Abstract
The activity of the renal thiazide-sensitive NaCl cotransporter (NCC) in the distal convoluted tubule plays a key role in defining arterial blood pressure levels. Increased or decreased activity of the NCC is associated with arterial hypertension or hypotension, respectively. Thus it is of major interest to understand the activity of NCC using in vivo models. Phosphorylation of certain residues of the amino-terminal domain of NCC has been shown to be associated with its activation. The development of phospho-specific antibodies against these sites provides a powerful tool that is helping to increase our understanding of the molecular physiology of NCC. Additionally, NCC expression in the plasma membrane is modulated by ubiquitylation, which represents another major mechanism for regulating protein activity. This work presents a review of our current knowledge of the regulation of NCC activity by phosphorylation and ubiquitylation.
Keywords: WNK4, distal tubule, ion transport, hypertension, diuretics
the renal thiazide-sensitive NaCl cotransporter (NCC) is the major salt transport and limiting step for salt reabsorption in the distal convoluted tubule (DCT) of mammalian kidneys. The role of NCC in the regulation of arterial blood pressure and in the renal ability for potassium, calcium, and proton excretion has been firmly established by the clinical effects of reduced or augmented activity of the cotransporter. Inactivating mutations in the SLC12A3 gene encoding NCC results in Gitelman's disease, in which patients exhibit hypokalemic metabolic alkalosis, arterial hypotension, and hypocalciuria. In contrast, an increased activity of NCC, as a result of mutations in the with no lysine kinases WNK1 or WNK4 genes, produces the mirror image condition: hyperkalemic metabolic acidosis, accompanied by arterial hypertension, and hypercalciuria. This condition is known as Gordon syndrome, pseudohypoaldosteronism type II (PHAII), or familial hyperkalemic hypertension (FHHt) (27). The thiazide-type diuretics that specifically inhibit NCC have been used for years and are recommended as the first-line pharmacological therapy for arterial hypertension (16). Finally, in an open population, rare inactivating mutations in one allele of NCC, which reduce the activity of the cotransporter (3), are associated with reduced blood pressure, lower risk for arterial hypertension, and no cardiovascular mortality (3, 40). Thus the activity of NCC plays a fundamental role in cardiovascular physiology and pathophysiology.
The study of NCC was practically impossible for years because of the lack of a native cell model purified from the DCT that exhibited thiazide-sensitive Na+ transport activity. The first tool for studying NCC using in vivo models was developed by Fanestill and coworkers (7) at the end of the 1980s: binding of the tracer [3H]metolazone to crude plasma membranes extracted from rat or mouse renal cortex was assessed. The authors demonstrated that [3H]metolazone binds to both a low- and a high-affinity site in the renal cortex. The high-affinity site showed several binding characteristics, demonstrating that the tracer was actually binding to NCC, then called the thiazide receptor. These characteristics included the absence of high-affinity sites in any other tissue, including the renal medulla; displacement of the tracer by different thiazide-type diuretics, but not by any other tested drug, with a similar potency to thiazide in clinical studies; and the localization of these sites only to the DCT as confirmed using autoradiography (22). A few years later, however, better tools were developed to study NCC in both in vitro and in vivo models. First, expression cloning was used to identify the cDNA encoding the NCC from winter flounder (Pseudopleuronectes americanus) urinary bladder (30). Next, the mammalian NCC cDNA was isolated using a homology approach, first from rat kidney (29) and later from human (53, 80), mouse (48), and rabbit (91) kidney. With the molecular identification of NCC, it became possible to generate antibodies against the cotransporter that allowed the immunolocalization of NCC specifically to the DCT, with more prominent expression in the early portion of the DCT (DCT1) than in the later portion (DCT2) (69). Together with probes and specific primers for assessing mRNA expression, the effects of several stimuli on NCC mRNA and protein expression levels (44) were studied using animal models to understand the role of NCC in physiological and pathophysiological conditions. NCC, and therefore DCT, was identified as a key site for modulation of NaCl transport and hence, for blood pressure regulation (21). However, it quickly became obvious that many stimuli do not affect NCC expression levels. Thus assessing the activity of the cotransporter, in addition to the expression level, would be required for a better understanding of the role of the cotransporter in many situations.
NCC Activity Correlates with Phosphorylation
The regulation of NCC activity by the phosphorylation of conserved threonine and serine residues in the amino-terminal domain was first demonstrated by Pacheco-Alvarez et al. (65). They first demonstrated in Xenopus laevis oocytes expressing NCC that the activity of NCC dramatically increased when the intracellular chloride concentration was lowered using two different techniques (Fig. 1A). One method involved low chloride hypotonic stress, in which incubation of oocytes in a light hypotonic medium (170 instead 210 mosmol/kgH2O) without chloride (substituted by isothaionate) promotes the opening of endogenous chloride channels (2) that efficiently decrease the intracellular chloride concentration (42). The other was the coinjection of oocytes with NCC cRNA, together with the cRNA of K+-Cl− cotransporter KCC2. This isoform of KCC remains active in isotonic conditions (83) and was therefore expected to promote a continuous extrusion of chloride ions from the oocytes during the incubation period. The efficiency of KCC2 in decreasing the intracellular chloride concentration was later demonstrated by Bertram et al. (8). At the time of these findings, there were no phospho-specific NCC antibodies. Therefore, the NKCC1 phospho-specific antibody (R5) developed by Forbush and coworkers (26) was used. However, for the R5 antibody to recognize phospho-NCC, a single amino acid change was made to rat NCC. A tyrosine residue in position 56 was changed to a histidine, which is the residue in the same position of Na+-K+-2Cl− cotransporters NKCC1 and NKCC2 that is recognized by R5 antibodies. It was observed that this single point mutation did not change the functional properties of NCC or its activation by intracellular chloride depletion.
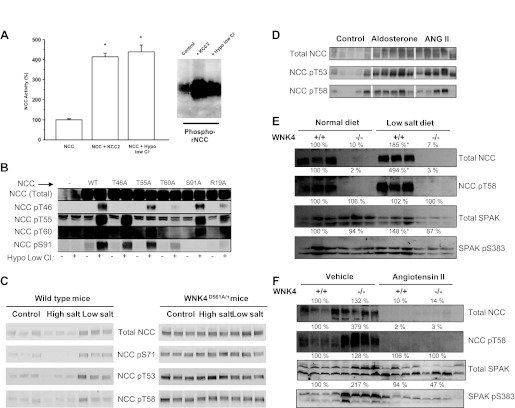
Fig. 1.
Activity of the renal thiazide-sensitive NaCl cotransporter (NCC) is modulated by phosphorylation. A: percent NCC activity in the heterologous expression system of Xenopus laevis oocytes is increased by 2 different maneuvers inducing intracellular chloride depletion (*P < 0.001 vs. NCC alone). This activation is associated with increased phosphorylation of threonine residues 53 and 58, as detected with the phospho-specific antibody R5 (modified from Ref. 65). B: detection of total NCC or phosphorylated NCC in threonine residues 46, 55, 60 or serine residue 91 using specific phospho-antibodies rose against each site, in extracted proteins from HEK-293 cells transiently transfected with wild-type or mutant NCC, as stated. The blots are shown in control conditions or after exposing of the cells to low chloride hypotonic stress, as stated (modified from Ref. 72 with permission). C: Western blot analysis of renal proteins from wild-type or with no lysine kinase WNK4D561A/+ mice exposed to control, a high-salt, or low-salt diet. Western blots were performed using the total anti-NCC antibody, or specific antibodies against phosphorylated threonine 53, threonine 58, or serine 71, as stated (modified from Ref. 15 with permission). D: NCC expression and phosphorylation at residues T53 or T58 in adrenalectomized rats treated with vehicle, aldosterone, or angiotensin II infusion (modified from Ref. 89 with permission). E: NCC expression and phosphorylation at T60 and Ste20-related proline/alanine-rich kinase (SPAK) expression and phosphorylation at S383, as stated, in wild-type or WNK4 total knockout mice exposed to a normal- or low-salt diet, as stated (modified from Ref. 12). F: NCC expression and phosphorylation at T60 and SPAK expression and phosphorylation at S383, as stated, in wild-type or WNK4 total knockout mice infused with vehicle or angiotensin II (modified from Ref. 12).
As shown in Fig. 1A, the increased activity of NCC induced by intracellular chloride depletion was associated with increased phosphorylation of threonine residues 53 and 58, as detected with the R5 antibody (65). An increased signal in activated NCC was not observed in the presence of alkaline phosphatase, indicating that it was a result of phosphorylation. Eliminating threonine residues 53 and 58, as well as serine residue 71, was associated with decreased basal activity of NCC and the complete prevention of the activation by intracellular chloride depletion, particularly when T58 was eliminated. Interestingly, the decreased activity observed in the triple mutant of NCC (T53A, T58A, and S71A) was not associated with a change in the expression level at the cell surface. Thus this study demonstrated that increased phosphorylation of amino-terminal threonine/serine residues in NCC are correlated with increased activity of the cotransporter. Therefore, “NCC activity” could be indirectly assessed by analyzing NCC phosphorylation with in vivo models. Moreover, a subsequent study showed that, in X. laevis oocytes, NCC activity is significantly reduced by coexpression with protein phosphatase 4 and that T58 is the target threonine for dephosphorylation by this phosphatase (32).
After this observation, specific phospho-antibodies for detecting equivalent threonine/serine residues in human or mouse NCC were raised (72, 99). Richardson et al. (72) used mass spectrophotometric analysis of NCC protein extracted from NCC-transfected HEK-293 cells and observed that low chloride hypotonic stress resulted in the phosphorylation of threonine residues 46, 55, and 60 and serine residue 91 of human NCC. This finding corroborated the importance of T53 and T58 of rat NCC (equivalent to 55 and 60 in human NCC) and added two new sites. Specific phospho-antibodies were raised for each site and used in transfected HEK-293 cells and in mpkDCT cells endogenously expressing NCC. Thus it was confirmed that the low chloride hypotonic stress increased the phosphorylation of these sites (Fig. 1B). There were two additional important observations from this study. One was that the R19A mutation, which eliminated a Ste20-related proline/alanine-rich kinase (SPAK) binding site in NCC, prevented the phosphorylation of these sites, implicating SPAK as the responsible kinase (Fig. 1B). The other was that eliminating threonine 60 (NCC-T60A) prevented or reduced the phosphorylation of the other sites, supporting a previous suggestion (65) that this threonine residue was key in the regulation of NCC activity.
Yang et al. (99) raised specific phospho-antibodies against serine 71. These antibodies were used to show in a knockin model of PHAII that, indeed, the mutation D561A in one allele of WNK4 resulted in increased phosphorylation of NCC in this residue (Fig. 1C). WNK4(D561A/+) mice exhibited increased expression of NCC in the apical membrane of DCT cells and increased phosphorylation of SPAK in renal tissue. These findings suggested that WNK4 mutations causing PHAII produce an increase in SPAK phosphorylation, which in turn induces an increase in NCC phosphorylation at serine 71 and in the surface expression of the cotransporter.
Two additional phosphorylation sites have been observed in NCC by using large-scale proteomics from human urine or rat renal cortex. In humans exosomes from urine, phosphorylation of NCC at serine 811 was observed (34). This serine is part of an exon that is present in humans, but not in the rat or mouse. Its significance is not known. In rat renal cortex, phosphorylation of serine 124 of NCC was also detected by this methodology (24). A recent study shows that this serine phosphorylation is increased by vasopressin and a low-salt diet (74).
Activation of NCC by a Low-Salt Diet
The studies discussed above revealed the close correlation between NCC activity and phosphorylation (65) and generated the tools for detecting NCC phosphorylation (72, 99). Thus it became possible to measure NCC activity using in vivo models. Table 1 shows a compilation of the studies in which the phosphorylation of NCC has been assessed. One of the first issues for researchers in the field was to analyze the role of NCC in salt handling when the salt content of a diet changed. Thus NCC phosphorylation was assessed in animals subjected to low- or high-salt diets. The first such study was performed by Chiga et al. (15), who observed that high- and low-salt diets were associated with decreased and increased phosphorylation of NCC, respectively, in threonine residues 53 and 58, as well as in serine residue 71 (Fig. 1C). The phosphorylation of serine 71 during a low-salt diet was partially prevented by spironolactone, suggesting that aldosterone modulates the phosphorylation of NCC. However, the ratio between phosphorylated NCC and total NCC was not presented. Thus the effect observed with spironolactone could be solely a consequence of NCC expression levels, which are affected by aldosterone (44). In another study, Vallon et al. (88) observed that the increased phosphorylation of NCC at the same residues during a low-salt diet was attenuated in serum glucocorticoid 1 (SGK1)-knockout mice. Because SGK1 is involved in the effects of aldosterone in the distal nephron, this observation supported the idea that increased phosphorylation of NCC during a low-salt diet is partially a result of aldosterone's effects. It was subsequently observed that NCC phosphorylation could be achieved independently by both angiotensin II and aldosterone. Angiotensin II modulates NCC trafficking in DCT cells from rats (78, 79) and increases the activity and phosphorylation of NCC in oocytes (77). Therefore, to distinguish between the effects of angiotensin II and aldosterone, Van der Lubbe et al. (89) performed a study in rats in which the adrenal glands were removed and subsequently treated with vehicle, aldosterone, low nonpressor doses of angiotensin II, or pressor doses of this hormone. Without adrenal glands, NCC expression and phosphorylation and SPAK phosphorylation in the rats were increased by either angiotensin II or aldosterone, indicating that angiotensin II affects NCC independently of aldosterone. Thus the increased phosphorylation of NCC during a low-salt diet is most likely a result of the combined effects of angiotensin II and aldosterone on DCT cells (Fig. 2). The aldosterone-sensitive distal nephron begins at DCT2 because 11-β-hydroxysteroid dehydrogenase type II enzyme is absent in DCT1, where NCC is more heavily expressed. This enzyme eliminates cortisol, preventing the promiscuous effect of the glucocorticoid on the aldosterone receptor (6). Thus the angiotensin II effects on NCC phosphorylation might occur in both DCT1 and DCT2, while the effect of aldosterone occurs only in the NCC of DCT2. No study thus far has specifically address this issue.
Table 1.
Phosphorylation of NCC in different sites and conditions
| Experimental System/Model | Stimulus | Effect | Phospho-Site* | Kinase Involved | Reference No. |
|---|---|---|---|---|---|
| Xenopus oocytes | Intracellular Cl− depletion | ↑↑ | T55, T60, S73 | ? | 65 |
| Mouse | WNK4(D561A/+) knockin | ↑↑ | S73 | WNK4 | 99 |
| HEK-293 cells | Intracellular Cl− depletion | ↑↑ | T46, T55, T60, S91 | SPAK | 72 |
| Rat | Low-salt diet | ↑↑ | T55,T60, S73 | ? | 15 |
| Human urine | None | ↑ | S811 | ? | 34 |
| Xenopus oocytes/mpkDCT cells | Angiotensin II | ↑↑ | T55, T60 | WNK4-SPAK | 77 |
| Mouse | Low-K+ diet | ↑ | T55, T60, S73 | SGK1 | 88 |
| Mouse | WNK4 hypomorphic | ↓ | T55, T60, S73 | WNK4 | 64 |
| Brattleboro rats | dDAVP | ↑↑ | T55, S73 | ? | 60 |
| Brattleboro rats/Wistar rats | dDAVP | ↑↑ | T55, T60 | SPAK | 67 |
| Mouse | SPAK knockin | ↓↓ | T55, T60, S91 | SPAK | 71 |
| mpkDCT cells/mouse | Angiotensin II/aldosterone | ↑↑ | T55, T60, S73 | SPAK | 86 |
| Adrenalectomized rats | Angiotensin II/aldosterone | ↑↑ | T55, T60 | WNK4-SPAK | 89 |
| Mouse | KS-WNK1 knockout | ↑ | T45, T55, T60, S73 | WNK1/WNK4 | 36 |
| Mouse | KS-WNK1 knockout | ↑ | T60 | WNK1/WNK4 | 51 |
| In tube | Incubation with MO25 | ↑ | T45, T55, T60, S91 | SPAK/OSR1 | 25 |
| Mouse | SPAK knockout | ↓↓ | T55, T60, S73 | SPAK | 98 |
| Mouse | SPAK knockout | ↓↓ | T55, S73 | SPAK | 54 |
| mDCT cells/rats | Cyclosporine | ↑ | T55, T60, S73 | ? | 56 |
| Mouse | Tacrolimus | ↑ | T55 | WNK3/WNK4 | 38 |
| Mouse | NCC transgenic | ⇆ | T55 | ? | 55 |
| Mouse | WNK4 Knockout | ↓↓ | T60 | WNK4/SPAK | 12 |
| mpkDCT cells/mouse | Insulin | ↑ | T55, T60, S73 | WNK4/SPAK | 81 |
| Xenopus oocytes Ex vivo kidney | Insulin | ↑ | T60 | WNK3 | 14 |
| ZO obese Zucker rats | Hyperinsulinism | ↑ | T55 | WNK4 | 47 |
| Mouse | KS-OSR1 knockout | ↑ | T55 | OSR1/SPAK | 50 |
| Mouse | Isoproterenol-salt-sensitive hypertension | ↑ | T55, S73 | WNK4 | 58 |
| In tube | WNK4/Ca2+ | ↑↑ | WNK4 | 61 | |
| Xenopus oocytes | WNK3 | ↑↑ | T60 | WNK3/SPAK | 66 |
| Rats | Large-scale proteomics | ↑ | S124 | ? | 24 |
| Brattleboro rats | dDAVP | ↑ | S124 | ? | 74 |
Numbers correspond to human Na-Cl cotransport (NCC) sequence. DCT, distal convoluted tubule; WNK, with no lysine kinase; SPAK, Ste20-related proline/alanine-rich kinase.
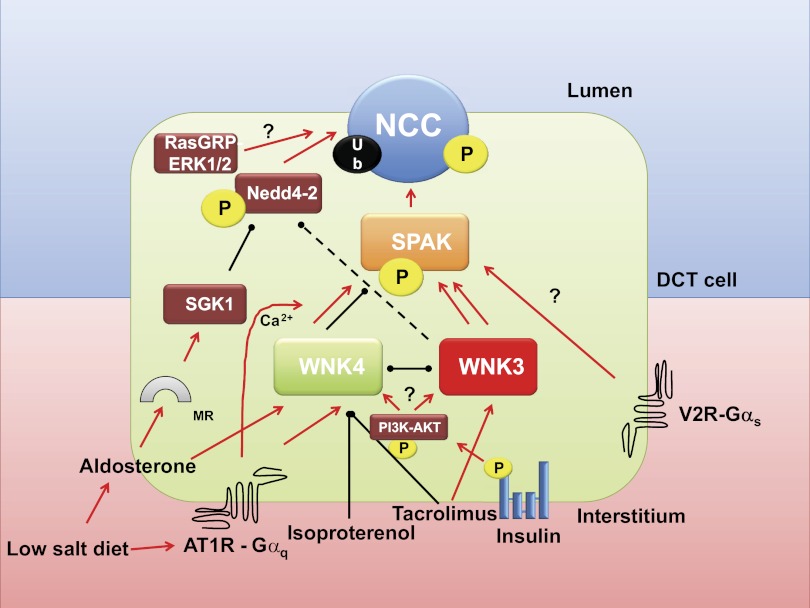
Fig. 2.
Regulation of NCC by ubiquitylation and phosphorylation. See text for explanations. MR, mineralocorticoid receptor; red arrows, activation; black lines, inhibition. To simplify the figure, WNK1 effects were not included.
Regulation of NCC by the WNKs-SPAK Complex
A decade ago, it was revealed that PHAII is a result of mutations in two genes encoding WNK1 and WNK4 (94). The PHAII clinical picture is the mirror image of Gitelman's disease and is reversed by low doses of thiazide-type diuretics. Therefore, it was suggested that WNK1 and WNK4 regulate NCC and that the pathophysiology of PHAII may be caused by impaired regulation of NCC by mutant WNK kinases. PHAII resulting from mutations in the PRKWNK1 gene is caused by intronic deletions that apparently increase the expression of wild-type WNK1. In contrast, PHAII associated with mutations in the PRKWNK4 gene is caused by missense mutations in a highly conserved acidic region of WNKs. Initial observations suggested that wild-type WNK4 reduces NCC activity and that this effect did not occur in WNK4 harboring PHAII-type missense mutations (11, 95, 96). Subsequently, it was demonstrated using in vivo models that extra-wild-type WNK4 activity (transgenic mice with 4 wild-type WNK4 alleles) is associated with reduced activity of NCC (Gitelman-like phenotype) (49). In contrast, mice with WNK4-PHAII-type mutant alleles (99), even in the presence of two normal WNK4 alleles (49), developed the PHAII phenotype associated with hypertrophy of the DCT and increased phosphorylation of NCC at serine 71. WNKs were found to lie upstream of other serine/threonine kinases of the STE-20 type known as SPAK and OSR1 (92), which appear to be the kinases that actually phosphorylate NCC (Fig. 2) (72). Supporting this hypothesis, WNKs induce phosphorylation of SPAK at threonine residue 243 (93). Additionally, the knockin mice in which this site in SPAK was substituted with alanine, SPAKT243A/T243A mice, developed a Gitelman-like syndrome, with arterial hypotension and a decreased phosphorylation of NCC at residues T53, T58, and S71 (71). Similar observations were obtained with the complete SPAK knockout colony (54, 98).
Experimental evidence in several models including X. laevis oocytes, mammalian epithelial cells, and genetically altered mice consistently shows that under certain circumstances, WNK4 behaves as an inhibitor of NCC. However, in the presence of PHAII-type mutations, WNK4 acts as an activator (13). This can be explained, at least in part, by the observation that the WNK4 effect on SPAK/NCC is modulated by angiotensin II (Fig. 2). This peptide hormone produces a positive effect on NCC trafficking toward the apical membrane of DCT cells (79) and induces increased thiazide-sensitive salt reabsorption in the distal nephron (101). In the X. laevis oocyte expression system, coexpression of WNK4 and NCC results in reduced activity of NCC (95, 96). This effect is reversed in the presence of angiotensin II and can be prevented by losartan, a specific AT1 receptor blocker (77). Interestingly, angiotensin II increased the activity of NCC when oocytes were coinjected with WNK4, but not in the absence of WNK4. This finding suggested that the effect of angiotensin II on NCC is WNK4 dependent. The activation of NCC by angiotensin II in the presence of WNK4 was also SPAK dependent and associated with increased phosphorylation of both SPAK and NCC, not only in oocytes but also in the mpkDCT cell line (77, 86). The requirement of WNK4 for the angiotensin II-induced activation of NCC has been confirmed in vivo. The NCC-induced phosphorylation in threonine 60 and SPAK at serine 383 by a low-salt diet or by chronic angiotensin II administration in wild-type animals was not observed in the WNK4−/− knockout mice (12) (Fig. 1, E and F). A potential biochemical explanation has been proposed. The WNK4-induced SPAK/OSR1 and NCC phosphorylation appears to be modulated by the calcium ion concentration. The NCC phosphorylation is higher when incubated in high (1 μM) than in low (10 nM) calcium concentrations. Interestingly, this difference does not occur when WNK4 constructs with PHAII-type mutations are used (61). Because the AT1 receptor is coupled to a Gαq type of G protein, angiotensin II binding induces an increase in calcium concentration in the cytoplasm by promoting the production of IP3 that stimulates the release of calcium by the smooth endoplasmic reticulum.
Considering all of these studies together, we have suggested that PHAII-type mutations in WNK4 are gain-of-function mutations, mimicking the effect of angiotensin II on the WNK4-SPAK-NCC (6, 12, 13, 77) and providing a pathophysiological explanation for PHAII. Because PHAII is a dominant disease, having one constitutively active allele of WNK4, the DCT behaves as if angiotensin II is present and explains the salt retention and hence arterial hypertension. This explanation also applies to hyperkalemia because increased salt reabsorption in the DCT reduces the salt delivery to the collecting duct, decreasing the possibility for Na+/K+ exchange via the epithelial sodium channel (ENaC)/renal outer medullary potassium channel (ROMK). Additionally, angiotensin II reduces the activity of ROMK by a WNK4-dependent mechanism (100).
Phosphorylation of NCC by WNK4/SPAK may be involved in at least two known models of arterial hypertension. Mu et al. (58) presented evidence supporting the hypothesis that salt-sensitive hypertension induced by isoproterenol administration is caused by an epigenetic-induced decrease in WNK4 expression, leading to an increased phosphorylation of NCC, thus increasing salt reabsorption in the DCT (Fig. 2). Similarly, two independent groups have shown that the arterial hypertension associated with calcineurin inhibitors, such as cyclosporine or tacrolimus, that are extensively used to prevent allograft rejection in organ transplantation, is associated with changes in WNK3 and WNK4 expression, and consequently in NCC phosphorylation (Fig. 2). This finding suggests that the arterial hypertension induced by these compounds is a result of increased salt reabsorption in the DCT (38, 56).
The effect of WNK1 on NCC activity remains elusive. No evidence of WNK1 effect on NCC has been reported. Instead, it was observed in X. laevis oocytes that WNK1 prevented the WNK4-induced inhibition of NCC activity (96), thus suggesting that increased WNK1 expression could exaggeratedly prevent the WNK4 inhibition of NCC, thus increasing the activity of the cotransporter. Later on it was observed that WNK1 produces a specific variant that is exclusively expressed in the kidney, particularly in DCT. This variant, named KS-WNK1 for kidney-specific WNK1, lacks the entire kinase domain and prevents the WNK1-induced inhibition of WNK4's negative effect on NCC (18, 64, 85). Thus it was proposed that under normal circumstances, KS-WNK1 modulates the effect of the longer WNK1 on WNK4-NCC. In this case, PHAII could be explained by deletion of intron 1 of WNK1 that results in increased expression of the longer form of WNK1, surpassing the inhibitory effect of KS-WNK1 and thus preventing the inhibitory effect of WNK4 on NCC. This proposal, however, awaits confirmation in animal models. On the one hand, deletion of intron 1 in mouse results in overexpression of both the long and the short WNK1 variants (17). On the hand, however, KS-WNK1 knockout mice exhibit increased expression and phosphorylation of NCC (Table 1) (36, 51).
WNK3 kinase is a powerful activator of NCC and consequently also phosphorylates threonine residue 58 in NCC (Fig. 2) (66). The positive effect is also associated with increased expression of NCC in the plasma membrane (33, 73). One group suggested that this effect is SPAK independent because coexpression of WNK3 and NCC with a dominant negative version of SPAK did not prevent the activation of NCC by WNK3 (33). However, the elimination of the SPAK binding site located at phenylalanine 242 of WNK3 completely prevents the activation of NCC, suggesting that a WNK3-SPAK interaction is required (66). Moreover, the requirement of SPAK for WNK3 modulation of other members of the SLC12 family, including NKCC1, NKCC2, and KCC4, has also been observed (66, 70).
Vasopressin Modulates NCC Activity
Vasopressin is a nanopeptide hormone produced in the hypothalamus and released in the posterior pituitary gland in response to increased plasma osmolarity or decreased blood pressure. Consequently, vasopressin promotes the formation of concentrated urine and increases blood pressure. Similarly to many other hormones (catecholamines, atrial natriuretic peptide, angiotensin II, aldosterone, etc.), vasopressin modulates blood pressure by modifying both vascular smooth muscle contraction and urinary salt and water reabsorption. For vasopressin, these effects are transduced in the smooth muscle and renal tubular cells through the Gα-coupled receptors V1 (Gαq) and V2 (Gαs), respectively. The role of antidiuretic hormone in modulating the activity of NKCC2, urea transporters, and aquaporin 2 in the kidney is very well known. However, these actions modulate extracellular osmolarity rather than arterial pressure. Early micropuncture studies from Elalouf et al. (20) in homozygous Brattleboro rats suggested that vasopressin increases salt reabsorption in the DCT. In addition, the expression of the V2 receptor in the DCT has been documented using in situ hybridization and immunohistochemistry of human, mouse, and rat kidney (59). Furthermore, by analyzing the phosphorylation of NCC, two groups have independently confirmed the effect of vasopressin on NCC (60, 67). Administering dDAVP to Brattleboro rats resulted in increased phosphorylation of NCC at residues T53, T58, and S71. Both groups showed that the effect occurs in the DCT, independently of other potential hormones such as angiotensin II or aldosterone. Pedersen et al. (67) suggested that vasopressin's effect could be through SPAK activation (Fig. 2). An interesting discrepancy regarding the effect of phosphorylation on NCC membrane trafficking is discussed below.
Insulin Modulates NCC Activity
There is a clear association between blood pressure levels and body weight. The higher the body mass index, the higher the prevalence of arterial hypertension. Metabolic syndrome, obesity, and diabetes mellitus type II are risk factors for the development of hypertension. One possibility is that hyperinsulinism that is often seen in these syndromes plays a critical role in the development of hypertension. It is known that obesity and hyperinsulinemic states are associated with increased expression of the distal nephron salt transporters (9, 43, 82, 87). In this regard, it has recently been shown by three independent groups that insulin or hyperinsulinemic states are associated with increased activity and phosphorylation of NCC (Table 1). Shoara et al. (81) observed in mpkDCT cells that insulin induces phosphorylation of SPAK and NCC by a phosphoinositol 3-kinase (PI3K)-dependent pathway and that this is reduced by knocking down WNK4, suggesting that it is WNK4 dependent. They also observed that intraperitoneal injection of insulin is associated with increased phosphorylation of SPAK and NCC in the kidney of wild-type mice but not in a WNK4 hypomorphic mouse. Komers et al. (47) demonstrated in obese Zucker rats that response to hydrochlorthiazide is increased, together with the phosphorylation of NCC at threonine 53. They also observed in vitro that insulin's effect on phosphorylating NCC can be prevented by PI3K blockers and suggested that WNK4 could be implicated in the insulin effect on NCC. Finally, the observation of Chavez-Canales et al. (14) in X. laevis oocytes showed that insulin increases the activity of NCC, assessed by thiazide-sensitive 22Na+ uptake and that this is associated with increased phosphorylation of threonine 60. Inhibitors of the PI3K, akt1, and mammalian target of rapamycin (mTOR)2 pathway prevented the insulin effect on NCC. It was also observed by using a kidney ex vivo perfusion technique that insulin perfusion into the kidney induces phosphorylation of the cotransporter. Thus insulin is an activator of NCC by inducing its phosphorylation in the amino-terminal domain acceptor sites (Fig. 2).
Do NCC Phosphorylation and Activity Correlate with Surface Expression?
A subject of debate is the location at which the phosphorylation that activates NCC occurs. Does it occur in the vesicles containing NCC copies, promoting their trafficking toward the plasma membrane, thus increasing surface expression of the cotransporter? Alternatively, does it occur at the NCC molecules that are already present in the plasma membrane, increasing their transport capacity as a consequence of the phosphorylation? As shown in Table 2, a few studies have addressed this issue. The data obtained from NCC cRNA-transfected X. laevis oocytes, a robust NCC expression system used to clone and study NCC properties (28), suggest that amino-terminal threonine phosphorylation occurs in the proteins that are already in the membrane. Intracellular chloride depletion increases the activity and phosphorylation of NCC, without changing the level of expression at the cell surface. Eliminating threonine 53 and 58, as well as the serine residue 71, dramatically reduced the basal activity of the cotransporter without affecting its cell surface expression (65). Supporting these observations, another study demonstrated that protein phosphatase 4 reduces NCC activity, presumably by dephosphorylation, without affecting NCC surface expression. In the same study, phosphorylation that mimicked NCC mutation T58D increased the basal activity of the cotransporter without affecting its expression levels at the cell surface (32).
Table 2.
Phosphorylation and cell surface expression of NCC
| Experimental Model | Stimulus | Phosphorylation/Activity | Surface Expression | Reference No(s). |
|---|---|---|---|---|
| Xenopus oocytes | Intracellular chloride depletion | ↑↑ | ⇆ | 65 |
| Xenopus oocytes | Protein phosphatase 4 | ↓↓ | ⇆ | 32 |
| Xenopus oocytes | WNK3 | ↑↑↑ | ↑↑↑ | 66, 73, 97 |
| Xenopus oocytes | Mutant NCC-T58D | ↑↑ | ⇆ | 32 |
| Xenopus oocytes | Mutant NCC-T58A | ↓↓ | ⇆ | 32, 65 |
| Mouse | WNK4(D561A/+) knockin | ↑↑ | ↑↑ | 99 |
| Brattleboro rats | dDAVP | ↑↑ | ↑↑ (no shift) | 67 |
| Brattleboro rats/Wistar rats | dDAVP | ↑↑ | ↑↑ (shift) | 60 |
| Mouse | SPAK knockout | ↓↓ | ? | 98 |
| Mouse | SPAK knockout | ↓↓ | ? | 54 |
The data from in vivo models are less clear regarding the relationship between NCC plasma membrane expression and phosphorylation. Using immunogold electron microscopy, Pedersen et al. (67) showed in rats that the vasopressin-induced increase in NCC phosphorylation apparently occurs in the NCC that is already present in the plasma membrane. In contrast, Mutig et al. (60) proposed that vasopressin-induced NCC phosphorylation occurs in the intracellular vesicles that then move toward the plasma membrane. This last possibility is supported by a previous study showing that vasopressin activation and phosphorylation of NKCC2 in the thick ascending limb of Henle's loop in mice is associated with increased expression of the cotransporter in the apical membrane (31). Thus in the heterologous expression system it appears as if phosphorylation occurs in NCCs that are already at the cell surface. However, the in vivo data do not yet allow a definitive conclusion.
With regard to the regulation of NCC by the WNKs-SPAK pathway, it appears as if both phosphorylation and trafficking occur, most likely because WNKs modulate either independently of each other. In oocytes, the activation of NCC by WNK3 is associated with both increased phosphorylation and cell surface expression (66, 73). Preliminary evidence suggests that WNK3, in addition to phosphorylating NCC (most likely via SPAK), also reduces the Nedd4–2-induced ubiquitylation of the cotransporter. Therefore, the increase in NCC expression in the plasma membrane could be explained by decreased NCC ubiquitylation (see below) rather than by increased NCC phosphorylation (5). In the WNK4(D561A/+) knockin mouse resembling PHAII, there is a clear increase in phosphorylation of NCC and in the expression at the apical surface of DCT cells (99). The elimination of the kinase SPAK, either by a knockout (54, 98) or by a knockin model in which SPAK is present but is unable to be activated by WNKs (71), is associated with a reduction in total NCC expression. Thus interpreting the decrease in both cell surface expression and phosphorylation of NCC in these models is difficult.
Modulation of NCC by Trafficking
In the cell, NCC resides within intracellular vesicles that undergo exocytosis and endocytosis. In this regard, one proposed mechanism is that WNK4, which is known to reduce NCC activity in basal conditions (95), reduces the delivery of NCC to the plasma membrane by redirecting NCC molecules from the Golgi complex to lysosomes, in which NCC is then degraded. Supporting this view, WNK4 stimulates the interaction between NCC and the AP3 protein complex that promotes the switching of proteins from endosomes to lysosomes (84). Another study showed that WNK4 also promotes the interaction between NCC and sortilin, a DCT protein that redirects peptides from the Golgi and other compartments to lysosomes (102). This degradation-promoting effect of WNK4 could explain, at least in part, the Gitelman-like effect in which wild-type WNK4 is overexpressed in a BAC transgenic model (49) that exhibits remarkable DCT hypotrophy. In addition, accelerated degradation in the endoplasmic reticulum appears to be the major mechanism for reducing the expression of NCC in the plasma membrane in Gitelman's disease (48, 62, 76).
Protein Ubiquitylation: A Novel Regulatory Mechanism for NCC
Adding the 76-amino acid protein ubiquitin to lysine residues in target proteins is a powerful way to modulate the activity of proteins because ubiquitylation marks the protein for destruction, modulating its half-life (90). There are two major types of ubiquitin ligases: HECT (for homologous to the E6-AP carboxyl terminus) and RING (for really interesting new genes). These ligases contain several members that, in conjunction with a variety of interacting proteins, constitute hundreds of possibilities for highly specific ubiquitylation of proteins at lysine residues, just as kinases induce phosphorylation of proteins at specific threonine, serine, or tyrosine residues. Several membrane proteins, including transporters and channels in the kidney, are regulated by ubiquitylation (75).
A well-known example of ubiquitylation-mediated regulation occurs with ENaC. This channel in the collecting duct is ubiquitylated by a HECT-type ubiquitin ligase known as Nedd4–2. This protein directly interacts with ENaC via a specific PY motif in the channel, inducing ubiquitylation of the channel. This ubiquitylation marks the channel to be removed from the apical plasma membrane and thus reduces sodium reabsorption. The mineralocorticoid hormone aldosterone increases ENaC activity, at least in part, by stimulating the expression of SGK1. SGK1 phosphorylates Nedd4–2 at serine residues 222 and 328, preventing Nedd4–2-ENaC interaction and thus ENaC ubiquitylation. As a consequence, the expression of the channel in the apical membrane is increased, as is the sodium reabsorption. The loss of the PY motif of the β- or γ-subunit of ENaC by specific mutations that occur in Liddle's syndrome provides a pathophysiological explanation for the disease. The absence of the PY motif in just one of the ENaC subunits is sufficient to preclude the Nedd4–2-induced ubiquitylation of the channel and thus chronically increase the expression/activity of the channel (41).
Modulation of NCC activity by ubiquitylation is an emerging field that is attracting the interest of researchers. The first observation suggesting that NCC is modulated by ubiquitylation was performed by Ko et al. (46) by searching for the mechanism by which phorbol esters reduce NCC activity via RasGRP1 and ERK1/2 activation (Fig. 2) (45). In that study, mDCT cells endogenously expressing NCC and Madin-Darby canine kidney cells transiently transfected with NCC cDNA were used. Exposing the cells to TPA resulted in increased ubiquitylation of NCC that could be prevented with UBEI-41, a compound that inhibits the activity of the E1 ligase, thus preventing TPA-induced ubiquitylation and reduction of NCC activity. The ubiquitin ligase responsible for NCC ubiquitylation was not elucidated. Subsequently, the SGK1-Nedd4–2 pathway that modulates ENaC activity was found to also regulate NCC (Fig. 2) (4). Aldosterone promotes increased NCC protein expression (44) by a mechanism that does not affect the NCC mRNA levels (1, 57), strongly suggesting that aldosterone increases NCC by a posttranslational effect. Supporting this hypothesis, Arroyo et al. (4) recently showed that Nedd4–2 induces an increase in NCC ubiquitylation, reducing the cotransporter in the plasma membrane, and thus its activity. Consistent with this observation, Nedd4–2 conditional knockout mice developed a remarkable increase in NCC expression a few days after the administration of doxycycline to induce the loss of Nedd4–2 in the kidney. Similarly to ENaC regulation, SGK1 prevented the Nedd4–2 effect in NCC. Supporting this, Faresse et al. (23) recently showed that inducible renal tubule-specific SGK1 knockout mice exhibit impaired Na+ reabsorption on a low-NaCl diet, and this was associated with decreased NCC abundance. Thus it is probable that the aldosterone activation of NCC is caused, at least in part, by decreasing the Nedd4–2-induced ubiquitylation of NCC in an SGK1-dependent mechanism. A recent study suggests that phosphorylation and ubiquitylation of NCC could interact with each other in such a way that phosphorylating NCC prevents ubiquitylation (39), and it has been shown in vitro that WNK1 and WNK4 are able to phosphorylate Nedd4–2 at sites overlapping with SGK1 (37). The significance of this finding is not known but could represent a possibility for regulating phosphorylation and ubiquitylation of NCC simultaneously.
Evidence suggests that NCC could be a substrate for other types of ubiquitin ligases. Degradation of NCC in the endoplasmic reticulum has been shown to be associated with the E3-ubiquitin ligase Hrd1 (62) and, as discussed below, NCC could be a target for the RING-type ubiquitin ligases.
Ubiquitylation is Also Involved in the Pathophysiology of PHAII
After the discovery of WNK1 and WNK4 as the causative genes of PHAII, it became clear that in several families this disease could occur in the absence of WNK1 or WNK4 mutations. Therefore, one or more unknown genes were capable of producing the same clinical outcome (19, 35). The causative genes have now been uncovered. Boyden et al. (10) observed that two genes encoding proteins that form a RING-type complex of ubiquitin ligase produce PHAII. These genes are known as Kelch-like 3 and Cullin 3. The human genome contains at least seven different cullin proteins; each constitutes the backbone of several multisubunit ubiquitin ligase complexes. In forming the complex, Cullin 3 specifically binds proteins, for example Kelch 3, that contain BTB domains. These two proteins, Kelch-like 3 and Cullin 3, form a complex that associates with a RING-type ubiquitin ligase (68). Kelch 3 or any of the BTB-containing domain proteins serves as the specific substrate recognition site of the complex, and the RING-type protein promotes the transfer of ubiquitin residues from E2 ligases onto the substrate recognized by the BTB protein. Using exome sequencing, mutations in Kelch 3 or Cullin 3 in 41 unrelated families with PHAII were identified (10). Although Kelch 3 mutations were either recessive or dominant, the Cullin 3 mutations were predominantly de novo. Interestingly, the PHAII patients with Cullin 3 mutations exhibited a more severe disease. As shown in Table 3, the severity of PHAII syndrome in terms of the mutated gene is Cullin 3 > Recessive Kelch 3 > Dominant Kelch 3 > WNK4 > WNK1. This ranking accounts for the age of diagnosis, the percentage of affected members under 18 yr of age exhibiting hypertension, the level of hyperkalemia, and the level of metabolic acidosis at the time of diagnosis.
Table 3.
Severity of PHAII stratified by genotype1
| Mutated Gene | No. of Families | No. of People Affected | HTA4 <age 18 yr, % | Age at Diagnosis, yr | K+,5 mM | HCO3−,6 mM |
|---|---|---|---|---|---|---|
| Cullin 3 | 17 | 21 | 94 | 9 ± 6 | 7.5 ± 0.9 | 15.5 ± 2.0 |
| Kelch Rec2 | 8 | 14 | 14 | 26 ± 14 | 6.8 ± 0.5 | 17.6 ± 1.5 |
| Kelch Dom3 | 16 | 40 | 17 | 24 ± 18 | 6.2 ± 0.6 | 17.2 ± 2.5 |
| WNK4 | 5 | 15 | 10 | 28 ± 18 | 6.4 ± 0.7 | 20.8 ± 2.3 |
| WNK1 | 2 | 23 | 13 | 36 ± 20 | 5.8 ± 0.8 | 22.4 ± 4.6 |
PHAII, pseudohypoaldosteronism type II; 1, modified from Boyden et al. (10); 2, recessive; 3, dominant; 4, hypertension; 5, serum potassium; 6, serum bicarbonate.
For the reasons discussed above, it is generally accepted that the level of activity of NCC plays a key role in the development of PHAII. Thus these observations suggest that Cullin 3 and Kelch 3 must be regulators of NCC, by ubiquitylation of the cotransporter or of proteins that in turn modulate NCC activity (such as WNKs, SPAK, and phosphatases). Louis-Dit-Picard et al. (52) also reported on PHAII families resulting from mutations in Kelch 3 and presented preliminary evidence suggesting that Kelch 3 may modulate NCC. They showed that Kelch 3 in the nephron is mostly expressed in the DCT. Additionally, in HEK-293 cells transiently transfected with NCC cDNA, treatment with Kelch 3 siRNA increased the presence of NCC protein in the plasma membrane. These observations do not necessarily probe the direct effect of Kelch 3 on NCC. However, these findings do support the hypothesis that by a direct or indirect pathway, NCC expression is most likely affected by the Cullin 3/Kelch 3 complex. Therefore, the disruption of NCC regulation by Cullin 3/Kelch 3 would most likely provide a pathophysiological explanation for PHAII. Because Cullin 3/Kelch 3 patients exhibit a more severe phenotype than WNK4 or WNK1 patients, it is possible that the effect of these ubiquitin ligases on NCC is more pronounced than that of WNKs or, alternatively, that in addition to NCC, ubiquitin ligases may affect other unknown players in the distal nephron for the development of PHAII, which has not become evident from studying WNKs. A decade has elapsed since the discovery of WNKs as causative genes for PHAII, but there are still several important unanswered questions about the mechanisms by which WNKs produces PHAII. Thus exciting times are ahead of us in defining the molecular mechanisms by which mutations in Cullin 3/Kelch 3 produce the disease.
GRANTS
The work performed in my laboratory has been possible thanks to support from the Consejo Nacional de Ciencia y Tecnología (CONACYT No. 165815), the Foundation Leducq Transatlantic Network on Hypertension, and the Wellcome Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: G.G. provided conception and design of research; G.G. performed experiments; G.G. analyzed data; G.G. interpreted results of experiments; G.G. prepared figures; G.G. drafted manuscript; G.G. edited and revised manuscript; G.G. approved final version of manuscript.
REFERENCES
- 1. Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na+/Cl− cotransporter abundance: role of aldosterone. J Am Soc Nephrol 12: 1335–1341, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol 103: 153–179, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acuna R, Martinez-de-la-Maza L, Ponce-Coria J, Vazquez N, Ortal-Vite P, Pacheco-Alvarez D, Bobadilla NA, Gamba G. Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. J Hypertens 29: 475–483, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Arroyo JP, Lagnaz D, Ronzaud C, Vazquez N, Ko BS, Moddes L, Ruffieux-Daidie D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O. Nedd4–2 modulates renal Na+-Cl− cotransporter via the aldosterone-SGK1-Nedd4–2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arroyo JP, Rojas-Vega L, Lagnaz D, Castaneda-Bueno M, Ronzaud C, Vazquez N, Staub O, Gamba G. WNK3 prevents the Nedd4–2 inhibition of the renal Na-Cl Cotransporter (NCC). FASEB J 26: 867.–834., 2011 [Google Scholar]
- 6. Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: diferential regulation of ion transport in distal nephron. Physiology (Bethesda) 26: 115–123, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Beaumont K, Vaughn DA, Fanestil DD. Thiazide diuretic receptors in rat kidney: identification with [3H]metolazone. Proc Natl Acad Sci USA 85: 2311–2314, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertram S, Cherubino F, Bossi E, Castagna M, Peres A. GABA reverse transport by the neuronal cotransporter GAT1: influence of internal chloride depletion. Am J Physiol Cell Physiol 301: C1064–C1073, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and β-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol 281: F639–F648, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castaneda-Bueno M, Gamba G. Mechanisms of sodium-chloride cotransporter modulation by angiotensin II. Curr Opin Nephrol Hypertens 21: 516–522, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Chavez-Canales M, Arroyo JP, Ko B, Vazquez N, Bautista R, Castañeda-Bueno M, Bobadilla NA, Hoover RC, Gamba G. Insulin increases the functional activity of the renal NaCl cotransporter. J Hypertens [Epub before print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. J Am Med Assoc 289: 2560–2571, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Delaloy C, Elvira-Matelot E, Clemessy M, Zhou XO, Imbert-Teboul M, Houot AM, Jeunemaitre X, Hadchouel J. Deletion of WNK1 first intron results in misregulation of both isoforms in renal and extrarenal tissues. Hypertension 52: 1149–1159, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Disse-Nicodeme S, Desitter I, Fiquet-Kempf B, Houot AM, Stern N, Delahousse M, Potier J, Ader JL, Jeunemaitre X. Genetic heterogeneity of familial hyperkalaemic hypertension. J Hypertens 19: 1957–1964, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Elalouf JM, Roinel N, de Rouffignac C. Effects of antidiuretic hormone on electrolyte reabsorption and secretion in distal tubules of rat kidney. Pflügers Arch 401: 167–173, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Ellison DH. Through a glass darkly: salt transport by the distal tubule. Kidney Int 79: 5–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fanestil DD, Tran JM, Vaughn DA, Maciejewski AR, Beaumont K. Investigation of the metolazone receptor. In: Diuretics III: Chemistry, Pharmacology and Clinical Applications, edited by Puschett JB, Greenberg A. New York: Elsevier, 1990, p. 195–204 [Google Scholar]
- 23. Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, Naray-Fejes-Toth A, Staub O. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol 302: F977–F985, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Feric M, Zhao B, Hoffert JD, Pisitkun T, Knepper MA. Large-scale phosphoproteomic analysis of membrane proteins in renal proximal and distal tubule. Am J Physiol Cell Physiol 300: C755–C770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filippi BM, De Los HP, Mehellou Y, Navratilova I, Gourlay R, Deak M, Plater L, Toth R, Zeqiraj E, Alessi DR. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J 30: 1730–1741, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Gamba G. Molecular physiology and pathophysiology of the electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Gamba G. The thiazide-sensitive Na+-Cl− cotransporter: molecular biology, functional properties, and regulation by WNKs. Am J Physiol Renal Physiol 297: F838–F848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994 [PubMed] [Google Scholar]
- 30. Gamba G, Saltzberg SN, Lombardi M, Miyanoshita A, Lytton J, Hediger MA, Brenner BM, Hebert SC. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci USA 90: 2749–2753, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Glover M, Mercier ZA, Figg N, O'Shaughnessy KM. The activity of the thiazide-sensitive Na+-Cl cotransporter is regulated by protein phosphatase PP4. Can J Physiol Pharmacol 88: 986–995, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Glover M, Zuber AM, O'Shaughnessy KM. Renal and brain isoforms of WNK3 have opposite effects on NCCT expression. J Am Soc Nephrol 20: 1314–1322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363–379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hadchouel J, Delaloy C, Faure S, Achard JM, Jeunemaitre X. Familial hyperkalemic hypertension. J Am Soc Nephrol 17: 208–217, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Hadchouel J, Soukaseum C, Busst C, Zhou XO, Baudrie V, Zurrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X. Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci USA 107: 18109–18114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem 285: 25161–25167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17: 1304–1309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hossain Khan MZ, Sohara E, Ohta A, Chiga M, Inoue Y, Isobe K, Wakabayashi M, Oi K, Rai T, Sasaki S, Uchida S. Phosphorylation of Na-Cl cotransporter by OSR1 and SPAK kinases regulates its ubiquitination. Biochem Biophys Res Commun 425: 456–461, 2012 [DOI] [PubMed] [Google Scholar]
- 40. Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kashlan OB, Kleyman TR. Epithelial Na+ channel regulation by cytoplasmic and extracellular factors. Exp Cell Res 318: 1011–1019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keicher E, Meech R. Endogenous Na+-K+ (or NH4+)-2Cl− cotransport in Rana oocytes; anomalous effect of external NH4+ on pHi. J Physiol 475: 45–57, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of the renal thiazide-sensitive Na-Cl cotransporter, blood pressure, and natriuresis in obese Zucker rats treated with rosiglitazone. Am J Physiol Renal Physiol 289: F442–F450, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104: 20120–20125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol 299: F300–F309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, Ellison DH. Enhanced phosphorylation of Na-Cl cotransporter in experimental metabolic syndrome—role of insulin. Clin Sci (Lond) 123: 635–647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kunchaparty S, Palcso M, Berkman J, Vélazquez H, Desir GV, Bernstein P, Reilly RF, Ellison DH. Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman's syndrome. Am J Physiol Renal Physiol 277: F643–F649, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS. Impaired phosphorylation of Na+-K+-2Cl− cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci USA 108: 17538–17543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 20: 855–866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012 [DOI] [PubMed] [Google Scholar]
- 53. Mastroianni N, DeFusco M, Zollo M, Arrigo G, Zuffardi O, Bettinelli A, Ballabio A, Casari G. Molecular cloning, expression pattern, and chromosomal localization of the human Na-Cl thiazide-sensitive cotransporter (SLC12A3). Genomics 35: 486–493, 1996 [DOI] [PubMed] [Google Scholar]
- 54. McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang CL, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McCormick JA, Nelson JH, Yang CL, Curry JN, Ellison DH. Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic hypertension. Hypertension 58: 888–894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Melnikov S, Mayan H, Uchida S, Holtzman EJ, Farfel Z. Cyclosporine metabolic side effects: association with the WNK4 system. Eur J Clin Invest 41: 1113–1120, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Moreno G, Merino A, Mercado A, Herrera JP, Gonzalez-Salazar J, Correa-Rotter R, Hebert SC, Gamba G. Electronuetral Na-coupled cotransporter expression in the kidney during variations of NaCl and water metabolism. Hypertension 31: 1002–1006, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17: 573–581, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Mutig K, Paliege A, Kahl T, Jons T, Muller-Esterl W, Bachmann S. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, Böhlick A, Bleich M, Shan Q, Bachmann S. Short-term stimulation of the thiazide-sensitive Na+-Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol 298: F502–F509, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Na T, Wu G, Peng JB. Disease-causing mutations in the acidic motif of WNK4 impair the sensitivity of WNK4 kinase to calcium ions. Biochem Biophys Res Commun 419: 293–298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Needham PG, Mikoluk K, Dhakarwal P, Khadem S, Snyder AC, Subramanya AR, Brodsky JL. The thiazide-sensitive NaCl cotransporter is targeted for chaperone-dependent endoplasmic reticulum-associated degradation. J Biol Chem 286: 43611–43621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S. Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion, and lowers blood pressure. Hum Mol Genet 18: 3978–3986, 2009 [DOI] [PubMed] [Google Scholar]
- 64. O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Pacheco-Alvarez D, San Cristobal P, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na-Cl cotransporter is activated and phosphorylated at the amino terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Pacheco-Alvarez D, Vazquez N, Castaneda-Bueno M, los Heros P, Cortes-Gonzalez C, Moreno E, Meade P, Bobadilla NA, Gamba G. WNK3-SPAK interaction is required for the modulation of NCC and other members of the SLC12 family. Cell Physiol Biochem 29: 291–302, 2012 [DOI] [PubMed] [Google Scholar]
- 67. Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Plotkin MD, Kaplan MR, Verlander JM, Lee WS, Brown D, Poch E, Gullans SR, Hebert SC. Localization of the thiazide sensitive Na-Cl cotransporter, rTSC1, in the rat kidney. Kidney Int 50: 174–183, 1996 [DOI] [PubMed] [Google Scholar]
- 70. Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, De Los HP, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Rinehart J, Kahle KT, De Los HP, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA 102: 16777–16782, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rosenbaek LL, Assentoft M, Pedersen NB, Macaulay N, Fenton RA. Characterization of a novel phosphorylation site in the sodium chloride cotransporter, NCC. J Physiol 2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rotin D, Staub O. Role of the ubiquitin system in regulating ion transport. Pflügers Arch 461: 1–21, 2011 [DOI] [PubMed] [Google Scholar]
- 76. Sabath E, Meade P, Berkman J, De Los HP, Moreno E, Bobadilla NA, Vazquez N, Ellison DH, Gamba G. Pathophysiology of functional mutations of the thiazide-sensitive Na-Cl cotransporter in Gitelman disease. Am J Physiol Renal Physiol 287: F195–F203, 2004 [DOI] [PubMed] [Google Scholar]
- 77. San Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol 291: F503–F508, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. Angiotensin II provokes acute trafficking of distal tubule NaCl cotransporter (NCC) to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Simon DB, Nelson-Williams C, Johnson-Bia M, Ellison D, Karet FE, Morey-Molina A, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitelman HJ, Lifton RP. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Gen 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 81. Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, Nomura N, Lin SH, Vandewalle A, Ohta E, Sasaki S, Uchida S. Acute insulin stimulation induces phosphorylation of the Na-Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS One 6: e24277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290: F1055–F1064, 2006 [DOI] [PubMed] [Google Scholar]
- 83. Song L, Mercado A, Vazquez N, Xie Q, Desai R, George AL, Gamba G, Mount DB. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Brain Res Mol Brain Res 103: 91–105, 2002 [DOI] [PubMed] [Google Scholar]
- 84. Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Talati G, Ohta A, Rai T, Sohara E, Naito S, Vandewalle A, Sasaki S, Uchida S. Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem Biophys Res Comm 393: 844–848, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol 293: F974–F984, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011 [DOI] [PubMed] [Google Scholar]
- 90. Varshavsky A. The ubiquitin system, an immense realm. Annu Rev Biochem 81: 167–176, 2012 [DOI] [PubMed] [Google Scholar]
- 91. Velazquez H, Bartiss A, Bernstein P, Ellison DH. Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 270: F211–F219, 1996 [DOI] [PubMed] [Google Scholar]
- 92. Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, Alessi DR. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J 397: 223–231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117: 3403–3411, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type ii: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 100. Yue P, Sun P, Lin DH, Pan C, Xing W, Wang W. Angiotensin II diminishes the effect of SGK1 on the WNK4-mediated inhibition of ROMK1 channels. Kidney Int 79: 423–431, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21: 82–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]