TS, Fann CS, Chung MY. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int 69: 165–172, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263: 681–684, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Nath KA, d'Uscio LV, Juncos JP, Croatt AJ, Manriquez MC, Pittock ST, Katusic ZS. An analysis of the DOCA-salt model of hypertension in HO-1−/− mice and the Gunn rat. Am J Physiol Heart Circ Physiol 293: H333–H342, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Nath KA, Grande JP, Kang L, Juncos JP, Ackerman AW, Croatt AJ, Katusic ZS. Beta-Catenin is markedly induced in a murine model of an arteriovenous fistula: the effect of metalloproteinase inhibition. Am J Physiol Renal Physiol 299: F1270–F1277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nath KA, Salahudeen AK. Induction of renal growth and injury in the intact rat kidney by dietary deficiency of antioxidants. J Clin Invest 86: 1179–1192, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phillips SA, Pechman KR, Leonard EC, Friedrich JL, Bian JT, Beal AG, Basile DP. Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries. Am J Physiol Regul Integr Comp Physiol 298: R1682–R1691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pires PW, Deutsch C, McClain JL, Rogers CT, Dorrance AM. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc Res 80: 445–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reddy MA, Sahar S, Villeneuve LM, Lanting L, Natarajan R. Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 29: 387–393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Schinstock C, Albright R, Williams A, Dillon J, Bergstralh E, Jenson B, McCarthy J, Nath KA. Outcomes of Arteriovenous Fistula Creation after the Fistula First Initiative. Clin J Am Soc Nephrol 6: 1996–2002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension 49: 355–364, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 37: 970–980, 2001 [DOI] [PubMed] [Google Scholar]
- 35. West N, Guzik T, Black E, Channon K. Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia: role of NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 21: 189–194, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Zielonka J, Zhao H, Xu Y, Kalyanaraman B. Mechanistic similarities between oxidation of hydroethidine by Fremy's salt and superoxide: stopped-flow optical and EPR studies. Free Radic Biol Med 39: 853–863, 2005 [DOI] [PubMed] [Google Scholar]